Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant
head and neck cancer that mainly occurs in China and southeast Asia
(1); intensity-modulated radiation
therapy (IMRT) is a major therapeutic approach for NPC. Although
ongoing improvement of precise radiation therapy has resulted in a
5-year survival rate of 70% for NPC patients in China, 30–40% of
the patients have a risk of tumour recurrence and metastasis after
the first diagnosis. For patients with a high risk of tumour
recurrence and metastasis, radiotherapy plus chemotherapy is a
highly recommended therapy. However, there remains significant room
to study the pathological and clinical features of the NPC patients
who enjoy better therapy effects from chemotherapy.
Recent studies have shown that CTCs, which could be
dispersed into circulation system by primary and metastatic
cancers, are closely correlated with recurrence and metastasis of
several tumour types, including breast cancer (2), lung cancer (3), gastric cancer (4), head and neck cancers (5–7), as well
as resistance to anti-tumour treatment. Additionally, it is of
significant clinical guiding value in auxiliary diagnosis,
therapeutic evaluation, recurrence and metastasis monitoring and
prognostic assessment of tumour patients. Wu et al (8) also found that the decrease of CTCs was
related with better therapeutic efficacy in NPC patients and the
CTC might be a predictive factor for clinical outcomes via
continuously detecting CTC count in a treatment (8). In our study, we also aim to analyse the
correlation between CTCs and the clinical features of NPC.
Materials and methods
Ethical approval
The study was approved by the Ethics Committee of
the First College of Clinical Medical Science, China Three Gorges
University and Yichang Central People's Hospital (Yichang, China),
and all patients provided written informed consent. All procedures
performed in studies involving human participants were in
accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards.
Subjects and samples
This study included 68 NPC patients and 10 healthy
individuals (undergoing routine physical examination) from Yichang
Central People's Hospital, Gezhouba Central Hospital, the Second
People's Hospital of Yichang and Renhe Hospital during the period
from March, 2014 to February, 2015. The NPC patients included 38
males and 30 females, whose ages were from 27 to 72 years with an
average of 50 years old. According to The 2008 TMN Staging System
for Nasopharyngeal Carcinoma (NPC), 5 were identified as stage I,
12 as stage II, 28 as stage III, 13 as stage IVa and 10 as stage
IVb. All the patients had been diagnosed by histopathological
diagnosis or fine-needle aspiration biopsy with clear TMN staging.
Those with other tumours were excluded. All participants lacked
active infection and malfunction or failure of important internal
organs, including the liver, kidney, heart, etc. CTCs of all
samples were detected and identified prior to providing the
patients with any treatment.
The patients in stages I–II received IMRT as the
first therapy (prescription dose 95%PGTVnx=72.6 Gy/2.2 Gy/fx33 F,
95%PGTVnd=72.6 Gy/2.2 Gy/fx33 F, 95%PTV1=62.04 Gy/1.88 Gy/fx33 F,
95%PTV2=56.76 Gy/1.72 Gy/fx33 F). The patients in stages III–IV
received a 2-period treatment of inductive chemotherapy (cisplatin
at 25 mg/m2, d1-d3; 5-fluoride of 600 mg/m2,
d1-d5) first. Then, they received a 2-period treatment of IMRT at
the same dose as above. One month after completion of IMRT, 31
patients received a 4-period chemotherapy (DF regimen). The
peripheral blood of 10 healthy volunteers was extracted to serve as
a control group. All patients underwent CT and MRI examination one
month after the treatment, and they underwent re-examination every
2–3 months. The results of a 12-month follow-up study were
recorded.
Enrichment and identification of
CTCs
The samples of 7.5 ml of peripheral blood were
incubated with anti-cluster of differentiation (CD)45 magnetic
nanoparticles and collected for subsequent CTC enrichment and
detection; then, CTCs were identified through the combination of
CD45 and CK18 with the FISH-centromere of chromosome 8 (CEP8) probe
method. Enriched cells were made into cell smears. In the following
steps, CEP8 probe was denatured at 75°C for 10 mins before it was
added to the slides. Afterwards, the slides were kept in a
hybridization oven for 2 h. After hybridization, an antibody
mixture of CD45 and CK18 was added to the slides with one-hour
incubation. In the final stage, DAPI was added and the CTCs were
analyzed with a fluorescence microscope (by immunostaining, not
western blotting and FACS).
Statistical analysis
Data were analyzed using statistic analysing
software SPSS 18.0. The χ2 test, t-test, and
Kaplan-Meier analysis were performed to analyse the data.
Results
Classification and identification of
CTCs
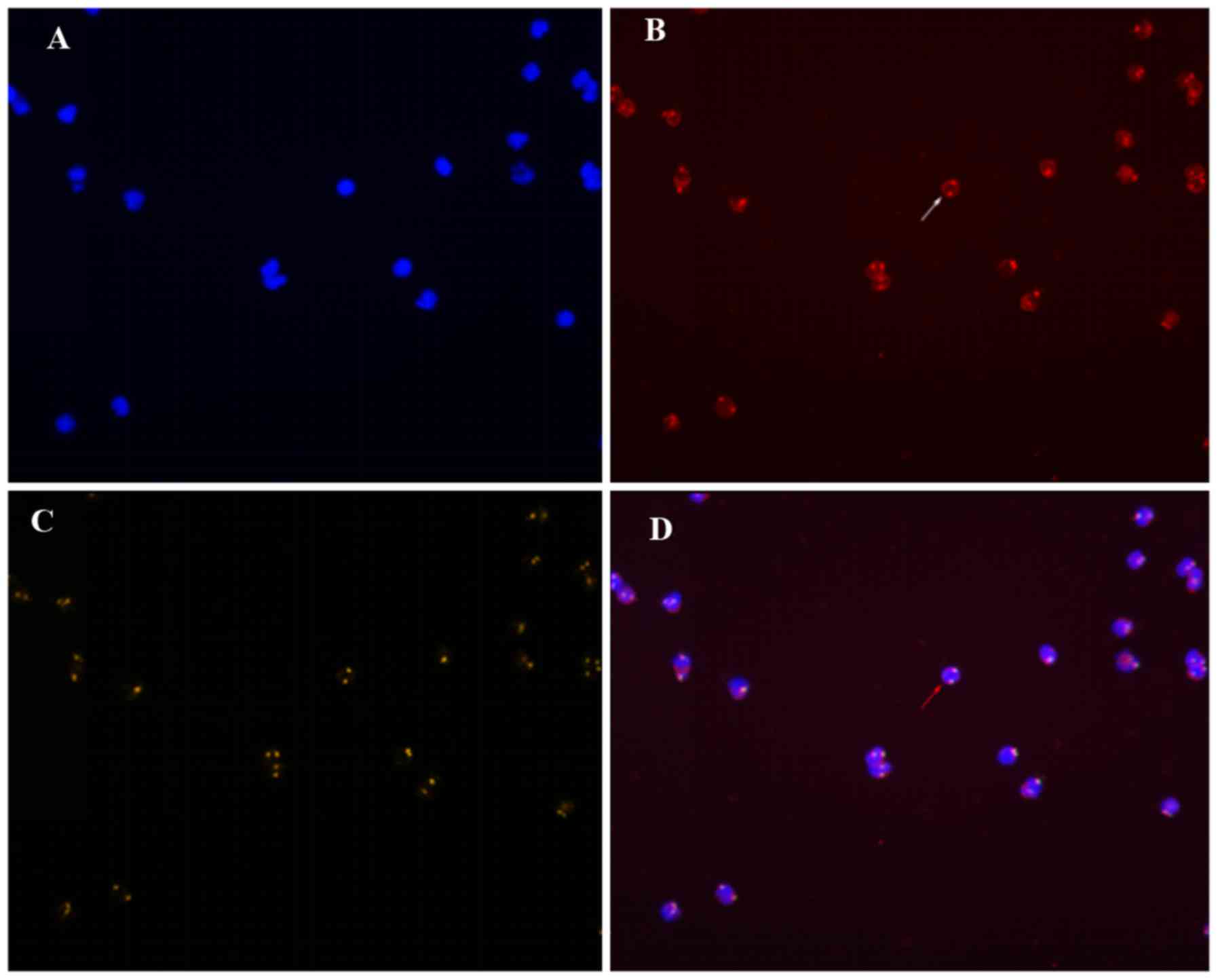
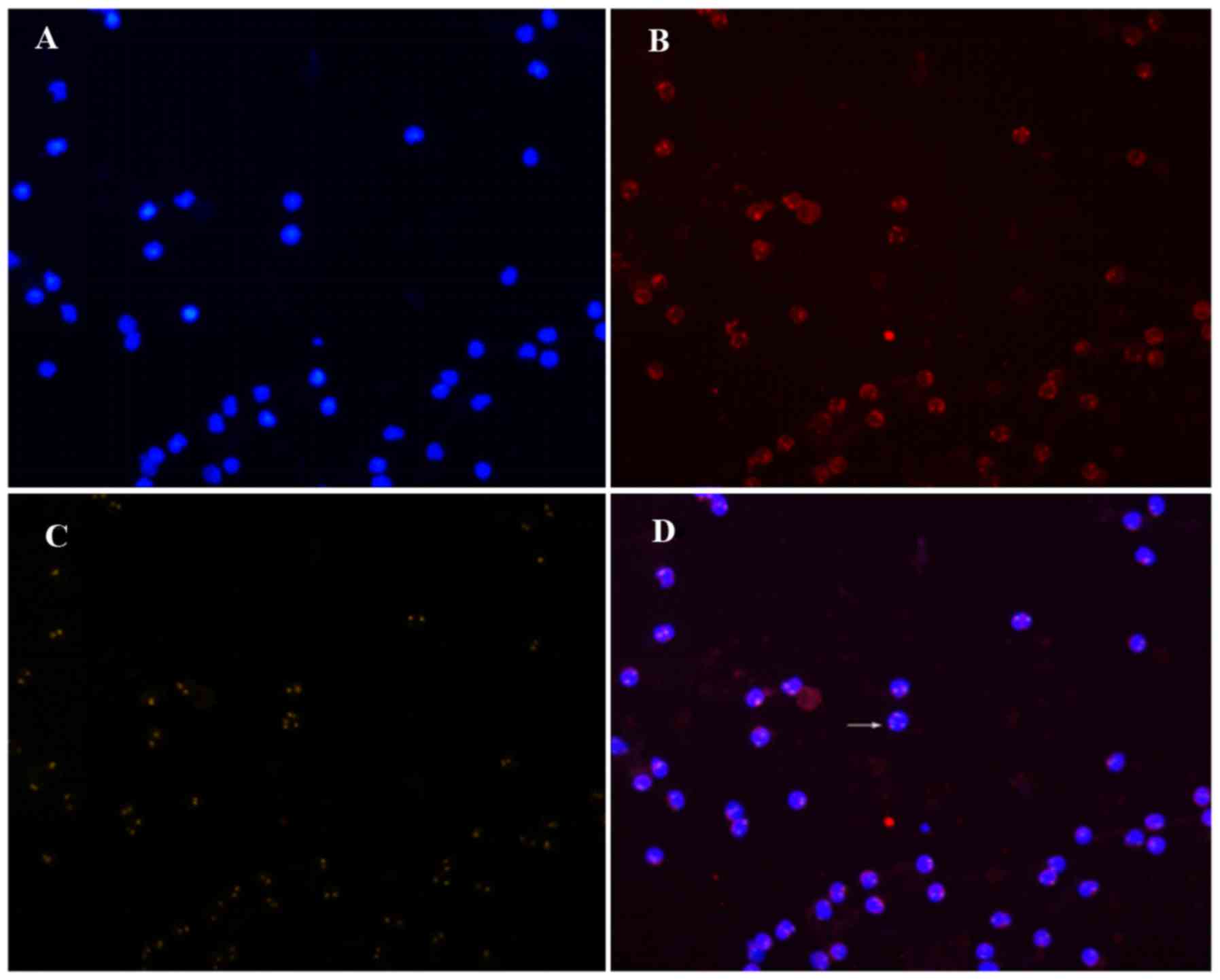
According to the theoretical criteria, the cells
under the microscope were classified as either CK18− or
CK18+, CD45+ or CD45−, and
CEP8>2 (the number of hybridization signals for CEP8>2) or
CEP8=2. Only three patterns are detected:
CD45+/CK18−/CEP8=2 (A pattern),
CD45−/CK18−/CEP8=2 (B pattern), and
CD45−/CK18−/CEP8>2 (C pattern) (Figs. 1–3). The
A pattern represented white blood cells, the C pattern indicated
CTCs, and the B pattern indicated suspicious CTCs, which were not
included in the CTC count. Cells of the C pattern and the B+C
pattern were detected in 67 of the 68 samples with a CTC detection
rate of 98.5%. According to the criterion that CTCs ≥3/7.5 ml was
considered positive [technically supported by the Kindstar Global
Co., Ltd. (Wuhan, China) and the standard set based on a large
number of pre-clinical screening], the positive rate of CTCs was
60.3% (41/68). No C-pattern cell was detected in the peripheral
blood of the healthy individuals.
CTC count in clinicopathologic
parameters
Based on the CTC evaluation criteria described
above, the number of CTCs detected in the peripheral blood of the
68 NPC patients ranged from 0 to 12 cells/7.5 ml with a median of
4.13±2.51/7.5 ml. The CTC number increased with an increase in the
pathological stages with mean counts of 3.71±2.33 (stages I–III)
and 4.96±2.69 (stage IV), respectively. However, the difference was
not statistically significant (P=0.052). The mean count of CTCs was
3.86±2.36 in the M0 stage, and it was 5.70±2.91 in the
M1 stage, which was significantly different (P=0.031;
Table I).
 | Table I.Circulating tumor cell numbers and
nasopharyngeal carcinoma clinical features. |
Table I.
Circulating tumor cell numbers and
nasopharyngeal carcinoma clinical features.
| Clinical feature | Range (n) | Circulating tumor
cell numbers (mean ± standard deviation) | P-value |
|---|
| Tumor stage |
|
| 0.052 |
|
I–III | 0–9 | 3.71±2.33 |
|
| IV | 1–12 | 4.96±2.69 |
|
| Primary tumor |
|
| 0.108 |
| T1 | 0–9 | 3.26±2.60 |
|
| T2 | 1–7 | 3.91±1.90 |
|
| T3 | 1–9 | 4.60±2.41 |
|
| T4 | 1–12 | 5.45±3.17 |
|
| Lymph nodes |
|
| 0.795 |
| N0 | 0–12 | 3.54±3.20 |
|
| N1 | 1–8 | 4.11±2.42 |
|
| N2 | 1–9 | 4.42±2.41 |
|
| N3 | 1–9 | 4.23±2.20 |
|
| Metastasis |
|
| 0.031 |
| M0 | 0–9 | 3.86±2.36 |
|
| M1 | 1–12 | 5.70±2.91 |
|
CTC-positive rates and
clinicopathologic parameters
The positive rates of CTCs in the peripheral blood
of the patients in stages I–III and stage IV were 51.1% (23/45) and
78.3% (18/23); the difference was statistically significant
(P=0.038). The positive rate of CTCs was 90.0% (9/10) in the
M1 stage and 55.2% (32/58) in the M0 stage;
the difference was also statistically significant (P=0.043). Among
the different groups of sexs, ages, pathological types, T stages
and N stages, the CTC-positive rates were not statistically
significant (P>0.05; Table
II).
 | Table II.Circulating tumor cell positive rate
and nasopharyngeal carcinoma clinical features. |
Table II.
Circulating tumor cell positive rate
and nasopharyngeal carcinoma clinical features.
| Clinical feature | Cases (n) | CTC positive/negative
(n) | CTC positive
rate | χ2 | P-value |
|---|
| Sex |
|
|
| 1.087 | 0.328 |
| Male | 38 | 25/13 | 65.8 |
|
|
|
Female | 30 | 16/14 | 53.3 |
|
|
| Age (years) |
|
|
| 0.911 | 0.455 |
|
>50 | 38 | 21/17 | 55.3 |
|
|
| ≤50 | 30 | 20/10 | 66.7 |
|
|
| Histological
type |
|
|
| 0.230 | 0.780 |
|
Non-keratinizing
carcinoma | 50 | 31/19 | 62.0 |
|
|
|
Keratinized squamous cell
carcinoma | 18 | 10/8 | 55.6 |
|
|
| Primary tumor |
|
|
| 6.373 | 0.096 |
| T1 | 19 | 7/12 | 36.8 |
|
|
| T2 | 23 | 15/8 | 65.2 |
|
|
| T3 | 15 | 11/4 | 73.3 |
|
|
| T4 | 11 | 8/3 | 72.7 |
|
|
| Lymph nodes |
|
|
| 4.666 | 0.194 |
| N0 | 13 | 5/8 | 38.5 |
|
|
| N1 | 18 | 10/8 | 55.6 |
|
|
| N2 | 24 | 16/8 | 66.7 |
|
|
| N3 | 13 | 10/3 | 76.9 |
|
|
| Metastasis |
|
|
| 4.322 | 0.043 |
| M0 | 58 | 32/26 | 55.2 |
|
|
| M1 | 10 | 9/1 | 90.0 |
|
|
| Tumor stage |
|
|
| 4.686 | 0.038 |
|
I–III | 45 | 23/22 | 51.1 |
|
|
| IV | 23 | 18/5 | 78.3 |
|
|
The prognostic value of CTC in
NPC
According to the data of a 12-month follow-up, 17
patients were found to have disease progression, and the one-year
progress free survival (PFS) rate was 74.6%. Among the cases with
disease progression, there was 1 patient in stages I–II (5.9%) and
16 in stages III–IV (32.0%). The disease-progression rates in
CTC-positive and CTC-negative patients were 34.1 and 11.5%,
respectively, and the difference was significantly different
(P=0.047; Table III). Among the 50
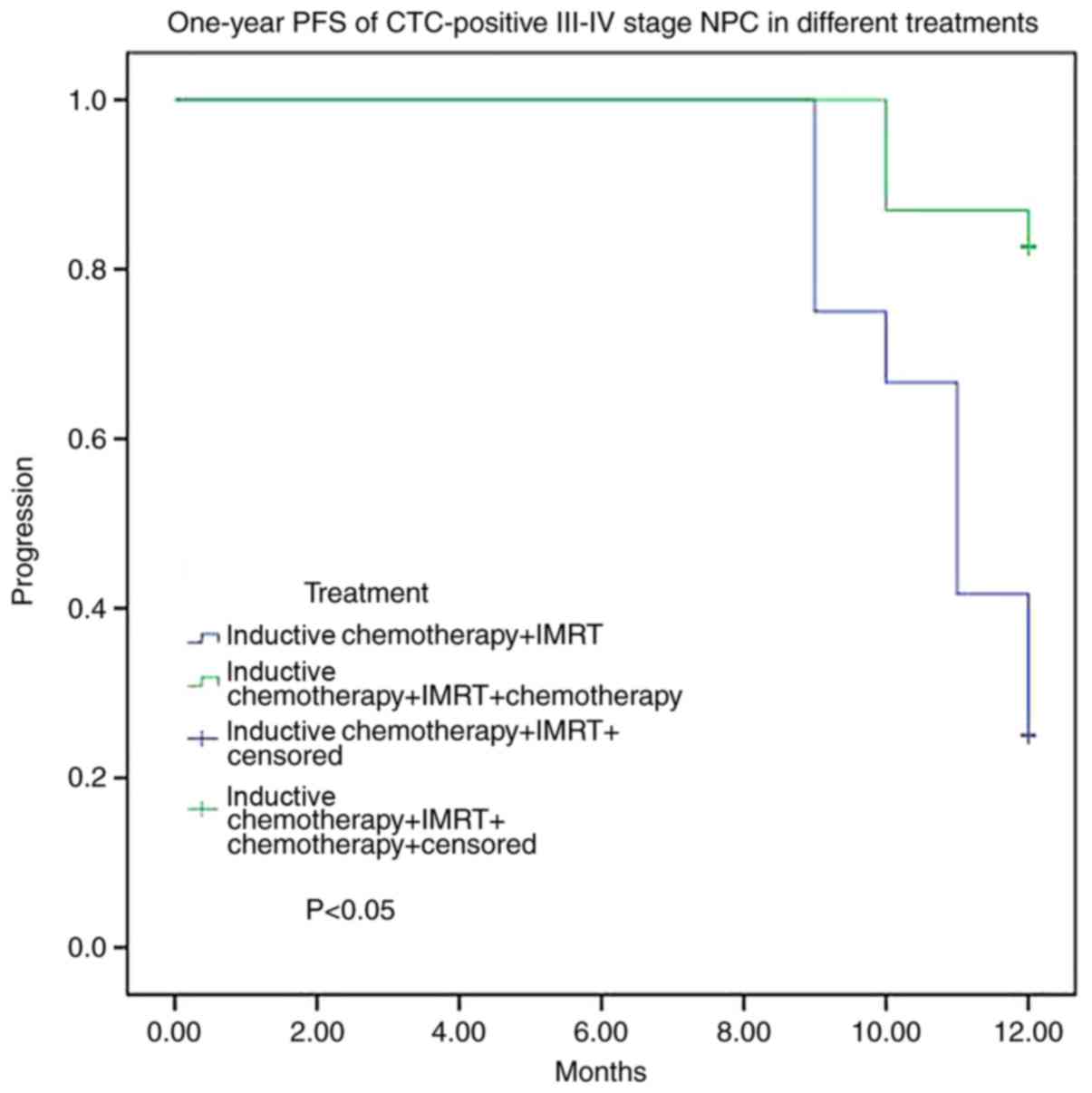
patients in stages III–IV, 31 received treatment with inductive
chemotherapy/IMRT/chemotherapy (A treatment) and 19 received
inductive chemotherapy/IMRT (B treatment). The disease-progression
rates were 16.1% with the A treatment and 57.9% with the B
treatment. For 35 CTC-positive stage III–IV cases, the
disease-progression rate was 83.3% with B treatment and 17.4% with
A treatment. There was a significant difference between the two
groups (P<0.05; Fig. 4).
 | Table III.One-year disease progression in
nasopharyngeal carcinoma. |
Table III.
One-year disease progression in
nasopharyngeal carcinoma.
| Clinical
feature | Cases (n) | Progression | Free-progress | Progress ratio | χ2 | P-value |
|---|
| Tumor stage |
|
|
|
| 4.570 | 0.050 |
|
I–II | 17 | 1 | 16 | 5.9 |
|
|
|
III–IV | 50 | 16 | 34 | 32.0 |
|
|
| CTC number |
|
|
|
| 4.295 | 0.047 |
| CTC
≥3 | 41 | 14 | 27 | 34.1 |
|
|
| CTC
<3 | 26 | 3 | 23 | 11.5 |
|
|
| Primary tumor |
|
|
|
| 3.359 | 0.364 |
| T1 | 19 | 3 | 16 | 15.8 |
|
|
| T2 | 22 | 5 | 17 | 22.7 |
|
|
| T3 | 15 | 4 | 11 | 26.7 |
|
|
| T4 | 11 | 5 | 6 | 45.4 |
|
|
| Lymph nodes |
|
|
|
| 4.370 | 0.125 |
|
N0-1 | 31 | 5 | 26 | 16.1 |
|
|
| N2 | 23 | 6 | 17 | 26.1 |
|
|
| N3 | 13 | 6 | 7 | 46.2 |
|
|
| Metastasis |
|
|
|
| 3.765 | 0.107 |
| M0 | 57 | 12 | 45 | 21.1 |
|
|
| M1 | 10 | 5 | 5 | 50.0 |
|
|
| Histological
type |
|
|
|
| 0.985 | 0.527 |
|
Keratinized squamous cell
carcinoma | 18 | 3 | 15 | 16.7 |
|
|
|
Non-keratinizing
carcinoma | 49 | 14 | 35 | 28.6 |
|
|
| Sex |
|
|
|
| 0.048 | 0.827 |
|
Male | 37 | 9 | 28 | 24.3 |
|
|
|
Female | 30 | 8 | 22 | 26.7 |
|
|
| Age (years) |
|
|
|
| 0.614 | 0.574 |
|
>50 | 37 | 8 | 29 | 21.6 |
|
|
|
≤50 | 30 | 9 | 21 | 30.0 |
|
|
Discussion
The methods for CTC detection remain inconsistent
with each other; the difference in the choice of tumour markers and
testing methods contributes to this. Comparatively, a relatively
mature method for CTC detection involves the use of EpCAM and CK
expression as indicators of tumour presence. However, some studies
have confirmed that the εpithelial-mesenchymal transition (EMT) of
CTCs may reduce the expression of epidermal protein, which can
reduce the efficiency of the testing method. Krebs et al
(9) found the diagnostic rate of lung
CTC is 30% in a test based on EpCAM and CK expression. So many new
technologies to detect CTCs are applied in recent years (6,7,10). unfortunately, there are a few
researches to compare the detection efficiency of the various new
technologies. In this study, CTCs are identified by combining
immunofluorescent staining of CK18 and CD45 and FISH with the
(CEP8) probe method followed by subtractive enrichment of CD45. The
subtractive enrichment approach is independent of down-regulation
or loss of EpCAM expression and increases the diagnostic rate
(10,11). CTCs are CD45(−) non-haematopoietic
cells, and the chromosomal instability of tumour cells causes
polyploidization of FISH-CEP8.
CD45−/CK18+/CEP8≥2 and
CD45−/CK18−/CEP8>2 are considered as
indicators of CTCs because of CK positivity or hyperdiploid. Our
collaborating laboratory discovered that the CK18-positive rate was
5–6% with this detection method based on testing of hundreds of
other cancer samples. In this study, only three patterns were
detected, CD45+/CK18−/CEP8=2 (A pattern),
CD45−/CK18−/CEP8=2 (B pattern) and
CD45−/CK18−/CEP8>2 (C pattern). The C
pattern suggested detection of CTCs, and the B pattern indicated
expressive attributes consistent with suspicious CTCs, which were
not included in the CTC count.
The detection rate of CTCs was 98.5% in the
peripheral blood of NPC patients, and the positive rate of CTC was
60.3%. No CTCs were detected in the peripheral blood of healthy
individuals. No CK-positive NPC patients were found in this study,
as supported the occurrence of CTC EMT. It also suggested that
further studies on different physiological states of CTCs could
promote a better understanding of the mechanism of tumour
development and provide a theoretical basis for the diagnosis and
treatment of tumours. Additionally, the cells of B pattern might be
white cells that have not been infected with CD45 antibodies or
they might be CK-negative diploid tumour cells.
The positive rates of CTCs were 51.1 and 78.3% in
the peripheral blood of NPC patients with stages I–III and IV,
respectively. The positive rate of CTCs was closely correlated to
the result of clinical staging; a later stage reveals a higher
positive rate of CTCs. The positive rate of CTCs in the
M1 stage was higher than that in the M0
stage. A higher CTC-positive rate could suggest a higher
probability of tumour recurrence. These were consistent with a poor
prognosis and high risk of metastasis for advanced patients.
Based on the study finding, it can be hypothesized
that the release of CTCs is a dynamic pathological process. The
level of released CTCs is affected by the tumour burden, and an
increase in the tumour burden might increase the number of CTCs. It
was found that the difference between the CTC-positive rates of the
T and N stages was not statistically significant. One reason might
be that the size of the primary tumour is a factor that modulates
the release of CTCs. Meanwhile, compared to other solid tumours,
the NPC location is relatively limited, limiting the growth of
primary tumours.
Considering tumour metastasis is a complex
multi-step process and positive detection of CTCs does not
guarantee the formation of metastases, we also analyzed the numbers
of CTCs in the peripheral blood of NPC patients. The mean count of
CTCs in the M0 stage was lower than that in the
M1 stage, due to the CTC numbers was really low, more
samples were needed if we analyzed the statistical
significance.
CTCs are killed via the body's immune response and
their own metabolism (12,13), resulting in the survival of some CTCs
that contribute to further development of specific conditions. The
more CTCs detected, the more CTCs might survive until the final
stage, increasing the risk of distant metastasis; this was
consistent with the study result.
Based on a 12-month follow-up survey, we found the
one-year progression free survival (PFS) rate was 74.6%, and the
disease-progression rate in CTC-positive cases was higher than that
in CTC-negative cases. A higher CTC-positive rate might mean a
poorer prognosis, which coincided with the hypothesis raised above.
At present, the therapeutic schedule for NPC patients with stages
I–II was relatively identical and consisted of IMRT. For advanced
(III–IV) NPC patients, the combination of IMRT and chemotherapy is
highly recommended. However, the specific patients who would
benefit more from the therapy is still not very clear. In 50 cases
of stages III–IV, the disease-progression rate of patients who
received treatment with inductive chemotherapy/IMRT (B treatment)
was higher than that who received inductive
chemotherapy/IMRT/chemotherapy (A treatment). Additionally, the A
treatment might be more effective. For CTC-positive cases of stages
III–IV, the disease-progression rate was higher with B treatment
than with A treatment. For advanced NPC patients, especially
CTC-positive cases, A treatment can be considered as the first
choice.
To conclude, we found that the positive rate of CTCs
was correlated with the clinical stages of NPC patients and distant
tumour metastasis. Treatment with inductive chemotherapy + IMRT +
chemotherapy might be more effective for CTC-positive patients with
NPC stages III–IV. Unfortunately, there are still many
insufficient. It has been documented that EB virus is associated
with NPC, we do not analyze the relationship of CTCs and EBV in our
study. With the advance of technology, newer or higher effective
detection techniques may be discovered. Besides, the number of
samples employed in this study is comparatively low, and the
follow-up period is somewhat short. As a result, we could not
comprehensively demonstrate the relationship between CTCs and the
prognosis of NPC patients. Because a larger sample size could
strengthen the conclusions, we will continue to enrol more cases
and follow-up the prognosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by Natural Science
Foundation of Hubei, China (grant no. 2014CFB312).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZC and LX made substantial contributions to the
study design and the acquisition, analysis and interpretation of
the data. CY took part in the analysis of the data and XX
contributed to the study conception and agreed to be accountable
for all aspects of the work.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First College of Clinical Medical Science, China Three Gorges
University and Yichang Central People's Hospital (Yichang, China),
and all patients provided written informed consent. All procedures
performed in studies involving human participants were in
accordance with the ethical standards of the institutional and/or
national research committee, and with the 1964 Helsinki Declaration
and its later amendments or comparable ethical standards.
Consent for publication
Patients provided written informed consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rack B, Schindlbeck C, Jückstock J,
Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H,
Fasching PA, et al: Circulating tumor cells predict survival in
early average-to-high risk breast cancer patients. J Natl Cancer
Inst. 106:pii: dju066. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen YY and Xu GB: Effect of circulating
tumor cells combined with negative enrichment and CD45-FISH
identification in diagnosis, therapy monitoring and prognosis of
primary lung cancer. Med Oncol. 31:2402014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kulasinghe A, Perry C, Jovanovic L, Nelson
C and Punyadeera C: Circulating tumour cells in metastatic head and
neck cancers. Int J Cancer. 136:2515–2523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ning N, Zhan T, Zhang Y, Chen Q, Feng F,
Yang Z, Liu Z, Xu D, Wang F, Guo Y, et al: Improvement of specific
detection of circulating tumor cells using combined CD45 staining
and fluorescence in situ hybridization. Clin Chim Acta. 433:69–75.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Si Y, Lan G, Deng Z, Wang Y, Lu Y, Qin Y,
Huang B, Yang Y, Weng J, Han X, et al: Distribution and clinical
significance of circulating tumor cells in nasopharyngeal
carcinoma. Jpn J Clin Oncol. 46:622–630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He C, Huang X, Su X, Tang T, Zhang X, Ma
J, Guo X and Lv X: The association between circulating tumor cells
and Epstein-Barr virus activation in patients with nasopharyngeal
carcinoma. Cancer Biol Ther. 18:888–894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Qian Y, Peng J, Wei X, Yuan Z, Wei S
and Hu D: Clinical evaluation of circulating tumor cells in locally
advanced nasopharyngeal carcinoma: A prospective study. J Clin
Oncol. 35:e175232017.
|
|
9
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of circulating tumor
cells in patients with non-small cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Wang F, Ning N, Chen Q, Yang Z,
Guo Y, Xu D, Zhang D, Zhan T and Cui W: Patterns of circulating
tumor cells identified by CEP8, CK and CD45 in pancreatic cancer.
Int J Cancer. 136:1228–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okabe H, Tsunoda S, Hosogi H, Hisamori S,
Tanaka E, Tanaka S and Sakai Y: Circulating tumor cells as an
independent predictor of survival in advanced gastric cancer. Ann
Surg Oncol. 22:3954–3961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HJ, Choi GS, Park JS, Park S, Kawai K
and Watanabe T: Clinical significance of thrombocytosis before
preoperative chemoradiotherapy in rectal cancer: Predicting
pathologic tumor response and oncologic outcome. Ann Surg Oncol.
22:513–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos MF, Mannam VK, Craft BS, Puneky LV,
Sheehan NT, Lewis RE and Cruse JM: Comparative analysis of innate
immune system function in metastatic breast, colorectal, and
prostate cancer patients with circulating tumor cells. Exp Mol
Pathol. 96:367–374. 2014. View Article : Google Scholar : PubMed/NCBI
|