Introduction
Gastric cancer (GC) is the fifth most common type of
cancer, and the third leading cause of cancer mortality in the
world (1). The 7th edition of the
tumor node metastasis (TNM) staging system indicates the importance
of the depth of invasion and the number of lymph nodes metastases
involved as major prognostic factors (2). Therefore, it is important to understand
the mechanisms involved in gastric cancer progression and lymphatic
metastasis.
The association between inflammation and cancer was
proposed >100 years ago, and experimental, clinical and
epidemiological studies have revealed that chronic inflammation
contributes to cancer progression and may increase predisposition
to different types of cancer (3,4).
Macrophages (Mφs) are one of the most important inflammatory cells
in the tumor microenvironment (4,5). There are
two types, M1 and M2, and they are derived from monocytes. M1
macrophages are produced by the classical complement pathway, and
are pro-inflammatory. M2 macrophages are activated through the
alternative complement pathway, and secrete anti-inflammatory
cytokines and participate in tissue repair (6). M1 Mφs produce large amounts of nitric
oxide by expressing inducible nitric oxide synthase (iNOS) and
tumor necrosis factor (TNF), and are essential for clearing
bacterial, viral and fungal infections (7). M2 Mφs serve a role in the response to
parasite infection, tissue remodeling, angiogenesis and tumor
progression (8). In a previous study
investigating GC, Ishigami et al (9) suggested that the expression of
tumor-associated Mφs (TAMs) was positively correlated with stomach
lesions, tumor staging and lymph node metastasis. The
differentiation of Mφs into the M1 or M2 phenotype is affected by
the microenvironment, and numerous types of cytokines present.
Amongst the tumor-derived factors that modulate myeloid cell
polarization, macrophage colony stimulation factor (M-CSF) promotes
the recruitment and survival rates of Mφ, and M-CSF enhances tumor
growth and aggressiveness by stimulating the protumor activities of
TAMs (10).
Interleukin (IL)-6 is a strong activator of signal
transducer and activator of transcription 3 (STAT3), and
IL-6-dependent STAT3 activation serves a pivotal role in tumor
progression. It has been demonstrated in previous studies that IL-6
is an independent risk factor for poor prognosis inpatients with GC
(11). Also, it has been reported
that interferon-regulatory factor 4 (IRF4) may regulate M2 Mφ
polarization, and contribute to the expression of M2 makers
(8,11). In the present study, the significance
of the correlation between IL-6 and TAMs, and their role in GC
progression and lymph node metastasis was investigated.
Furthermore, it was demonstrated that M-CSF and IL-6 drive the
differentiation of M2 polarization of TAMs through the
IL-6/STAT3/IRF4 signaling pathway, and that in vitro induced
Mφs express high levels of IL-10, transforming growth factor
(TGF)-β and vascular endothelial growth factor (VEGF)-C. The
elevated expression level of STAT3 indicates the activation of the
IL-6 signaling pathway. Collectively, these data suggest that the
IL-6/STAT3/IRF4 axis may facilitate tumor progression and lymph
node metastasis in the GC microenvironment.
Materials and methods
Clinical data
GC tissue was collected from 60 patients by
gastrectomy, subsequently embedded in paraffin, and diagnosed at
the Southwest Hospital of Third Military Medical University
(Chongqing, China). None of the patients had received radiation or
chemotherapy prior to surgery, and clinicopathological
classification was confirmed by a pathologist. The age of the
patients ranged between 28 to 82 years, and the median age was 54
years. There were 33 males and 27 females. According to the 2010
edition of Union for International Cancer Control staging of GC
(12), there were 7 patients with
stage I, 14 patients with stage II, 34 patients with stage III and
5 patients with stage IV. According to tumor location, there were
11 patients with gastric cardia, 24 patients with gastric body and
25 patients with antrum. There were 42 patients with lymph node
metastasis and 18 patients without lymph node metastasis, as
summarized in Table I. The present
study was approved by the Ethics Review Board at the Third Military
Medical University, and written informed consent was obtained from
all patients.
 | Table I.Association of IL-6+ cells and CD68+
cells with clinicopathological characteristics. |
Table I.
Association of IL-6+ cells and CD68+
cells with clinicopathological characteristics.
|
| CD68 expression | IL-6 expression |
|---|
|
|
|
|
|---|
| Clinical
parameter | Low, n | High, n | P-value | Low, n | High, n | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<54 | 10 | 16 | 0.181 | 14 | 12 | 0.228 |
| ≥54 | 19 | 15 |
| 13 | 21 |
|
| Sex |
|
|
|
|
|
|
| Male | 18 | 15 | 0.287 | 16 | 17 | 0.586 |
|
Female | 11 | 16 |
| 15 | 12 |
|
| Location |
|
|
|
|
|
|
|
Cardia | 4 | 7 | 0.408 | 6 | 5 | 0.607 |
| Body | 14 | 10 |
| 11 | 13 |
|
|
Sinuses | 11 | 14 |
| 15 | 10 |
|
| TNM Stage |
|
|
|
|
|
|
| I+II | 16 | 5 | 0.007 | 14 | 7 | 0.004 |
|
III+IV | 14 | 25 |
| 11 | 28 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
| Yes | 12 | 30 | 0.018 | 14 | 28 | 0.017 |
| No | 11 | 7 |
| 12 | 6 |
|
Immunohistochemical staining
The GC tissues were fixed with 10% paraformaldehyde,
and the paraffin-embedded specimens were then cut into 5 µm
sections. Subsequent to dewaxing and rehydration, the sections were
submerged in EDTA buffer and heated in a microwave oven (100°C) for
antigenic retrieval. The sections were the treated with 3% hydrogen
peroxide in methanol to prohibit the endogenous peroxidase
activity, and incubated with normal goat serum at 37°C for 30 min.
Rabbit antibodies against IL-6 (1:400; cat. no. ab6672) and CD68
(1:400; cat. no. Ab125212; Abcam, Cambridge, MA, USA) were
incubated with the sections overnight at 4°C. Subsequent to
washing, the tissue sections were treated with horseradish
peroxidase labeled anti-rabbit antibody (1:100; cat. no. A0208;
Beyotime Institute of Biotechnology, Haimen, China) at 37°C for 30
min. The sections were immersed in 3-amino-9-ethyl carbazole (DAB;
OriGene Technologies). Subsequent to washing, the tissue sections
were counterstained with 10% hematoxylin, and mounted in
Clear-Mount. The sections were then evaluated at magnification,
×100, and 5 representative areas where high densities (>5
cells/field) of IL-6 or CD68 marker (brown) accumulation at were
identified. They were then counted at magnification, ×200 in each
case, and the average value was used. All protocols were performed
by 2 researchers (Third Military Medical University) who were
blinded to the groups, with assistance from a professional
pathologist.
Cell culture
The blood samples were obtained from the Department
of Hematology, Southwest Hospital, Chongqing, China. The peripheral
blood mononuclear cells (PBMC) were separated by centrifugation at
20°C and 2,000 × g for 20 min with lymphocyte separation medium (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). CD14+ cells were
isolated from the PBMC with the Human CD14 Positive Selection kit
(Stemcell Technologies, Inc., Vancouver, Canada). The purified
monocytes were then cultured with RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Hyclone, Logan, UT, USA) and 100 ng/ml of M-CSF.
Fresh media and cytokines were added on day 3. Subsequent to 6 days
of induction, the cells were stimulated for 24 h with or without 50
or 100 ng/ml human recombinant IL-6 (PeproTech, Inc., Rocky Hill,
NJ, USA). All cells were grown at 37°C in a humidified atmosphere
of 5% CO2.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol® reagent according to the manufacturers' protocol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
RNA (1,000 ng in 10 µl volume) was reverse transcribed with a
reverse transcription kit (Takara Bio., Inc., Otsu, Japan) at 37°C
for 15 min, then 85°C for 12 sec. cDNA was obtained and diluted
with 20 µl nuclease free water. qPCR was performed on a BIO-RAD
CFX96-Tm Real-Time System (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) by mixing 2 µl diluted cDNA with SYBR Green Master Mix
(Applied Biosystems, MA, USA). The reaction conditions were as
follows: 95°C for 2 min, 40 cycles at 95°C for 5 sec, 55°C for 30
sec, and 60°C for 2 min. The forward and reverse primers (Shanghai
GenePharma Co., Ltd., Shanghai, China) listed in Table II. The relative expression levels of
the target mRNAs (IL-10, IL-12, VEGF-C and TGF-β) were using the
2−ΔΔCq method, using β-actin as a calibrator (13).
 | Table II.Primers for quantitative expression of
IL-10, IL-12, VEGF-C and TGF-β. |
Table II.
Primers for quantitative expression of
IL-10, IL-12, VEGF-C and TGF-β.
| Cytokine | Forward | Reverse | Length, bp |
|---|
| IL-10 |
5′-GCTGTCATCGATTTCTTCCC-3′ |
5′-CTCATGGCTTTGTAGATGCCT-3′ | 103 |
| IL-12 |
5′-AGGGCCGTCAGCAACATG-3′ |
5′-TCTTCAGAAGTGCAAGGGTAAAATTC-3′ | 68 |
| VEGF-C |
5′-CAGCACGAGCTACCTCAGCAAG-3′ |
5′-TTTAGACATGCATCGGCAGGAA-3′ | 117 |
| TGF-β |
5′-AACTACTGCTTCAGCTCCAC-3′ |
5′-TGTGTCCAGGCTCCAAATGTA-3′ | 155 |
ELISA analysis
The cytokines IL-10, IL-12, TGFβ and VEGF-C in the
supernatants of the cultured monocytes, centrifuged at 500 × g for
5 min at 20°C, were examined using an ELISA, following the protocol
of the manufacturer (BioLegend, Inc., San Diego, CA, USA). All of
the samples were measured in triplicate.
Transwell analysis
Subsequent to 6 days of differentiation of the
monocytes with M-CSF, IL-6 was added to a final concentration of
100 ng/ml and they were cultured for an additional 24 h.
Subsequently, 1×105 MGC-803 cells were seeded in the
upper well of the Transwell in RPMI 1640 with 5% fetal calf serum
(FCS) and 1×105 Mφs or IL-6 induced Mφs were seeded on
the lower surface of the inverted filter membrane in RPMI 1640 with
5% FCS. Subsequent to 1 day of incubation at 37°C, the cells fixed
with 3.7% paraformaldehyde in CS buffer, 0.1 M piperazine-N,N'-bis
(2-ethanesulfonic acid; PIPES), 1 mM EGTA, 4% polyethylene glycol
800 and 0.1 M NaOH, for 20 min at room temperature for subsequent
crystal violet staining. The number of MGC-803 cells that
penetrated the micropores were then counted at magnification ×200
with a light-microscope (Nikon Corporation, Tokyo, Japan) (14).
Western blot analysis
Total protein was extracted from the cells cultured
for 7 days with or without IL-6 stimulation, and the concentration
of proteins was determined using a BCA protein assay kit (Nanjing
KeyGen Biotech., Co., Ltd., Nanjing, China). Equal quantities of
protein were separated by 10% SDS-PAGE gels and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Subsequent to blocking for 1 h with 3% bovine serum albumin,
the membranes were incubated overnight at 4°C with primary antibody
against STAT3 (dilution, 1:1,000; cat. no. ab68153), P-STAT3
(dilution, 1:1,000; cat. no. ab76315), IRF4 (dilution, 1:1,000;
cat. no. ab133590; all Abcam), and β-actin (dilution, 1:2,000; cat.
no. sc-130656; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Subsequently, the membranes were washed with 0.1% TBS-Tween-20 and
incubated with goat anti-mouse or rabbit horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:5,000;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for
1 h at room temperature. The blots were detected using the
SuperSignal™ West Dura Extended Duration Substrate enhanced
chemiluminescence western blotting kit (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
The statistical analysis between the groups was
performed by the Mann-Whitney U test. Overall survival (OS) rates
of patients and their differences were determined by Kaplan-Meier
method and log-rank test. Analyses were performed using GraphPad
Prism software, version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference. All of the data are presented as the mean ±
standard deviation.
Results
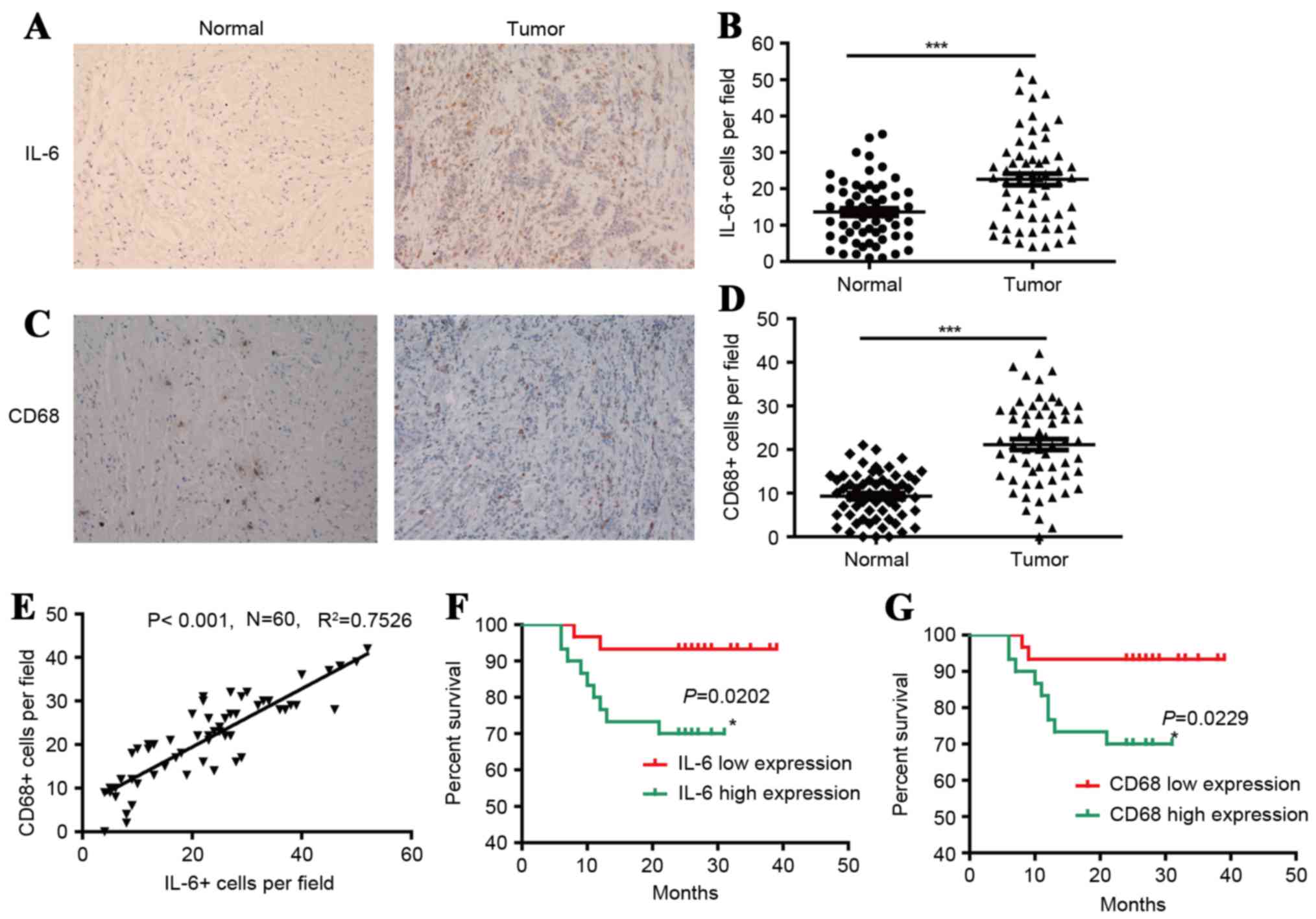
The level of IL-6+ and CD68+ Mφs were
higher in the tumor regions of gastric tissues
Mφs exhibit diverse phenotypes during inflammation
and tumor pathogenesis. To evaluate the types of Mφ phenotype and
distribution patterns in human GC tissues, the present study used
immunostaining to investigate IL-6+ and CD68+ Mφs in situ.
IL-6+ Mφs were revealed to be distributed throughout the tissues,
and were more frequent in the tumor regions compared with non-tumor
regions of gastric tissues, 23±13 and 13±8 cells/field,
respectively, as demonstrated in Fig.
1. It was also demonstrated that CD68+ Mφs were distributed
throughout the tissues, and were more frequent in tumor regions
than non-tumor regions of gastric tissues, 21±10 and 9±5
cells/field, respectively, as illustrated in Fig. 1. Additionally, the frequencies of
IL-6+ cells were positively associated with the frequencies of
CD68+ cells. These results indicate that the level of IL-6+Mφs and
CD68+ Mφs is higher in the tumor regions of gastric tissues.
IL-6 and CD68 were correlated with
poor clinical pathological features and prognosis of GC
patients
As demonstrated in Table
I, the expression levels of IL-6 and CD68 were positively
associated with lymph node metastasis and TNM stages. The
difference was statistically significant (P<0.05). In addition,
to investigate the associations between IL-6 and CD68 with GC
progression, 60 patients with GC were divided into 2 groups based
on the median frequencies of IL-6+ and CD68+ Mφs, respectively.
Kaplan-Meier survival curves were then plotted to investigate the
association with OS rate, as demonstrated in Fig. 1. The log-rank statistic was used to
compare OS rates between the low and high IL-6 or CD68 expression
groups. There was a markedly positive association between OS and
the densities of IL-6+ (P=0.0202) and CD68+ Mφs (P=0.0229), as
illustrated in Fig. 1F and G,
respectively. These results indicate that IL-6 and CD68 exhibit a
positive correlation with poor clinical pathological features and
prognosis of patients with GC.
Expression levels of IL-10, IL-12,
VEGF-C and TGF-β in the CD14+ monocytes and in supernatants of
cultured monocytes stimulated with M-CSF alone, or M-CSF and
IL-6
As M-CSF may induce monocyte differentiation into
Mφs, it has been demonstrated that M1 Mφs produce pro-inflammatory
cytokines such as IL-12 and exert an antitumor effect, whilst M2
Mφs produce IL-10, VEGF-C and TGF-β and promote tumor progression.
Therefore, CD14+ monocytes were cultured for 6 days with 100 ng/ml
M-CSF and stimulated for 24 h with or without 100 ng/ml IL-6. The
Mφs induced by IL-6 exhibited an M2 polarized phenotype,
IL-10high, IL-12low, and high expression
levels of lymph angiogenesis-promoting l factor VEGF-C and tumor
progression-promoting factor TGF-β compared with the control group,
which was cultured with M-CSF, as demonstrated in Fig. 2A. In addition, the differentiation of
Mφs was also detected with ELISA analysis. CD14+ monocytes from the
IL-6-treated model exhibited higher IL-10 and TGF-β expression
levels but lower IL-12 level compared with the control group, as
illustrated in Fig. 2, which supports
the hypothesis that IL-6 treatment promotes the differentiation of
M2 Mφs. The IL-6-treated M2 Mφs also generated a high expression
level of VEGF-C, which supported the hypothesis of the ability of
M2 Mφs to induce the formation of lymphatic fluid. To determine the
effect of TAMs on VEGF/VEGF-C production, the expression levels of
VEGF and VEGF-C in Mφs induced and not induced by IL-6 were
examined. In the IL-6-inducted model, a significant increase in the
expression of VEGF and VEGF-C in the Mφs was detected, indicating
that the TAMs were a source of VEGF and VEGF-C in the tumor
microenvironment.
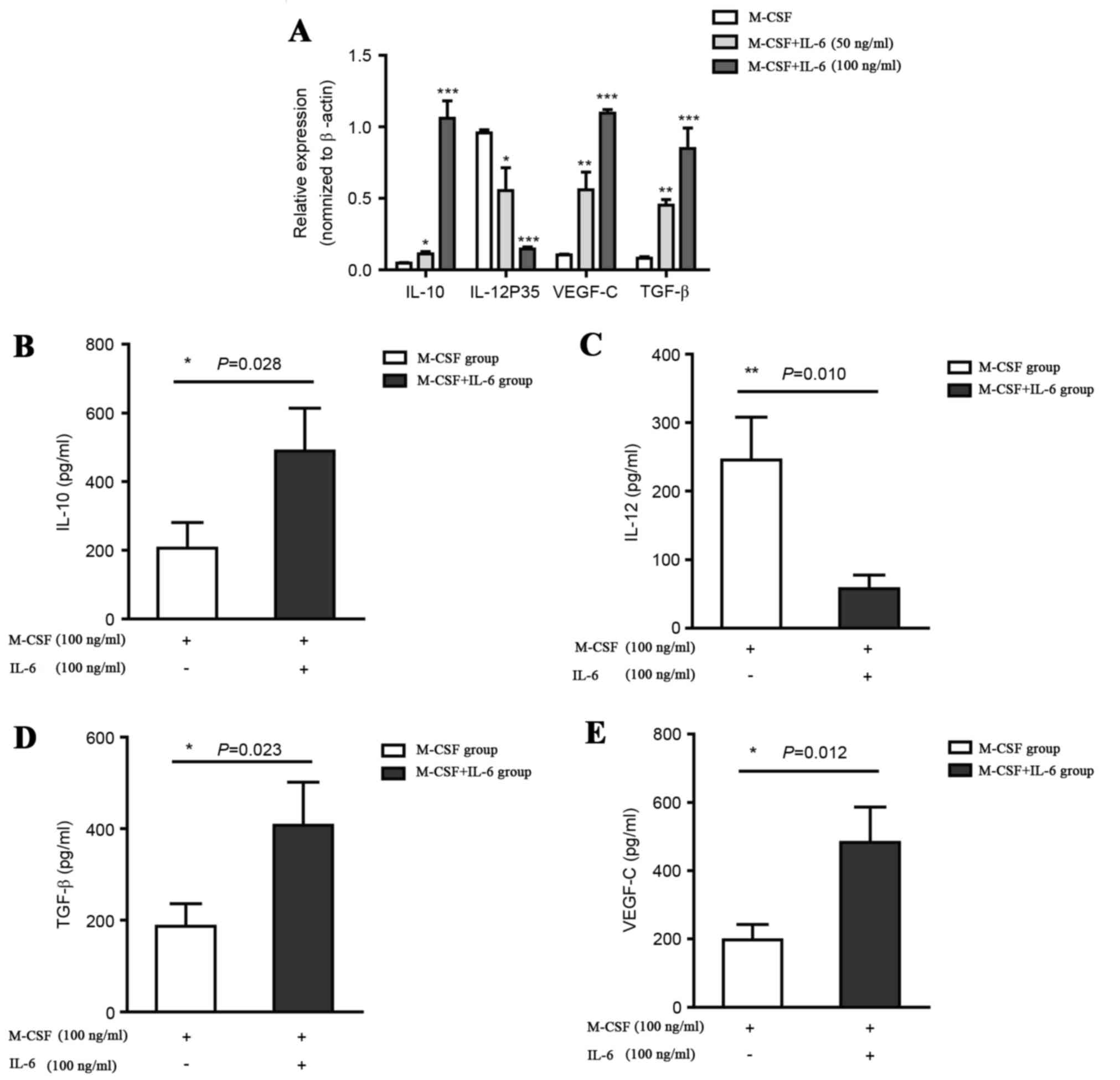 | Figure 2.IL-10, VEGF-C and TGF-β levels
increase and IL-12 levels decrease in CD14+ monocytes and
supernatants of cultured monocytes stimulated with M-CSF alone, or
M-CSF and IL-6. (A) Relative levels of IL-10, IL-12, VEGF-C and
TGF-β in CD14+ monocytes stimulated with M-CSF alone, or M-CSF and
IL-6 at concentration of 50 and 100 ng/ml, quantified by reverse
transcription quantitative polymerase chain reaction. Data are
presented as the mean ± standard deviation (n=6, n, number of
samples). (B) Measurements of IL-10 (C) IL-12 (D) VEGF-C and (E)
TGF-β in supernatants of cultured monocytes stimulated with M-CSF
alone, or M-CSF and 100 ng/ml IL-6 were performed using ELISA in
triplicate. *P<0.05, **P<0.01, ***P<0.001 vs. M-CSF.
M-CSF, macrophage colony stimulation factor; IL, interleukin;
TGF-β, transforming growth factor β; VEGF-C, vascular endothelial
growth factor C. |
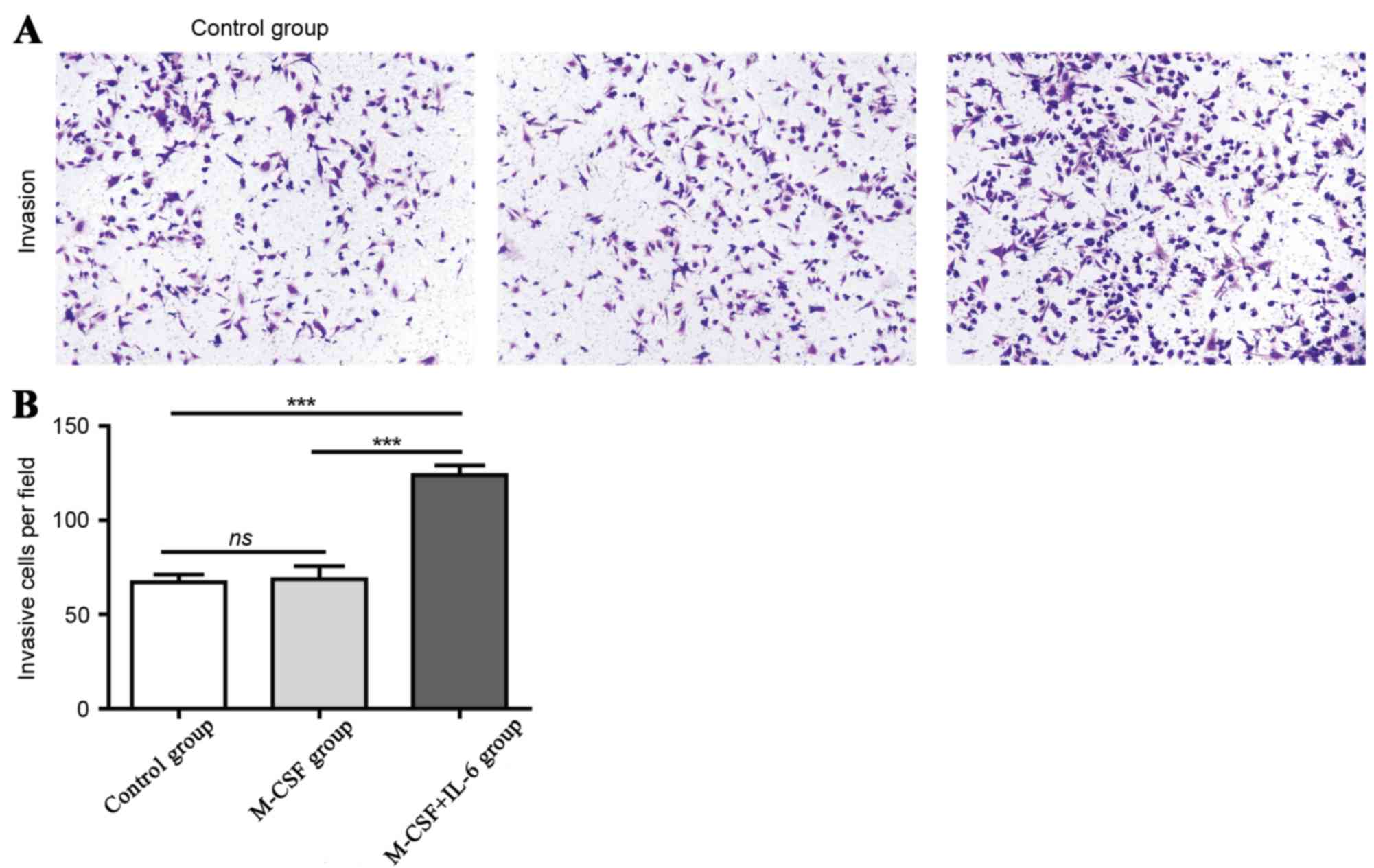
TAMs from IL-6-treated Mφs promote the
invasion and metastasis capacity of tumor cells
It has been suggested that TAMs are critical
regulators of the tumor microenvironment and directly affect the
growth, survival, invasion and metastasis of tumor cells. In the
Transwell assay analysis, it was identified that there were ~70
cells/field in the control and the Mφs-elicited groups. In
contrast, there were ~130 cells per field in the IL-6 induced M2
Mφs group, which supports the hypothesis that TAMs may promote the
invasion of tumor cells, as demonstrated in Fig. 3A and B.
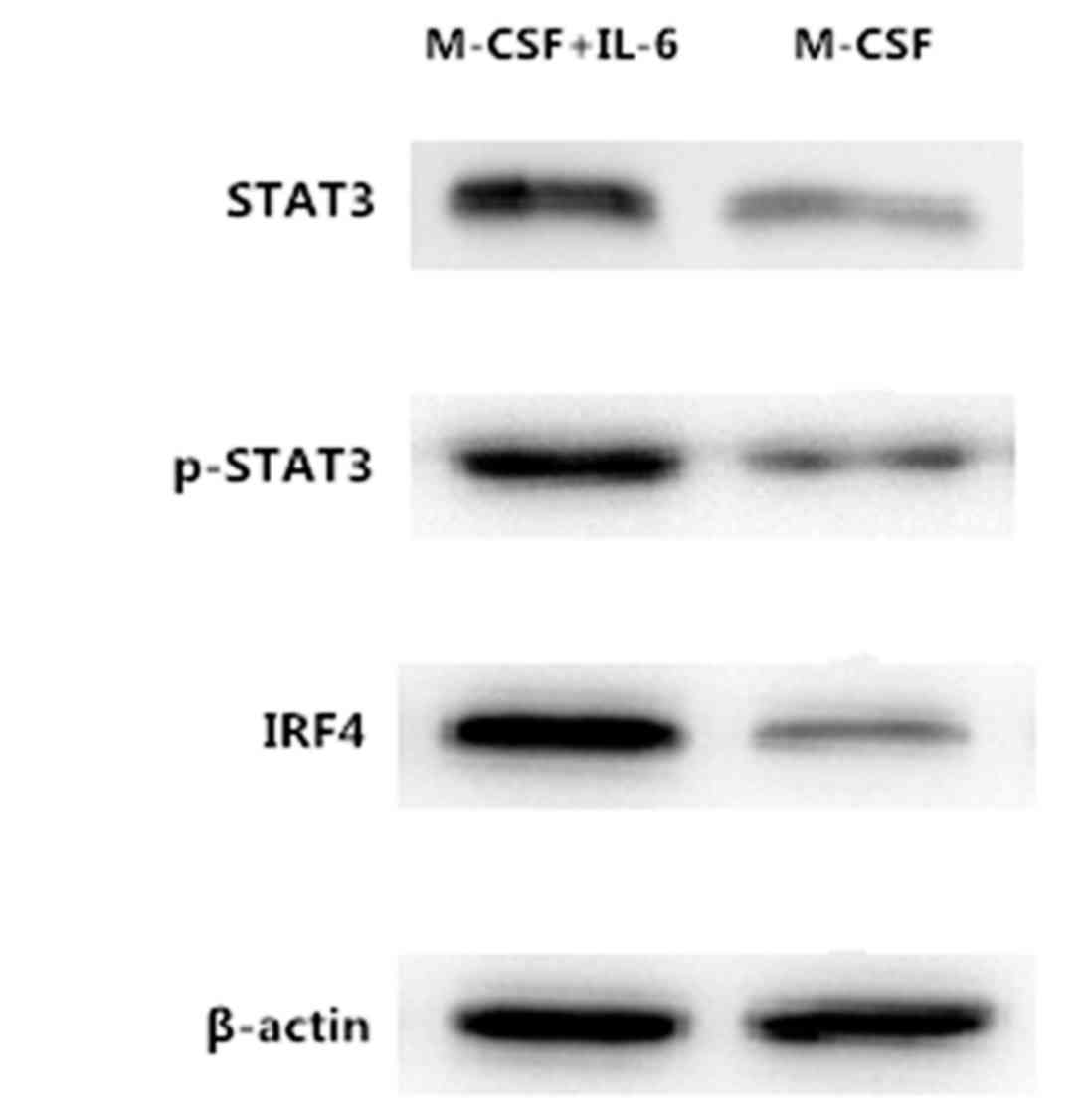
Janus kinase/signal transducers and
activators of transcription 3 (JAK/STAT3) signal pathway was
activated by IL-6 and resulted in the activation of IRF4
IL-6 is a major cancer-promoting cytokine, which
induces several pathways that lead to tumor growth, survival,
angiogenesis, and drug resistance. In the present study, it was
identified that IL-6 induced Mφ differentiation into the M2
phenotype, but the mechanism of this method of differentiation
remains unknown. To additionally explore the mechanism of IL-6
inducing Mφs polarized in the in vitro culture system, the
present study used western blot analysis to detect the production
of IRF4, STAT3 and P-STAT3. The results from the western blot
analysis demonstrated that the levels of expression of IRF4, STAT3
and P-STAT3 proteins in M2 Mφ cells was markedly elevated compared
with non-IL-6-induced Mφs, as illustrated in Fig. 4.
Discussion
GC is one of the most common types of malignant
tumor in the digestive tract, with high rates of incidence and
mortality. The global incidence rate is 952,000 cases of GC and
723,000 incidences of mortality in 2012 (1,15,16). The formation of lymphatic vessels and
subsequent lymphatic metastasis is the primary cause of mortality
in patients with GC (17). Therefore,
studies investigating the mechanisms of lymphangiogenesis may
identify novel methods for improving the effects of treatment and
prognosis of GC. The present study investigated IL-6 and CD68+ Mφs
in the GC microenvironment, and the correlation between prognosis.
In addition, aspects of the IL-6-dependent induction of CD14+
monocytes Mφ differentiation into M2-like Mφs in vitro have
been revealed.
The tumor microenvironment consists of tumor cells,
mesenchymal cells and the infiltrated immune cells, and supports
tumor growth and progression. It has been demonstrated that the
infiltration of inflammatory immune cells into the tumor
microenvironment is important in the mechanisms of tumor
progression, invasion and metastasis. Previous studies have
identified TAMs as a type of inflammatory immune cell present in
tumor tissues, and that the number of TAMs is positively correlated
with tumor lymphatic metastasis and poor prognosis (18–20).
Analysis of immunohistochemical staining of GC and normal tissues
has revealed that the expression levels of VEGF-C and IL-6 are
positively correlated with the levels of CD68+ Mφs in GC tissues,
that the expression of VEGF-C in GC tissues is significantly higher
compared with normal tissues and the level of VEGF-C is positively
correlated with the density of lymphatic vessels, levels of lymph
node metastasis of GC tissue and poor prognosis of patients.
IL-6 is a major cancer-promoting cytokine, which
induces several pathways that lead to tumor growth, survival,
angiogenesis and drug resistance. The immunohistochemical staining
results of the present study demonstrate that the level of IL-6
increased in the tumor microenvironment, which induces Mφ
differentiation into TAMs, consequently promoting tumor
progression. The present study also demonstrated that the
expression levels of IL-6, CD68+ Mφs and VEGF-C are positively
correlated with the number of new lymphatic vessels, the depth of
GC invasion, lymph node metastasis and TNM stages. The results of
the immunohistochemical double-staining analysis indicated that
IL-6 is expressed in TAMs, whilst a previous study (21) has demonstrated that there is an IL-6
receptor expressed on the surface of TAMs. The present study
speculates that IL-6 may induce the differentiation of Mφs to the
M2-like phenotype with high expression levels of IL-10, TGF-β and
VEGF-c, and low expression levels IL-12 through paracrine
signaling.
It was revealed that the Mφs induced by IL-6
exhibited an M2-like polarized phenotype (IL-10high,
IL-12low) and high expression levels of
lymphangiogenesis-promoting factor VEGF-C and tumor
progression-promoting factor TGF-β compared with the control group,
which was cultured with M-CSF. The supernatants were collected to
detect expression levels of IL-10, IL-12, TGF-β and VEGF-C. The
results suggests a M2 phenotype, which indicates that IL-6
treatment promoted the differentiation of M2-like Mφs.
Subsequently, a Transwell experiment was used to determine if TAMs
from IL-6-treated Mφs exhibited a similar capacity. Subsequent to
crystal violet staining, there were ~130 cells/field in the IL-6
induced M2-like Mφs group, but ~70 cells/field in the control
group, which supported the hypothesis that IL-6 induced M2-like Mφs
may promote the invasion of tumor cells.
A previous study identified that the activation of
the JAK/STAT3 signaling pathway as one of the carcinogenic
mechanisms of IL-6 (22), and that
transcription factor IRF4 is an important molecule that controls M2
Mφ polarization (8). To examine the
mechanism of IL-6 induction of Mφ polarization in vitro,
western blot analyses were used to detect the production of IRF4,
STAT3 and P-STAT3. The results demonstrated that the levels of
IRF4, STAT3 and P-STAT3 proteins in IL-6 induced Mφs was markedly
elevated compared with non-IL-6-induced Mφs, which indicates that
IL-6 may elicit the phosphorylation of STAT3 and the activation of
the STAT3 signaling pathway, promote the transcription of IRF4 and
regulate the production of IL-10 or IL-12, resulting in the M2-like
phenotype.
Acknowledgements
The present study would like to thank the Department
of Pathology, Southwest Hospital, Third Military Medical
University, Chongquing, China, for their technical assistance.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81372560).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
YLZ and PWY designed the experiment, CYS and XLF
performed the experiment, WD processed the data and CYS wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board at The Third Military Medical University, and written
informed consent was obtained from all patients.
Consent for publication
Patient, parent or next of kin (in the case of
deceased patients) provided written informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare they they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
Statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biondi A and Hyung WJ: Seventh edition of
TNM classification for gastric cancer. J Clin Oncol. 29:4338–4342.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anestakis D, Petanidis S, Kalyvas S, Nday
CM, Tsave O, Kioseoglou E and Salifoglou A: Mechanisms and
applications of interleukins in cancer immunotherapy. Int J Mol
Sci. 16:1691–1710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Zhang Y, Yao G and Gao J, Yang B,
Zhao Y, Rao Z and Gao J: M2-polarized macrophages promote
metastatic behavior of Lewis lung carcinoma cells by inducing
vascular endothelial growth factor-C expression. Clinics(Sao
Poulo). 67:901–906. 2012.
|
|
6
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benoit M, Desnues B and Mege JL:
Macrophage polarization in bacterial infections. J Immunol.
181:3733–3739. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Satoh T, Takeuchi O, Vandenbon A, Yasuda
K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh
T, et al: The Jmjd3-Irf4 axis regulates M2 macrophage polarization
and host responses against helminth infection. Nat Immunol.
11:936–989. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishigami S, Natsugoe S, Tokuda K, Nakajo
A, Okumura H, Matsumoto M, Miyazono F, Hokita S and Aikou T:
Tumor-associated macrophage (TAM) infiltration in gastric cancer.
Anticancer Res. 23:4079–4083. 2003.PubMed/NCBI
|
|
10
|
Dominguez-Soto A, Sierra-Filardi E,
Puig-Kroeger A, Pérez-Maceda B, Gomez-Aguado F, Corcuera MT,
Sánchez-Mateos P and Corbí AL: Dendritic cell-specific
ICAM-3-grabbing nonintegrin expression on M2-polarized and
tumor-associated macrophages is macrophage-CSF dependent and
enhanced by tumor-derived IL-6 and IL-10. J Immunol. 186:2192–2200.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB, Byrd DB, Compton CC, Fritz A,
Balch CM, Haller DG, et al: AJCC cancer staging manual. 7th. New
York: Springer-Verlag; pp. P117–P126. 2009
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasper JY, Hermanns MI, Unger RE and
Kirkpatrick CJ: A responsive human triple-culture model of the
air-blood barrier: Incorporation of different macrophage
phenotypes. J Tissue Eng Regen Med. 11:1285–1297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Milne AN, Carneiro F, O'Morain C and
Offerhaus GJA: Nature meets nurture: Molecular genetics of gastric
cancer. Hum Genet. 126:615–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu JW, Wu JG, Tajima Y, Li XQ, Du GY,
Zheng LH, Zhang B, Ni XC and Jiang BJ: Study on lymph node
metastasis correlated to lymphangiogenesis, lymphatic vessel
invasion and lymph node micrometastasis in gastric cancer. J Surg
Res. 168:188–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda K, Kobayashi A and Watabe K: The
role of tumor-associated macrophage in tumor progression. Front
Biosci (Schol Ed). 4:787–798. 2012.PubMed/NCBI
|
|
19
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, et al:
M2-polarized tumor-associated macrophage infiltration of regional
lymph nodes is associated with nodal lymphangiogenesis and occult
nodal involvement in pN0 pancreatic cancer. Pancreas. 42:155–159.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang BC, Gao J, Wang J, Rao ZG, Wang BC
and Gao JF: Tumor-associated macrophages infiltration is associated
with peritumoral lymphangiogenesis and poor prognosis in lung
adenocarcinoma. Med Oncol. 28:1447–1452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao RH, Björndahl MA, Religa P, Clasper S,
Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et
al: PDGF-BB induces intratumoral lymphangiogenesis and promotes
lymphatic metastasis. Cancer Cell. 6:333–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tacke RS, Tosello-Trampont A, Nguyen V,
Mullins DW and Hahn YS: Extracellular hepatitis c virus core
protein activates STAT3 in human monocytes/macrophages/dendritic
cells via an IL-6 autocrine pathway. J Biol Chem. 286:10847–10855.
2011. View Article : Google Scholar : PubMed/NCBI
|