Introduction
Radiofrequency ablation (RFA) has been validated as
a safe and efficient treatment for hepatocellular carcinoma (HCC)
and focal metastatic liver tumors in patients who are not
candidates for hepatic resection or who refuse surgery (1). However, clinical studies have identified
a high rate of unsuccessful RFA treatment for large tumors
(diameter >3 cm) and also for local tumors with a high
recurrence rate (2). A typical lesion
created by a linear RFA ablation probe may be modeled as a
three-dimensional spheroid, which has one major and two minor axes,
with the major axis lying parallel to the probe shaft. The extent
of the long axis of the lesion may be controlled by selecting
different lengths of electrodes, but controlling the short axis
remains an issue that limits the success of ablation. A number of
strategies currently exist for overcoming this limitation and
increasing the ablated volume. Firstly, the electrode surface is
enhanced by increasing the probe gauge (3), using several applicators in a cluster
(4), a multiprobe array (5) and expandable applicators (6). Secondly, the power output may be
increased and combined with strategies for reducing probe heating,
such as pulsing the energy application (7). Thirdly, energy application may be
affected at the interface between the applicator and tissue by
preventing tissue dehydration and carbonization, using actively
cooled applicators with closed circuit water cooling (8), perfusion (9) or cryogenic cooling (10). Studies have demonstrated that saline
or hypertonic saline (HS)-enhanced bipolar RFA creates larger
lesions compared with monopolar RFA, due to the high electrical
conductivity of saline (11–15). However, studies assessing the
association between the concentration of the perfused NaCl solution
and the diameter of the coagulation necrosis caused by ablation are
scarce (16). There has been little
comparative data to validate these results on tissue coagulation.
Concurrently, there is a large clinical demand to improve RFA
efficacy to create larger coagulation necrosis areas (17,18).
In the present study, increasing the electrode
surface (multiprobe array) and continuous infusion of electrical
conductivity fluid during RFA were combined to enlarge the ablation
volume. The sizes, shapes and pathology data of the ablated lesions
created by an internally cool-tip electrode multipolar RFA
applicator with a perfused HS-augmented and normal saline
(NS)-augmented needles, and conventional internally cool-tip
electrode multipolar RF, were compared. The results of the present
study demonstrated that using perfused hypertonic-saline-augmented
needle combined with the RFA instrument may enlarge the ablation
zones and improve the efficacy for treatment of large tumors.
Materials and methods
RFA system
An RF generator (Radionics Inc., Burlington, MA,
USA) capable of producing 200 W was used with a 21-gauge needle
(21Gx200 mm; Hakko Co., Ltd., Nagano, Japan) with a 3-cm
exposed-tip internally water-cooled electrode [multipolar (three
applicators) Cool-tip™ RFA Cluster Electrode kit;
Covidien IIc, Mansfield, MA, USA] (Fig.
1). Needle cooling was achieved by a continuous internal
perfusion of the applicator (Microinfusion pump WZS-50F2; Zhejiang
Medical University Equipment Co., Ltd, Hangzhou, China). A
mechanical pump (Radionics Cool-tip RF system; Radionics Inc.) was
used to perfuse the electrode with 0°C saline solution at a rate of
110 ml/min in a closed perfusion circuit prior to each ablation, in
order to cool the electrode tip and reduce the charring of the
surrounding tissue that may decrease tissue conductivity and block
RF energy. The generator was used in impedance control mode
delivering a pulsed RF application. RF was applied to each
electrode for 15 min under 50-W conditions. A total of 3 ablation
zones were created under each condition. A total of 18 ablations
were performed. In the experiments of the present study, in case of
a fast increase of impedance, if the tissue impedance rose 10 Ω
beyond the baseline value (60 Ω), the current switched off
automatically for 15 sec. Auto-regulation of the generator was
current-based and impedance-controlled; full impedance was defined
as the point when the generator automatically sensed that the
resistance was too high, and power was no longer deposited
efficiently in the tissues. At this point, the generator was turned
off. Following a pause of 15 sec, the system was switched on
again.
RFA protocol
Conventional RFA group
Lesions were created in fresh (within 1 h of
mortality) room-temperature swine livers obtained from the local
butcher. The room-temperature livers were positioned on a
10-cm2 grounding pad that was placed at least 30 cm away
from the electrode. The electrodes were placed 3 cm into the
livers.
Perfused NS and HS-augmented RFA groups
The experiments were performed in the same manner as
the conventional RFA group, with the exception of a 21-gauge open
perfused electrode located in the middle of the needle being placed
into the porcine livers; the NaCl solution was instilled into the
tissue through the electrode, with an injection rate of 60 ml/h.
The concentrations of the NaCl solutions perfused were 0.9 and 6%
in the NS and HS-augmented RFA groups, respectively (16).
Evaluating the size and shape of ablation
lesions
The dimensions of the ablation zones were compared
among the groups. The ablated lesions exhibited an elliptical shape
and were well-demarcated within the normal liver tissue. The
ablated lesions were evaluated macroscopically and histologically.
Dimensions of the lesions were compared among the conventional-RFA,
0.9% NS-RFA and 6% HS-augmented-RFA groups. Each post-RF ablation
coagulation specimen was cut along the long axis of the coagulation
(ablation lesion along the axis of the electrode or needle
insertion); therefore, only the length and one diameter (90° to the
electrode or needle) measurement were utilized and measured with a
ruler by two investigators who reached consensus on each dimension.
The length and each diameter of the white central zone of necrosis
were measured with a ruler to within 1 mm. It was not possible to
measure the other diameter. However, in a pre-experiment, a
separate section of tissue along the other axis was measured, and
it was identified that the diameters were usually symmetrical in
this ex vivo experiment. Therefore, the single diameter was
used twice in volume calculations. The volume of the ablation
lesion was calculated by approximating it to an ellipsoid using the
following formula: V=1/6 × π × LD × TD2, where V is
volume, π is the circumference of a circle divided by its diameter,
LD is longitudinal diameter and TD is transverse diameter (9,18). The
representative tissue specimens of each ablation zone containing
the central area of necrosis and the transition zone towards the
healthy liver tissue were selected. Representative tissue was
maintained in 10% formaldehyde solution at 28°C for 24 h. Following
dehydration with 70% xylene and 100% absolute ethanol (1:1), tissue
was embedded in paraffin for 130 min at 58°C. Histological features
were assessed from 4-µm thick slides with hematoxylin and eosin
(H&E) staining (1% H&E, 25 min, 26°C) under light
microscopy (magnifications, ×40, ×200 and ×400).
Statistical analysis
Data are presented as the mean ± standard deviation.
Ablation lesion dimensions were compared among conventional RFA,
NS-RFA and 6% perfused HS-augmented RFA with the Mann Whitney
U-test with Bonferroni correction for several independent samples
using SPSS 15 (SPSS Inc., Chicago, IL, USA). A two-sided P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
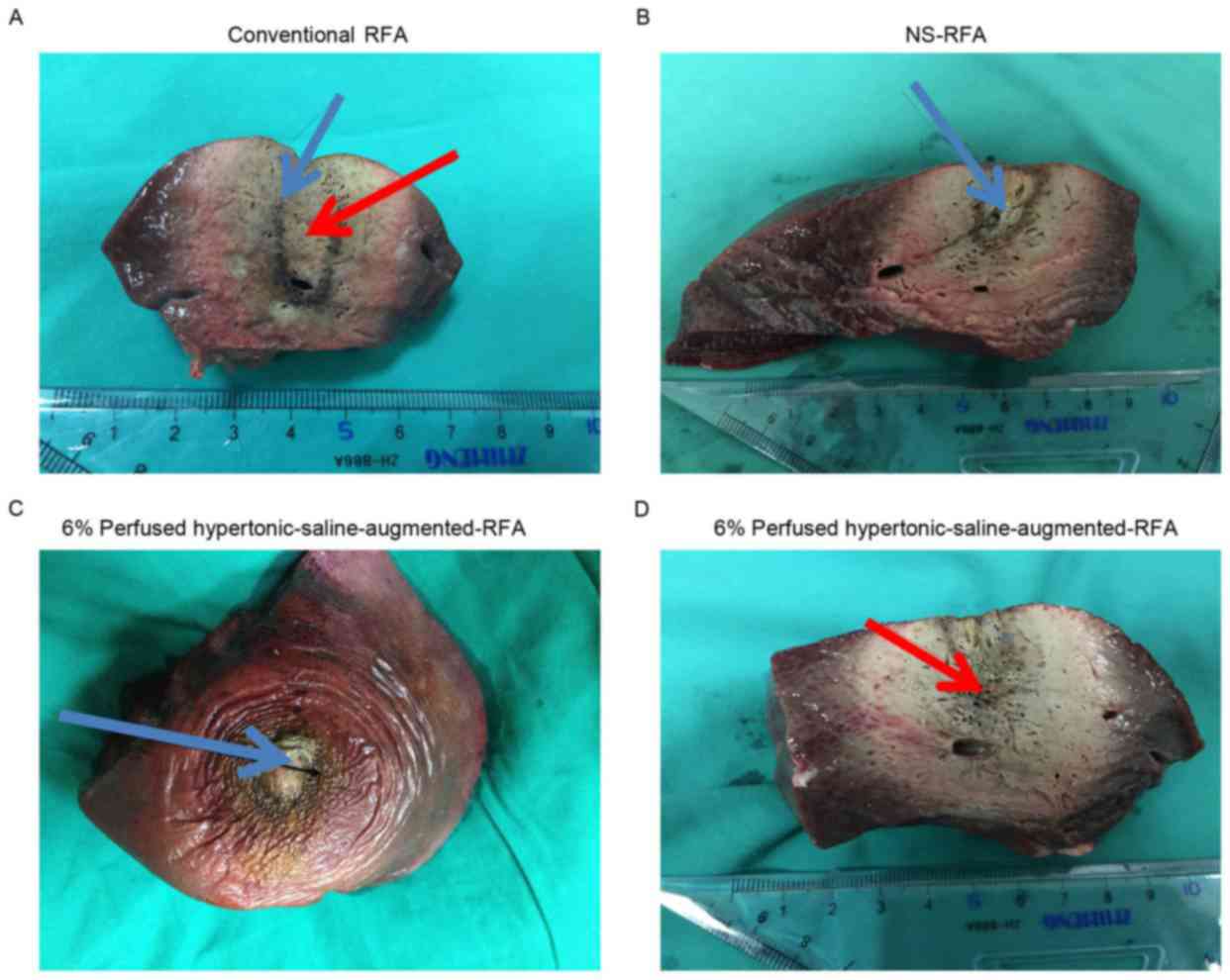
Macroscopic data
All procedures were successful, and 18 lesions were
created by conventional-RFA, NS-RFA and 6% perfused HS-augmented
RFA. Subsequent to slicing, all ablation zones macroscopically
demonstrated a black-brown-yellow appearance in their center
corresponding to the necrotic ablation, clearly demarcated by a red
hyperemic rim (Fig. 2).
The conventional-RFA lesions appeared elliptical in
shape and were well-demarcated within the normal liver tissue.
There were central cavities representing the needle tract
surrounded by pale coagulation necrosis, and the outer coagulation
necrosis appeared as a brownish color (Fig. 2A).
The NS-RFA lesions were approximately elliptical in
shape and were in well-demarcated areas within the liver tissue.
The post-RFA liver surface has four poles. The outer three poles
corresponded to the RFA probes and the middle pole was used to
inject 0.9% NaCl liquid. The central zone, whose diameter was
slightly larger compared with the channel, was a hollow space
created by the vaporization of tissue and withdrawal of the
channel. Beyond the central area was a region of brown coagulated
tissues, and reddish tissue was in the outermost region of the
lesion, which was bordered by normal liver tissue (Fig. 2B).
By contrast, 6% perfused HS-augmented RFA lesions
were also elliptical in shape, but the central portion was
jelly-like, and the track of the RF electrode was clearer.
Surrounding the jelly-like tissue was off-white or dust-colored
coagulation necrosis, and beyond this was an area of reddish
coagulated tissues that was bordered by normal liver tissue
(Fig. 2C and D).
Sizes of ablation lesions created by
conventional RFA, NS-RFA and HS-augmented-RFA
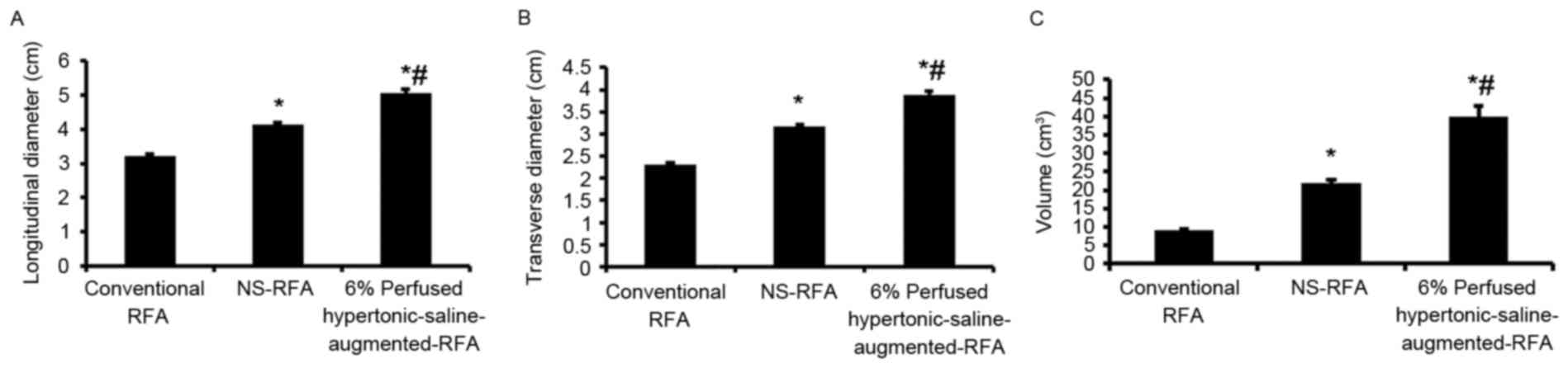
Ablation lesions (Table
I) created by 6% perfused HS-augmented RFA were significantly
larger compared with those created by conventional RFA or NS-RFA
(P<0.05) (Fig. 3). Oval-shaped
ablation zones were created with a larger transverse diameter at
the midpoint compared with either the conventional or NS-RF
application. Specifically, the mean volumes (Fig. 3C) of 8.99±0.52, 21.79±1.05 and
40.01±2.86 cm3 were obtained for ablation lesions
created by conventional RFA, NS-RFA and HS-augmented-RFA,
respectively. Concordantly, longitudinal (Fig. 3A) and transverse (Fig. 3B) diameters were significantly
different in the three treatments groups (conventional RFA,
3.22±0.06 and 2.31±0.04 cm, respectively; NS-RFA, 4.13±0.08 and
3.17±0.05 cm, respectively; HS-augmented-RFA, 5.05±0.12 and
3.89±0.09 cm, respectively).
 | Table I.Dimensions and volume of ablation
lesions created in excised porcine livers by conventional RFA,
NS-RFA and 6% perfused hypertonic-saline-augmented-RFA. |
Table I.
Dimensions and volume of ablation
lesions created in excised porcine livers by conventional RFA,
NS-RFA and 6% perfused hypertonic-saline-augmented-RFA.
| Dimension | Conventional RFA | NS-RFA | 6% Perfused
HS-augmented-RFA | P-value |
|---|
| Longitudinal
diameter, cm |
3.22±0.06 |
4.13±0.08 |
5.05±0.122 | 0.006 |
| Transverse diameter,
cm |
2.31±0.04 |
3.17±0.05 |
3.89±0.09 | 0.003 |
| Volume,
cm3 |
8.99±0.52 |
21.79±1.05 |
40.01±2.86 |
0.0002 |
Histopathological data
Microscopically, the conventional-RFA lesions
demonstrated centralized, complete destruction of the parenchyma.
Coagulation necrosis created by conventional RFA revealed a zone of
altered cellular morphology, which was best characterized as a heat
effect that consisted of degenerated shrunken hepatocytes with
pyknotic nuclei of the liver tissue. This region did not meet the
classical criteria of coagulation necrosis defined by previous
studies (19–21), but the cells were clearly different
from normal hepatocytes. There was no marked difference between
ablated lesions and normal liver tissue (Fig. 4A).
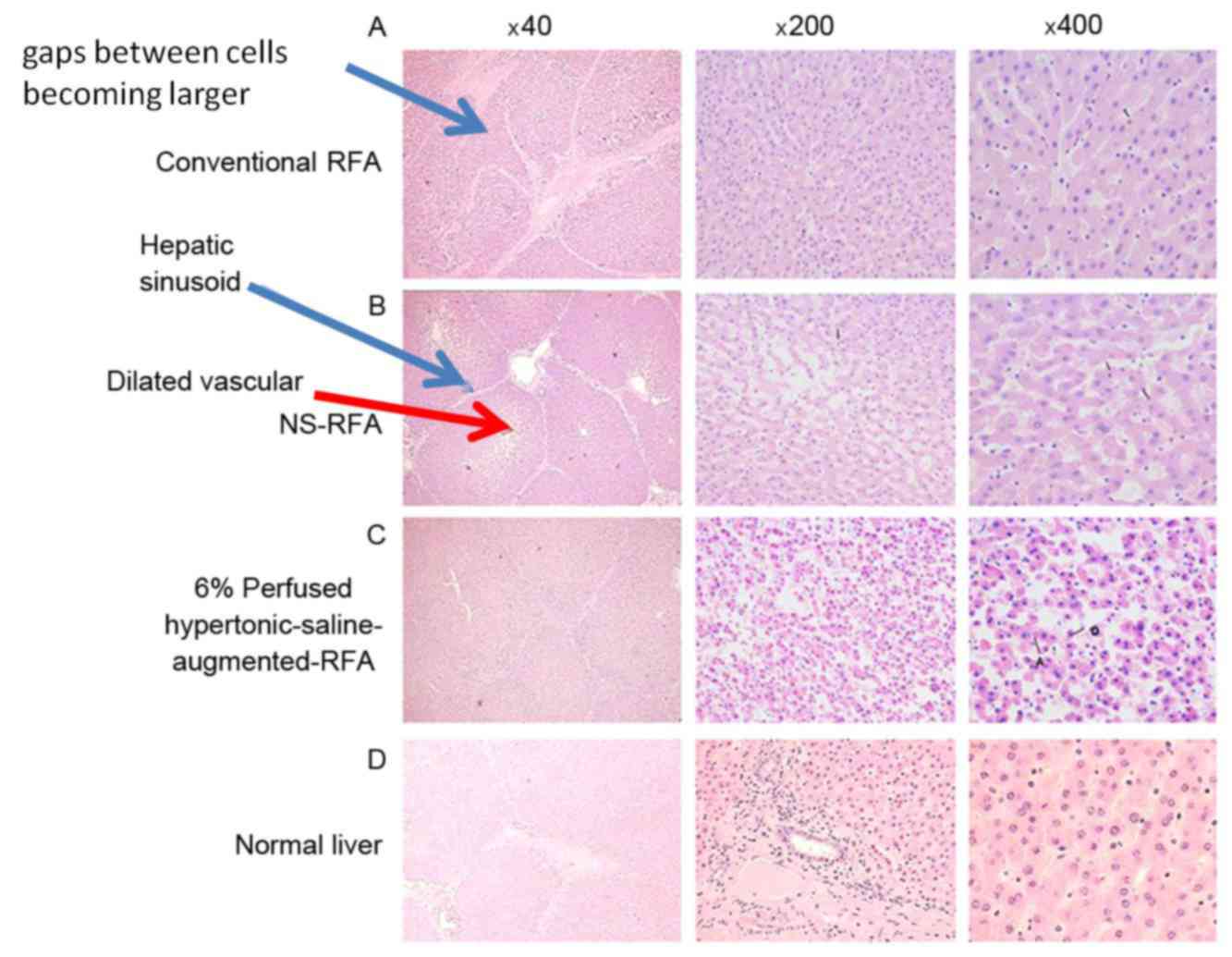 | Figure 4.Micrographs of the coagulated ablation
lesions created by conventional RFA, NS-RFA and 6% perfused
hypertonic-saline-augmented-RFA. (A) Left panel (H&E;
magnification, ×40) demonstrates relatively sparse hepatocytes,
with gaps between cells becoming larger. Liver borders and hepatic
sinusoid remained normal, with dilated vessels. Central (H&E;
magnification, ×200) and right (H&E; magnification, ×400)
panels indicate coagulation necrosis and degenerated shrunken
hepatocytes with pyknotic nuclei. (B) Left panel (H&E;
magnification, ×40), morphology was slightly altered but normal
cells were observed, similar to the control cells. Liver borders
were ruptured, with hepatic sinusoid becoming sparse (blue arrow),
larger gaps between cells and dilated vessels (red arrow). Central
(H&E; magnification, ×200) and right (H&E; magnification,
×400) panels demonstrate more degenerated shrunken hepatocytes
appearing with pyknotic nuclei compared with that demonstrated by
the conventional and NA-RFA groups. Inflammatory cells and focal
infiltration were observed. Red blood cells were also observed in
the hepatic sinusoid. (C) Left panel (H&E; magnification, ×40)
demonstrates damage to or disappearance of the structure of the
liver borders and hepatic sinusoid. Hepatocytes are arranged in a
disorderly manner. Central (H&E; magnification, ×200) and right
(H&E; magnification, ×400) panels demonstrate the majority of
hepatocytes becoming necrotic, deformed or ruptured. Apoptosis of
hepatocytes was observed. Degenerated shrunken hepatocytes appeared
with pyknotic nuclei and cytoplasmic condensation. Hepatocyte
spacing was not evident. (D) Micrographs of the normal liver
(H&E; magnifications, ×40, ×200 and ×400, respectively). NS,
normal saline; RFA, radiofrequency ablation; H&E, hematoxylin
and eosin; NA-RFA, 0.9% saline-enhanced RFA group. |
The coagulation lesions created by NS-RFA were
similar to those of conventional RFA, but demonstrated a small
blank area (central charred zone) where tissue was necrotic.
Surrounding the central portion was coagulation necrosis and a
peripheral hemorrhagic rim. Coagulation necrosis created by NS-RFA
also appeared as irregular, degenerated and shrunken hepatic cells
with pyknotic nuclei. However, the extent of hepatocyte damage in
the lesions induced by NS-RFA was more substantial compared with
the lesions produced by conventional RFA, and there was a marked
difference between ablated lesions and normal liver tissue
(Fig. 4B).
By contrast, the damage of liver cells created by 6%
perfused HS-augmented-RFA was more severe compared with that
induced by conventional RFA or NS-RFA. The central jelly-like
tissue appeared as a homogeneous red dye-like substance, and
coagulation necrosis in the outer layer of 6% perfused
HS-augmented-RFA lesions was similar to that in NS-RFA lesions. A
marked difference was observed between the ablated lesions and
normal liver tissue (Fig. 4C). The
normal liver H&E staining images were used as controls
(Fig. 4D).
Discussion
In the present study, multipolar RFA with 6% NaCl
demonstrated improved efficacy in creating a larger ablation zone
compared with the conventional and 0.9% NaCl modes.
In total, 70–85% of patients with liver cancer are
not candidates for surgery (19–21). RFA
destroys biological tissues with electromagnetic waves at
frequencies between 460 and 500 kHz, which is a high enough range
to result in molecular frictional heating without stimulating a
neuromuscular reaction and electrolysis, and which is low enough to
confine the transmission of energy to a tissue mass in a more
controlled manner, without excessive radiation being created
(22,23).
The major limitation of this approach is reaching an
acceptable coagulation size (24).
Also, abutting large blood vessels (≥3 mm) reduces the
effectiveness of the heat produced by RF due to perfusion-mediated
tissue cooling within the area to be ablated, which consequently
lowers RFA efficacy (25). The
development of a number of strategies has improved tissue-energy
interactions for thermal ablation therapy by increasing the region
of induced coagulation (11,13,26). One
of the most effective approaches is the injection of saline or HS
into the tissue during RFA via an electrode, which increases
electrical conductance and thermal conductivity. The greater saline
conductivity confers less resistive heating around the electrode
tip (11,26).
Lee et al (27)
suggested that bipolar RFA resulted in larger, short-axis diameters
of coagulation necrosis with 6% NaCl solution compared with those
created with 0.9% NaCl solution. However, the results of the
present study demonstrated that NaCl solution concentrations of
>6% did not additionally increase the extent of coagulation
necrosis. In addition, 6% NaCl administered at a rate of 1.0 ml/min
in bipolar RFA yielded larger necrosis diameters compared with
values obtained with 0.5 ml/min (data not shown). In the present
study, NaCl solution was introduced into the area of ablation
during RFA using three-polar perfusion electrodes to enlarge the
ablation volume, which achieved safe thermal ablation. The results
demonstrated that 6% perfused HS-augmented-RFA lesions were
significantly larger compared with the 0.9% NS-RFA or conventional
RFA lesions. The most important factor that may explain this
difference is that the ionic concentration of 6% perfused
HS-augmented-RFA is much higher compared with that of NS (0.9%),
and therefore, the perfusion of the solution of NaCl during 6%
perfused HS-augmented-RFA potentially increased the electrical
conductivity of the tissue. Therefore, compared with NS-RFA, 6%
perfused HS-augmented-RFA may have created increased molecular
friction, which could generate more energy and enlarge the ablated
volume. In the pre-experiment of the present study, it was observed
that NaCl concentrations >6% do not enlarge the ablated lesions.
This confirms the results identified by Lee et al (27). Although it has been demonstrated that
hydrochloric acid RFA may be used to produce a larger ablated
lesion size, the safety of diluted hydrochloric acid remains a
concern (27). Solutions of 6%
perfused HS-augmented NaCl are much safer compared with using
diluted hydrochloric acid, and NaCl does not require additional
costs or medical fees.
Several studies have indicated that following RFA,
the tissue appears to be almost unchanged microscopically (23,28).
Thermal coagulation necrosis induced by RFA differs from classic
coagulation necrosis in that cells retain their original shape and
the nuclei do not change, which makes it difficult to visualize by
H&E staining. In fact, these results were described in the
literature as a ‘ghost phenomenon’ and cells exhibiting thermal
coagulation necrosis were referred to as ‘ghost cells’ (23,29–31).
Nicotinamide adenine dinucleotide (NADH) vital staining may be used
to identify the viability of hepatocytes with normal morphology
(23,31,32).
However, NADH vital staining is more complicated than H&E
staining. In the present study, using H&E staining with careful
observation, it was identified that the majority of hepatocytes
killed by NS-RFA appeared as coagulation necrosis microscopically.
The results also demonstrated that 6% perfused HS-augmented-RFA
produced more evident coagulation necrosis compared with NS-RFA.
The coagulation necrosis created with 6% perfused HS-augmented-RFA
was easily observed using H&E staining, and therefore, NADH
vital staining was not performed to identify cell viability.
A theoretical, yet unevaluated, oncological concern
regarding the perfusion electrodes used in RFA is leakage from the
electrode track of saline that is contaminated with viable tumor
cells, resulting in peritoneal or track seeding. An additional
uninvestigated concern is that the saline may increase the
intra-tumoral pressure and force tumor cells into the circulation,
thus resulting in lymphatic or hematogenous seeding (23,33,34).
However, 6% perfused HS-augmented-RFA may reduce these risks, as
tumor cells cannot survive easily in this high concentration
liquid; however, this requires additional confirmation.
Limitations of the present study include the fact
that normal tissues in ex vivo porcine livers, and not in
vivo or metastatic HCC tumor tissues, were used. Therefore, the
distribution of infused liquid (NS or 6% perfused HS-augmented)
differed due to the different tissue architecture. Also, without
the cooling effect of blood flow, the ablated volume in ex
vivo tissues may be larger compared with that produced in in
vivo tissue. Additionally, other parameters of RFA treatment
efficacy, such as tumor number and location, body tolerance to the
procedure and associated complications (e.g., liver failure), were
not assessed in the present ex vivo study. Therefore, the
results are preliminary and provide a reference for future
investigations. Although 6% perfused HS-augmented is safe and
efficient when it is used in ablation, the safety of 6% perfused
HS-augmented requires additional investigation, which will be
addressed in a future study.
In conclusion, the present study demonstrated that
6% perfused HS-augmented-RFA creates larger ablation zones compared
with either the conventional method or NS-RF application. These
data suggest that 6% perfused HS-augmented-RFA may be superior to
conventional or NS-RF applications, with the potential to improve
the results of RFA for the treatment of larger tumors.
References
|
1
|
Ikeda K, Kobayashi M, Kawamura Y, Imai N,
Seko Y, Hirakawa M, Hosaka T, Sezaki H, Akuta N, Saitoh S, et al:
Stage progression of small hepatocellular carcinoma after radical
therapy: Comparisons of radiofrequency ablation and surgery using
the Markov model. Liver Int. 31:692–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Duijnhoven FH, Jansen MC, Junggeburt
JM, van Hillegersberg R, Rijken AM, van Coevorden F, van der Sijp
JR, van Gulik TM, Slooter GD, Klaase JM, et al: Factors influencing
the local failure rate of radiofrequency ablation of colorectal
liver metastases. Ann Surg Oncol. 13:651–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berber E and Siperstein A: Local
recurrence after laparoscopic radiofrequency ablation of liver
tumors: An analysis of 1032 tumors. Ann Surg Oncol. 15:2757–2764.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldberg SN, Gazelle GS, Dawson SL,
Rittman WJ, Mueller PR and Rosenthal DI: Tissue ablation with
radiofrequency using multiprobe arrays. Acad Radiol. 2:670–674.
1995.PubMed/NCBI
|
|
5
|
Goldberg SN, Solbiati L, Hahn PF, Cosman
E, Conrad JE, Fogle R and Gazelle GS: Large-volume tissue ablation
with radio frequency by using a clustered, internally cooled
electrode technique: Laboratory and clinical experience in liver
metastases. Radiology. 209:371–379. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldberg SN, Gazelle GS, Dawson SL,
Rittman WJ, Mueller PR and Rosenthal DI: Tissue ablation with
radiofrequency: Effect of probe size, gauge, duration, and
temperature on lesion volume. Acad Radiol. 2:399–404. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Baere T, Denys A, Wood BJ, Lassau N,
Kardache M, Vilgrain V, Menu Y and Roche A: Radiofrequency liver
ablation: Experimental comparative study of water-cooled versus
expandable systems. AJR Am J Roentgenol. 176:187–192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solazzo SA, Ahmed M, Liu Z, Hines-Peralta
AU and Goldberg SN: High-power generator for radiofrequency
ablation: Larger electrodes and pulsing algorithms in bovine ex
vivo and porcine in vivo settings. Radiology. 242:743–750. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lorentzen T: A cooled needle electrode for
radiofrequency tissue ablation: Thermodynamic aspects of improved
performance compared with conventional needle design. Acad Radiol.
3:556–563. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo RG, Gao F, Gu YK, Huang JH and Li CL:
Radioablation settings affecting the size of lesions created ex
vivo in porcine livers with monopolar perfusion electrodes. Acad
Radiol. 17:980–984. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rempp H, Voigtländer M, Clasen S, Kempf S,
Neugebauer A, Schraml C, Schmidt D, Claussen CD, Enderle MD,
Goldberg SN and Pereira PL: Increased ablation zones using a
cryo-based internally cooled bipolar RF applicator in ex vivo
bovine liver. Invest Radiol. 44:763–768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burdio F, Güemes A, Burdío JM, Navarro A,
Sousa R, Castiella T, Cruz I, Burzaco O, Guirao X and Lozano R:
Large hepatic ablation with bipolar saline-enhanced radiofrequency:
An experimental study in in vivo porcine liver with a novel
approach. J Surg Res. 110:193–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gangi A, Guth S and Imbert J: Interest of
radiofrequency liver tissue ablation with a bipolar-wet electrode.
Eur Radiol. 133:4772003.
|
|
14
|
Lee JM, Han JK, Kim SH, Sohn KL, Lee KH,
Ah SK and Choi BI: A comparative experimental study of the in-vitro
efficiency of hypertonic saline-enhanced hepatic bipolar and
monopolar radiofrequency ablation. Korean J Radiol. 4:163–169.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt D, Trübenbach J, Brieger J, Koenig
C, Putzhammer H, Duda SH, Claussen CD and Pereira PL: Automated
saline-enhanced radiofrequency thermal ablation: Initial results in
ex vivo bovine livers. AJR Am J Roentgenol. 180:163–165. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hänsler J, Frieser M, Schaber S, Kutschall
C, Bernatik T, Müller W, Becker D, Hahn EG and Strobel D:
Radiofrequency ablation of hepatocellular carcinoma with a saline
solution perfusion device: A pilot study. J Vasc Interv Radiol.
14:575–580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JM, Kim SH, Han JK, Sohn KL and Choi
BI: Ex vivo experiment of saline-enhanced hepatic bipolar
radiofrequency ablation with a perfused needle electrode:
Comparison with conventional monopolar and simultaneous monopolar
modes. Cardiovasc Intervent Radiol. 28:338–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seong NJ, Yoon CJ, Kang SG, Chung JW, Kim
HC and Park JH: Effects of arsenic trioxide on radiofrequency
ablation of VX2 liver tumor: Intraarterial versus intravenous
administration. Korean J Radiol. 13:195–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McWilliams JP, Yamamoto S, Raman SS, Loh
CT, Lee EW, Liu DM and Kee ST: Percutaneous ablation of
hepatocellular carcinoma: Current status. J Vasc Interv Radiol.
21(8 Suppl): S204–S213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhim H, Choi D, Kim YS, Lim HK and Choe
BK: Ultrasonography-guided percutaneous radiofrequency ablation of
hepatocellular carcinomas: A feasibility scoring system for
planning sonography. Eur J Radiol. 75:253–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garrean S, Hering J, Saied A, Helton WS
and Espat NJ: Radiofrequency ablation of primary and metastatic
liver tumors: A critical review of the literature. Am J Surg.
195:508–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiina S: Image-guided percutaneous
ablation therapies for hepatocellular carcinoma. J Gastroenterol.
44 Suppl 19:S122–S131. 2009. View Article : Google Scholar
|
|
23
|
Goldberg S Nahum and Dupuy DE:
Image-guided radiofrequency tumor ablation: Challenges and
opportunities-part I. J Vasc Interv Radiol. 12:1021–1032. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni Y, Mulier S, Miao Y, Michel L and
Marchal G: A review of the general aspects of radiofrequency
ablation. Abdom Imaging. 30:381–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruners P, Pfeffer J, Kazim RM, Günther
RW, Schmitz-Rode T and Mahnken AH: A newly developed perfused
umbrella electrode for radiofrequency ablation: An ex vivo
evaluation study in bovine liver. Cardiovasc Intervent Radiol.
30:992–998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamaki K, Shimizu I, Oshio A, Fukuno H,
Inoue H, Tsutsui A, Shibata H, Sano N and Ito S: Influence of large
intrahepatic blood vessels on the gross and histological
characteristics of lesions produced by radiofrequency ablation in a
pig liver model. Liver Int. 24:696–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JM, Kim YK, Lee YH, Kim SW, Li CA and
Kim CS: Percutaneous radiofrequency thermal ablation with
hypertonic saline injection: In vivo study in a rabbit liver model.
Korean J Radiol. 4:27–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo RG, Fao F, Huang JH, Gu YK, Jiang XY
and Huang YJ: Diluted hydrochloric acid generates larger
radiofrequency ablation lesions in excised porcine livers. Diagn
Interv Radiol. 19:145–149. 2013.PubMed/NCBI
|
|
29
|
Miao Y, Ni Y, Mulier S, Yu J, De Wever I,
Penninckx F, Baert AL and Marchal G: Treatment of VX2 liver tumor
in rabbits with ‘wet’ electrode mediated radio-frequency ablation.
Eur Radiol. 10:188–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldberg SN, Girnan GD, Lukyanov AN, Ahmed
M, Monsky WL, Gazelle GS, Huertas JC, Stuart KE, Jacobs T,
Torchillin VP, et al: Percutaneous tumor ablation: Increased
necrosis with combined radio-frequency ablation and intravenous
liposomal doxorubicin in a rat breast tumor model. Radiology.
222:797–804. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hänsler J, Neureiter D, Wasserburger M,
Janka R, Bernatik T, Schneider T, Müller W, Frieser M, Schaber S,
Becker D, et al: Percutaneous US-guided radiofrequency ablation
with perfused needle applicators: Improved survival with the VX2
tumor model in rabbits. Radiology. 230:169–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen P, Geisinger KR, Zagoria R and Levine
EA: Pathologic correlation study of microwave coagulation therapy
for hepatic malignancies using a three-ring probe. J Gastrointest
Surg. 11:603–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simon CJ, Dupuy DE, Iannitti DA, Lu DS, Yu
NC, Aswad BI, Busuttil RW and Lassman C: Intraoperative triple
antenna hepatic microwave ablation. AJR Am J Roentgenol.
187:W333–W340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ni Y, Miao Y, Mulier S, Yu J, Baert AL and
Marchal G: A novel ‘cooled-wet’ electrode for radiofrequency
ablation. Eur Radiol. 10:852–854. 2000. View Article : Google Scholar : PubMed/NCBI
|