Introduction
Malignant mesothelioma is an asbestos-associated
pleural malignancy that arises from serosal cells and presents a
poor prognosis. Statistical surveys conducted by the Japanese
Ministry of Health, Labor and Welfare reported that 1,410
fatalities were caused by mesothelioma in 2013 in Japan, double the
number of fatalities in 1999 (www.mhlw.go.jp/toukei/saikin/hw/jinkou/tokusyu/chuuhisyu15/dl/chuuhisyu.pdf).
Although therapies including surgery, chemotherapy and radiotherapy
have been adopted, the prognosis remains poor. Since the
combination of an anti-folate reagent, pemetrexed, and cisplatin
was demonstrated to prolong survival for longer than cisplatin
alone (1), this combination has been
frequently used, but its effects remain limited. Second-line
therapy for tumor recurrence has not been established. Newer
therapies based on an improved molecular understanding of
mesothelioma are therefore required.
Wnt signaling, which activates a canonical pathway
through β-catenin, is aberrantly activated in a wide range of
tumors (2). Numerous types of tumor,
including colon cancer, undergo aberrant activation of this
canonical pathway and Wnt may also activate non-canonical pathways
(2). Our previous study identified
that Wnt signaling is activated in mesothelioma cells, and that
blockade of Wnt signaling may be achieved with antibodies against
Wnt-1 or −2, Wnt-1 or −2 small interfering RNAs (siRNAs), or
dominant-negative dishevelled (Dvl), leading to suppressed
viability or tumorigenesis of mesothelioma cells in athymic mice
(3–7).
Notably, the mesothelioma H28 cell line exhibits Wnt signal
activity, which blocks apoptosis, without expression of β-catenin,
due to a homozygous deletion of the β-catenin gene and activation
of the aforementioned non-canonical pathways (8). Wnt signaling has been revealed to have a
crucial function in maintaining cancer stem cells, which are highly
resistant to chemotherapy (9).
The epidermal growth factor receptor (EGFR)-tyrosine
kinase inhibitors (TKIs) gefitinib, erlotinib and afatinib are
promising anticancer drugs for the treatment of patients with
non-small cell lung cancer (NSCLC) with a specific EGFR mutation
(10). EGFR has been revealed to be
expressed in 68% of paraffin-embedded mesothelioma specimens
(11). However, EGFR-TKIs alone are
not effective in the treatment of mesothelioma (12). In NSCLC, tankyrase, an upregulator of
the canonical Wnt signaling pathway, has been revealed to protect
lung cancer cells from EGFR inhibition (13). Inhibition of β-catenin with EGFR-TKIs
was reported to be able to enhance the anticancer effect or to
overcome the resistance to EGFR-TKIs (14–16). In
the present study, the effect of suppression of Wnt signaling with
Dvl-3 siRNA and of inhibition of EGFR with gefitinib on
mesothelioma cell viability were investigated.
Materials and methods
Cell lines and cell culture
Mesothelioma NCI-H28 (H28), NCI-H2452 (H2452) and
MSTO-211H (211H) cell lines [American Type Culture Collection
(ATCC), Manassas, VA, USA] were cultured in RPMI-1640 complete
medium (ATCC) containing 10% fetal bovine serum (FBS; ATCC) in a 75
cm2 tissue culture flask in a 37°C 5% CO2
incubator for 3 days. Medium was changed every 3 days. Once cells
had reached 80% confluence, they were treated with 0.5%
trypsin/0.2% EDTA solution (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and were passaged 1:4.
Western blot analysis
Cells were removed by scraping, washed with TBS [25
mM Tris-HCl (pH 8.0) and 150 mM NaCl] containing 0.1 mM
phenylmethylsulfonyl fluoride and centrifuged at 1,000 × g for 5
min at room temperature. Cells were lysed using mammalian protein
extraction reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and were centrifuged at 10,000 × g for 5 min at 4°C. Protein
concentrations were determined using the Pierce™ BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). The supernatant was mixed
with an equal volume of 4% SDS-containing 10% 2-mercaptoethanol.
Whole cell lysate aliquots (20 µg) were separated on 4–15% gradient
SDS-polyacrylamide gels, subjected to 10% SDS-PAGE and were
electrotransferred onto an Immun-Blot™ polyvinylidene difluoride
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for
protein blotting. Blots were incubated overnight at 4°C with
antibodies against Dvl-3 (1:1,000; 4D3; cat. no. sc-8027; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), β-catenin (1:1,000;
cat. no. 610153; BD Biosciences, San Jose, CA, USA), EGFR (1:1,000;
cat. no. sc-03; Santa Cruz Biotechnology, Inc.), glycogen synthesis
kinase-3β (GSK3β) (1:5,000; cat. no. 9832; Cell Signaling
Technology, Inc., Danvers, MA, USA) or phosphorylated (p)-GSK3β
(Ser9) (1:5,000; cat. no. 5558; Cell Signaling
Technology, Inc.), and anti-β-actin (1:1,000; cat. no. sc-47778;
Santa Cruz Biotechnology, Inc.) was used as an antibody for the
reference protein. Antigen-antibody complexes were detected using
an enhanced chemiluminescence blotting analysis system (GE
Healthcare Life Sciences, Little Chalfont, UK).
Transfection with Dvl-3 siRNA
Dvl-3 siRNA (Stealth RNAi™) was prepared by
Invitrogen; Thermo Fisher Scientific, Inc. The sequence of the
siRNA and the methods of transfection have been described
previously (17). Briefly, cells were
seeded onto 35-mm dishes at 1×104 cells/dish, and were
transfected 24 h later with 4 pmol siRNA using 4 ml Lipofectamine™
2000 (Thermo Fisher Scientific, Inc.). Cells were then incubated
for 24 h at 37°C, washed once with PBS, then incubated with
RPMI-1640 medium containing 10% FBS at 37°C for >24 h for
western blot analysis and >14 days for colony formation assays
on 35-mm dishes, or seeded onto 96-well plates for cytotoxicity
assays.
Cytotoxicity assays
Cell viability was assessed using a modification of
the MTT assay using the Cell Counting Kit-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) containing
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
and monosodium salt (WST-8) dye. Cells were seeded onto 96-well
plates at 5×103 cells/well 24 h after transfection with
siRNA. Cell viability was estimated at 24, 48, 72 and 96 h after
plating. WST-8 dye was added 2 h prior to the end of culture and
absorbance was measured at 450 nm using a Multiskan JX instrument
(Thermo Fisher Scientific, Inc.). Experiments were performed ≥6
times. At 24 h after plating, cells were exposed to 5, 10 or 30 µM
gefitinib (Sigma-Aldrich; Merck KGaA) or dimethylsulfoxide (DMSO)
as a control, and cell viability was estimated in the
aforementioned manner. Gefitinib was dissolved in DMSO and controls
for all experiments were created by adding equivalent volumes of
DMSO. Each drug concentration was added to three replicate wells
and each experiment was performed 4 times.
Colony formation assays
After 24 h of siRNA transfection, 400 cells were
spread onto 35-mm dishes with RPMI-1640 medium and 10% FBS, with 5
µM gefitinib or DMSO as a control. After 14 days, cells were
stained with 0.5% methylene blue for 24 h at room temperature and
colonies were counted visually. Colony assays were performed ≥4
times and results are reported as the mean.
Cell cycle analysis
After 24 h of siRNA transfection, 5 µM gefitinib or
DMSO was added to the medium. A further 24 h later, cells were
collected and a CycleTest PLUS DNA Reagent kit (BD Biosciences, San
Jose, CA, USA) was used according to the manufacturer's protocol.
Cell cycle analysis was determined using a flow cytometer (BD
FACSVerse™; version 1.0.3.2942; BD FACSuite software; BD
Biosciences).
Statistical analysis
Results are expressed as the mean ± standard
deviation. Data between two groups were compared using a two-tailed
unpaired Student's t-test. Analysis of variance (ANOVA), followed
by Dunnett's test, was used to compare multiple groups. For
cytotoxicity assays comparing concentrations of gefitinib, cells
treated with DMSO were used as a control and the viability of other
cells were compared using ANOVA followed by Dunnett's test.
Viability of cells transfected with control siRNA was compared with
that of those transfected with Dvl-3 siRNA using a two-sided
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using a commercial statistical software package (version
21; SPSS Statistics; IBM Corp., Armonk, NY, USA).
Results
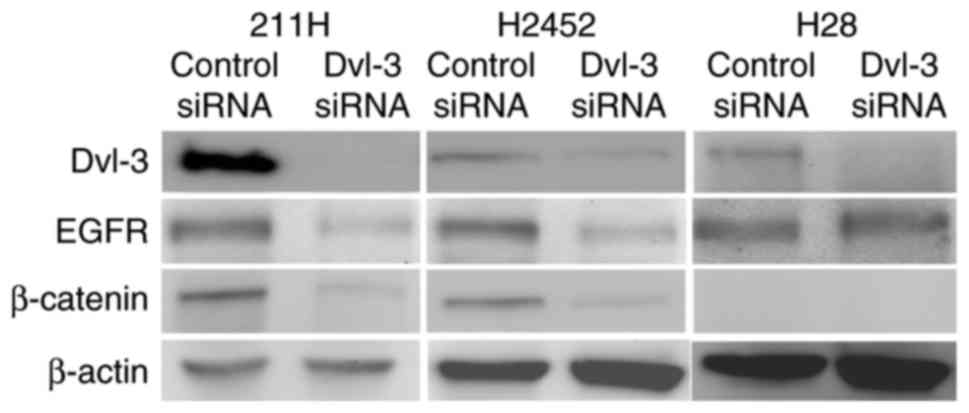
Dvl-3 siRNA downregulates expression
of Dvl-3 in mesothelioma cells
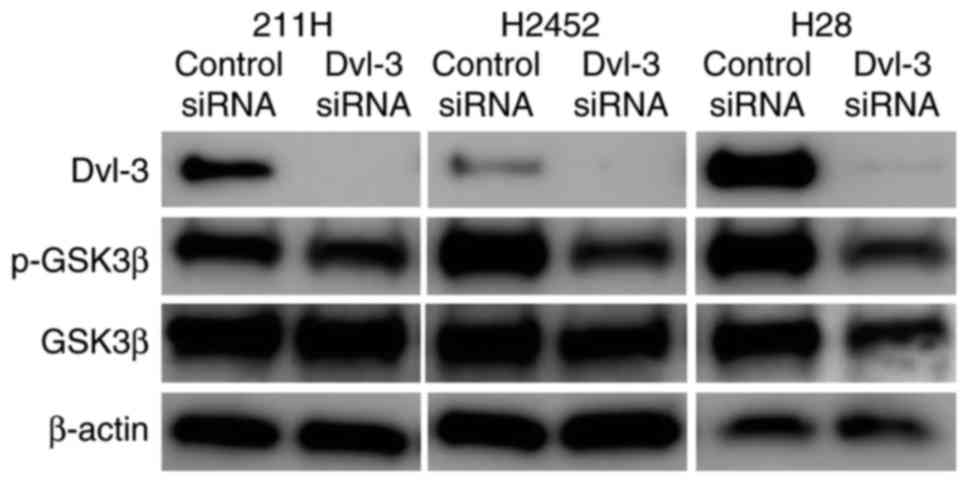
Mesothelioma 211H, H2452 and H28 cells express Dvl-3
and EGFR. Whereas H28 cells expressed no β-catenin due to a
homozygous deletion of the β-catenin gene, 211H and H2452 cells
did. At 48 h after transfection with Dvl-3 siRNA, expression of
Dvl-3 was downregulated in 211H, H2452 and H28 cells (Fig. 1).
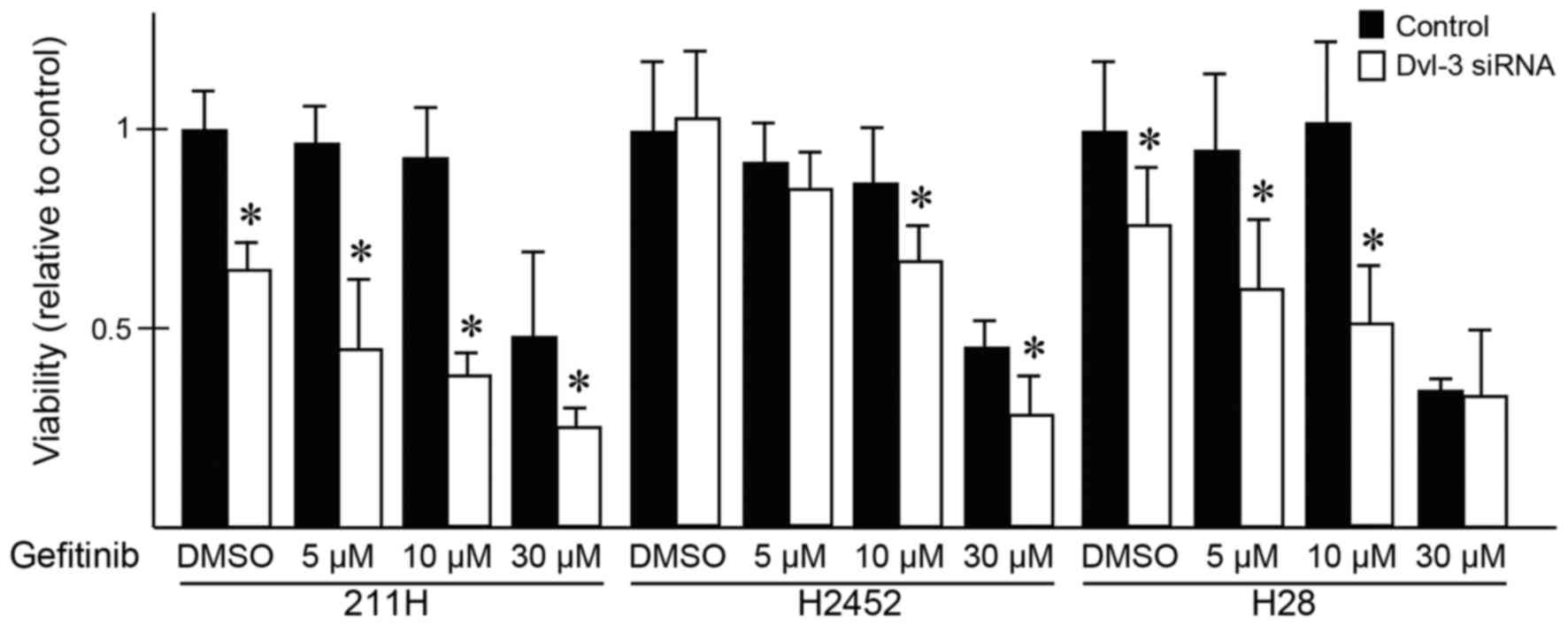
Downregulation of Dvl-3 and treatment
with gefitinib suppresses the viability of mesothelioma cells
synergistically
After 24 h of transfection with Dvl-3 siRNA or
control siRNA, gefitinib or DMSO was added to the medium. Following
transfection with Dvl-3 siRNA, the viability of H28 and 211H cells
was significantly suppressed compared with that of the cells
transfected with control siRNA, but the viability of H2452 cells
was not (Fig. 2). At 48 h after the
addition of gefitinib or DMSO, the viability of H28 cells
transfected with control siRNA was not suppressed in the presence
of 5 or 10 µM gefitinib; however, the viability of H28 cells
transfected with Dvl-3 siRNA was significantly suppressed following
treatment with these concentrations of gefitinib compared with
those treated with DMSO. The viability of 211H cells was
significantly suppressed following treatment with 30 µM gefitinib.
At 5, 10 or 30 µM gefitinib, the viability of 211H cells were
significantly suppressed synergistically with Dvl-3 siRNA
transfection compared with that of cells transfected with control
siRNA. In H2452 cells, 10 and 30 µM gefitinib significantly
suppressed the viability of cells transfected with Dvl-3 siRNA,
compared with that of those transfected with control siRNA.
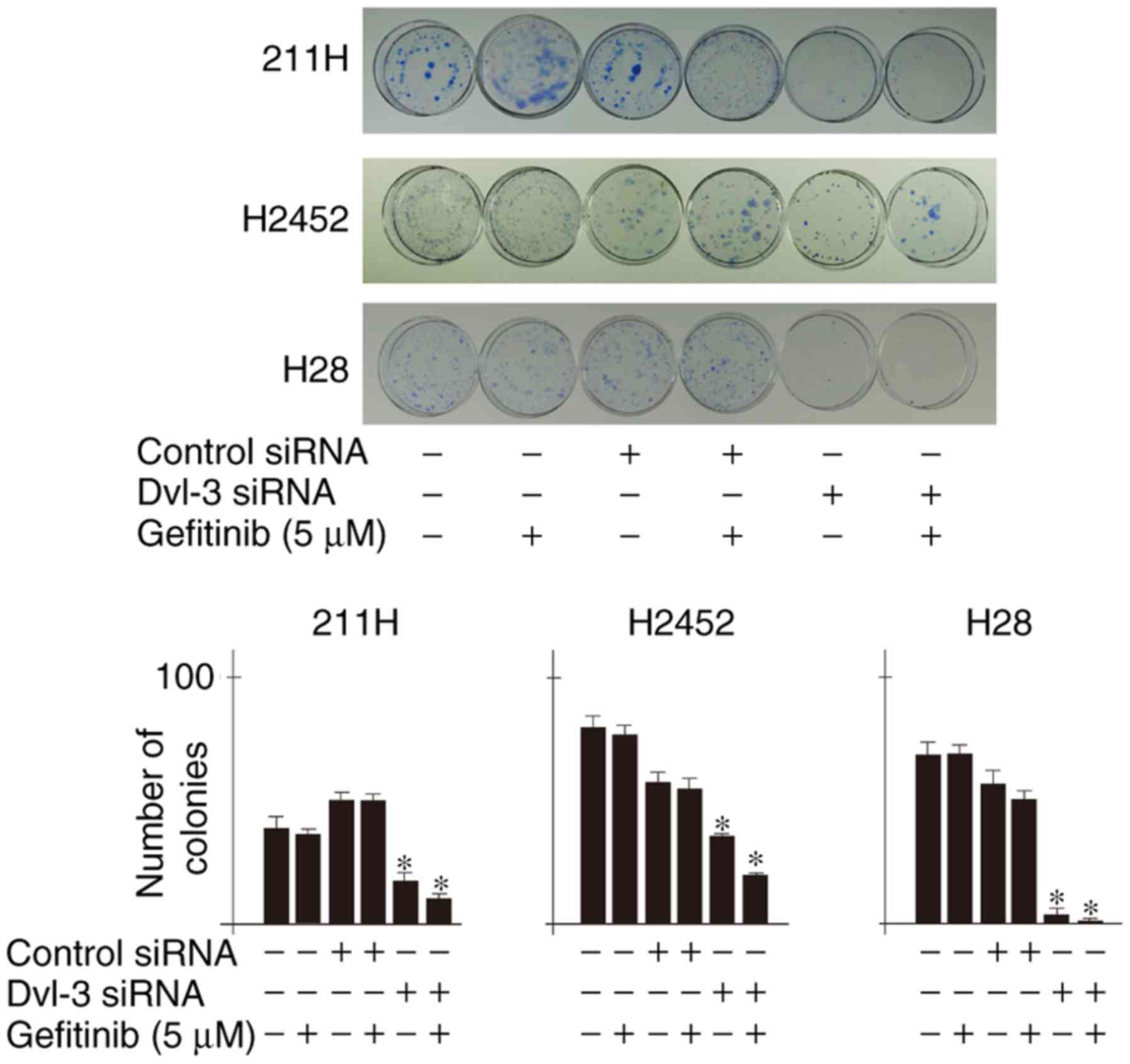
Downregulation of Dvl-3 suppresses
colony formation of mesothelioma cells
Colony counts of 211H, H2452 and H28 cells were
significantly decreased following transfection with Dvl-3 siRNA,
and were further significantly decreased following treatment with 5
µM gefitinib, compared with respective controls (Fig. 3).
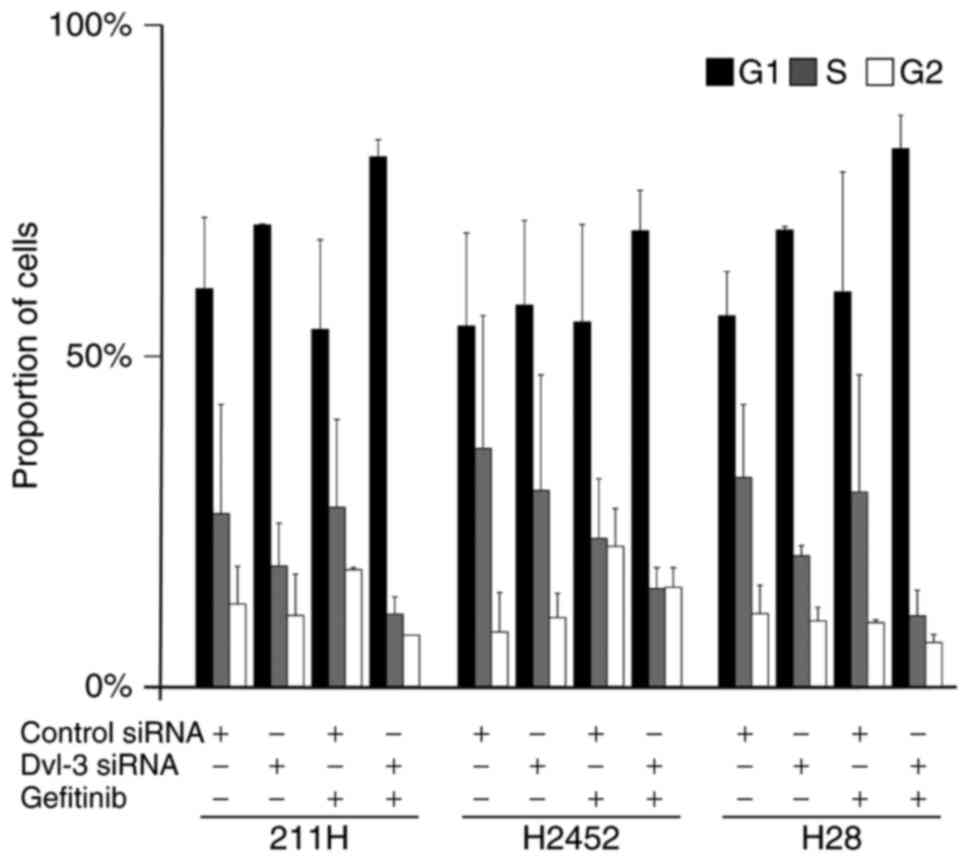
Downregulation of Dvl-3 and treatment
with gefitinib induces G1 population increase
After 48 h of transfection with Dvl-3 siRNA, the
population of 211H, H2452 and H28 cells in G1 phase tended to
increase (Fig. 4). At a further 24 h
after the addition of gefitinib to the medium, after 24 h of
transfection with Dvl-3 siRNA, the population of 211H, H2452 and
H28 cells in G1 phase tended to increase further, although these
results were not statistically significant (P>0.05; Fig. 4).
Downregulation of Dvl-3 decreases
phosphorylation of GSK3β
Phosphorylation of GSK3β was decreased in 211H and
H2452 cells following downregulation of Dvl-3 (Fig. 5). In H28 cells, expression of p-GSK3β
and total expression of GSK3β were decreased.
Discussion
The results of the present study indicate that
downregulation of Dvl-3 induced suppression of cell viability and
that the addition of the EGFR-TKI gefitinib acted synergistically,
resulting in colony formation with a tendency to persist in the G1
phase of the cell cycle. Of all pleural mesotheliomas, ~70% exhibit
high levels of EGFR expression (11).
Jänne et al (18) demonstrated
that 10 µM gefitinib suppressed the viability and colony formation
of mesothelioma cell lines in soft agarose. It has been
demonstrated that 10 µM gefitinib exceeds the effective dose in
NSCLC (13). In the present study,
inhibition of Dvl-3 enhanced inhibition of viability at 10 µM in
all three mesothelioma cell lines. In H28 cells, downregulation of
Dvl-3 suppressed cell viability, an effect which was enhanced 48 h
after treatment with 5 or 10 µM gefitinib. At 30 µM gefitinib, H28
cell viability was markedly decreased, but it was not affected by
downregulation of Dvl-3. Nutt et al (19) demonstrated that H28 cell viability was
completely suppressed 72 h after the addition of 30 µM gefitinib. A
concentration of 30 µM gefitinib is more toxic to H28 cells
compared with a concentration of 5 or 10 µM, and this toxicity may
not be associated with signaling pathways affected by the
downregulation of Dvl-3. The aim of colony formation assay
performed in the present study was to investigate the temporary
effect of suppression of Dvl-3 combined with treatment with an
EGFR-TKI on colony formation of mesothelioma cells. As colony
formation was suppressed in the present study, suppression of Dvl-3
may be associated with the initial expansion of cells. A limitation
of the present study is that the siRNA had no function after 14
days of transfection. It was confirmed that temporary transfection
of siRNA did not suppress Dvl-3 expression after 14 days (data not
shown). Future studies are required to examine colony formation
using short hairpin RNA in order to elucidate the effect on other
signaling pathways of continuous suppression of Dvl-3. In cell
cycle analysis, 5 µM gefitinib was used, and this dose did not
inhibit cell viability effectively 24 h after the addition.
Downregulation of Dvl-3 by siRNA usually induced G1 phase, which
tended to be enhanced by gefitinib, although these results were not
statistically significant. These results suggest that blockade of
the EGF signaling pathway by gefitinib or other EGFR-TKIs, and of
Wnt signaling by Dvl-3 suppression may be a useful combination for
the treatment of mesothelioma.
p-GSK3β (Ser9), which is the inactive
form of GSK3β and a regulator of Wnt signaling, and EGFR were
revealed to be negatively associated with survival of patients with
lung cancer, indicating that EGFR may phosphorylate GSK3β into
inactive p-GSK3β (20). GSK3β
participates in various critical cellular processes, one of which
is the formation of the β-catenin destruction complex (21). When Wnt signaling is not activated,
GSK3β is able to phosphorylate β-catenin, resulting in its
ubiquitination. Dvl family members inhibit activation of GSK3β and
degradation of β-catenin, which is translocated to the nucleus and
interacts with transcription factors, resulting in the expression
of target genes (21). The results of
the present study indicate that downregulation of Dvl-3 decreased
phosphorylation of GSK3β in 211H and H2452 cells. However, H28
cells without β-catenin expression exhibited a decrease in p-GSK3β
levels and total expression of GSK3β following downregulation of
Dvl-3. In 211H and H2452 cells, synergistic inhibition of cell
viability by Dvl-3 downregulation and gefitinib may be associated
with p-GSK3β. However, the precise function of GSK3β in EGFR and
Wnt signaling pathways in mesothelioma cells requires further
elucidation.
In NSCLC, Wnt signaling protects cells from
EGFR-TKIs via tankyrase or β-catenin (13–16). An
interaction between EGFR and Wnt signaling has been identified
(22,23). Numerous studies reviewed in Paul et
al (22) have demonstrated that
downregulation of β-catenin leads to a decreased expression of
EGFR, signal transducer and activator of transcription 3, cyclin
D1, matrix metalloproteinase (MMP)2, MMP9 and protein kinase B. In
mesothelioma cells, Wnt signaling and EGF signaling pathways may
support each other against cytotoxicity.
Dvl proteins relay Wnt signals from receptors to
downstream effectors, which activate either the canonical Wnt
pathway or the β-catenin-independent non-canonical pathway,
depending on the nuclear translocation of β-catenin (24). Previous studies have reported that the
suppression of Dvl inhibits the viability or tumorigenesis of
mesothelioma cells (3–7), and the cell viability of lung cancer
(4). Furthermore, our previous
studies demonstrated that mesothelioma cells express Dvl-3 and that
the inhibition of Dvl-3 suppressed mesothelioma cell viability,
including that of H28 cells, which do not express β-catenin
(8,17). This suggests that the activation of
Wnt signaling in mesothelioma cells may utilize the
β-catenin-independent non-canonical pathway. Zhao et al
(25) demonstrated that Dvl-3 induced
upregulation of p120-catenin, which is associated with cell
viability, invasion and metastasis of lung cancer. In lung cancer
cells, cytosolic transmembrane protein 88 was revealed to interact
with Dvl family members independently of β-catenin, which promoted
invasion and metastasis by activating p38-GSK3β-Snail signaling
(26).
The underlying molecular mechanism that led to these
results may be attributed to a reciprocal interaction of the EGFR
and Wnt signaling pathways. The function of Dvl-3 may differ among
cell lines utilizing the canonical and non-canonical pathways.
Notably, H28 cells do not express β-catenin, which is suspected to
be associated with a different signaling pathway from those
utilized by other mesothelioma cells. Although Dvl-3 is unable to
affect EGFR directly in H28 cells, downregulation of Dvl-3 may
inhibit other pathways to compensate for the negative effect
induced by inhibition of the EGFR pathway in H28 cells. However, in
order to fully understand these mechanisms, further studies are
required. Furthermore, the mechanism of cross-talk between these
pathways in mesothelioma cells remains to be elucidated.
Glossary
Abbreviations
Abbreviations:
|
Dvl
|
dishevelled
|
|
EGFR
|
epidermal growth factor receptor
|
|
GSK3β
|
glycogen synthesis kinase-3β
|
|
NSCLC
|
non-small cell lung cancer
|
|
siRNA
|
small interfering RNA
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uematsu K, Kanazawa S, You L, He B, Xu Z,
Li K, Peterlin BM, McCormick F and Jablons DM: Wnt pathway
activation in mesothelioma: Evidence of dishevelled overexpression
and transcriptional activity of β-catenin. Cancer Res.
63:4547–4551. 2003.PubMed/NCBI
|
|
4
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: Evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He B, You L, Uematsu K, Xu Z, Lee AY,
Matsangou M, McCormick F and Jablons DM: A monoclonal antibody
against Wnt-1 induces apoptosis in human cancer cells. Neoplasia.
6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You L, He B, Xu Z, Uematsu K, Mazieres J,
Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, et al:
An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant
melanoma cells and inhibits tumor growth. Cancer Res. 64:5385–5389.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujii N, You L, Xu Z, Uematsu K, Shan J,
He B, Mikami I, Edmondson LR, Neale G, Zheng J, et al: An
antagonist of dishevelled protein-protein interaction suppresses
β-catenin-dependent tumor cell growth. Cancer Res. 67:573–579.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You L, He B, Uematsu K, Xu Z, Mazieres J,
Lee A, McCormick F and Jablons DM: Inhibition of Wnt-1 signaling
induces apoptosis in β-catenin-deficient mesothelioma cells. Cancer
Res. 64:3474–3478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dazzi H, Hasleton PS, Thatcher N, Wilkes
S, Swindell R and Chatterjee AK: Malignant pleural mesothelioma and
epidermal growth factor receptor (EGF-R). Relationship of EGF-R
with histology and survival using fixed paraffin embedded tissue
and the F4, monoclonal antibody. Br J Cancer. 61:924–926. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Govindan R, Kratzke RA, Herndon JE II,
Niehans GA, Vollmer R, Watson D, Green MR and Kindler HL; Cancer
and Leukemia Group B (CALGB 30101), : Gefitinib in patients with
malignant mesothelioma: A phase II study by the cancer and leukemia
group B. Clin Cancer Res. 11:2300–2304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Casás-Selves M, Kim J, Zhang Z, Helfrich
BA, Gao D, Porter CC, Scarborough HA, Bunn PA Jr, Chan DC, Tan AC
and DeGregori J: Tankyrase and the canonical Wnt pathway protect
lung cancer cells from EGFR inhibition. Cancer Res. 72:4154–4164.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fong JT, Jacobs RJ, Moravec DN, Uppada SB,
Botting GM, Nlend M and Puri N: Alternative signaling pathways as
potential therapeutic targets for overcoming EGFR and c-Met
inhibitor resistance in non-small cell lung cancer. PLoS One.
8:e783982013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Togashi Y, Hayashi H, Terashima M, de
Velasco MA, Sakai K, Fujita Y, Tomida S, Nakagawa K and Nishio K:
Inhibition of β-catenin enhances the anticancer effect of
irreversible EGFR-TKI in EGFR-mutated non-small-cell lung cancer
with a T790M mutation. J Thorac Oncol. 10:93–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhang X, Huang J and Dong Q: Wnt
signaling regulation of stem-like properties in human lung
adenocarcinoma cell lines. Med Oncol. 32:1572015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uematsu K, Seki N, Seto T, Isoe C,
Tsukamoto H, Mikami I, You L, He B, Xu Z, Jablons DM and Eguchi K:
Targeting the wnt signaling pathway with dishevelled and cisplatin
synergistically suppresses mesothelioma cell growth. Anticancer
Res. 27:4239–4242. 2007.PubMed/NCBI
|
|
18
|
Jänne PA, Taffaro ML, Salgia R and Johnson
BE: Inhibition of epidermal growth factor receptor signaling in
malignant pleural mesothelioma. Cancer Res. 62:5242–5247.
2002.PubMed/NCBI
|
|
19
|
Nutt JE, O'Toole K, Gonzalez D and Lunec
J: Growth inhibition by tyrosine kinase inhibitors in mesothelioma
cell lines. Eur J Cancer. 45:1684–1691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng H, Saito H, Masuda S, Yang X and
Takano Y: Phosphorylated GSK3beta-ser9 and EGFR are good prognostic
factors for lung carcinomas. Anticancer Res. 27:3561–3569.
2007.PubMed/NCBI
|
|
21
|
McCubrey JA, Rakus D, Gizak A, Steelman
LS, Abrams SL, Lertpiriyapong K, Fitzgerald TL, Yang LV, Montalto
G, Cervello M, et al: Effects of mutations in Wnt/β-catenin,
hedgehog, notch and PI3K pathways on GSK-3 activity-diverse effects
on cell growth, metabolism and cancer. Biochim Biophys Acta.
1863:2942–2976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paul I, Bhattacharya S, Chatterjee A and
Ghosh MK: Current understanding on EGFR and Wnt/β-catenin signaling
in glioma and their possible crosstalk. Genes Cancer. 4:427–446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu T and Li C: Convergence between
Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 9:2362010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao H, Zhao Y, Jiang G, Zhang X, Zhang Y,
Dong Q, Luan L, Papavassiliou P and Wang E: Dishevelled-3 activates
p65 to upregulate p120-catenin transcription via a p38-dependent
pathway in non-small cell lung cancer. Mol Carcinog. 54:E112–E121.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Yu X, Jiang G, Miao Y, Wang L,
Zhang Y, Liu Y, Fan C, Lin X, Dong Q, et al: Cytosolic TMEM88
promotes invasion and metastasis in lung cancer cells by binding
DVLs. Cancer Res. 75:4527–4537. 2015. View Article : Google Scholar : PubMed/NCBI
|