Introduction
Acute leukemia is characterized as a heterogeneous
clonal disease with undifferentiated malignant growth. In total,
~140,000 people are diagnosed with acute leukemia worldwide
annually (1). Acute myeloid leukemia
(AML) is the most common type of acute leukemia in aging adults and
is considered responsible for the highest annual mortality rate by
leukemia globally (2). However, AML
remains one of the most difficult malignant hematological diseases
to treat, and has the lowest survival rate among all types of
leukemia (3). Therefore, a deeper
insight into the molecular etiology of AML will allow the
development of an effective natural anti-tumor drug.
Several recent developments have been instigated for
high efficacy and low toxicity antitumor drugs. Securinega
alkaloids have been isolated from the Euphorbiaceae family
of plants. Typically, securinine, an alkaloid from the leaves of
Securinega suffruticosa, was initially isolated by a Russian
scientist, Ia A, in 1956. Securinine has been structurally
characterized into two optical isomers: L-securinine and
virosecurinine, by Chinese scientists in 1963 (4). As a natural product, securinine was
observed to exert several important roles in biological systems
(5). Securinine is able to act as a
γ-aminobutyric acid receptor antagonist (6) and exhibit antimalarial (7) and antibacterial activities (8). Li et al (9) have demonstrated that virosecurinine is
able to exhibit apoptotic activity in human breast cancer MCF-7
cells, whereas Zhang et al (10) have demonstrated its apoptotic activity
in human chronic myeloid leukemia K562 cells. Therefore,
virosecurinine may be potentially used for cancer treatment.
Apoptosis is a physiological cell removal mechanism
that is critical in the cancer cell cycle (11). Cancer cells have evolved multiple
molecular mechanisms against the onset of apoptosis, and therefore
the signaling pathways induced by natural products, including
L-securinine, betulinic acid and resveratrol, may serve as key
factors for antitumor activities (12). One such example is the
phosphatidylinositol 3-kinase/protein kinase B/mammalian target of
rapamycin (PI3K/AKT/mTOR) signaling pathway, which is crucial for
proliferation, development and cell death (13). Constitutive activation of the
PI3K/AKT/mTOR signaling pathway is associated with the progression
and pathogenesis of a broad spectrum of various types of human
cancer, including acute leukemia (14–16).
Therefore, investigating novel approaches for inhibiting this
signaling pathway in order to develop targeted therapeutics while
limiting the side effects is vital for increasing treatment
efficacy and improving prognosis in patients (17).
The present study investigated the effects and
underlying mechanisms of virosecurinine on apoptosis in human AML
THP-1 cells. Furthermore, the present study also searched for
natural anti-tumor drugs that exhibit a high efficacy and low
toxicity.
Materials and methods
Chemicals
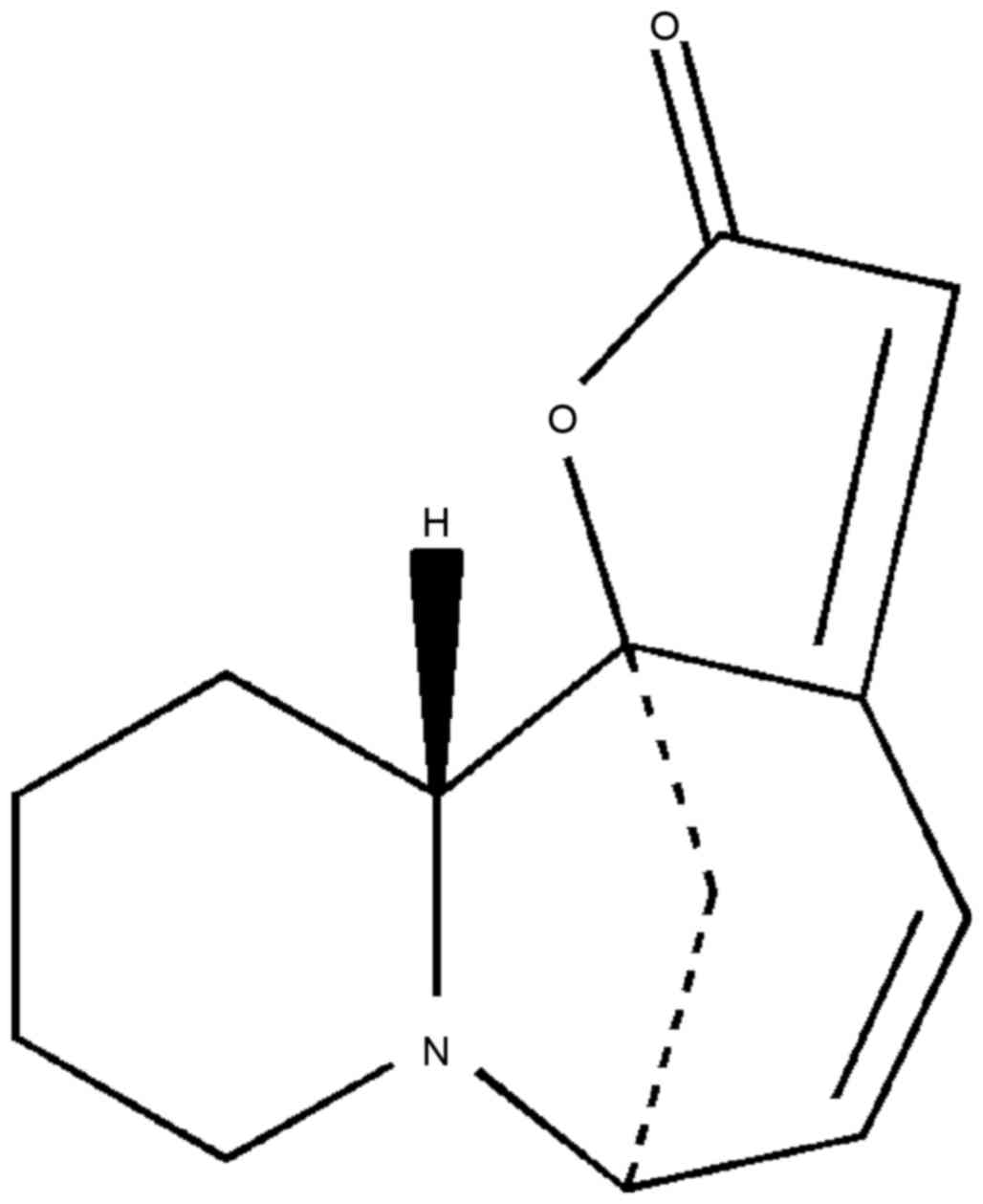
Virosecurinine (Fig.
1) was provided by the Institute of Traditional Chinese
Medicine and Natural Products, Jinan University (Guangzhou, China).
Cell Counting Kit-8 (CCK-8) was purchased from Nanjing KeyGen
Biotech Co., Ltd., (catalog no. KGA317; Nanjing, China). The cell
culture media (RPMI-1640) and solutions were bought from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell line and culture
Human AML THP-1 cell lines were obtained from the
Key Gen Serving Science Company and were grown in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS;
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China), 100 IU/ml penicillin, and 100 µg/ml streptomycin.
The cells were grown and maintained at 37°C in a humidified
incubator with 5% CO2.
Cell viability analysis
Cell Counting Kit-8 assay (CCK-8; catalog no.
KGA317; Nanjing KeyGen Biotech Co., Ltd.) was used to measure cell
viability. THP-1 cells in the exponential phase were plated in 100
µl into 96-well plates (Corning Incorporated, Corning, NY, USA) at
a density of 5×103 cells/well. After 24 h at 37°C, THP-1
cells were replenished with RPMI-1640 medium containing 10% FBS and
treated with virosecurinine (200 µl/well) at concentrations ranging
from 0 to 200 µmol/l. Then, the plates were incubated in a
humidified incubator for 24, 48, and 72 h at 37°C. A total of 10 µl
CCK-8 solution was added to each well three hours prior to
measurement of absorbance. The optical density was measured at 450
nm with a microplate reader (EL-x 800; BioTek Instruments, Inc.,
Winooski, VT, USA). Relative cell proliferation inhibition rate
(IR) was calculated using the following formula: IR=(absorbance of
the control group-absorbance of the experimental group)/(absorbance
of the control group-absorbance of the blank control group)x100%.
All experiments were performed four times.
Transmission electron microscopy
THP-1 cells were seeded at a density of
5×105 cells/well in 6-well plates (Corning Incorporated)
with or without 12.5 µmol/l virosecurinine for 48 h at 37°C.
Subsequently, the cells were harvested and fixed for 2 h at 4°C in
2.5% chilled glutaraldehyde followed by three washes in 0.1 mol/l
PBS (Nanjing KeyGen Biotech Co., Ltd.). The cells were then
post-fixed at 4°C in 1% osmium tetroxide for 2 h, dehydrated
sequentially in 50, 70, 90 and 100% ethanol for 15 min each (three
times in 100% ethanol), and embedded in epoxy resin. Consequently,
the embedded cells were sliced into 50–60 nm sections and stained
with uranyl acetate for 30 min at room temperature and lead citrate
for 30 min at room temperature, and subsequently observed under a
transmission electron microscope (TEM-1011; JEOL, Ltd., Tokyo,
Japan).
Apoptosis analysis
For apoptosis analysis, THP-1 cells were cultured in
6-well plates at a density of 3.0×105 cells/well for 24
h, and then treated with various concentrations of virosecurinine,
6.25, 12.5 and 25 µmol/l, respectively, for 48 h. The cells were
washed twice with cold PBS and gently resuspended in 500 µl binding
buffer. Thereafter, 5 µl annexin V-fluorescein isothiocyanate
(FITC) (KGA105; Nanjing KeyGen Biotech Co., Ltd.) and propidium
iodide (KGA511; Nanjing KeyGen Biotech Co., Ltd.) were added. After
15 min incubation at room temperature in the dark, the cells were
subjected to flow cytometric analysis. The fluorescence was
measured using a flow cytometer (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA) equipped with an argon laser (488 nm). The
percentage of apoptotic cells was calculated using the FACScan
software (version 6.0; BD Biosciences).
Cell cycle analysis
For cell cycle analysis, THP-1 cells were cultured
in 6-well plates and treated with virosecurinine as described
above. The cells were then washed, harvested and fixed with 70%
ethanol at 4°C overnight. Subsequently, the cells were treated with
Tris-HCl buffer (pH 7.4) containing 1% RNase A (KGA511; Nanjing
KeyGen Biotech Co., Ltd.) and stained with propidium iodide (PI, 5
mg/ml; Nanjing KeyGen Biotech Co., Ltd.). The distribution of cells
with different DNA contents was determined by flow cytometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR assays were performed on THP-1 cells treated
with or without virosecurinine in order to evaluate the expression
of PI3K, AKT, mTOR and phosphatase and tensin homolog (PTEN). Total
RNA was isolated from THP-1 cells with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. A two-step reverse transcription PCR was performed.
First-strand cDNA synthesis was performed using the ProSTARt First
Strand RT-PCR kit (catalog no. PC0002; Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
efficiency of cDNA synthesis from each sample was estimated by PCR
with GAPDH-specific primers. CK8 and GAPDH genes were quantified by
RT-PCR for mRNA level and as an endogenous control, respectively.
Subsequently, 20 µl reaction mixture was used for qPCR, using a
qPCR kit (catalog no. DA7600; OriGene Technologies, Inc.,
Rockville, MD, USA), which consisted of 40 cycles: Denaturation (15
sec at 94°C), annealing (20 sec at 60°C) and extension (40 sec at
72°C). The reaction mixture (catalog no. EP0702; Fermentas; Thermo
Fisher Scientific, Inc.) contained 10 µM of each primer, 2 µl of 2×
QuantiTect SYBR Green RT-PCR Master Mix, 10 µl QuantiTect reverse
transcriptase mix, and 8 µl nuclease-free water. The relative
quantification was analyzed by the 2−ΔΔCq method
(18) with GAPDH as the housekeeping
gene and the control cells as the baseline. The results were
expressed as fold changes. Each experiment was repeated three
times. The sequences of the primers used are as follows: PI3K
forward, 5′-GGGGATGATTTACGGCAAGATA-3′ and reverse,
5′-CACCACCTCAATAAGTCCCACA-3′; AKT forward,
5′-GCAGCACGTGTACGAGAAGA-3′ and reverse, 5′-GGTGTCAGTCTCCGACGTG-3′;
mTOR forward, 5′-ATTTGATCAGGTGTGCCAGT-3′, and reverse,
5′-GCTTAGGACATGGTTCATGG-3′; PTEN forward,
5′-CAAGATGATGTTTGAAACTATTCCAATG-3′, and reverse,
5′-CCTTTAGCTGGCAGACCACAA-3′; GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′, and reverse,
5′-CTCCACGACGTACTCAGCG-3′.
Statistical analysis
All data are represented as the mean ± standard
deviation. SPSS (version 18.0; SPSS Inc., Chicago, IL, USA) was
used for statistical analyses. Statistically significant
differences between the groups were analyzed by Student's t-test,
and multiple comparisons were performed using one-way analysis of
variance followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Virosecurinine treatment inhibits
growth THP-1 cells in vitro
In order to determine the mechanistic effects of
virosecurinine-induced apoptosis, CCK-8 assay was employed to
investigate the proliferation of THP-1 cells. The inhibitory effect
on proliferation was determined by treating cells with a range of
virosecurinine concentrations ranging from 0 to 200 µmol/l for 24,
48 and 72 h, respectively. The assay revealed that treatment with
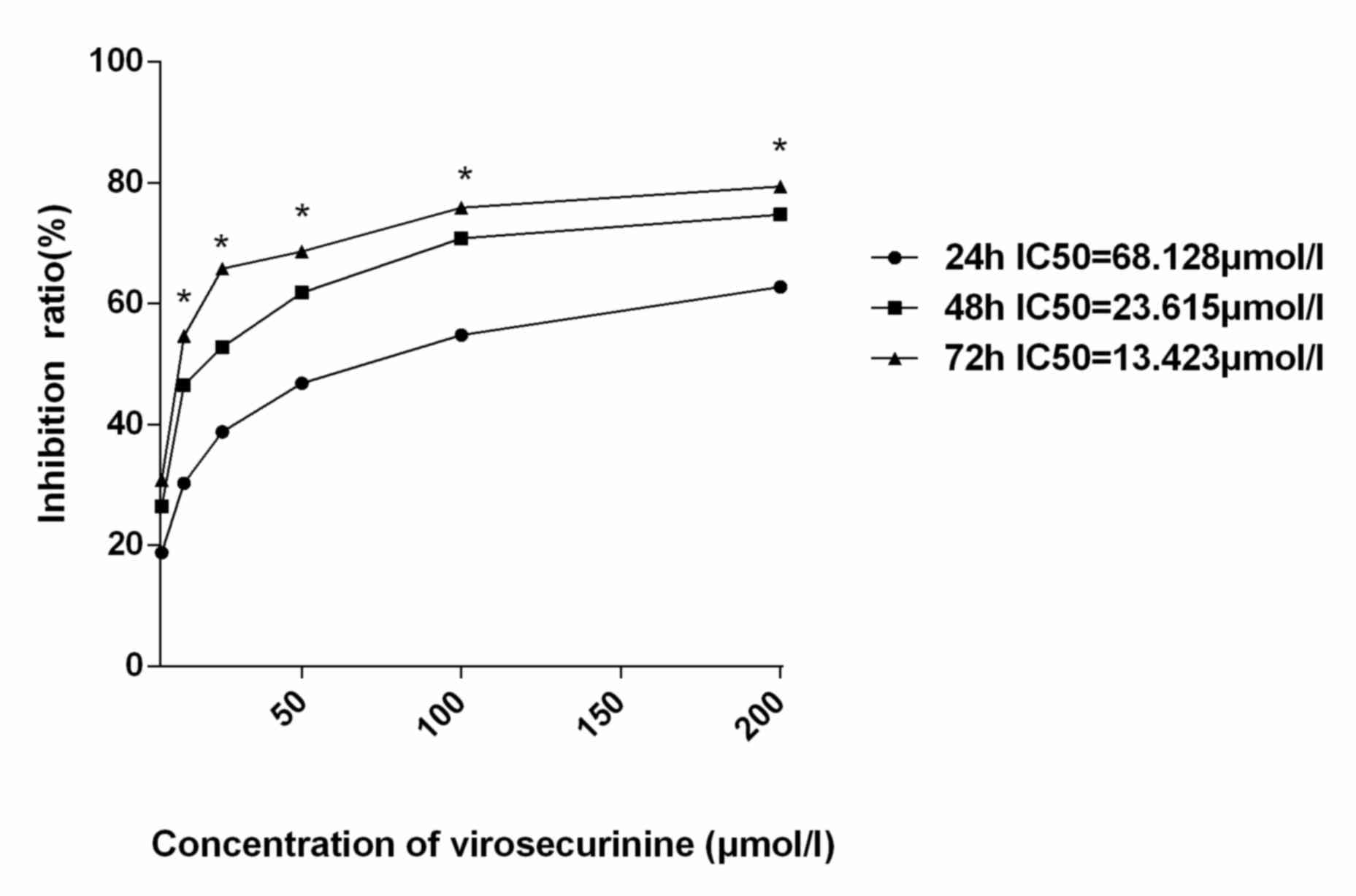
virosecurinine was able to significantly inhibit the proliferation
of THP-1 cells in a dose- and time- dependent manner (Fig. 2). The IC50 values at 24, 48
and 72 h following treatment were 68.128, 23.615 and 13.423 µmol/l,
respectively.
Ultrastructure of THP-1 cells
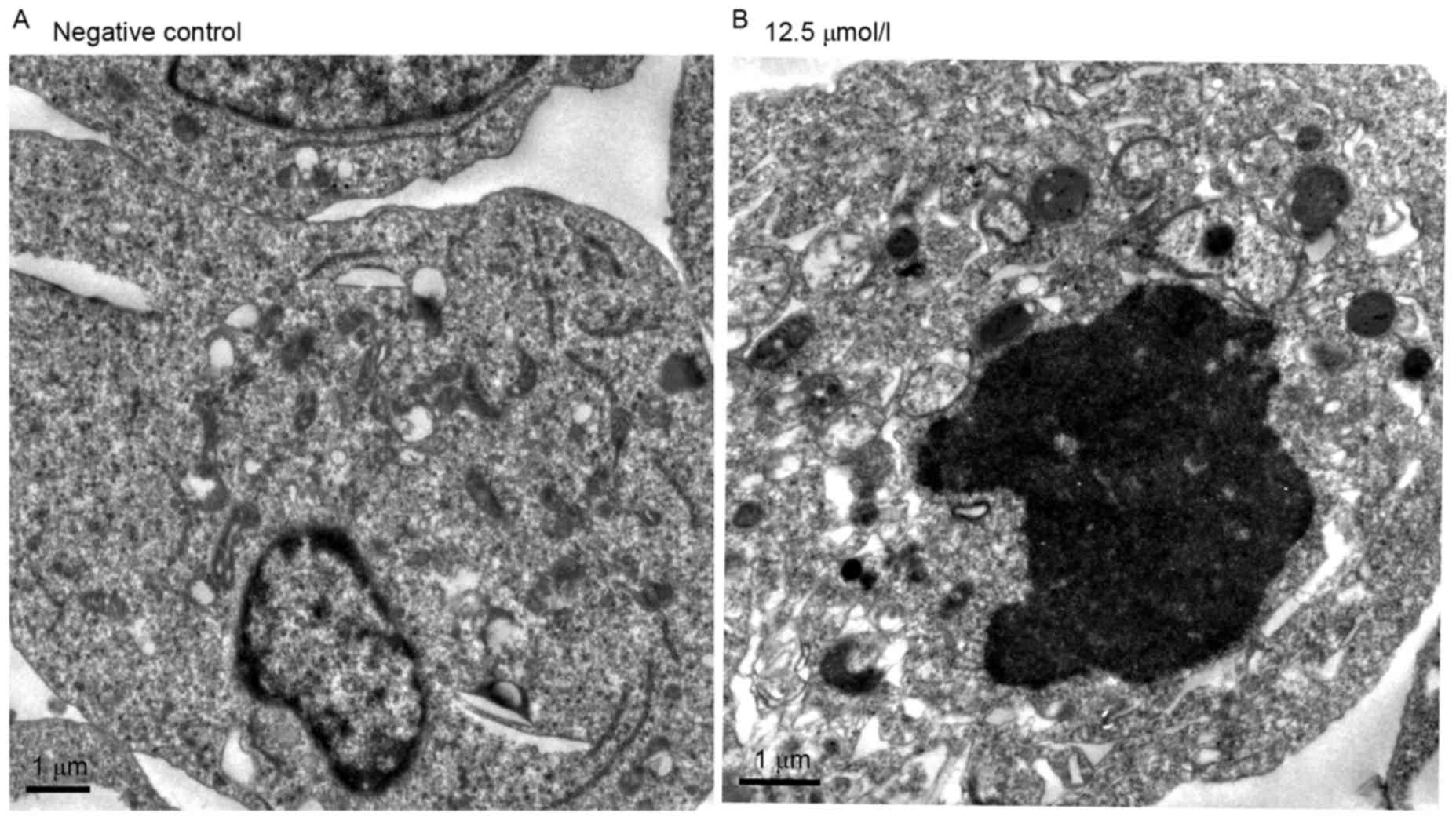
The ultra-structural analysis of
virosecurinine-induced apoptosis in THP-1 cells was carried out by
electron microscopy (Fig. 3). The
appearance of apoptotic bodies in THP-1 cells that were treated
with 12.5 µmol/l virosecurinine for 48 h (Fig. 3B) confirmed that apoptosis was taken
place in these treated cells. Similar results were not observed in
untreated cells (Fig. 3A).
Virosecurinine treatment inhibits cell
cycle progression in THP-1 cells
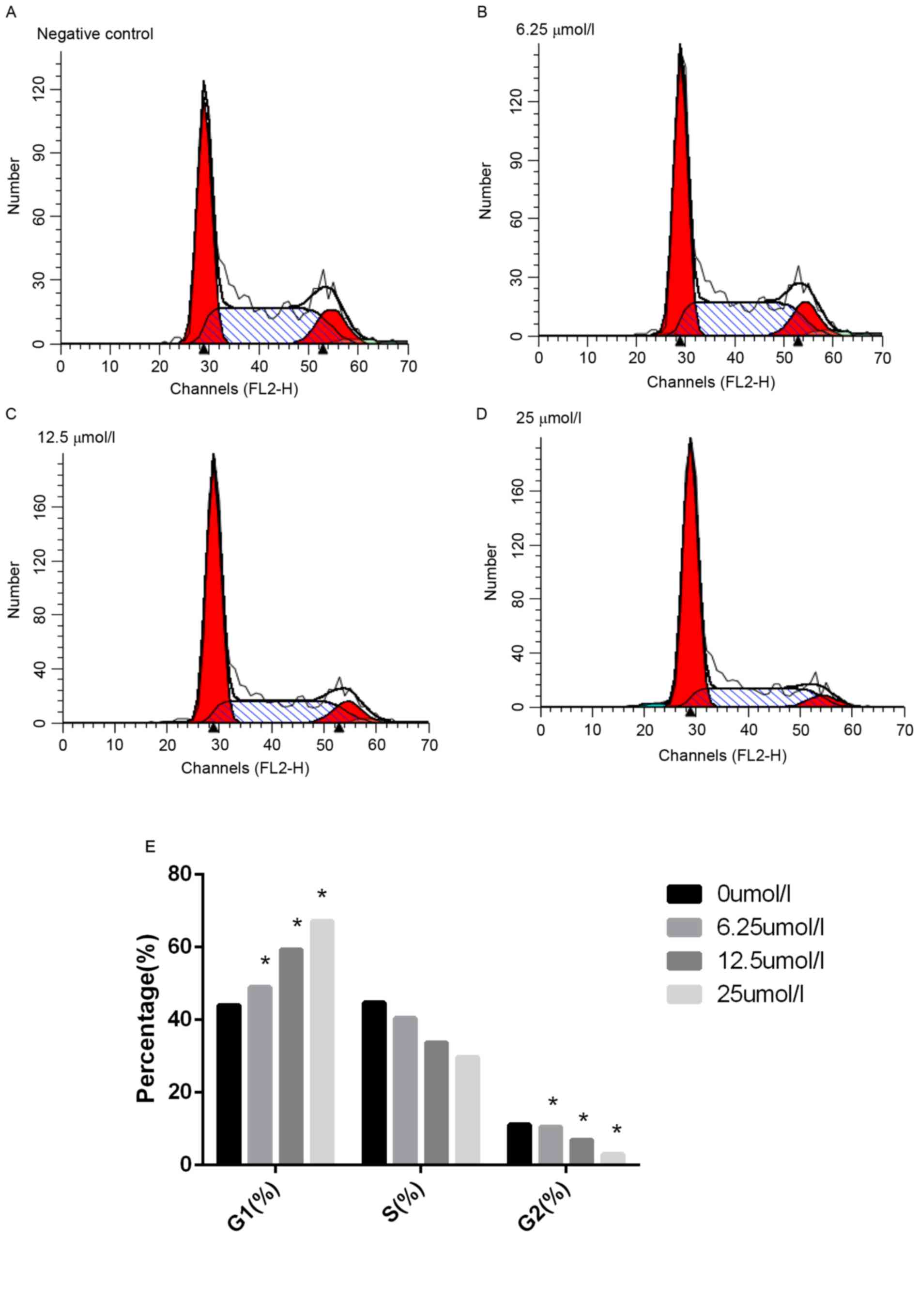
To understand the mechanisms of
virosecurinine-induced THP-1 cell apoptosis, flow cytometric cell
cycle analysis of THP-1 cells following treatment with 0, 6.25,
12.5 and 25 µmol/l virosecurinine, respectively, for 48 h was
performed. The results demonstrated that treatment with
virosecurinine resulted in cell-cycle arrest in the G1 phase.
(Fig. 4). Notably, a sub-G1 peak was
observed, which represented an apoptotic population as a response
to virosecurinine treatment.
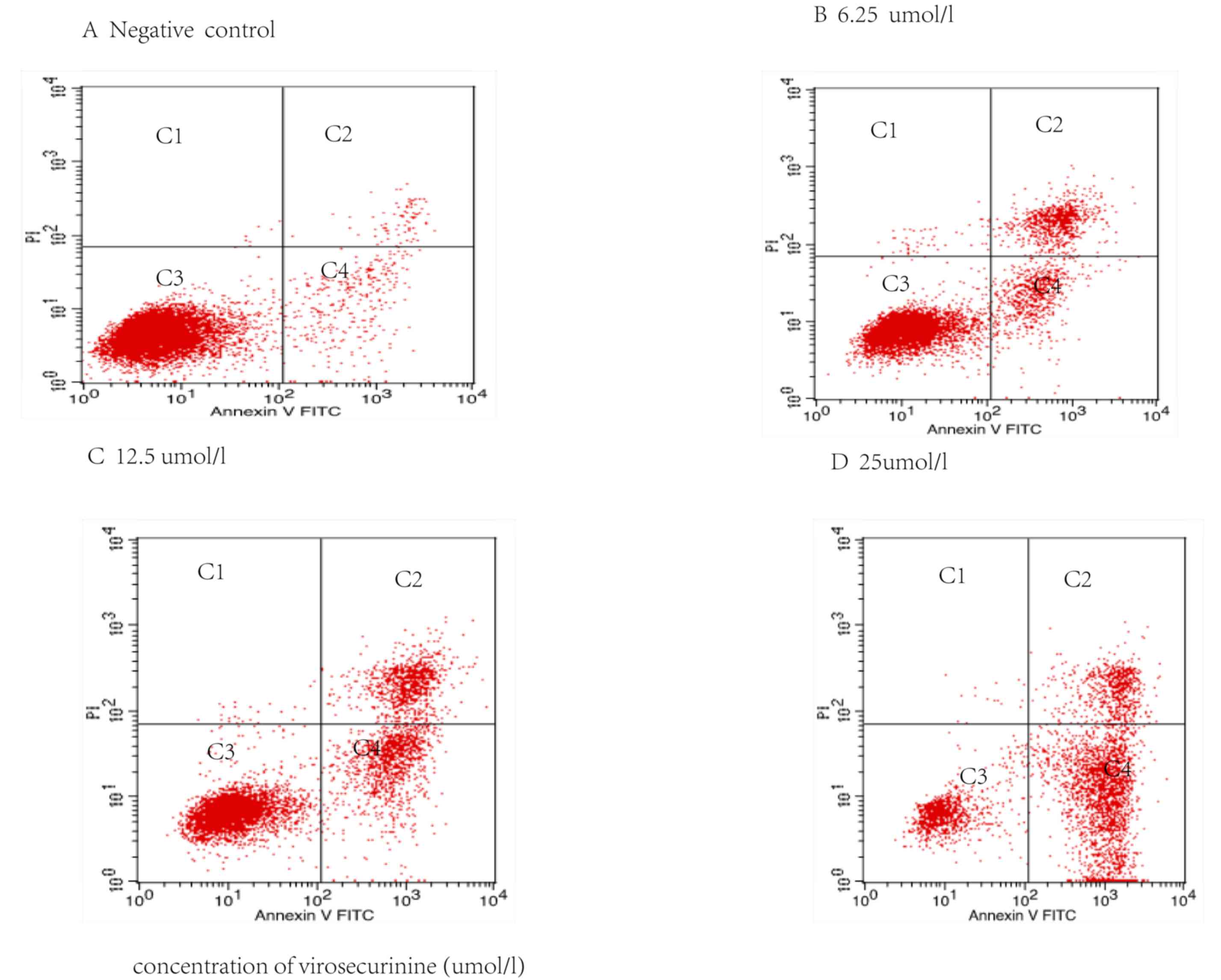
Virosecurinine treatment induces
apoptosis in THP-1 cells in vitro
Apoptotic rate in THP-1 cells treated with
virosecurinine was determined by FITC-annexin V and PI double
staining followed by flow cytometric analysis. The quantification
of cells in each quadrant in Fig. 5
are representative of necrosis (C1), late apoptosis (C2), live
cells (C3) and early apoptosis (C4). This indicated that
virosecurinine concentration was directly proportional to the rate
of apoptosis in THP-1 cells. The proportion of apoptotic cells
treated with 6.25, 12.5 and 25 µmol/l of virosecurinine for 48 h
was 25.47, 38.29 and 64.31%, respectively (Fig. 5).
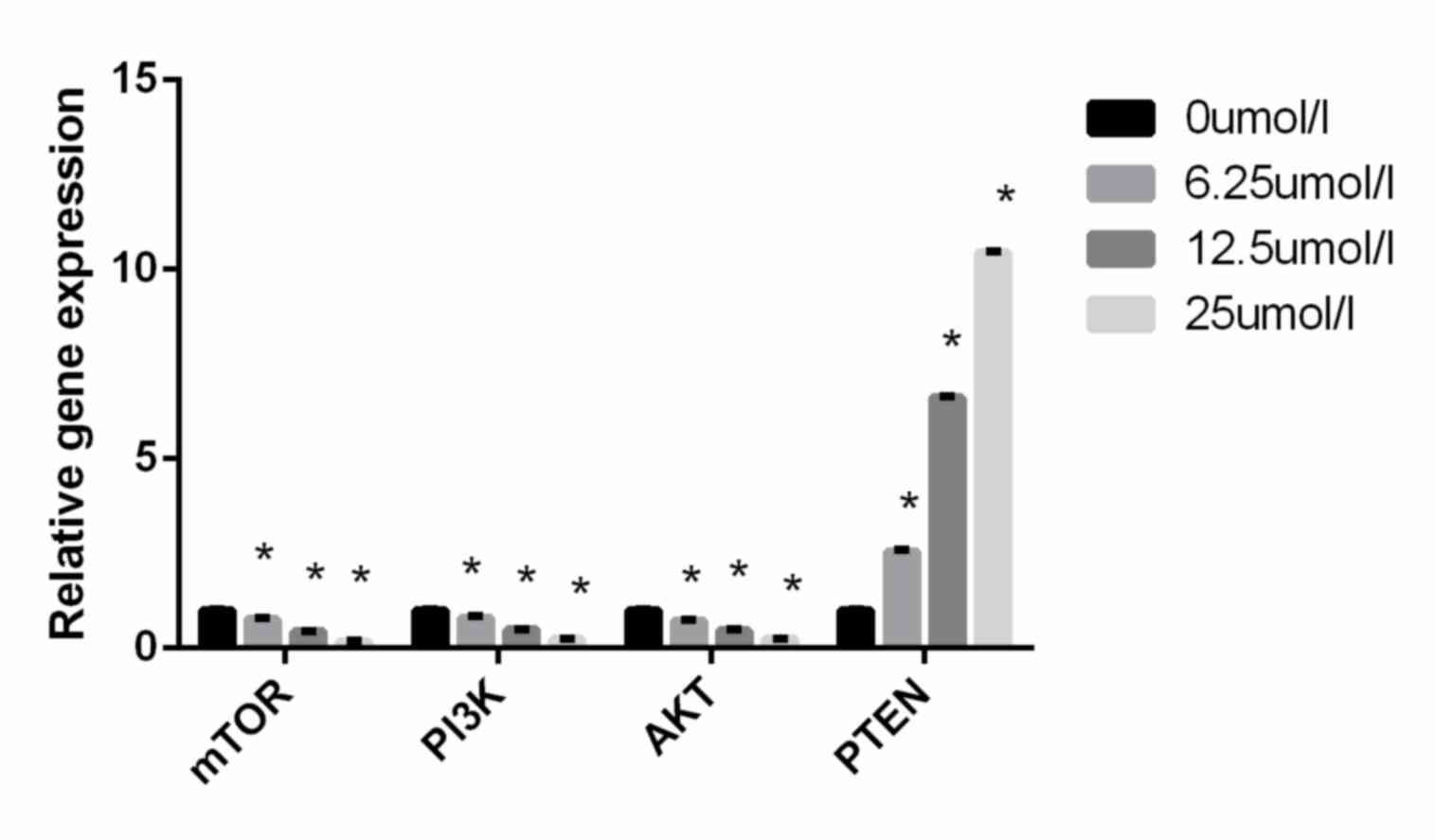
Virosecurinine treatment affects the
expression of genes in the PI3K/AKT/mTOR signaling pathway in THP-1
cells
To further elucidate the mechanism underlying
virosecurinine-induced apoptosis in THP-1 cells, the expression of
PI3K, AKT, mTOR and PTEN in treated and control THP-1 cells was
evaluated. RT-qPCR analysis revealed that treatment with
virosecurinine was able to downregulate the level of PI3K, AKT and
mTOR expression and upregulate the expression of PTEN (P<0.05;
Fig. 6). These results suggested that
virosecurinine-induced apoptotic cell death was associated with the
activation of PI3K, AKT, mTOR and PTEN.
Discussion
AML treatment remains a major challenge due to poor
efficacy of the current chemotherapeutics. Therefore, investigating
natural plants as resources for antitumor agents is an increasingly
important topic in cancer research. Herein, the effect of
virosecurinine on the proliferation of human AML THP-1 cells was
investigated. In the present study, it was demonstrated that
virosecurinine was able to inhibit proliferation of THP-1 cells at
low concentrations. The IC50 values were determined to
be 68.128, 23.615, and 13.423 µmol/l, respectively at 24, 48 and 72
h post-treatment. The US National Cancer Institute Plant Screening
Program demonstrated in vitro cytotoxicity of a crude
extract, with a IC50 value of <20 µg/ml (919 µmol/l)
following incubation between 48 and 72 h (19). Therefore, the present study exhibited
the in vitro cytotoxic activity of virosecurinine in THP-1
cells. This result also illustrated that the proliferation of THP-1
cells was markedly inhibited by virosecurinine in a dose- and
time-dependent manner. Moreover, apoptosis was also confirmed by
the appearance of apoptotic bodies, and a sub-G1 peak was observed
in THP-1 cells that were treated with 25 µmol/l virosecurinine for
48 h.
Several studies demonstrated that apoptosis and
autophagy are two predominant cell death routes in various types of
cancer (20,21). Apoptosis or programmed cell death can
be activated by anti-neoplastic drugs, which interfere with cell
proliferation mediated by (22). In
agreement with previous studies, the present study also
demonstrated that treatment with virosecurinine was able to induce
apoptosis in AML cell line THP-1 via an inhibitory effect on the
cell apoptosis. The PI3K/AKT/mTOR signaling pathway is one of the
major intracellular pathways, which are tightly regulated under
normal physiological conditions. However, the PI3K/AKT/mTOR
signaling pathway is frequently activated in human cancer types
(23,24). Upon stimulation by receptor tyrosine
kinases or G-protein coupled receptors, PI3K is translocated to the
plasma membrane, resulting in the phosphorylation of
phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol
3,4,5-triphosphate. In previous studies, dysregulation of
PI3K/AKT/mTOR signaling has been observed to exhibit a vital role
in the onset of various types of human cancer (25,26). This
may be an innate characteristic of the signaling pathway or the
consequence of mutations that are able to activate or regulate the
PI3K/AKT/mTOR signaling pathway. Some of these mutations include
activating mutations in Fms-like tyrosine kinase, N- or KRAS, and
c-kit tyrosine kinase receptor (27–29). The
phosphatase, PTEN, functions as a tumor suppressor through negative
regulation of the PI3K/AKT/mTOR signaling pathway (30). Inactivation of PTEN leads to increased
ATP-binding cassette transporter G2 expression, which inhibits the
PI3K/AKT/mTOR signaling pathway, thereby designating it as a
potential therapeutic target in the treatment of AML (31). The factors modulating PI3K and mTOR
have been accentuated to function in a synergistic association with
the current chemotherapeutic drugs in the treatment of AML
(32,33).
To further investigate the molecular etiology,
analysis of the four apoptosis-linked genes demonstrated that
treatment with virosecurinine was able to downregulate PI3K, AKT
and mTOR gene expression and upregulate the PTEN expression in
THP-1 cells. Herein, to the best of our knowledge, it was
demonstrated for the first time that virosecurinine is able to
induce apoptosis in THP-1 cells, which is regulated by altered
expression of PI3K, AKT, mTOR and PTEN. These results suggest that
virosecurinine is an effective agent for suppressing the
proliferation of AML THP-1 cells and that this may be partially
mediated by the downregulation of PI3K, AKT and mTOR and
upregulation of PTEN.
In the present study, it was indicated that
virosecurinine may be a potential therapeutic for the prevention
and treatment of AML.
Acknowledgements
The present study was supported by the Jiaxing
Haematology Key Discipline Fund (grant no. 04-Z-13). The authors
would like to thank the Institute of Traditional Chinese Medicine
& Natural Products, Jinan University (Guangdong, China) for
providing a pure sample of virosecurinine.
References
|
1
|
Elert E: Living with leukaemia. Nature.
498:S2–S3. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Donnell MR, Tallman MS, Abboud CN,
Altman JK, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE,
Damon LE, et al: Acute myeloid leukemia, version 2.2013. J Natl
Compr Canc Netw. 11:1047–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deschler B and Lübbert M: Acute myeloid
leukemia: Epidemiology and etiology. Cancer. 107:2099–2107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito S, Kotera K, Shigematsu N, Ide A,
Sugimoto N, Horii Z, Hanaoka M, Yamawaki Y and Tamura Y: Structure
of securinine. Tetrahedron. 19:2085–2099. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CR, Xia YH, Yao SY, Zhang Q, Wang Y
and Ji ZN: Virosecurinine induces apoptosis by affecting Bcl-2 and
Bax expression in human colon cancer SW480 cells. Pharmazie.
67:351–354. 2012.PubMed/NCBI
|
|
6
|
Beutler JA, Karbon EW, Brubaker AN, Malik
R, Curtis DR and Enna SJ: Securinine alkaloids: A new class of GABA
receptor antagonist. Brain Res. 330:135–140. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weenen H, Nkunya MH, Bray DH, Mwasumbi LB,
Kinabo LS, Kilimali VA and Wijnberg JB: Antimalarial compounds
containing an alpha, beta-unsaturated carbonyl moiety from
Tanzanian medicinal plants. Planta Med. 56:371–373. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mensah JL, Lagarde I, Ceschin C, Michel G,
Gleye J and Fouraste I: Antibacterial activity of the leaves of
Phyllanthus discoideus. J Ethnopharmacol. 28:129–133. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li M, Han S, Zhang G, Wang Y and Ji Z:
Antiproferative activity and apoptosis-inducing mechanism of
L-securinine on human breast cancer MCF-7 cells. Pharmazie.
69:217–223. 2014.PubMed/NCBI
|
|
10
|
Zhang G, Li M, Han S, Chen D, Wang Y, Ye
W3 and Ji Z: Induction of human chronic myeloid leukemia K562 cell
apoptosis by virosecurinine and its molecular mechanism. Mol Med
Rep. 10:2365–2371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lima RT, Busacca S, Almeida GM, Gaudino G,
Fennell DA and Vasconcelos MH: MicroRNA regulation of core
apoptosis pathways in cancer. Eur J Cancer. 47:163–174. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fulda S: Modulation of apoptosis by
natural products for cancer therapy. Planta Med. 76:1075–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt-Kittler O, Zhu J, Yang J, Liu G,
Hendricks W, Lengauer C, Gabelli SB, Kinzler KW, Vogelstein B, Huso
DL and Zhou S: PI3Kα inhibitors that inhibit metastasis.
Oncotarget. 1:339–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng C, Chen Y, Li D and Li S: Role of
PTEN in leukemia stem cells. Oncotarget. 1:156–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kharas MG, Okabe R, Ganis JJ, Gozo M,
Khandan T, Paktinat M, Gilliland DG and Gritsman K: Constitutively
active Akt depletes hematopoietic stem cells and induces leukemia
in mice. Blood. 115:1406–1415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dancey JE, Bedard PL, Onetto N and Hudson
TJ: The genetic basis for cancer treatment decisions. Cell.
148:409–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castello-Branco MVS, Tavares JF, Silva MS,
Barbosa Filho JM, Anazetti MC, Frungillo L, Haun M, Melo Diniz MF
and Melo PS: Xylodiol from Xylopia langsdorfiana induces apoptosis
in HL60 cells. Rev Bras Farmacogn. 21:1035–1042. 2011. View Article : Google Scholar
|
|
20
|
Subramaniya BR, Srinivasan G, Sadullah SS,
Davis N, Subhadara LB, Halagowder D and Sivasitambaram ND:
Apoptosis inducing effect of plumbagin on colonic cancer cells
depends on expression of COX-2. PLoS One. 6:e186952011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YC, He SM, He ZX, Li M, Yang Y, Pang
JX, Zhang X, Chow K, Zhou Q, Duan W, et al: Plumbagin induces
apoptotic and autophagic cell death through inhibition of the
PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells.
Cancer Lett. 344:239–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nahata A, Saxena A, Suri N, Saxena AK and
Dixit VK: Sphaeranthus indicus induces apoptosis through
mitochondrial-dependent pathway in HL-60 cells and exerts cytotoxic
potential on several human cancer cell lines. Integr Cancer Ther.
12:236–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L and Vogt PK: Class I PI3K in
oncogenic cellular transformation. Oncogene. 27:5486–5496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sokolosky ML, Stadelman KM, Chappell WH,
Abrams SL, Martelli AM, Stivala F, Libra M, Nicoletti F, Drobot LB,
Franklin RA, et al: Involvement of Akt-1 and mTOR in sensitivity of
breast cancer to targeted therapy. Oncotarget. 2:538–550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Altman JK, Sassano A and Platanias LC:
Targeting mTOR for the treatment of AML. New agents and new
directions. Oncotarget. 2:510–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muranyi AL, Dedhar S and Hogge DE:
Combined inhibition of integrin linked kinase and FMS-like tyrosine
kinase 3 is cytotoxic to acute myeloid leukemia progenitor cells.
Exp Hematol. 37:450–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Faderl S, Pal A, Bornmann W, Albitar M,
Maxwell D, Van Q, Peng Z, Harris D, Liu Z, Hazan-Halevy I, et al:
Kit inhibitor APcK110 induces apoptosis and inhibits proliferation
of acute myeloid leukemia cells. Cancer Res. 69:3910–3917. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Birkenkamp KU, Geugien M, Schepers H,
Westra J, Lemmink HH and Vellenga E: Constitutive NF-kappaB
DNA-binding activity in AML is frequently mediated by a
Ras/PI3-K/PKB-dependent pathway. Leukemia. 18:103–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maiuri MC, Tasdemir E, Criollo A, Morselli
E, Vicencio JM, Carnuccio R and Kroemer G: Control of autophagy by
oncogenes and tumor suppressor genes. Cell Death Differ. 16:87–93.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY,
Li WJ, Chen XP, Zhao XL, Chen FP and Zeng H: Inactivation of PTEN
increases ABCG2 expression and the side population through the
PI3K/Akt pathway in adult acute leukemia. Cancer Lett. 336:96–105.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Q, Thompson JE and Carroll M: mTOR
regulates cell survival after etoposide treatment in primary AML
cells. Blood. 106:4261–4268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Batista A, Barata JT, Raderschall E,
Sallan SE, Carlesso N, Nadler LM and Cardoso AA: Targeting of
active mTOR inhibits primary leukemia T cells and synergizes with
cytotoxic drugs and signaling inhibitors. Exp Hematol.
39:457–472.e3. 2011. View Article : Google Scholar : PubMed/NCBI
|