Introduction
Salivary gland adenoid cystic carcinoma (ACC)
accounts for ~10% of cases of epithelial salivary tumors and has a
low 5-year survival rate (<20% in patients with highly
metastatic tumors) (1,2). The development of ACC involves the
interaction of oncogenes and tumor suppressor genes, similar to
most other types of tumor (3).
However, the precise mechanisms underlying ACC carcinogenesis
remain to be elucidated (4,5).
PIM kinases are oncogenic and are known to
phosphorylate numerous substrates to exert their functions and have
important roles in numerous malignancies. PIM-1, Pim-1
proto-oncogene, serine/threonine kinase (PIM-1) is upregulated in a
number of cancer subtypes and the overexpression of PIM-1 is
thought to be involved in cancer-specific apoptosis signaling
pathways (6–12). Apoptosis is modulated by complex
pathways that involve a series of apoptosis-associated proteins. As
a substrate of PIM-1 kinase, Forkhead box O3a (FOXO3a) is a
proapototic transcription factor and regulates the expression of
numerous apoptosis-associated genes to induce apoptosis (13). It has previously been revealed that
the invalidation of FOXO3a by PIM-1 may downregulate its
transcriptional function and aid cell survival (14). It has been previously established that
the B cell lymphoma-2 (BCL-2) family includes the most well-known
apoptosis-associated proteins (15).
As an anti-apoptotic factor of the BCL-2 family, BCL-2 is able to
restrain the mitochondrial permeability transformation and interact
with proapoptotic proteins to inactivate them. BCL-2-associted
agonist of cell death (BAD), a proapoptotic member of the BCL-2
family, normally binds to the BCL-2/BCL-X complex and triggers
apoptosis. A previous study demonstrated that PIM-1 physically
interacted with BAD and was suggested to be an essential molecular
mechanism underlying PIM-1-modulated cell apoptosis (16). This evidence indicated that FOXO3a,
BAD and BCL-2 were involved in the PIM-1-associated apoptosis
process (13–16).
The present study evaluated protein expression
levels of PIM-1 and apoptosis-associated proteins, including
FOXO3a, BAD and BCL-2 in 60 ACC tissues by immunohistochemistry
(IHC). Terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling (TUNEL) assay was performed to evaluate the apoptosis rate
in ACC tissues. The associations between PIM-1 and apoptotic status
and apoptosis-associated proteins were deduced. Furthermore,
associations between PIM-1, apoptotic status, apoptosis-associated
proteins and clinical parameters, including prognosis, were
analyzed.
Materials and methods
Tissue specimens
The present study was approved by the Ethics
Committee of Zhejiang Cancer Hospital (Hangzhou, China). A total of
60 paraffin-embedded ACC tissue samples, 4 fresh ACC tissues and 4
fresh normal salivary gland tissues were obtained from Zhejiang
Cancer Hospital between November 2002 and April 2013. These were
taken from the hospital bank archive, and subsequently were
‘freshly prepared’ between the years 2002–2013, and so they were
still covered by the retrospective approval form provided. There
were 23 male, 37 female patients and the mean age was 51 (range
between 28 and 78). All patients underwent surgical treatment and
all tumor samples were histopathologically confirmed to be ACC.
Western blot analysis
ACC tissue samples were ground with Tissue Lyser-II
(Qiagen GmbH, Hilden, Germany) and lysed with
radioimmunoprecipitation assay lysis buffer (P10013B; Beyotime
Institute of Biotechnology, Haimen, China). Subsequently, the BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China) was used to evaluate the protein concentration. All samples
were maintained at −70°C prior to electrophoresis. Each sample
containing 50 µg of protein was separated using 12% SDS-PAGE.
Following electrophoresis, the proteins were transferred from the
gel to nitrocellulose membrane (Immobilon-PSQ Transfer
Membrane; EMD Millipore, Billerica, MA, USA). Membranes were
blocked in TBS buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.6)
supplemented with 5% non-fat dry milk at room temperature for 3 h.
Subsequently, the membranes were incubated with primary antibodies
against PIM-1 (EP2645Y; 1:1,000 dilution; Novus Biologicals, Ltd.,
Cambridge, UK) and GAPDH (R1208-3; 1:2,000 dilution; Huabio
Technology, Hangzhou, China) at 4°C overnight prior to incubation
with horseradish peroxidase-labeled Goat Anti-Rabbit IgG (G+L)
(HA1001; 1:2,000 dilution; Huabio Technology) at room temperature
for 3 h. Subsequently, the membranes were washed in TBST (B1009;
Applygen, Beijing, China) and exposed to 2 ml enhanced
chemiluminesence reagent (TJWBKLS0100; Tiengene, Guangzhou, China).
The images were captured and analyzed using Bio-Rad GelDoc XR
(Image Lab 4.1; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
IHC
Sections (4-µm thick) of paraffin-embedded tissues
were cut, mounted on glass slides (MS-coated glass; Matsunami Glass
Ind., Ltd., Kishiwada, Japan) and dried overnight at 37°C.
Following deparaffinization and antigen retrieval in 0.01 M citrate
buffer, the slides were inactivated for endogenous peroxidase
activity in 3% H2O2/methanol and incubated
with antibodies for PIM-1 (EP2645Y; 1:200 dilution; Novus
Biologicals, Ltd.), FOXO3a (10849–1-AP; 1:200 dilution; ProteinTech
Group, Inc., Chicago, IL, USA), BCL-2 (15071; 1:400 dilution; Cell
Signaling Technology, Inc., Danvers, MA, USA) or BAD (ab32445;
1:200 dilution; Abcam, Cambridge, UK) at 4°C overnight. The
streptavidin-biotin peroxidase staining kit (Histofine Simple Stain
Max PO Multi; Nichirei, Tokyo, Japan) and DAB solution (Simple
Stain DAB; Nichirei) were used to detect immunoreactivity. Images
were captured under a light microscope at ×200 magnification.
Evaluation of IHC staining
The staining results of IHC for no staining, light
yellow, yellow-brown and brown were defined as the mean of 0, 1, 2
and 3 staining intensity score, respectively. The staining
distribution scores were presented as the percentage of nuclei
staining positive cells and the total cells as 0, 1–25, 26–50,
51–75 and >75% and defined as 0, 1, 2, 3 and 4, respectively
(17). The criterion of final scores
was evaluated by multiplying the staining intensity score and the
staining distribution score. Those with a score of <5 were
defined as ‘low expression’ and those with a score of ≥5 were
considered as ‘high expression’.
TUNEL assay
TUNEL staining was performed to quantify apoptosis.
Sections (4-µm thick) of paraffin-embedded ACC tissues were cut and
stained with the TUNEL kit (Roche Applied Science, Madison, WI,
USA), according to the manufacturer's instructions. Briefly, tissue
sections were incubated with proteinase K for 20 min at 37°C, and
rinsed twice with PBS (5 min each). Subsequently, TUNEL reaction
mixture (terminal deoxynucleotidyl transferase (TdT) buffer: TdT
end-labeling cocktail=1:9) was added to the samples at 37°C for 60
min and washed with PBS. Next, sections were treated with converter
POD at 37°C for 30 min and colored with DAB.
Cancer cells with dark brown nuclei were considered
apoptotic when investigated under a light microscope at ×200
magnification. The TUNEL index for ACC tissues was evaluated by the
percentage of positive cells in one field of vision. At least three
fields were randomly selected and 200 cells in each area were
counted per slide.
Statistical analysis
SPSS version 10.0 (SPSS, Inc., Chicago, IL, USA) was
used to analyze all experimental data. The data are presented as
‘high’ and ‘low’ levels. Associations between PIM-1 and FOXO3a,
BCL-2, BAD expression levels, apoptotic rate and the clinical
parameters in ACC tissues were analyzed using the χ2
test or Fisher's exact test. The Kaplan-Meier method was used to
perform survival analysis and significant differences were
evaluated by means of the log-rank test. Multivariate analysis with
the Cox regression model was used to determine the prognostic
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
Protein expression levels of PIM-1 in
ACC tissues and corresponding normal salivary gland tissues
A total of 4 matched pairs of ACC tissue and
corresponding normal salivary gland tissue were randomly selected
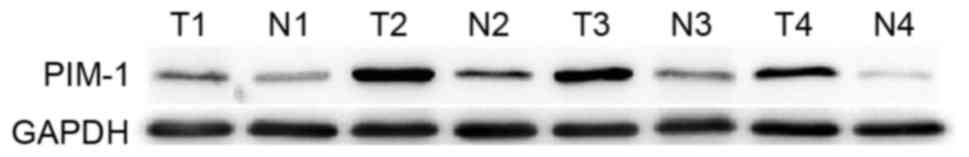
for western blot analysis. As presented in Fig. 1, PIM-1 protein expression was higher
in tumor tissues compared with in matched normal tissues.
Expression levels of PIM-1, FOXO3a,
BCL-2 and BAD in ACC tissues
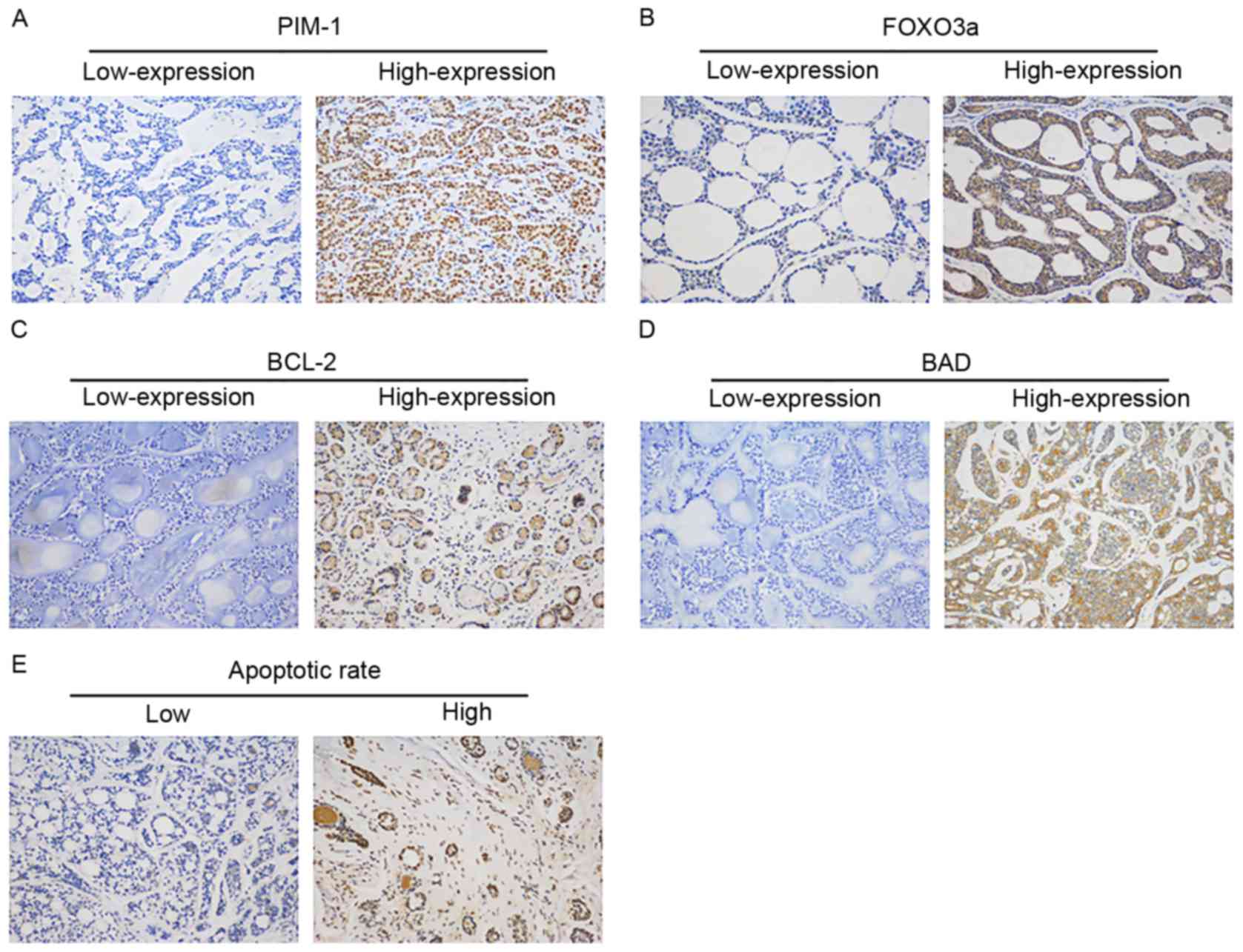
IHC staining for protein expression levels of PIM-1,
FOXO3a, BCL-2 and BAD in ACC tissues are shown in Fig. 2. High expression ratios of PIM-1,
FOXO3a, BCL-2 and BAD were 33.33% (20/60), 51.67% (31/60), 51.67%
(31/60) and 55% (33/60), respectively. Table I presents significant associations
between the expression of PIM-1 and FOXO3a (P=0.018). Furthermore,
the expression of BCL-2 also had a significant association with the
expression of PIM-1 (P=0.044). There was no association between the
expression of PIM-1 and BAD.
 | Table I.Association between the expression
levels of PIM-1 and FOXO3a and BCL-2 and BAD in ACC tissues. |
Table I.
Association between the expression
levels of PIM-1 and FOXO3a and BCL-2 and BAD in ACC tissues.
|
|
| PIM-1 expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Variables | Low 40 | High 20 | Kappa value | P-value |
|---|
| FOXO3a |
|
|
|
|
|
| Low | 29 | 15 | 14 | −0.286 | 0.018a |
| High | 31 | 25 | 6 |
|
|
| BCL-2 |
|
|
|
|
|
| Low | 29 | 23 | 6 |
0.242 | 0.044a |
| High | 31 | 17 | 14 |
|
|
| BAD |
|
|
|
|
|
| Low | 27 | 15 | 12 | −0.194 | 0.099 |
| High | 33 | 25 | 8 |
|
|
TUNEL staining indicates apoptosis
status of ACC tissues
The apoptotic rate analyzed by a TUNEL assay in ACC
tissues was 36.65±23.94 (range from 5 to 94). Those with a score
<36.65 were defined as ‘low apoptotic rate’ and those with a
score ≥36.65 were considered to have a ‘high apoptotic rate’.
Fig. 2E presents the TUNEL assay
stain results of low/high apoptotic rate tissues. As presented in
Table II, the apoptotic rate was
significantly associated with PIM-1, FOXO3a, BCL-2 and BAD
expression levels. Patients with higher apoptotic rates tended to
have lower PIM-1 and BCL-2 expression levels, and higher FOXO3a and
BAD expression levels. Furthermore, the present study investigated
the associations between apoptotic rate and clinical parameters,
including sex, age, T-status, tumor node metastasis (TNM) stage,
tumor location, tumor size, histological type, lymph node
involvement, nerve invasion and distant metastasis. However,
apoptotic rate had no significant associations with any clinical
index.
 | Table II.Association between the apoptotic
rate and PIM-1, FOXO3a, BCL-2 and BAD expression levels in ACC
tissues. |
Table II.
Association between the apoptotic
rate and PIM-1, FOXO3a, BCL-2 and BAD expression levels in ACC
tissues.
|
|
| Apoptotic rate |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Variables | Low 34 | High 26 | Kappa value | P-value |
|---|
| PIM-1 |
|
|
|
|
|
|
Low | 40 | 17 | 23 | −0.395 | 0.002a |
|
High | 20 | 17 | 3 |
|
|
| FOXO3a |
|
|
|
|
|
|
Low | 29 | 21 | 8 |
0.303 | 0.017a |
|
High | 31 | 13 | 18 |
|
|
| BCL-2 |
|
|
|
|
|
|
Low | 29 | 12 | 17 | −0.294 | 0.021a |
|
High | 31 | 22 | 9 |
|
|
| BAD |
|
|
|
|
|
|
Low | 27 | 20 | 7 | 0.309 | 0.014a |
|
High | 33 | 14 | 19 |
|
|
Association between the expression of
PIM-1, FOXO3a, BCL-2 and BAD levels, and the clinical
characteristics in ACC tissues
As presented in Table
III, PIM-1 levels were significantly associated with tumor
size, lymph node involvement, nerve invasion and distant
metastasis, whereas theywere weakly associated with TNM stage.
FOXO3a expression level was closely associated with T-status, tumor
size and lymph node involvement. There were significant
associations between BAD level and TNM stage and distant
metastasis. BCL-2 expression level revealed no significant
associations with any clinical index.
 | Table III.Associations between PIM-1, FOXO3a,
BCL-2 and BAD expression levels and the apoptotic rate and clinical
characteristics in ACC tissues. |
Table III.
Associations between PIM-1, FOXO3a,
BCL-2 and BAD expression levels and the apoptotic rate and clinical
characteristics in ACC tissues.
|
|
| PIM-1 |
| FOXO3a |
| BCL-2 |
| BAD |
| Apoptotic rate |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | Patients
(total=60) | Low 40 | High 20 | P-value | Low 29 | High 31 | P-value | Low 29 | High 31 | P-value | Low 27 | High 33 | P-value | Low 34 | High 26 | P-value |
|---|
| Sex |
|
|
| 0.133 |
|
| 0.098 |
|
| 0.039 |
|
| 0.729 |
|
| 0.580 |
|
Male | 23 | 18 | 5 |
| 8 | 15 |
| 15 | 8 |
| 11 | 12 |
| 12 | 11 |
|
|
Female | 37 | 22 | 15 |
| 21 | 16 |
| 14 | 23 |
| 16 | 21 |
| 22 | 15 |
|
| Age |
|
|
| 0.584 |
|
| 0.196 |
|
| 0.796 |
|
| 0.436 |
|
| 0.435 |
|
<52 | 30 | 21 | 9 |
| 17 | 13 |
| 14 | 16 |
| 15 | 15 |
| 19 | 11 |
|
|
≥52 | 30 | 19 | 11 |
| 12 | 18 |
| 15 | 15 |
| 12 | 18 |
| 15 | 15 |
|
| T-status |
|
|
| 0.333 |
|
| 0.044a |
|
| 0.465 |
|
| 0.099 |
|
| 0.713 |
|
T1-2 | 20 | 15 | 5 |
| 6 | 14 |
| 11 | 9 |
| 6 | 14 |
| 12 | 8 |
|
|
T3-4 | 40 | 25 | 15 |
| 23 | 17 |
| 18 | 22 |
| 21 | 19 |
| 22 | 18 |
|
| TNM stage |
|
|
| 0.050a |
|
| 0.511 |
|
| 0.650 |
|
| 0.031a |
|
| 0.581 |
|
I–II | 19 | 16 | 3 |
| 8 | 11 |
| 10 | 9 |
| 5 | 14 |
| 12 | 7 |
|
|
III–IV | 41 | 24 | 17 |
| 21 | 20 |
| 19 | 22 |
| 23 | 18 |
| 22 | 19 |
|
| Tumor location |
|
|
| 0.232 |
|
| 0.693 |
|
| 0.866 |
|
| 0.533 |
|
| 0.909 |
| Major
salivary | 18 | 10 | 8 |
| 8 | 10 |
| 9 | 9 |
| 7 | 11 |
| 10 | 8 |
|
| Minor
salivary | 42 | 30 | 12 |
| 21 | 21 |
| 20 | 22 |
| 20 | 22 |
| 24 | 18 |
|
| Tumor size |
|
|
| 0.015a |
|
| 0.009a |
|
| 0.951 |
|
| 0.379 |
|
| 0.276 |
| <3
cm | 37 | 29 | 8 |
| 13 | 24 |
| 18 | 19 |
| 12 | 25 |
| 23 | 14 |
|
| ≥3
cm | 23 | 11 | 12 |
| 16 | 7 |
| 11 | 12 |
| 15 | 8 |
| 11 | 12 |
|
| Histotype |
|
|
| 0.281 |
|
| 0.853 |
|
| 0.219 |
|
| 0.635 |
|
| 0.636 |
|
Cribiform | 31 | 23 | 8 |
| 15 | 16 |
| 14 | 17 |
| 14 | 17 |
| 17 | 14 |
|
|
Tubula | 14 | 7 | 7 |
| 6 | 8 |
| 5 | 9 |
| 5 | 9 |
| 7 | 7 |
|
|
Solid | 15 | 10 | 5 |
| 8 | 7 |
| 10 | 5 |
| 8 | 7 |
| 10 | 5 |
|
| Lymph node
involvement |
|
|
| 0.015a |
|
| 0.020a |
|
| 0.421 |
|
| 0.469 |
|
| 0.387 |
|
Yes | 13 | 5 | 8 |
| 10 | 3 |
| 5 | 8 |
| 7 | 6 |
| 6 | 7 |
|
| No | 47 | 35 | 12 |
| 19 | 28 |
| 24 | 23 |
| 20 | 27 |
| 28 | 19 |
|
| Nerve invasion |
|
|
| 0.018a |
|
| 0.599 |
|
| 0.119 |
|
| 0.622 |
|
| 0.414 |
|
Yes | 29 | 15 | 14 |
| 13 | 16 |
| 11 | 18 |
| 15 | 14 |
| 18 | 11 |
|
| No | 31 | 25 | 6 |
| 16 | 15 |
| 18 | 13 |
| 13 | 18 |
| 16 | 15 |
|
| Distant
metastasis |
|
|
| 0.002a |
|
| 0.193 |
|
| 0.758 |
|
| 0.021a |
|
| 0.402 |
|
Yes | 7 | 1 | 6 |
| 5 | 2 |
| 3 | 4 |
| 6 | 1 |
| 5 | 2 |
|
| No | 53 | 39 | 14 |
| 24 | 29 |
| 26 | 27 |
| 21 | 32 |
| 29 | 24 |
Survival analysis
In the present study, patients' average follow-up
time was 68.23±38.69 months (mean ± standard deviation; range from
15 to 156 months). Patients with a follow-up of >5 years
accounted for 36.7% of all patients. At the end of the follow-up, 4
patients (6.7%) were not included as data was not available, 10
patients (16.7%) experienced disease recurrence, 20 patients
(33.3%) had passed away and 36 patients (60%) remained alive.
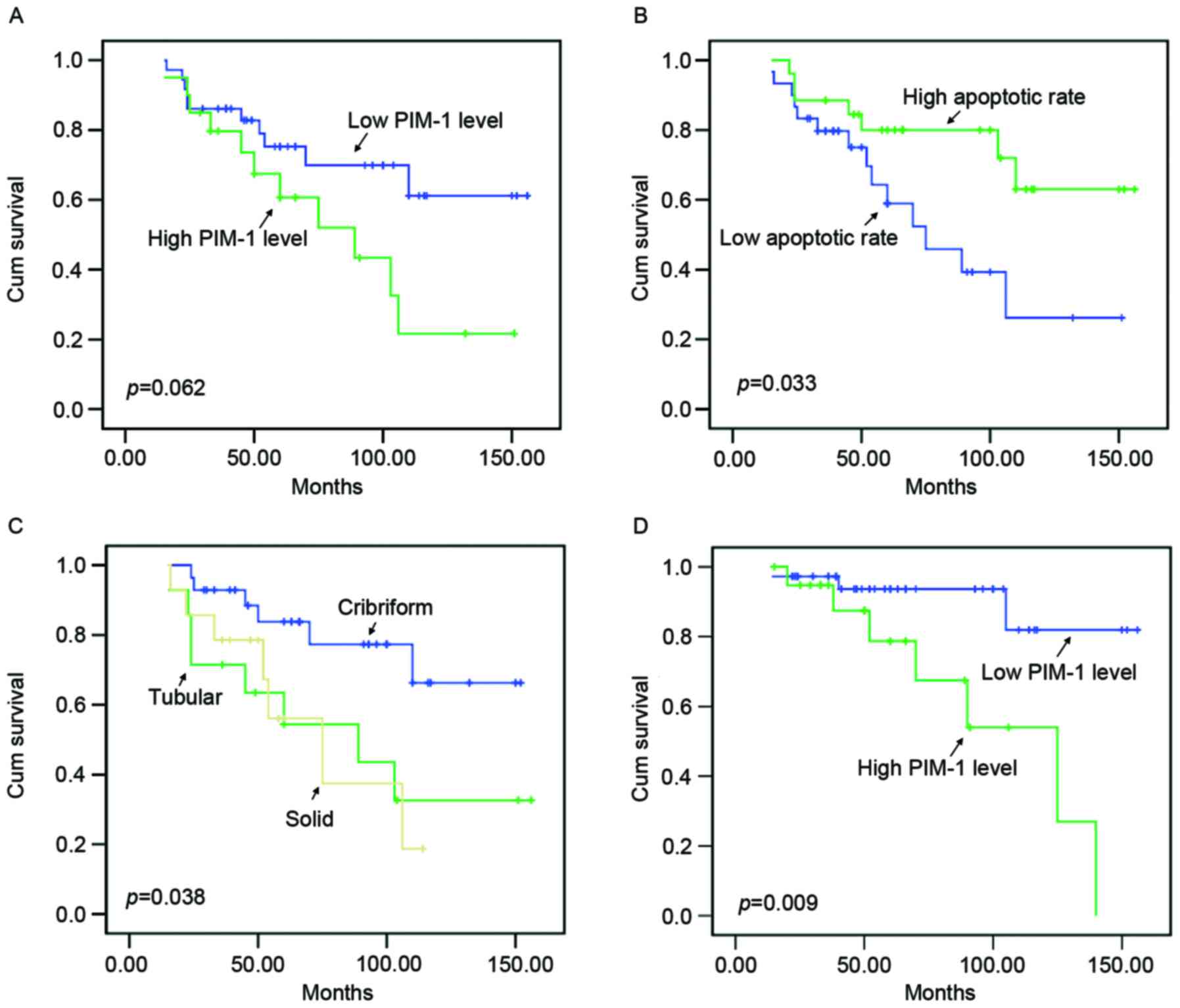
Kaplan-Meier survival curves (Fig. 3A) demonstrated that the PIM-1 level
was weakly associated with the overall survival of patients with
ACC (P=0.062). patients with higher PIM-1 expression levels had a
poorer prognosis compared with patients with lower PIM-1 expression
levels. Fig. 3B revealed that
apoptotic rate, reflected by TUNEL results, had a significant
impact on the prognosis of patients (P=0.033). The outcomes for
patients with higher apoptotic rates were more favorable compared
with those with lower apoptotic rates. Additionally, the present
study revealed that there was a significant association between
tumor histotype and the survival of patients with ACC (P=0.038;
Fig. 3C). However, other
clinicopathological parameters, including sex, age, T-status, TNM
stage, tumor location, tumor size, lymph node involvement, nerve
invasion, distant metastasis and other proteins, including FOXO3a,
BCL-2 and BAD levels, had no significant impact on the overall
survival of patients with ACC. Furthermore, the present study
demonstrated that PIM-1 expression level was significantly
associated with disease-free survival of patients with ACC
(P=0.009; Fig. 3D). It is worth
noting that Cox regression multivariate analysis revealed that
histotype, distant metastasis and apoptotic rate were independent
prognosis factors for patients with ACC (P<0.05; Table IV).
 | Table IV.Multivariate survival analysis of
clinicopathologic data of the adenoid cystic carcinoma patients
(Cox regression hazards model). |
Table IV.
Multivariate survival analysis of
clinicopathologic data of the adenoid cystic carcinoma patients
(Cox regression hazards model).
|
| Multivariate
analysis |
|---|
|
|
|
|---|
|
| 95% CI for
Exp(B) |
|
|---|
|
|
|
|
|---|
| Variable | Lower | Higher | P-value |
|---|
| Sex | 0.529 | 6.074 | 0.348 |
| Age | 0.385 | 5.051 | 0.613 |
| T-status | 0.173 | 9.338 | 0.812 |
| TNM stage | 0.055 | 2.668 | 0.333 |
| Tumor location | 0.388 | 6.233 | 0.533 |
| Tumor size | 0.134 | 1.694 | 0.252 |
| Histotype | 0.056 | 0.778 | 0.020a |
| Lymph node
involvement | 0.110 | 2.073 | 0.324 |
| Nerve invasion | 0.671 | 10.995 | 0.161 |
| Distant
metastasis | 1.348 | 81.307 | 0.025a |
| PIM-1 | 0.319 | 5.055 | 0.734 |
| FOXO3a | 0.090 | 1.302 | 0.116 |
| BCL-2 | 0.346 | 3.220 | 0.923 |
| BAD | 0.550 | 5.614 | 0.342 |
| Apoptotic rate | 1.787 | 57.428 | 0.009a |
Discussion
Previous studies have suggested that PIM-1 is
important for carcinogenesis and metastasis in numerous types of
human cancer (6–12). The present study selected 4 pairs of
ACC tissue and corresponding normal tissues and revealed that PIM-1
was overexpressed in ACC. IHC results demonstrated that PIM-1 was
highly expressed in 33.33% (20/60) of ACC tissues. Furthermore,
PIM-1 expression was significantly associated with tumor size,
lymph node involvement, nerve invasion and distant metastasis.
Patients with larger, lymph node involvement positive, nerve
invasion positive and distant metastasis positive ACC tissues
revealed higher PIM-1 levels. There was a weak association between
the expression level of PIM-1 and ACC TNM stage. Survival analysis
demonstrated that patients with higher PIM-1 levels had a shorter
disease-free survival time and poorer prognosis. This suggested
that upregulation of PIM-1 may promote the disruption of cell
proliferation and homeostasis to drive malignant aggression and
lead to a poor outcome.
Dysregulation of apoptosis is a vital feature of
oncogenes and a number of previous studies have revealed that PIM-1
has an important role in apoptosis (18–20). The
present study demonstrated that the apoptotic rate had a
significant association with PIM-1 expression level in ACC
patients' tissues. Patients with higher PIM-1 levels tended to have
lower apoptotic rates. These results suggested the importance of
PIM-1 in tumorigenesis of ACC, which involved the imbalance of
apoptotic events. The associations between apoptosis, degree of
malignancy and the prognosis of cancer patients have been
well-studied (21–26). Patients with high apoptotic rates have
a more favorable outcome compared with those with low apoptotic
rates (21–26). In the present study, the apoptotic
rate analyzed by TUNEL assay was associated with a significant
impact on the patients' prognosis and was revealed to be an
independent prognostic factor. These results are consistent with
previous studies and demonstrated the importance of apoptosis in
the prediction of outcome in ACC (21–26).
As a proapototic transcription factor, FOXO3a is a
phosphorylation substrate of PIM-1. FOXO3a may upregulate
proapototic proteins, including Bim and FAS ligand to trigger
apoptosis (13). The present study
revealed that PIM-1 protein expression level had an inverse
association with FOXO3a expression level in ACC tissues. The
significant association between FOXO3a level and apoptosis was
observed in the present study. Furthermore, IHC results
demonstrated that FOXO3a expression levels were closely associated
with clinical parameters, including TNM stage, tumor size and lymph
node involvement. Previous studies revealed that FOXO3a was
significantly associated with clinical stage and lymph node
involvement in nasopharyngeal carcinoma and ovarian cancer, which
were in agreement with our current findings (27,28).
The findings obtained from the ACC tissues in the
present study also demonstrated that BCL-2 protein expression
levels had a significant association with PIM-1 expression level,
whereas BAD protein expression level had no association with PIM-1
level. Furthermore, the present study revealed that BAD protein
expression level was significantly associated with TNM stage and
distant metastasis. Taken together, these results suggest PIM-1 may
exert its oncogenic function by regulating apoptosis, which
involves the interaction of BCL-2 family and FOXO3a proteins.
As a frequently occurring malignant epithelial
neoplasm, ACC originates from the salivary glands (1,2). The
growth modes of ACCs are histologically categorized into three
types: Cribriform, tubular and solid (3,4). It has
been previously established that solid types of tumor are markedly
more malignant compared with the other two types (29). The present study did not observe
associations between histotype and PIM-1, FOXO3a, BCL-2, BAD levels
or apoptotic rate. However, the survival analysis demonstrated that
tumor histotype was significantly associated with patient
prognosis. Furthermore, Cox regression results revealed that
histotype was an independent prognosis factor. The results of the
present study were in line with previous studies, that suggested
histotypes have important roles in the aggressive behavior of ACC
(30,31).
The results of the present study confirmed that
PIM-1 was overexpressed in ACC tissues and associated with FOXO3a,
BCL-2 expression and apoptotic rate. PIM-1 was revealed to be
significantly associated with tumor size, lymph node involvement,
nerve invasion and distant metastasis, and is weakly associated
with patient survival. Furthermore, the present study determined
that apoptotic rates were significantly associated with PIM-1,
FOXO3a, BCL-2 and BAD expression levels in ACC tissues. The results
of the present study also revealed that histotype, distant
metastasis and apoptotic rate were independent prognosis factors.
Considered together, the current findings suggested that PIM-1
kinase is a novel molecular biomarker and a promising prognostic
marker for ACC.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (grant no. 81202127), the Subproject of
National High Technology Research and Development Program of China
(grant no. 2014AA022402) and Zhejiang Province Natural Science
Foundation (grant no. LY14H160014).
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adenoid cystic carcinoma
|
|
IHC
|
immunohistochemistry
|
|
TUNEL
|
terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
|
References
|
1
|
International Head and Neck Scientific
Group, . Cervical lymph node metastasis in adenoid cystic carcinoma
of the sinonasal tract, nasopharynx, lacrimal glands and external
auditory canal: A collective international review. J Laryngol Otol.
130:1093–1097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Wal JE, Becking AG, Snow GB and
van der Waal I: Distant metastases of adenoid cystic carcinoma of
the salivary glands and the value of diagnostic examinations during
follow-up. Head Neck. 24:779–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gondivkar SM, Gadbail AR, Chole R and
Parikh RV: Adenoid cystic carcinoma: A rare clinical entity and
literature review. Oral Oncol. 47:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Shao C, Tan ML, Mu D, Ferris RL and
Ha PK: Molecular biology of adenoid cystic carcinoma. Head Neck.
34:1665–1677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moskaluk CA: Adenoid cystic carcinoma:
Clinical and molecular features. Head Neck Pathol. 7:17–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarado Y, Giles FJ and Swords RT: The
PIM kinases in hematological cancers. Expert Rev Hematol. 5:81–96.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan B, Yau EX, Samanta S, Ong CW, Yong KJ,
Ng LK, Bhattacharya B, Lim KH, Soong R, Yeoh KG, et al: Clinical
and therapeutic relevance of PIM1 kinase in gastric cancer. Gastric
Cancer. 15:188–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim J, Roh M and Abdulkadir SA: Pim1
promotes human prostate cancer cell tumorigenicity and c-MYC
transcriptional activity. BMC Cancer. 10:2482010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li S, Xi Y, Zhang H, Wang Y, Wang X, Liu H
and Chen K: A pivotal role for Pim-1 kinase in esophageal squamous
cell carcinoma involving cell apoptosis induced by reducing Akt
phosphorylation. Oncol Rep. 24:997–1004. 2010.PubMed/NCBI
|
|
10
|
Malinen M, Jääskeläinen T, Pelkonen M,
Heikkinen S, Väisänen S, Kosma VM, Nieminen K, Mannermaa A and
Palvimo JJ: Proto-oncogene PIM-1 is a novel estrogen receptor
target associating with high grade breast tumors. Mol Cell
Endocrinol. 365:270–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin Y, Tong DY, Tang LY, Chen JN, Zhou J,
Feng ZY and Shao CK: Expressions of osteopontin (OPN), ανβ3 and
Pim-1 associated with poor prognosis in Non-small cell lung cancer
(NSCLC). Chin J Cancer Res. 24:103–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weirauch U, Beckmann N, Thomas M,
Grünweller A, Huber K, Bracher F, Hartmann RK and Aigner A:
Functional role and therapeutic potential of the pim-1 kinase in
colon carcinoma. Neoplasia. 15:783–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nawijn MC, Alendar A and Berns A: For
better or for worse: The role of Pim oncogenes in tumorigenesis.
Nat Rev Cance. 11:23–34. 2011. View
Article : Google Scholar
|
|
14
|
Morishita D, Katayama R, Sekimizu K,
Tsuruo T and Fujita N: Pim kinases promote cell cycle progression
by phosphorylating and down-regulating p27Kip1 at the
transcriptional and posttranscriptional levels. Cancer Res.
68:5076–5085. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magnuson NS, Wang Z, Ding G and Reeves R:
Why target PIM1 for cancer diagnosis and treatment? Future Oncol.
6:1461–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling ZQ, Guo W, Lu XX, Zhu X, Hong LL,
Wang Z, Wang Z and Chen Y: A Golgi-specific protein PAQR3 is
closely associated with the progression, metastasis and prognosis
of human gastric cancers. Ann Oncol. 25:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nensa F, Stattaus J, Morgan B, Horsfield
MA, Soria JC, Besse B, et al: Dynamic contrast-enhanced MRI
parameters as biomarkers for the effect of vatalanib in patients
with non-small-cell lung cancer PIM kinases: An overview in tumors
and recent advances in pancreatic cancer PIM kinases: An overview
in tumors and recent advances in pancreatic cancer. Future Oncol.
10:823–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Xiong G, Cao Z, Huang H, Wang T, You
L, Zhou L, Zheng L, Hu Y, Zhang T and Zhao Y: PIM-1 contributes to
the malignancy of pancreatic cancer and displays diagnostic and
prognostic value. J Exp Clin Cancer Res. 35:1332016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matou-Nasri S, Rabhan Z, Al-Baijan H,
Al-Eidi H, Yahya WB, Al Abdulrahman A, Almobadel N, Alsubeai M, Al
Ghamdi S, Alaskar A, et al: CD95-mediated apoptosis in Burkitt's
lymphoma B-cells is associated with Pim-1 down-regulation. Biochim
Biophys Acta. 1863:239–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Cai ZD, Lou LM and Zhu YB:
Expressions of p53, c-MYC, BCL-2 and apoptotic index in human
osteosarcoma and their correlations with prognosis of patients.
Cancer Epidemiol. 36:212–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Y, Dong B, Tang L, Liu Y, Du H, Yuan
P, Wu A and Ji J: Apoptosis index correlates with chemotherapy
efficacy and predicts the survival of patients with gastric cancer.
Tumour Biol. 33:1151–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adell GC, Zhang H, Evertsson S, Sun XF,
Stål OH and Nordenskjöld BA: Apoptosis in rectal carcinoma:
Prognosis and recurrence after preoperative radiotherapy. Cancer.
91:1870–1875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu DR, Kato D, Watabe A, Endo Y and
Kadosawa T: Prognostic utility of apoptosis index, Ki-67 and
survivin expression in dogs with nasal carcinoma treated with
orthovoltage radiation therapy. J Vet Med Sci. 76:1505–1512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reséndiz-Martínez J, Asbun-Bojalil J,
Huerta-Yepez S and Vega M: Correlation of the expression of YY1 and
Fas cell surface death receptor with apoptosis of peripheral blood
mononuclear cells, and the development of multiple organ
dysfunction in children with sepsis. Mol Med Rep. 15:2433–2442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamazaki K, Hasegawa M, Ohoka I, Hanami K,
Asoh A, Nagao T, Sugano I and Ishida Y: Increased E2F-1 expression
via tumour cell proliferation and decreased apoptosis are
correlated with adverse prognosis in patients with squamous cell
carcinoma of the oesophagus. J Clin Pathol. 58:904–910. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shou Z, Lin L, Liang J, Li JL and Chen HY:
Expression and prognosis of FOXO3a and HIF-1α in nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 138:585–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu M, Zhao Y, Xu F, Wang Y, Xiang J and
Chen D: The expression and prognosis of FOXO3a and Skp2 in human
ovarian cancer. Med Oncol. 29:3409–3415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradley PJ: Adenoid cystic carcinoma of
the head and neck: A review. Curr Opin Otolaryngol Head Neck Surg.
12:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Dai J, Li T, Zhang P, Ma Q, Li Y,
Zhou J and Lei D: Expression of EMMPRIN in adenoid cystic carcinoma
of salivary glands: Correlation with tumor progression and
patients' prognosis. Oral Oncol. 46:755–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Peng B and Chen X: Expressions of
nuclear factor kappaB, inducible nitric oxide synthase, and
vascular endothelial growth factor in adenoid cystic carcinoma of
salivary glands: Correlations with the angiogenesis and clinical
outcome. Clin Cancer Res. 11:7334–7343. 2005. View Article : Google Scholar : PubMed/NCBI
|