Introduction
Breast cancer, with increasing rates of incidence
and mortality worldwide, is a major cause of mortality within
female malignancies (1,2). Due to its late disease presentation,
breast cancer exhibits poor prognosis and frequently presents with
distant metastases (3). Clinically,
it is difficult to treat advanced breast cancer due to the
inability to completely resect the diffused tumor cells and to
overcome the chemoresistance of cancer cells to chemotherapy.
Paclitaxel (Taxol®) is a widely used chemotherapeutic
drug for the treatment of breast cancer, and acts through the
induction of proapoptotic signaling, blocking of the cell cycle in
the G2-M phases and stabilization of the microtubule
(4,5).
Although breast cancer cells demonstrate high sensitivity to
Taxol®, the prognosis of patients with advanced disease
remains poor due to chemoresistance to Taxol®.
Therefore, it is important to study the underlying mechanisms
involved in the development of Taxol® resistance, to
improve the effectiveness of chemotherapy.
Twist is a member of the basic helix-loop-helix
(bHLH) transcription factor family. It includes a bHLH domain that
mediates heterodimerization or homodimerization and a DNA binding
domain, which combines with DNA sequences (6). Functionally, Twist was primitively
identified as a potential oncogene (6,7), and
previous studies have identified that Twist also contributed to
acquired Taxol® resistance (8) and metastasis in cancer (9). In addition, a previous study indicated
that the upregulation of Twist was positively associated with the
level of disease aggression and poor survival rate (10), suggesting Twist may be a potential
target for cancer therapy. Although elevated expression of Twist
was revealed to be associated with Taxol® resistance,
the molecular mechanism remains unclear. Notably, a series of
studies demonstrated that multidrug resistance (MDR)-associated
proteins served a critical role in chemical resistance, such as
Taxol® resistance (11,12).
Previously, lung resistance-related protein (LRP), topoisomerase
IIα (TOPO IIα), MDR-associated protein (MRP) and P-glycoprotein
(P-gp) have attracted attention for their functions as
MDR-associated proteins (13), which
may induce MDR in chemotherapy through increasing or decreasing
drug efflux, inactivation of drug and alteration of drug targets
(13). LRP, a major vault protein,
pumps drugs away from intracellular targets to trigger drug
resistance (14,15). TOPO IIα, a nuclear enzyme, regulates
the topology of DNA and maintains genomic integrity (16). A previous study suggested that the
overexpression of TOPO IIα is markedly associated with alterations
in tumor behavior and chemotherapeutic resistance via the
inhibition of apoptosis (17). MRP
and P-gp, two important adenosine 5-triphosphate (ATP)-binding
cassette transporter proteins, mediated intracellular drug influx
or efflux to alter the concentration of drugs, which increased
chemoresistance to therapeutic agents, including Taxol®
and anthracyclines (18). Although
Twist also affects drug resistance, no studies have explored the
association between Twist and MDR proteins. Therefore, the present
study aimed to examine the association between Twist and MDR
proteins in order to identify a novel mechanism of
chemoresistance.
The present study was performed to investigate the
association between Twist and MDR-associated proteins. In order to
identify how Twist increases drug resistance in cancer, a MCF-7
cell model of Taxol® resistance was generated by
repeatedly exposing MCF-7 cells to Taxol®, and a
293-cell model of Twist overexpression was created through
transfecting pcDNA5/FRT/TO-Twist vectors into 293 cells.
Concurrently, the expression of Twist, LRP, MRP, TOPO IIα and P-gp
were detected by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) or immunohistochemistry in these two cell
models to determine the clear association between Twist and these 4
types of MDR-associated proteins, and to uncover the underlying
molecular mechanisms.
Materials and methods
Cell culture
The MCF-7 cell line was a gift from the Infection
and Immunology Laboratory of Southwest Medical University (Luzhou,
China). MCF-7 cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc.),
1×105 U/l penicillin and 100 mg/l streptomycin at 37°C
with 5% CO2. The 293 cell line was also a gift from the
Genetic Laboratory of Southwest Medical University. 293 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FCS,
1×105 U/l penicillin and 100 mg/l streptomycin at 37°C
with 5% CO2. The morphology of 293 cell was observed
using a light microscope (Olympus Corporation, Tokyo, Japan) at
×400 magnification, and the evaluation of shape and size of
293-Twist and 293-vector were based on microscopic examination.
Construction of MCF-7 cell model of
Taxol® resistance
The Taxol®-resistant breast cancer cell
line was established by exposing the Taxol®-sensitive
mammary cell line MCF-7 to 15 µg/m Taxol® (Taiji
Industry (Group) Co., Ltd., Chongqing, China), as follows: MCF-7
cells were cultured in RPMI-1640 medium with 15 µg/ml
Taxol® for 24 h at 37°C with 5% CO2, followed
by washing with warm phosphate-buffered saline (PBS) for 2 min
three times and culturing in RPMI-1640 medium without
Taxol® at 37°C with 5% CO2 until cells grew
normally. These cells were labeled MCF-7/Taxol® I.
Subsequent to culturing in RPMI-1640 medium without
Taxol® for 10 days at 37°C, the MCF-7/Taxol®
I cells were cultured with 15 µg/ml Taxol® for 24 h at
37°C, followed by washing with warm PBS for 2 min three times and
culturing in RPMI-1640 medium without Taxol® at 37°C
until the cells grew normally, these cells were labeled
MCF-7/Taxol® II. Cells from step 2
(MCF-7/Taxol® II cells) were cultured with 15 µg/ml
Taxol® for 24 h at 37°C after 10 days of normal culture
without Taxol®, followed by washing with warm PBS for 2
min three times and culturing in RPMI-1640 medium without
Taxol® at 37°C until cells grew normally, these cells
were labeled to MCF-7/Taxol® III.
MCF-7/Taxol® IV cells were generated by exposing
MCF-7/Taxol® III to Taxol® as aforementioned
(15 µg/ml Taxol® for 24 h at 37°C). The half maximal
inhibitory concentration (IC50) and Taxol®
resistance index (RI) of MCF-7 and MCF-7/Taxol® cell
lines were calculated based on an MTT assay as follows: Cell growth
inhibition ratio (%)=[optical density
(OD)control-ODexperiment]/ODcontrol
× 100%; and RI=resistance cell IC50/parent cell
IC50. Cells in 96-well plates were treated with 0.5% MTT
(Affymetrix; Thermo Fisher Scientific, Inc.) for 4 h at 37°C,
followed by the addition of 150 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA) to dissolve the purple formazan. Cells
were then agitated for 10 min at room temperature and the
absorbance at 570 nm was read using a reference filter of 630 nm.
MCF-7/Taxol® IV and MCF-7 cells were treated with
vincristine (0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10 and 15 µg/ml)
(Guangdong Lingnan Pharmaceutical Co., Ltd., Guangzhou, China),
cisplatin (data not shown; Sigma-Aldrich; Merck KGaA), fluorouracil
(0, 0.625, 1.25, 2.5, 5, 10, 20 and 40 µg/ml) (5-FU; data not
shown; Xian Haixin Pharmaceutical Co., Ltd., Xi'an, China) and
mitomycin (data not shown; Sigma-Aldrich; Merck KGaA) to determine
the ability of these cells to acquire multidrug resistance.
Overexpression of Twist in 293
cells
The plasmid pcDNA5/FRT/TO (1 µg; Invitrogen; Thermo
Fisher Scientific, Inc.) was digested with 10 U restriction enzymes
NotI and BamHI (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C for 6 h to harvest the vector backbone. Concurrently,
1 µg plasmid pcDNA3-Twist (a gift from the Faculty of Medical
Molecular Biology, Southwest Medical University, Sichuan, China)
was digested with NotI and BamHI to obtain the hTwist
fragment. These two fragments were ligated to pcDNA5/FRT/TO-Twist
by 1 U T4 ligase (Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature for 1 h. Following this, 7.5 µg pOG44 (Invitrogen;
Thermo Fisher Scientific, Inc.) and 0.75 µg pcDNA5/FRT/TO-Twist
were co-transfected into Flp-In-T-Rex-293 cells by using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C for 24 h to generate the 293-Twist cells, and 7.5 µg pOG44 and
0.75 µg pcDNA5/FRT/TO were also co-transfected into
Flp-In-T-Rex-293 cells to serve as negative control. Following
transfection for 24 h, the medium containing 5 µg/ml blasticidin
(Invitrogen; Thermo Fisher Scientific, Inc.) was changed and the
cells were cultured for 48 h at 37°C. Subsequently, 200 µg/ml
Hygromycin B (Invitrogen; Thermo Fisher Scientific, Inc.) was added
to the medium following the transfer of the cells to a new plate at
25% confluence for 3-h incubation. Once the anti-Hygromycin B cell
clones had grown, they were seeded onto a 24-well plate in DMEM
medium with 200 µg/ml Hygromycin B, 5 µg/ml blasticidin and 10% FCS
at 37°C for expansion. Finally, the 293-Twist cells and the
293-vector cells were successfully constructed. Prior to performing
western blot analysis and immunohistochemistry, 293-Twist and
293-vector cells were cultured with 0.1 µg/ml tetracycline.
293-Twist and 293-vector cells were treated with Trichostatin A
(TSA; 1, 4 or 8 µmol/l; Beyotime Institute of Biotechnology,
Haimen, China), 5-FU (2.5, 12, 62.5 or 312.5 µmol/l) and Taxol
(0.276, 1.38, 6.9 or 34.5 µmol/l) to test the multidrug resistance
of these cells.
Immunohistochemistry
Human breast cancer tissues were obtained from the
Affiliated Hospital of Southwest Medical University between Jan
2016 and Apr 2016. A total of 32 patients, aged between 37 and 69
years (48.32±8.41), who had not received preoperative radiation
therapy and chemotherapy prior to breast cancer surgery were
included in the present study. The present study obtained ethical
approval from the Ethics Committee of Southwest Medical University
and written informed consent was obtained from all participants.
All human breast cancer tissues were fixed in 10% formalin (Chengdu
KeLong Chemical Co., Ltd., Chengdu, China) at room temperature
overnight and embedded in paraffin. The paraffin-embedded tissues
were sliced into 4-µm thick sections. Subsequent to dewaxing with
100% xylene for 10 min three times, the section was rehydrated with
gradient (100, 90, 80 and 70%) ethanol for 1 min. Antigen retrieval
was performed by boiling at 98°C for 10 min in 10 mM citric acid
solution (pH 6.0). For cell samples, cells were cultured on glass
coverslips and fixed with 4% paraformaldehyde for 10 min at room
temperature followed by permeabilization with 0.25% Triton X-100
for 10 min at room temperature. Immunohistochemistry was conducted
with an ABC staining kit (OriGene Technologies, Inc., Beijing,
China) according to the manufacturer's protocol. Briefly, following
antigen retrieval or Triton X-100 treatment, sections or cells were
blocked at room temperature with 10% goat serum (cat. no. 16210072;
Gibco; Thermo Fisher Scientific, Inc.) for 30 min and incubated
with the indicated primary antibodies at the indicated dilution
rate overnight at 4°C. Subsequent to washing with PBS twice for 5
min, biotinylated goat anti-mouse (dilution, 1:150; cat. no.
ZB-2055; OriGene Technologies, Inc., Beijing, China) or
biotinylated goat anti-rabbit (dilution, 1:150; cat. no. ZB-2011;
OriGene Technologies, Inc.) secondary antibodies were applied to
recognize the primary antibody at 37°C for 30 min. Then, the
sections were washed again with PBS twice prior to being incubated
with Vectastain ABC reagent (dilution, 1:50; Vector Laboratories,
Inc., CA, USA) at 37°C for 20 min. Chromogenesis was performed with
DAB for 10–15 min. The primary antibodies used in the present study
included: Mouse anti-TOPO IIα antibody (dilution, 1:200; cat. no.
ZM-0245; OriGene Technologies, Inc.); mouse anti-MRP antibody
(dilution, 1:200; cat. no. ZM-0345; OriGene Technologies, Inc.);
rabbit anti-P-gp antibody (dilution, 1:500; cat. no. ab103477;
Abcam, Cambridge, UK); mouse anti-LRP antibody (dilution, 1:200;
cat. no. ZM-0325; OriGene Technologies, Inc.); and rabbit
anti-Twist antibody (dilution, 1:500; cat. no. ab49254; Abcam).
Images from immunohistochemistry were captured using a Nikon
Eclipse 50i microscope (Nikon Corporation, Tokyo, Japan) at ×400
magnification.
RT-qPCR
Total RNA was extracted from the MCF-7 and 293 cells
using an RNAprep pure Cell/Bacteria kit (Tiangen Biotech Co., Ltd.,
Beijing, China). cDNA was synthesized from total RNA using a
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China). Amplification of harvested cDNA (1 µg) was
performed in real time within a 20-µl reaction system consisting of
SYBR Green PCR Master mix (Toyobo Life Science, Osaka, Japan) at
99°C for 10 min, followed by 40 cycles of: 30 sec denaturation at
94°C, 30 sec annealing at 58°C and 30 sec extension at 72°C.
Primers used in this reaction were as follows: Twist, forward
5′-GTGCGCAGTCTTACGAGGAG-3′ and reverse
5′-GCTTGAGGGTCAGAATCTTGCT-3′; and GAPDH, forward
5′-ATGCTGGCGCTGAGTACGTC-3′ and reverse
5′-GGTCATGAGTCCTTCCACGATA-3′. The results were analyzed using the
2−ΔΔCq method (19).
Western blot analysis
Western blot analysis was performed as previously
described (20). The primary
antibodies used in the present study were rabbit anti-Twist
antibody (dilution, 1:1,000; cat. no. ab49254; Abcam) and rabbit
anti-β-actin antibody (dilution, 1:3,000; cat. no. PR-0255; OriGene
Technologies, Inc.), and the secondary antibodies were horseradish
peroxidase-conjugated secondary goat anti-rabbit antibody
(dilution, 1:5,000; cat. no. ZB-2301; OriGene Technologies, Inc.).
The relative protein level of Twist was normalized to β-actin.
Statistical analysis
All data were presented as the mean ± standard
deviation and all results were analyzed using SPSS statistical
software (version 19.0; IBM Corp., Armonk, NY, USA). Multivariate
comparisons of the means were performed using one-way analysis of
variance followed by the Student-Newman-Keuls post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
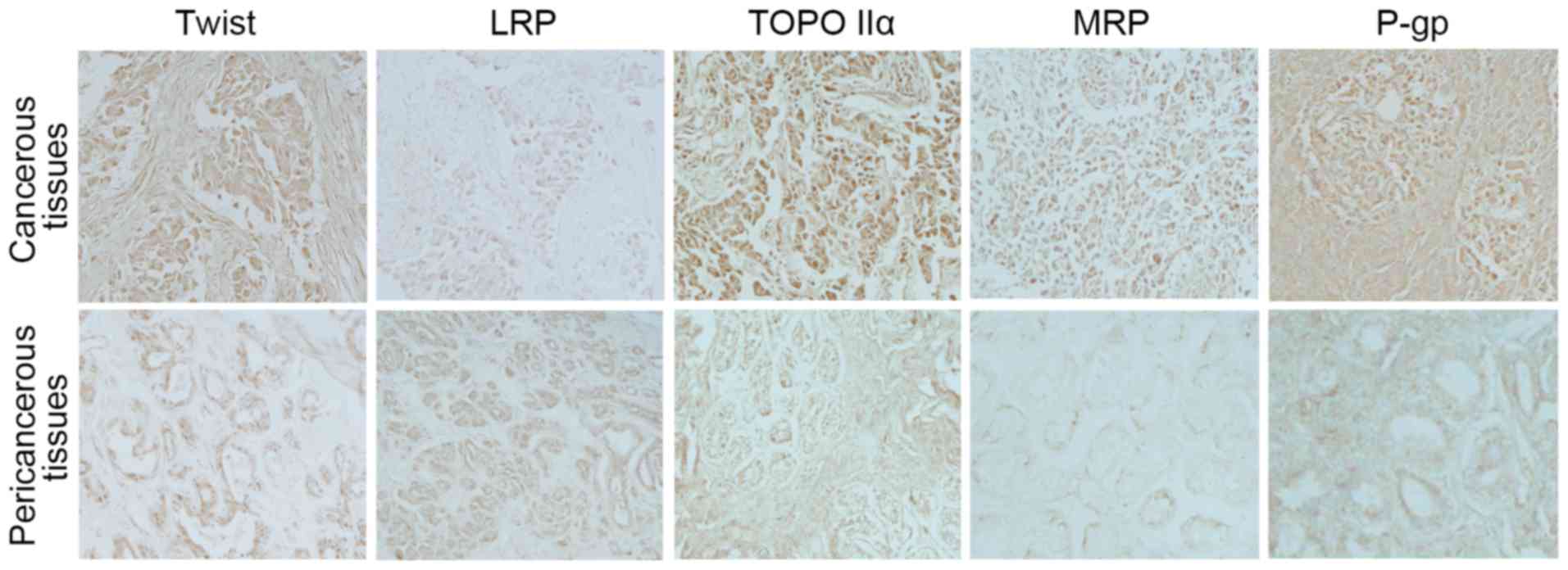
Expression of Twist and MDR-associated
proteins in human breast cancer
Immunohistochemical analysis was performed to detect
the expression levels of Twist and MDR-associated proteins in human
breast cancer samples. It was identified that the expression of
Twist was largely increased in the cancerous tissues of human
breast cancer compared with the pericancerous tissues. Notably, the
expression levels of TOPO IIα, MRP and P-gp in cancerous tissues
were concomitantly increased with the expression of Twist (Fig. 1). However, the expression of LRP was
reduced in cancerous tissues compared with the pericancerous
tissues.
Establishment of
Taxol®-resistant MCF-7 cell lines
To simulate Taxol® resistance in cells,
MCF-7 cells were repeatedly exposed to high concentrations of
Taxol® to establish the Taxol®-resistant
MCF-7 cells. Notably, the four types of Taxol®-resistant
MCF-7 cells (MCF-7/Taxol® I–IV) exhibited a gradually
reduced growth inhibition ratio to Taxol® (Fig. 2A), and an increased IC50
value and RI (Table I), which
suggests that the Taxol®-resistant cell models were
successfully established. Notably, compared with the MCF-7 cells,
MCF-7/Taxol® IV cells also exhibited lower growth
inhibition ratios to other chemotherapy medications, including
vincristine (Fig. 2B and Table II), cis-platinum (data not shown),
5-FU (data not shown) and mitomycin (data not shown), suggesting
that Taxol®-resistant cells may have generated MDR.
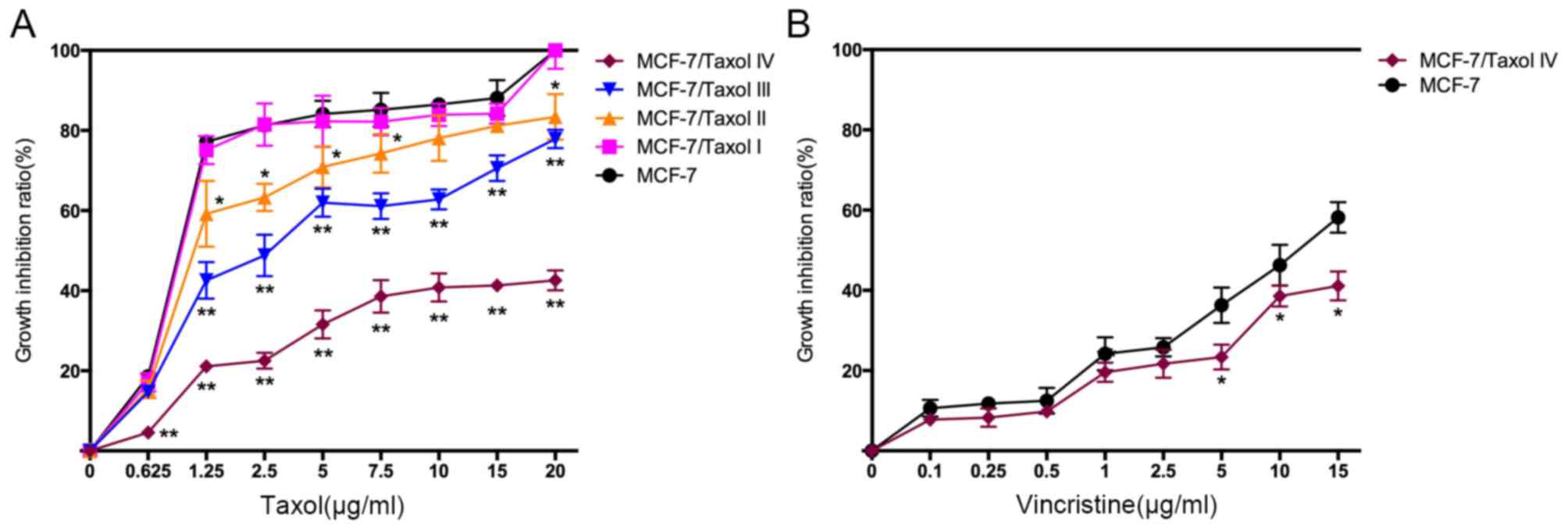 | Figure 2.MCF-7/Taxol® cells exhibit
Taxol® and vincristine resistance. (A) Four levels of
Taxol®-resistant cells were established by repeatedly
exposing cells to 15 µg/ml Taxol®. Cell growth
inhibition ratios of these cells from different concentrations of
Taxol® (0, 0.625, 1.25, 2.5, 5, 7.5, 10, 15 and 20
µg/ml) were calculated based on MTT assay data. Compared with MCF-7
cells, MCF-7/Taxol® IV cells demonstrated a much lower
cell growth inhibition ratio to Taxol. The cell growth inhibition
ratio
(%)=(ODcontrol-ODexperiment)/ODcontrol
×100%. (B) Cell growth inhibition ratios of various concentrations
of vincristine (0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10 and 15 µg/ml). The
results were from MCF-7 and MCF-7/Taxol® IV cells.
MCF-7/Taxol® IV cells exhibited lower growth inhibition
ratios following vincristine treatment compared with MCF-7 cells.
OD, optical density. *P<0.05 and **P<0.01 vs. MCF-7
cells. |
 | Table I.IC50 and RI of MCF-7 and
MCF-7/Taxol® cells treated with Taxol®. |
Table I.
IC50 and RI of MCF-7 and
MCF-7/Taxol® cells treated with Taxol®.
| Cell
population |
IC50 | RI |
|---|
| MCF-7 | 3.83±0.04 | 1.00 |
|
MCF-7/Taxol® I | 3.76±0.07 | 0.94±0.04 |
|
MCF-7/Taxol® II |
10.41±0.27a |
2.36±0.33a |
|
MCF-7/Taxol® III |
24.82±0.32b |
7.51±1.23b |
|
MCF-7/Taxol® IV |
107.05±1.79b |
28.83±1.05b |
 | Table II.IC50 and RI of MCF-7 and
MCF-7/Taxol® IV cells treated with vincristine. |
Table II.
IC50 and RI of MCF-7 and
MCF-7/Taxol® IV cells treated with vincristine.
| Cell
population |
IC50 | RI |
|---|
| MCF-7 | 12.86±0.34 | 1.00 |
|
MCF-7/Taxol® IV |
22.06±1.12a |
1.71±0.13a |
Expression of Twist and MDR-associated
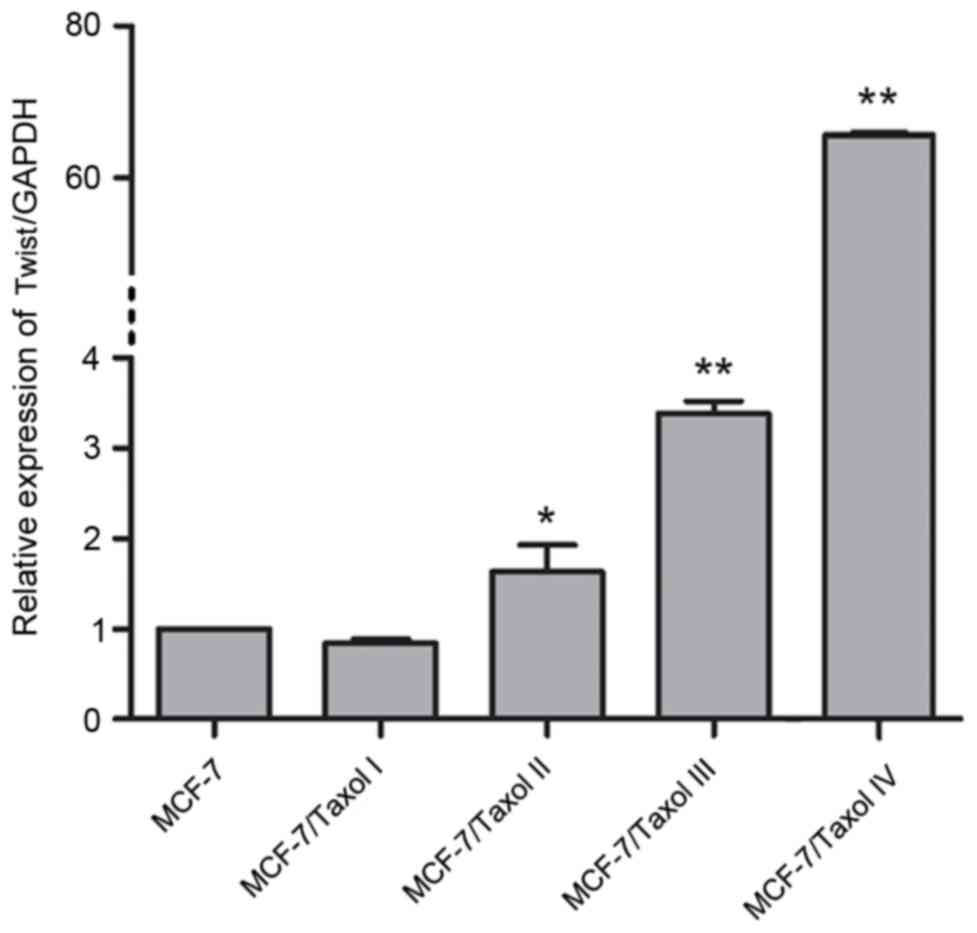
proteins in Taxol®-resistant cells
To investigate the expression of Twist in
Taxol®-resistant cells, RT-qPCR was firstly performed to
examine the mRNA expression levels of Twist in MCF-7 cells and
MCF-7/Taxol® I–IV cells. Compared with the MCF-7 cells,
the mRNA expression of Twist was significantly increased in the
second, third and fourth levels of Taxol®-resistant
cells (MCF-7/Taxol® II–IV; Fig. 3). Notably, compared with the MCF-7
cells, the greatest increase in the mRNA expression level of Twist
was observed in the fourth level of Taxol®-resistant
cells (MCF-7/Taxol® IV), with the expression level being
upregulated by >60-fold compared with the level in the MCF-7
cells (Fig. 3). This may partially
suggest that Twist is associated with Taxol® resistance.
Furthermore, immunohistochemistry was performed to investigate the
expression of MDR-associated proteins in each level of
Taxol®-resistant cells (Fig.
4). According to the results of immunohistochemistry, it was
identified that the expression of Twist, TOPO IIα, MRP and P-gp
were increased in Taxol®-resistant cells, particularly
in MCF-7/Taxol® IV cells (Table III). Conversely, the expression of
LRP was reduced in Taxol®-resistant cells (Table III). These results were consistent
with the expression of Twist, LRP, TOPO IIα, MRP and P-gp in
cancerous tissues of breast cancer.
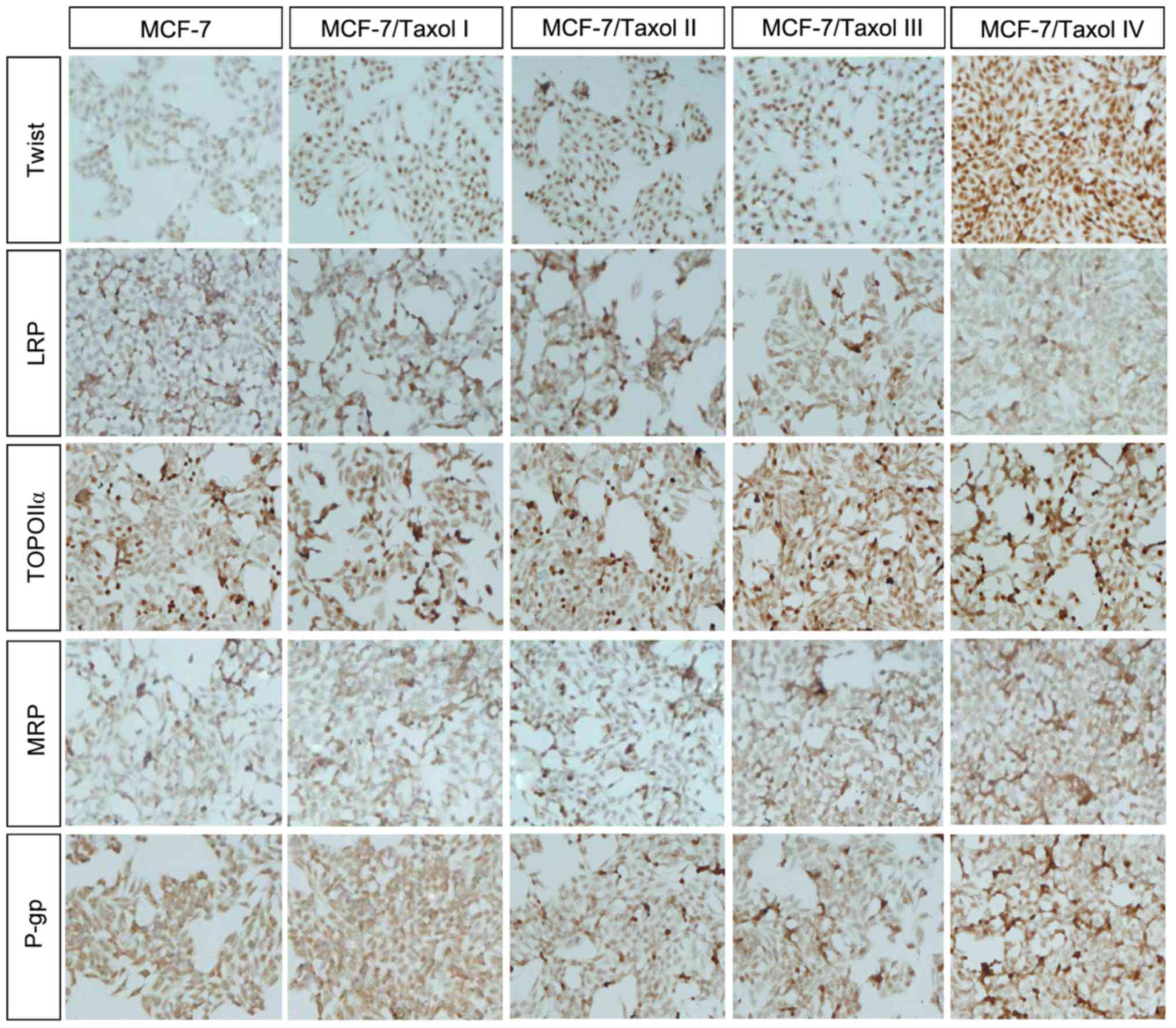 | Figure 4.Taxol® resistance
increases the protein expression of Twist, TOPO IIα, MRP and P-gp
in MCF-7/Taxol® cells. Immunohistochemistry results
(magnification, ×400) demonstrated that the protein expression of
Twist, TOPO IIα, MRP and P-gp were increased in
Taxol®-resistant cells, whereas the protein expression
of LRP was reduced. TOPO IIα, topoisomerase IIα; MRP, multidrug
resistant-associated protein; P-gp, P-glycoprotein; LRP, lung
resistance-related protein. |
 | Table III.Immunohistochemistry analysis of LRP,
TOPO IIα, MRP, P-gp and Twist in Taxol-resistant breast cancer cell
lines. |
Table III.
Immunohistochemistry analysis of LRP,
TOPO IIα, MRP, P-gp and Twist in Taxol-resistant breast cancer cell
lines.
|
| Proteins |
|---|
|
|
|
|---|
| Cell
populations | LRP | TOPO IIα | MRP | P-gp | Twist |
|---|
| MCF-7 |
44,541.00±2,778.42 |
26,378.60±1,574.08 |
34,513.80±4,343.94 |
33,550.20±5,850.43 |
11,911.20±3,679.47 |
| MCF-7/Taxol I |
38,349.80±6,402.81 |
34,582.40±4,560.87a |
68,355.60±6,911.49a |
35,952.80±4,961.75 |
20,463.50±6,344.64 |
| MCF-7/Taxol II |
32,210.80±6,751.98a |
36,871.60±3,966.03a |
64,004.40±11,964.28b |
46,370.60±18,817.21 |
25,191.40±6,876.17b |
| MCF-7/Taxol
III |
28,835.00±4,177.66a |
37,207.60±3,915.78a |
116,419.00±18,992.03a |
50,992.40±13,135.89 |
28,575.40±6,421.50a |
| MCF-7/Taxol IV |
15,225.80±2,219.39a |
49,597.20±3,256.65a |
151,732.40±50,276.11a |
344,788.00±71,465.02a |
88,988.20±7,431.32a |
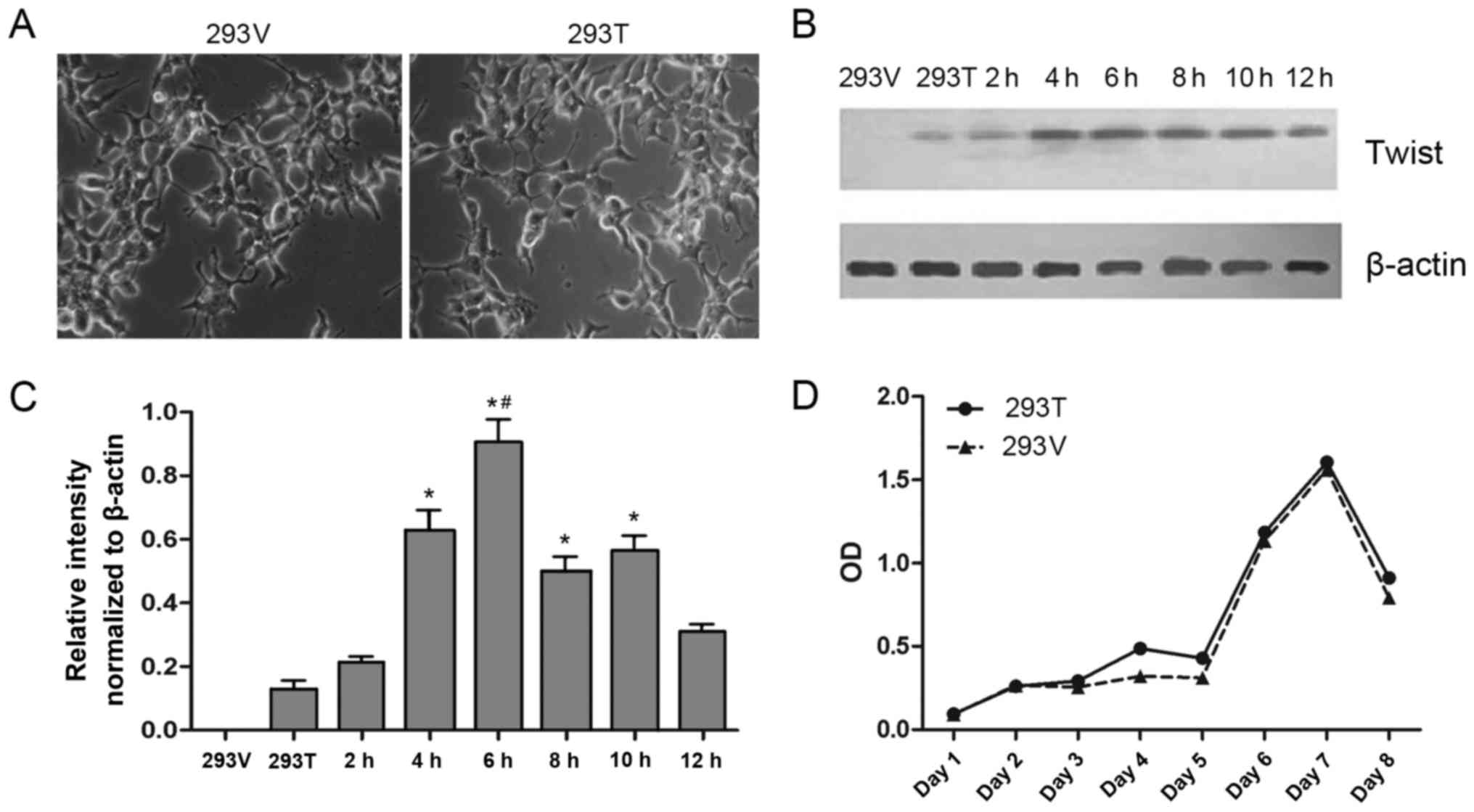
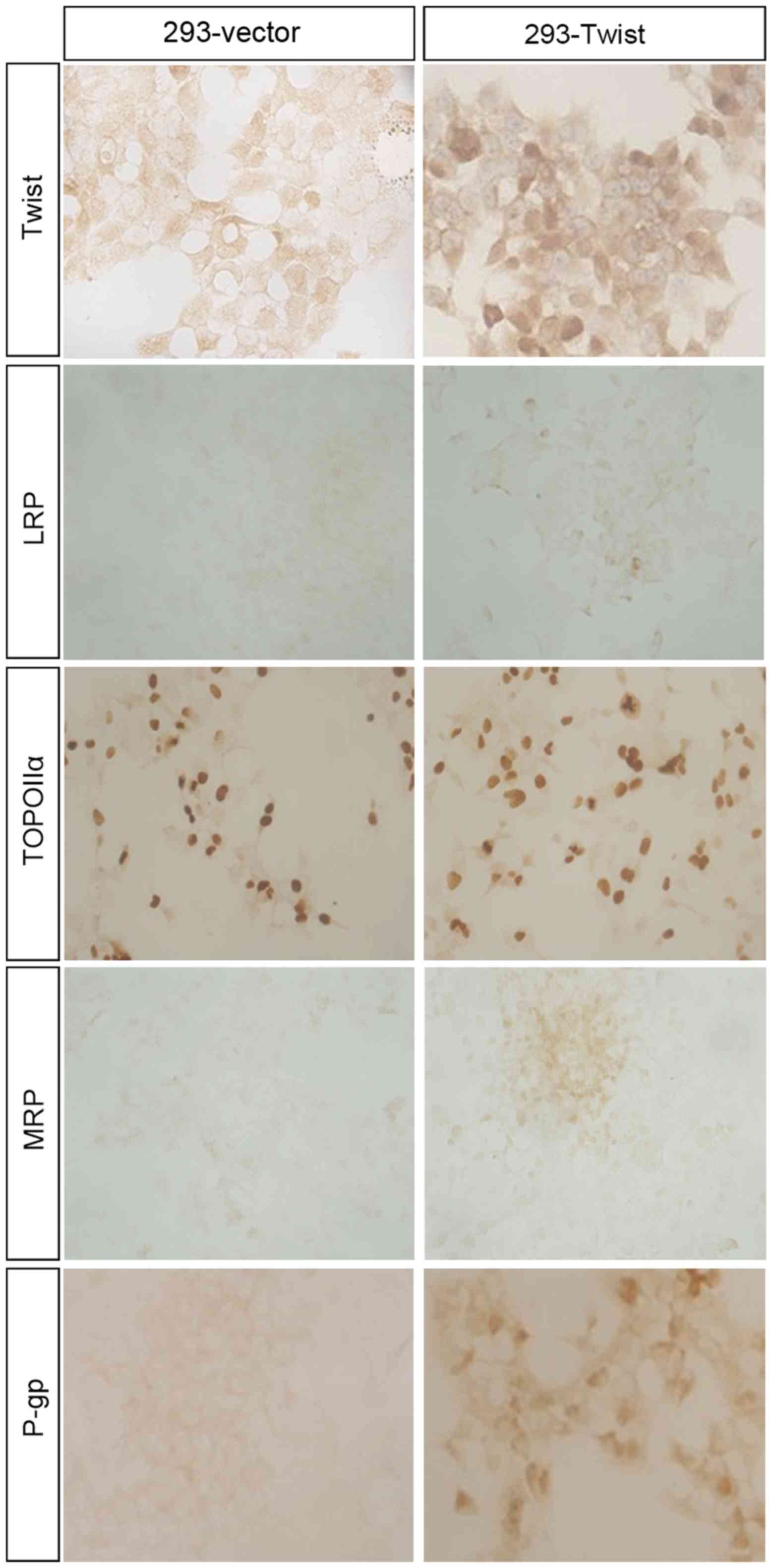
Overexpression of Twist in 293
cells
Although the expression of Twist was increased in
Taxol®-resistant cells, it was unclear if overexpression
of Twist was responsible for the drug resistance. Therefore, a
Twist-overexpressing cell line, 293-Twist, was constructed by
transfecting a pcDNA5/FRT/TO-Twist vector into Flp-In-T-Rex-293
cells; a negative control cell line, 293-vector, was also
constructed. No differences in the morphology of 293-vector and
293-Twist cells were observed (Fig.
5A). However, the results of western blotting demonstrated that
the expression of Twist was gradually upregulated by treatment with
tetracycline for 0–6 h, and then gradually downregulated from 6–12
h (Fig. 5B and C). Concurrently, the
growth curves of 293-vector and 293-Twist cells were not different
(Fig. 5D), suggesting that
transfection with the pcDNA5/FRT/TO-Twist vector may increase the
expression of Twist in 293 cells and not lead to cell damage in the
Twist-overexpressing cells.
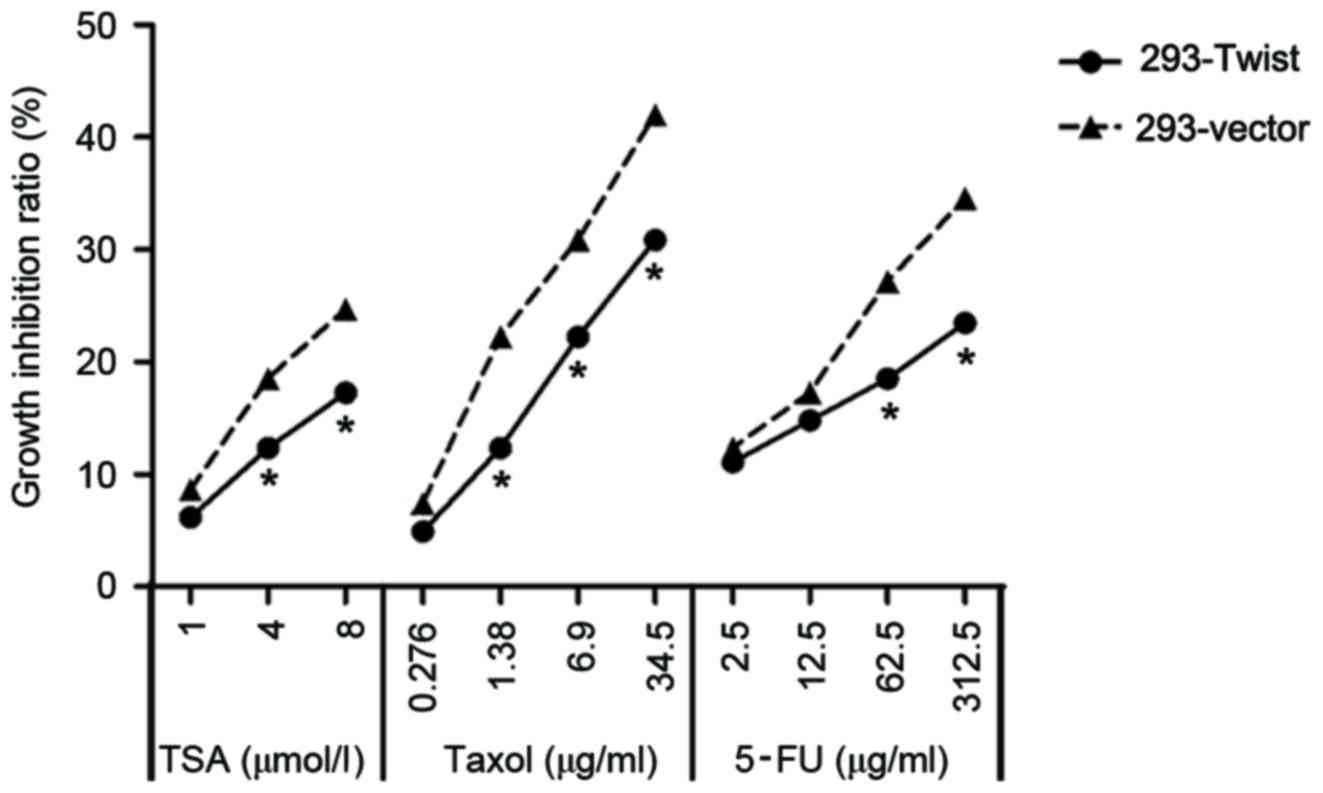
Overexpression of Twist leads to MDR
in 293-Twist cells
MDR was measured by exposing 293-Twist cells to
different concentrations of TSA, 5-FU and Taxol®. TSA is
an anticancer drug, which promotes the expression of
apoptosis-associated genes, resulting in a decrease in the survival
rates of the cancerous cells (21).
5-FU is also widely used as an anticancer drug (22). 293-Twist cells were exposed to 1, 4
and 8 µmol/l TSA; 0.276, 1.38, 6.9 and 34.5 µg/ml
Taxol®; and 2.5, 12.5, 62.5 and 312.5 µg/ml 5-FU. The
results indicated that the growth inhibition ratios of TAS,
Taxol® and 5-FU were significantly decreased in
293-Twist cells compared with that in 293-vector cells (Fig. 6). These data suggest that the
overexpression of Twist increased the level of MDR in 293
cells.
Expression of drug resistance-related
proteins in 293-vector and 293-Twist cells
To demonstrate the association between Twist
overexpression and the expression of MDR-associated proteins,
immunohistochemistry was performed in 293-vector and 293-Twist
cells (Fig. 7). The results indicated
that, compared with the 293-vector cells, expression levels of MRP
and P-gp were upregulated in 293-Twist cells, which was consistent
with the expression of these proteins in
Taxol®-resistant MCF-7 cells and breast cancer tissues;
however, the expression of TOPO IIα and LRP were equivalent in
293-vector and 293-Twist cells. These data suggest that the
overexpression of Twist may lead to MDR through regulating the
expression of MDR-associated proteins.
Discussion
In this present study, Taxol®-resistant
and Twist overexpression cell models were established to
investigate the role of Twist in drug resistance in cancer therapy.
It was identified that the expression of Twist was significantly
amplified in Taxol®-resistant cells, accompanied by
increased expression of TOPO IIα, MRP and P-gp, and a decreased
expression of LRP. Concurrently, vincristine resistance was also
identified in these cells. Conversely, overexpression of Twist
augmented Taxol®, TSA and 5-FU resistance in 293 cells,
and upregulated the expression of MDR-associated proteins, such as
MRP and P-gp. All results from the present study suggested that
Twist increased drug resistance, and may be associated with the
alteration of expression of MDR-associated proteins.
Drug resistance is a major limitation of anticancer
therapy, including in breast cancer treatment. Unfortunately, poor
prognoses are often observed in patients with advanced breast
cancer, due to severe drug resistance to chemotherapeutics
(23). Therefore, it is important to
reveal the mechanisms of drug resistance in order to identify
potential targets to limit the increasing chemoresistance observed
in cancer therapy. According to the features of drug resistance in
cancer, there are two types of drug resistance: Primary drug
resistance and MDR (24). MDR in
cancer therapy refers to cancer cells that are resistant to certain
types of chemotherapy drug, and that also exhibit cross-resistance
to other drugs that have different mechanisms of pharmacology
(11,25). Technically, MDR has been divided into
four types according to the different intracellular targets of
chemotherapeutic drugs: Type 1, classical MDR, mediated by P-gp;
type 2, non-P-glycoprotein MDR, mediated by MDR-associated
proteins; type 3, atypical MDR, mediated by TOPO II; and type 4,
mediated by LRP (26). Although a
previous study demonstrated that acquired Taxol®
resistance is associated with a high expression of P-gp in breast
cancer (27), the overall
interactions between drug resistance and MDR-associated proteins
have not been clearly demonstrated. Notably, a previous study
indicated that Twist, a transcription factor, was identified to
promote Taxol® resistance in the nasopharyngeal
carcinoma HNE1-T3 cell line by performing comparative genomic
hybridization analysis (8).
Furthermore, it was also revealed that overexpression of Twist
elevated Taxol® resistance in gallbladder carcinoma,
ovarian cancer and prostate cancer cells, suggesting that the
ectopic expression of Twist may lead to drug resistance in
different cell lines (8). An
additional study indicated that Twist reduced the cell apoptotic
rate and increased Taxol® resistance by upregulating the
expression of RAC-β serine/threonine-protein kinase in the breast
cancer MCF-7 cell line (28); this
was also observed in a nasopharyngeal carcinoma cell line (29). In addition, Twist-1, as a downstream
target gene of nuclear factor (NF)-κB, may block programmed cell
death induced by daunorubicin or tumor necrosis factor when NF-κB
is deficient (30), and RNA
interference of Twist-1 may improve chemotherapeutic effectiveness
in breast cancer (31). Indeed, these
data demonstrate that Twist may lead to MDR in numerous types of
cancer; however, whether the augmentation of drug resistance by
Twist is associated with the expression level of MDR-associated
proteins remains unclear, and requires additional examination.
In the present study, Taxol® resistance
in breast cancer was simulated by repeatedly exposing MCF-7 cells
to Taxol®; this resulted in an increase in the
expression levels of Twist, P-gp, MRP and TOPO IIα and a decrease
in the expression level of LRP in MCF-7/Taxol® cells.
Notably, the different expression pattern of LRP compared with the
other three MDR-associated proteins may due to the following
reasons: i) Although LRP protein is an efflux protein, it does not
belong to the ATP binding cassette transporter family; or ii) the
mechanism of MDR mediated by LRP is different from those mediated
by P-gp and MRP. However, the overexpression of Twist identified in
Taxol®-resistant cells did not sufficiently explain the
association between Twist and MDR-associated proteins. Therefore,
an inverse cell model was established through transfecting a
pcDNA5/FRT/TO-Twist vector into Flp-In-T-Rex-293 cells, a
transfection-sensitive cell line, to generate a Twist
overexpression cell line, which was used to examine the potential
drug resistance and altered expression of MDR-associated proteins.
In this cell model, elevated drug resistance to Taxol, TSA and
5-FU, and an increased expression of MRP and P-gp, was detected.
Nevertheless, the expression levels of LRP and TOPO IIα in
293-Twist cells were not different to those in 293-vector cells,
which may be due to the fact that the transfected 293 cell was not
a cancer cell line. However, this result may suggest that the
overexpression of Twist in 293 cells increases drug resistance
through altering the expression of MRP and P-gp. Notably, the
results of the immunohistochemistry analysis in the human breast
cancer tissues demonstrated a lower expression of LRP compared with
the pericancerous tissue, suggesting that an increase in the level
of malignancy of the tumor tissue corresponded to a notable
downregulation of LRP expression.
In summary, the data from the present study
indicated that with the gradual formation of Taxol®
resistance, the expression levels of Twist and MDR-associated
proteins gradually increased in Taxol®-resistant cells,
suggesting that Twist serves a crucial role in the process of
acquiring Taxol® resistance in breast cancer cells.
Notably, the overexpression of Twist in 293 cells also resulted in
MDR, and increased the expression of MDR-associated proteins. The
present study may assist in understanding the association between
Twist and MDR-associated proteins, and contribute to identifying
novel chemotherapeutic targets.
Acknowledgements
The present study was supported by grants from the
Science and Technology Support Program of Sichuan Province (grant
nos. 2013JY0076 and 14ZC0024) and the Education Department Program
of Sichuan Province (grant nos. 15TD0020 and 12ZB064).
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhoo-Pathy N, Yip CH, Hartman M, Uiterwaal
CS, Devi BC, Peeters PH, Taib NA, van Gils CH and Verkooijen HM:
Breast cancer research in Asia: Adopt or adapt Western knowledge?
Eur J Cancer. 49:703–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bedard PL, Di Leo A and Piccart-Gebhart
MJ: Taxanes: Optimizing adjuvant chemotherapy for early-stage
breast cancer. Nat Rev Clin Oncol. 7:22–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murray S, Briasoulis E, Linardou H,
Bafaloukos D and Papadimitriou C: Taxane resistance in breast
cancer: Mechanisms, predictive biomarkers and circumvention
strategies. Cancer Treat Rev. 38:890–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maestro R, Dei Tos AP, Hamamori Y,
Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH and
Hannon GJ: Twist is a potential oncogene that inhibits apoptosis.
Genes Dev. 13:2207–2217. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valsesia-Wittmann S, Magdeleine M,
Dupasquier S, Garin E, Jallas AC, Combaret V, Krause A, Leissner P
and Puisieux A: Oncogenic cooperation between H-Twist and N-Myc
overrides failsafe programs in cancer cells. Cancer Cell.
6:625–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Ling MT, Guan XY, Tsao SW, Cheung
HW, Lee DT and Wong YC: Identification of a novel function of
TWIST, a bHLH protein, in the development of acquired taxol
resistance in human cancer cells. Oncogene. 23:474–482. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of TWIST in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunjachan S, Rychlik B, Storm G, Kiessling
F and Lammers T: Multidrug resistance: Physiological principles and
nanomedical solutions. Adv Drug Deliv Rev. 65:1852–1865. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu D, Shi HC, Wang ZX, Gu XW and Zeng YJ:
Multidrug resistance-associated biomarkers PGP GST-pi, Topo-II and
LRP as prognostic factors in primary ovarian carcinoma. Br J Biomed
Sci. 68:69–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheffer GL, Schroeijers AB, Izquierdo MA,
Wiemer EA and Scheper RJ: Lung resistance-related protein/major
vault protein and vaults in multidrug-resistant cancer. Curr Opin
Oncol. 12:550–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scheffer GL, Wijngaard PL, Flens MJ,
Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC and
Scheper RJ: The drug resistance-related protein LRP is the human
major vault protein. Nat Med. 1:578–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang JC: Cellular roles of DNA
topoisomerases: A molecular perspective. Nat Rev Mol Cell Biol.
3:430–440. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coss A, Tosetto M, Fox EJ, Sapetto-Rebow
B, Gorman S, Kennedy BN, Lloyd AT, Hyland JM, O'Donoghue DP,
Sheahan K, et al: Increased topoisomerase IIalpha expression in
colorectal cancer is associated with advanced disease and
chemotherapeutic resistance via inhibition of apoptosis. Cancer
Lett. 276:228–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: The multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Mao N, Tan RZ, Wang HL, Wen J, Liu
YH, Furhad M and Fan JM: Ginsenoside Rg1 reduces
aldosterone-induced autophagy via the AMPK/mTOR pathway in NRK-52E
cells. Int J Mol Med. 36:518–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drummond DC, Noble CO, Kirpotin DB, Guo Z,
Scott GK and Benz CC: Clinical development of histone deacetylase
inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol.
45:495–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atalay C, Deliloglu Gurhan I, Irkkan C and
Gunduz U: Multidrug resistance in locally advanced breast cancer.
Tumour Biol. 27:309–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kovalev AA, Tsvetaeva DA and Grudinskaja
TV: Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the
development of primary and acquired multiple drug resistance in
patients with early and metastatic breast cancer. Exp Oncol.
35:287–290. 2013.PubMed/NCBI
|
|
25
|
Yoneyama H, Takizawa-Hashimoto A, Takeuchi
O, Watanabe Y, Atsuda K, Asanuma F, Yamada Y and Suzuki Y: Acquired
resistance to gemcitabine and cross-resistance in human pancreatic
cancer clones. Anticancer Drugs. 26:90–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scagliotti GV, Novello S and Selvaggi G:
Multidrug resistance in non-small-cell lung cancer. Ann Oncol. 10
Suppl 5:S83–S86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mechetner E, Kyshtoobayeva A, Zonis S, Kim
H, Stroup R, Garcia R, Parker RJ and Fruehauf JP: Levels of
multidrug resistance (MDR1) P-glycoprotein expression by human
breast cancer correlate with in vitro resistance to taxol and
doxorubicin. Clin Cancer Res. 4:389–398. 1998.PubMed/NCBI
|
|
28
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Wang Q, Ling MT, Wong YC, Leung
SC and Wang X: Anti-apoptotic role of TWIST and its association
with Akt pathway in mediating taxol resistance in nasopharyngeal
carcinoma cells. Int J Cancer. 120:1891–1898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pham CG, Bubici C, Zazzeroni F, Knabb JR,
Papa S, Kuntzen C and Franzoso G: Upregulation of Twist-1 by
NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs.
Mol Cell Biol. 27:3920–3935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar : PubMed/NCBI
|