Introduction
Bladder cancer is one of the most common malignant
tumors (1). It is estimated that
bladder cancer accounts for 38,600 new cases and causes ~15,000
mortalities worldwide annually (2).
Approximately 75% of the cases are non-muscle-invasive bladder
cancer (NMIBC), which commonly recurs but rarely progresses.
However, the remaining cases are muscle-invasive bladder cancer
(MIBC), which have a worse prognosis and ultimately endanger the
lives of patients (3,4). At present, little is known regarding the
molecular mechanisms of bladder cancer, and no sensitive prognostic
biomarker has been identified (5).
Therefore, it is imperative to investigate the underlying mechanism
and identify novel prognostic biomarkers of bladder cancer.
Long noncoding RNAs (lncRNAs) are an important type
of noncoding RNA (ncRNA); they are >200 mucleotides in length
but lack protein-coding capacity (6).
An increasing amount of evidence has demonstrated that lncRNAs
exhibit crucial regulatory roles in the biological processes of
cancer, including genomic imprinting, chromatin modification and
post-transcriptional processing (7–10).
Increasingly, studies have indicated that lncRNAs may be used as
potential prognostic biomarkers and therapeutic targets (11,12).
Gastric adenocarcinoma-associated, positive CD44 regulator, long
intergenic noncoding RNA (GAPLINC) has been demonstrated to be
aberrantly expressed in gastric and colorectal cancer (13,14).
However, the clinical significance and biological functions of
GAPLINC in bladder cancer remain unknown.
In the present study, GAPLINC expression level in
bladder cancer tissues was investigated using RNA sequencing data
from The Cancer Genome Atlas (TCGA) database the result was
validated in a cohort of 80 pairs of bladder cancer tissues and
normal adjacent tissues. The present study aimed to determine the
relationship between GAPLINC expression and the prognosis for
patients with bladder cancer, and explore the role of GAPLINC in
bladder cancer cell proliferation, cell cycle, migration and
invasion.
Materials and methods
Patients and tissue samples
A total of 80 paired bladder cancer and adjacent
normal tissue samples were collected from patients who underwent
cystectomy in Sun Yat-sen Memorial Hospital of Sun Yat-sen
University (Guangzhou, China). All tissue samples were collected
with written consent from the patients and were approved by the
Hospital Ethics Review Committee. All tissues samples were stored
in RNA later (Ambion; Thermo Fisher Scientific, Waltham, MA, USA)
at −80°C until extraction. All tissue samples were graded according
to the World Health Organization (WHO) grading system and were
staged according to the tumor-node-metastasis (TNM) classification
system (15). The patients were
divided into a high (n=40) and low (n=40) GAPLINC expression groups
(n=40) according to GAPLINC expression. The median value of GAPLINC
expression was used as the cut-off value.
TCGA bladder cancer RNA sequencing
data
TCGA database contains RNA sequencing data for
multiple types of cancer. The RNA sequences of 251 bladder cancer
tissue and 19 normal tissues were downloaded from the Atlas of
Noncoding RNAs in Cancer (TANRIC) database (http://ibl.mdanderson.org/tanric/_design/basic/index.html)
(16).
Cell culture and small interfering RNA
(siRNA) transfection
The human bladder cancer cell lines UM-UC-3, T24,
J82, 5637, RT4 and the normal urothelium cell line SV-HUC-1 were
obtained from American Type Culture Collection (ATCC; Manassas, VA,
USA). T24 and 5637 were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; HyClone;
GE Healthcare Life Sciences, Logan, UT, USA) in a humidified
incubator with 5% at 37°C. UM-UC-3, J82 and RT4 were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; GE Healthcare
Life Sciences, Little Chalfont, UK) in a humidified incubator with
5% CO2 at 37°C. siRNAs were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai GenePharma Co., Ltd., Shanghai,
China). siRNA transfection was conducted using
Lipofectamine® RNAiMAX Reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. In
total, 5 µl siRNA per well was used in each transfection in a
6-well plate. The siRNA sequences were as follows: Si#1 for
GAPLINC, 5′-GCAGGGUAUGCACAGAUGUTT-3′, and Si#2 for GAPLINC
5′-GGACAGAGGCCAGAACAAUTT-3′. Negative control siRNA for GAPLINC
5′-UUCUCCGAACGUGUCACGUTT-3′.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction RT-qPCR
Total RNA of UM-UC-3, T24, J82, 5637 and RT4 cancer
cells was extracted using TRIzol reagent (Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNA using PrimeScript RT Master
Mix (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. RT was performed with the following protocol: 37°C for 15
min, 85°C for 5 sec. With the cDNA of cancer cells (UM-UC-3, T24,
J82, 5637, RT4), we performed qPCR to examine the gene expression
using SYBR-Green PCR Master mix (Roche Diagnostics, Basel,
Switzerland) in a LightCycler 96 Real-Time PCR instrument (Roche
Diagnostics, Basel, Switzerland). Relative gene expression level
was determined using β-actin as a normalizer. The qPCR reaction
included a preincubation step (95°C for 300 sec), 40 cycles of
amplification step (95°C for 15 sec, 56°C for 15 sec and 72°C for
15 sec), a melting step (95°C for 10 sec, 65°C for 60 sec, 97°C for
1 sec) and a cooling step (37°C for 30 sec). The sequences of
primers were as follows, β-actin forward,
5′-ACTGGAACGGTGAAGGTGAC-3′; β-actin reverse,
5′-AGAGAAGTGGGGTGGCTTTT-3′. GAPLINC forward,
5′-TGGACTCAGGCACGTTTACAG-3′; and GAPLINC reverse,
5′-TCATTGTTCTGGCCTCTGTCC-3′. All fold changes were calculated using
the comparative Cq (2−ΔΔCq) method (17).
Protein extraction and western blot
analysis
Cells were washed and lysed in RIPA buffer
containing fresh protease and phosphatase inhibitor cocktails
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total protein
was extracted according to the manufacturer's protocol. The Bio-Rad
assay system (Bio-Rad Laboratories, Hercules, CA, USA) was used to
measure the total protein concentration. For western blot analysis,
equal amounts of protein (30 µg/well) was separated by 10%
SDS-PAGE, and transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). Blotted membranes were blocked
in 5% skimmed milk in TBST, followed by incubation with following
primary antibodies overnight at 4°C: Cyclin D1 (1:1,000; cat. no.
2922; Cell Signaling Technology, Inc., Danvers, MA, USA), CDK4
(1:1,000; cat. no. 12790; Cell Signaling Technology, Inc.), p18
(1:1,000; cat. no. 2896; Cell Signaling Technology, Inc.) and GAPDH
(1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.).
Membranes were then washed with TBST and were incubated with
horseradish peroxidase-conjugated secondary goat anti-rabbit
antibody (catalog no., sc-2004, 1:5,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at room temperature for 1 h. The bound
antibodies were detected using enhanced chemiluminescence kit (EMD
Millipore, Billerica, MA, USA) with Genesys 2.0 (Syngene Europe,
Cambridge, UK). GAPDH and β-tubulin were used as internal
controls.
Flow cytometric analysis of cell
cycle
A flow cytometric assay was conducted to analyze the
cell cycle of bladder cancer cells. At 48 h after siRNA
transfection, cells were collected, washed with PBS, fixed with 7%
ice-cold ethanol overnight, and stained with propidium iodide (PI).
The cell cycle was detected by FACSVerse™ flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA).
Cell proliferation assay
One Solution Cell Proliferation assay (Promega
Corporation, Madison, WI, USA) and a colony formation assay were
conducted to determine the cell proliferation. For One Solution
Cell Proliferation assay, 1×103 cells were planted in
96-well plates with >5 replicate wells. Absorbance was measured
with the multifunctional microplate reader SpectraMax M5 (Molecular
Devices, LLC, Sunnyvale, CA, USA) at 490 nm. The measurement of
absorbance was conducted every 24 h for a total of 5 days. For the
colony formation assay, 1×103 cells were plated in
6-well plates at 48 h after RNA transfection and cultured in
corresponding medium. After 1 week, cells were washed twice with
PBS, fixed with 4% paraformaldehyde for 30 min, and then stained
with 0.1% crystal violet for 30 min for visualization and
counting.
Wound healing assay
The cells were plated into 6-well plates at 48 h
after RNA transfection. After 24 h, the transfected cells were
wounded with a 200 µl pipette tip. Following scratching, the cells
were cultured in serum-free medium (Gibco; Thermo Fisher
Scientific, Inc.). Images of the wound were captured at 0 and 24 h
with inverted microscope (×100). The wound closure rate
(%)=migrated cell surface area/total surface area ×100.
Cell migration and invasion assay
The migration assay was performed using 24-well
Transwell insert chambers (Corning Incorporated, Corning, NY, USA)
with 8-µm pore size polycarbonate filters. Approximately
1×104 cells were seeded into the upper chamber with
serum-free medium and the lower chamber was filled with 600 µl
medium with 10% FBS. For the invasion assay, Matrigel Invasion
Chambers in the 24-well plates (BD Biosciences, Franklin Lakes, NJ,
USA) were used. A total of 1×105 cells were seeded into
the upper chamber with serum-free medium and the lower chamber was
filled with 600 µl medium with 10% FBS. After 24 h, cells in upper
chambers were removed, and migratory or invasive cells were fixed
with 4% paraformaldehyde and stained with 0.1% crystal violet. The
number of migratory or invasive cells was counted in 5 randomly
selected fields under an inverted fluorescence microscope (×100,
magnification).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference. The significance
of the differences was analyzed by the Student's t-test or
χ2 test when only two groups were compared. The
significance of the differences was analyzed by one-way analysis of
variance (ANOVA) using Bonferroni post hoc test in the case of
multiple comparisons. Kaplan-Meier analysis was used to analyze the
patients' survival. All the experiments were performed a minimum of
three times.
Results
Upregulation of GAPLINC in bladder
cancer and cell lines
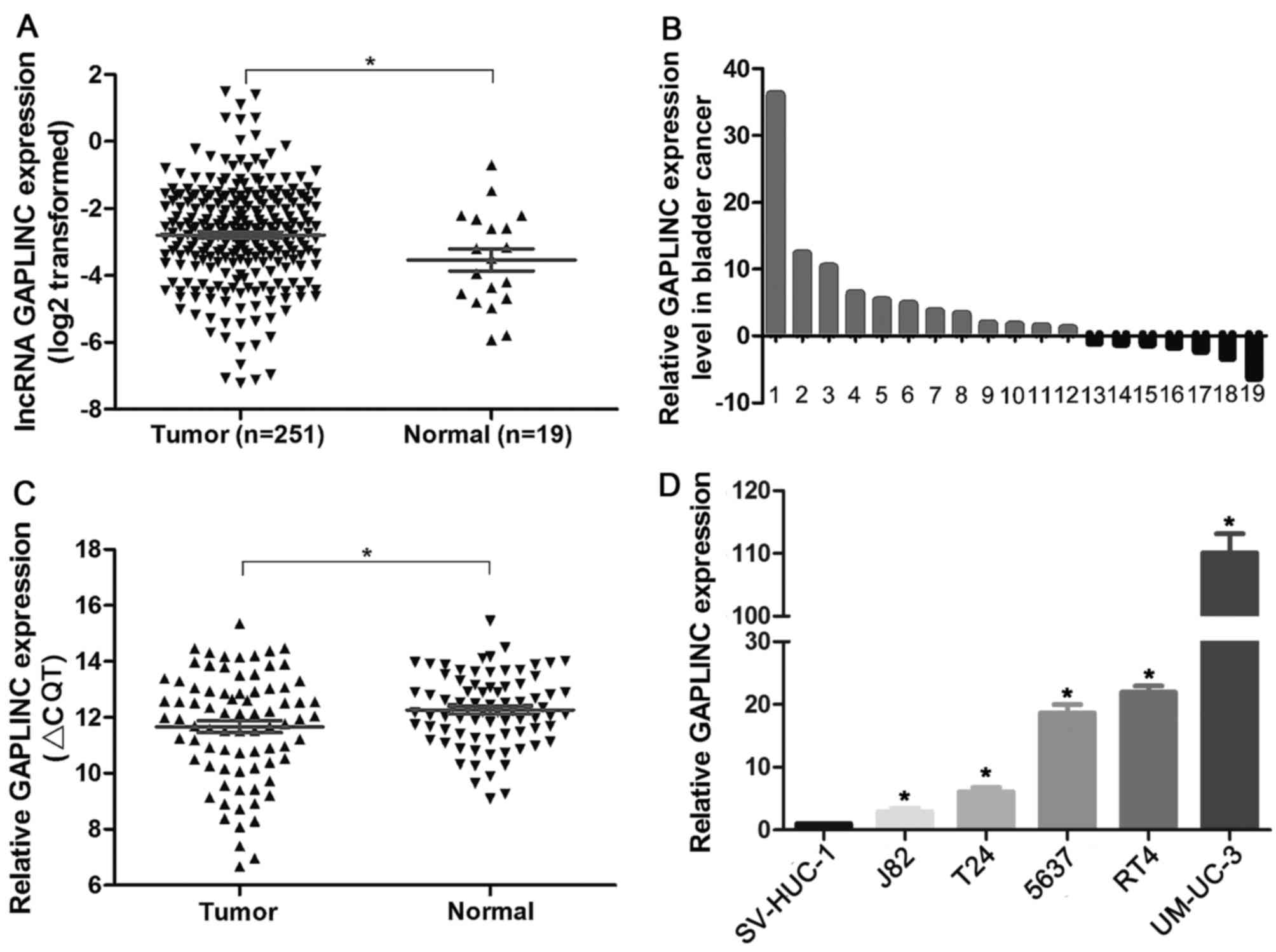
To investigate the GAPLINC expression level in
bladder cancer tissues and normal tissues, we first analyzed the
RNA sequencing data from TCGA database was initially analyzed. The
results demonstrated that GAPLINC was significantly upregulated in
251 bladder cancer tissues compared with 19 normal tissues
(Fig. 1A and B; P=0.039). To validate
the findings from TCGA database, the present study further examined
the GAPLINC expression level in 80 pairs of bladder cancer tissues
and adjacent normal tissues by RT-qPCR, using β-actin as a
reference gene (Fig. 1C; tumor vs.
normal P=0.021). The results supported a strong associated between
the RT-qPCR results and the TCGA database analysis. Furthermore,
the expression pattern of GAPLINC in bladder cancer cell lines
(UM-UC-3, T24, J82, RT4 and 5637) and a normal urothelium cell line
SV-HUC-1 were determined. Similar to the changes in tissue samples,
the results indicated the GAPLINC expression level was
significantly higher in bladder cancer cell lines than in normal
urothelial cells (Fig. 1D). Based on
the expression pattern of GAPLINC, we hypothesized that GAPLINC may
be a potential oncogene in bladder cancer.
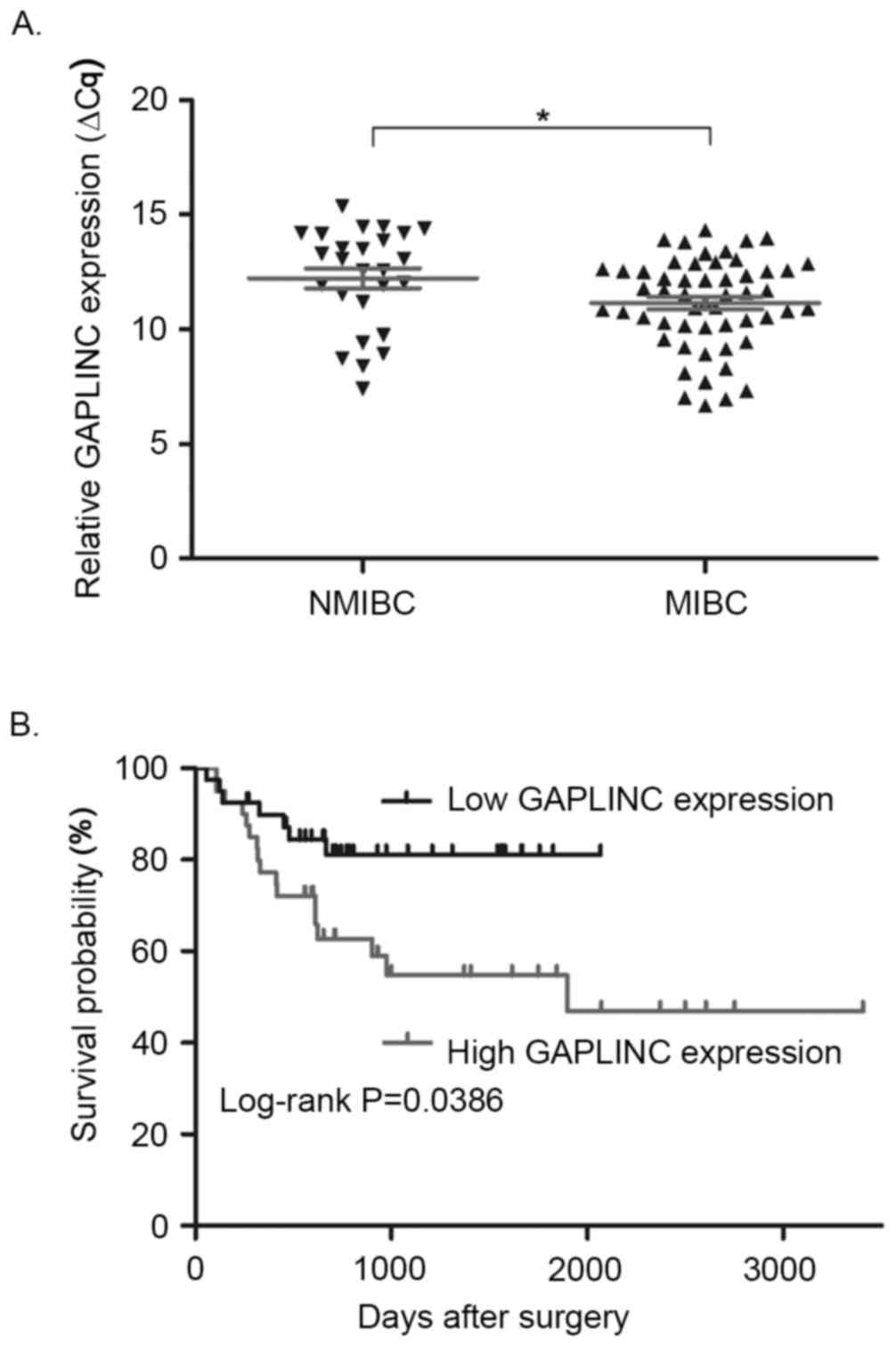
Association between GAPLINC expression
and clinical characteristics
The association between GAPLINC expression and
clinical characteristics of patients with bladder cancer was
analyzed (Table I). According to the
cut-off value of GAPLINC expression, bladder cancer patients were
classified into the high (n=40) or the low GAPLINC expression
groups (n=40). Statistical analysis revealed that GAPLINC
expression level was associated with tumor stage. The GAPLINC
expression level was significantly higher in MIBC tissues compared
with that in NMIBC tissues (Fig. 2A;
P=0.017). However, no significant association was identified
between GAPLINC expression and age, sex, tumor size, N stage or
tumor grade. Additionally, Kaplan-Meier analysis demonstrated that
patients in the high GAPLINC expression group had significantly
shorter overall survival times than patients in the low GAPLINC
expression group (Fig. 2B;
P=0.0386).
 | Table I.Association between GAPLINC expression
and clinical characteristics (n=80). |
Table I.
Association between GAPLINC expression
and clinical characteristics (n=80).
|
|
| GAPLILNC
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | Cases | Low | High | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.058 | 0.809 |
|
<60 | 25 | 12 | 13 |
|
|
|
>60 | 55 | 28 | 27 |
|
|
| Sex |
|
|
| 0.032 | 0.858 |
|
Female | 15 | 7 | 8 |
|
|
|
Male | 65 | 33 | 32 |
|
|
| Tumor size
(cm) |
|
|
| 0.056 | 0.813 |
|
<3 | 27 | 14 | 13 |
|
|
|
>3 | 53 | 26 | 27 |
|
|
| T stage |
|
|
| 5.689 | 0.017a |
| T1 | 26 | 18 | 8 |
|
|
|
T2-4 | 54 | 22 | 32 |
|
|
| N stage |
|
|
| 2.451 | 0.117 |
| N0 | 68 | 37 | 31 |
|
|
| N1 | 12 | 3 | 9 |
|
|
| Grade |
|
|
| 0.069 | 0.793 |
|
Low | 19 | 10 | 9 |
|
|
|
High | 61 | 30 | 31 |
|
|
Knockdown of GAPLINC inhibits bladder
cancer cell proliferation
Due to the upregulation of GAPLINC in bladder cancer
tissues and cell lines, we hypothesized that GAPLINC may be
involved in a number of critical biology processes, including
proliferation and invasion. To determine the role of GAPLINC on
bladder cancer biological behavior, siRNAs were designed to knock
down GAPLINC. The RT-qPCR results demonstrated that GAPLINC was
sufficiently silenced in UM-UC-3 and T24 cells (Fig. 3A). The One Solution Cell Proliferation
assay and colony formation assay were employed to evaluate the
effect of GAPLINC on the proliferation of bladder cancer cells.
Evident inhibitory effects on cell proliferation were observed in
the GAPLINC knockdown group (Fig. 3B and
C). The colony formation assay demonstrated that the colonies
in the GAPLINC knockdown group were smaller and fewer compared with
the normal control group (Fig. 3D and
E).
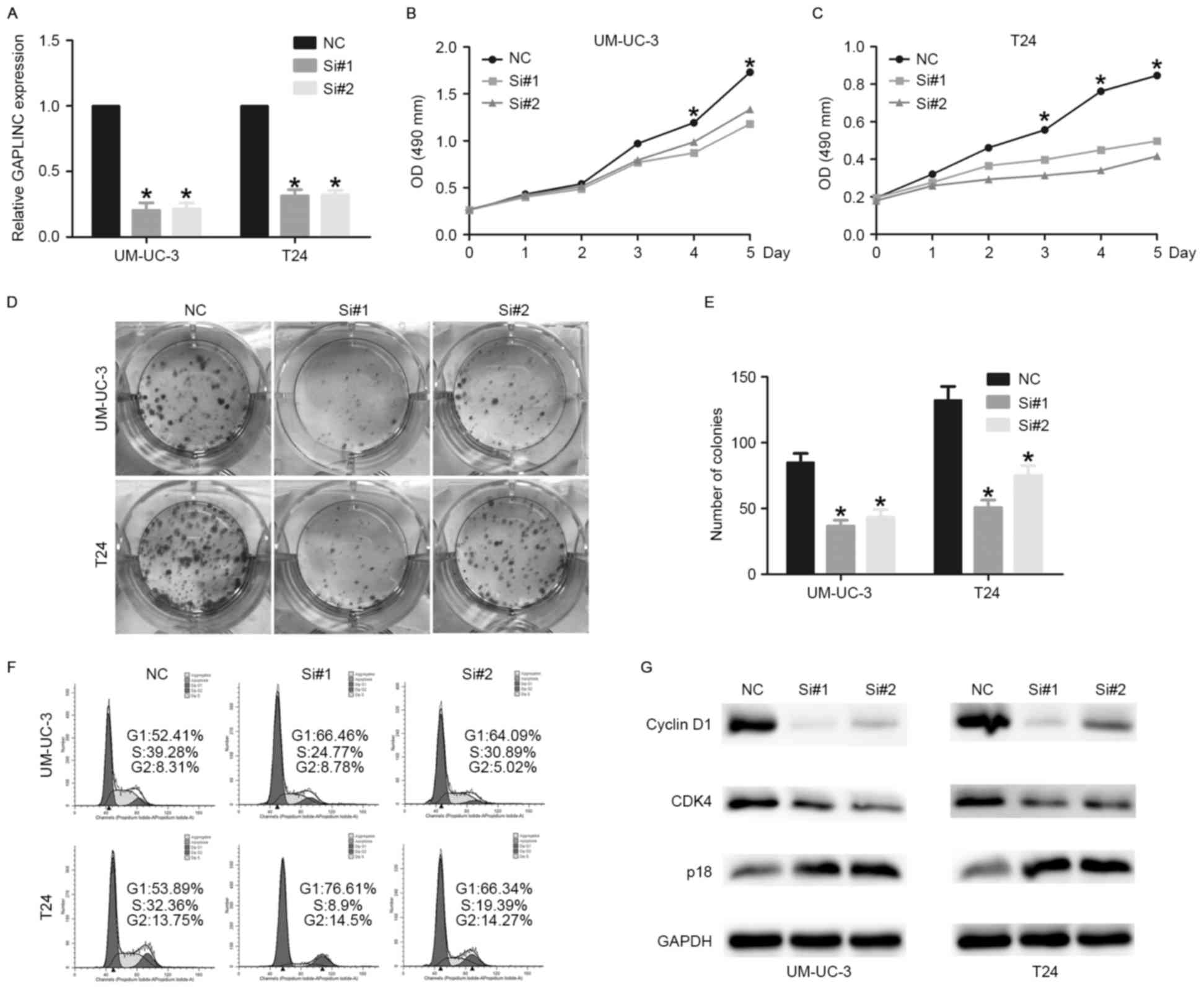 | Figure 3.Knockdown of GAPLINC inhibits bladder
cancer cell proliferation. (A) GAPLINC was sufficiently silenced by
siRNAs (*P<0.05 vs. NC). (B and C) The One Solution Cell
Proliferation assay indicated that knockdown of GAPLINC inhibited
bladder cancer cell proliferation (*P<0.05 vs. NC). (D and E)
Colony formation assay indicated that knockdown of GAPLINC
inhibited bladder cancer cell proliferation (*P<0.05 vs. NC).
(F) Cell cycle analysis revealed that GAPLINC influenced the
proliferation of UM-UC-3 and T24 cells by regulating the cell
cycle. (G) Western blotting revealed that GAPLINC silencing
promotes cell cycle arrest through regulating the expression of
cell cycle-associated proteins. Data are shown as means ± SD are
shown. Statistical analysis was conducted by one-way ANOVA. GAPLIN,
gastric adenocarcinoma associated, positive CD44 regulator, long
intergenic noncoding RNA; siRNA, short interfering RNA; si#1, short
interfering RNA 1; si#2, short interfering RNA 2; SD, standard
deviation; OD, optical density; NC, negative control; CDK4, cyclin
dependent kinase 4. |
GAPLINC silencing promotes cell cycle
arrest at G1 phase by regulating cell cycle-associated genes
Flow cytometric analysis was performed to evaluate
the effects of GAPLINC on the cell cycle. A significant cell cycle
arrest was observed at G1 phase, with an evident decrease in S
phase following GAPLINC silencing (Fig.
3F). To validate the flow cytometry results, western blot
analysis was performed to determine the alteration of G1/S
checkpoint-associated proteins. The results identified that the
expression of cyclin D1 and cyclin-dependent kinase 4 (CDK4) were
decreased in the GAPLINC knockdown group. Furthermore, the
expression of p18, one of the inhibitors of CDK4, was increased
following GAPLINC knockdown (Fig.
3G). Taken together, the results suggest that GAPLINC silencing
promotes cell cycle arrest, potentially through regulating the
expression of cell cycle-associated proteins.
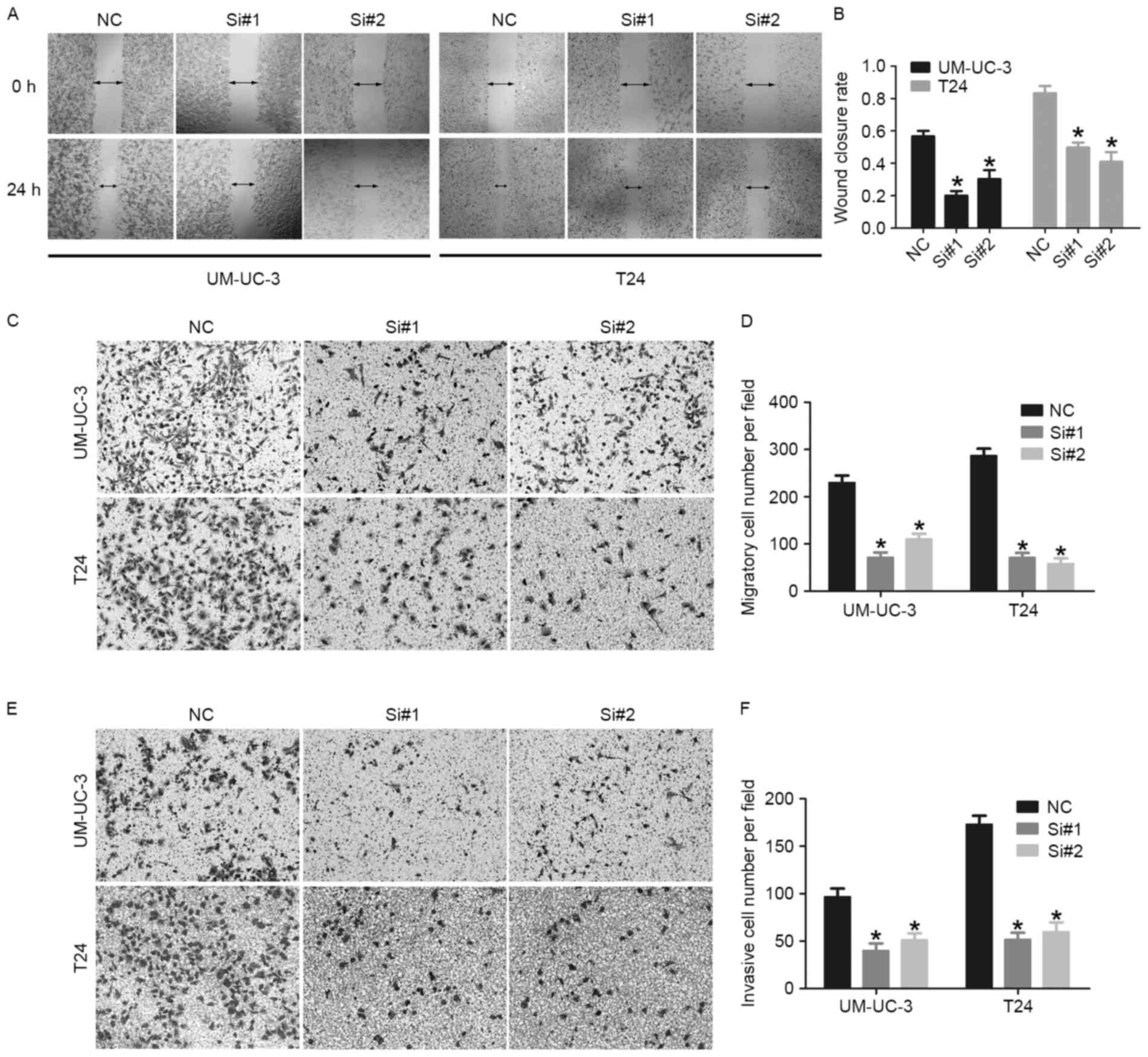
Knockdown of GAPLINC inhibits bladder
cancer cell migration and invasion
A previous study identified that GAPLINC promoted
migration and invasion in gastric cancer and colorectal cancer
(13,14). However, the role of GAPLINC in
migration and invasion of bladder cancer remains unknown. The
effect of GAPLINC on migratory and invasive ability of bladder
cancer cells was investigated using wound-healing assays and
Transwell assays. The wound-healing assay indicated that the
GAPLINC knockdown inhibited the healing of bladder cancer cells
compared with the normal control group (Fig. 4A and B). The Transwell assays revealed
that the GAPLINC knockdown group exhibited a significantly reduced
number of migratory and invasive cells (Fig. 4C-F). Overall, these results indicated
that GAPLINC serves an important role in the migration and invasion
of bladder cancer cells.
Discussion
It has been demonstrated that the human
transcriptome comprises not only many types of protein-coding RNAs,
but also a large number of ncRNAs, including lncRNAs (18,19). An
increasing amount of evidence has indicated that certain lncRNAs
exhibit crucial roles in human cancers, acting as oncogenes or
tumor suppressors (20,21). The lncRNA GAPLINC was previously
reported to be overexpressed in gastric cancer and colorectal
cancer. GAPLINC is associated with poor prognosis of gastric cancer
and regulates gastric cancer cells invasiveness (13,22).
GAPLINC also interacts with PTB-associated splicing factor (PSF)
and non-POU domain-containing octamer-binding (NONO) protein, and
ultimately promotes invasion in colorectal cancer (14). However, little is known about the
association between GAPLINC and bladder cancer.
To the best of our knowledge, this is the first
report of GAPLINC being involved in the progression of bladder
cancer. In the present study, GAPLINC was demonstrated to be
frequently upregulated in bladder cancer tissues and its
upregulation was associated with poor prognosis of bladder cancer
patients. The GAPLINC expression level was significantly higher in
MIBC tissues compared with that in NMIBC tissues. These results
imply that GAPLINC might be a potential diagnostic biomarker and
act as an oncogene in bladder cancer, and may also be able to
distinguish NMIBC from MIBC, which requires more complementary
therapy.
The dysregulation of cell proliferation is one of
the causative factors of bladder cancer (23,24). In
the present study, the One Solution Cell Proliferation assay and
colony formation assay demonstrated that GAPLINC knockdown
significantly inhibited the proliferation of UM-UC-3 and T24 cells.
The flow cytometric analysis indicated that GAPLINC knockdown
inhibited cells proliferation through inducing cell cycle arrest at
the G1 phase. Western blot analysis was performed to determine the
alteration of G1/S checkpoint-associated proteins and identified
that the expression of cyclin D1 and CDK4 were decreased and the
expression of p18 was increased following GAPLINC knockdown.
Previous studies have identified that the complex of cyclin D1-CDK4
serves an important role in cell cycle regulation (25,26) and
p18 is one of the inhibitors of CDK4 (27). Therefore, the most likely mechanism
underlying the dysregulation of proliferation involves a decrease
in p18 expression, which leads to an increase of the complex of
cyclin D1-CDK4, and then ultimately promotes bladder cancer cells
to go progress through the G1 phase to the S phase of the cell
cycle.
Due to the importance of invasiveness and metastasis
on prognosis of bladder cancer patients (28,29), the
present study further explored the role of GAPLINC on the invasive
ability of bladder cancer cells. The wound healing assay and
Transwell assays demonstrated that GAPLINC knockdown significantly
inhibited the migratory and invasive abilities of bladder cancer
cells. These observations may explain why GAPLINC was upregulated
in MIBC tissues compared with NMIBC tissues, and were in agreement
with the results of other studies (13,14) on
gastric cancer and colorectal cancer.
To conclude, the present study demonstrated that
GAPLINC expression level was markedly increased in bladder cancer
tissues and cell lines, and was associated with the tumor stage and
prognosis in bladder cancer patients. Furthermore, knockdown of
GAPLINC significantly suppressed the bladder cancer cells
proliferation, migration and invasion. The findings of the present
study indicate that GAPLINC serves an important fucntion in bladder
cancer and may serve a novel therapeutic target.
Acknowledgements
We acknowledge financial support from the following
sources: The National Natural Science Foundation of China (grant
no. 81672534 and 81472388), and the Guangdong Science and
Technology Department Foundation (grant no. 2013B021800110).
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNAs
|
long noncoding RNAs
|
|
GAPLINC
|
gastric adenocarcinoma associated,
positive CD44 regulator, long intergenic noncoding RNA
|
|
NMIBC
|
non-muscle invasive bladder cancer
|
|
MIBC
|
muscle-invasive bladder cancer
|
|
TCGA
|
The Cancer Genome Atlas
|
|
TANRIC
|
The Atlas of Noncoding RNAs in
Cancer
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
siRNAs
|
small interfering RNAs
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmstrom PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kluth LA, Black PC, Bochner BH, Catto J,
Lerner SP, Stenzl A, Sylvester R, Vickers AJ, Xylinas E and Shariat
SF: Prognostic and prediction tools in bladder cancer: A
comprehensive review of the literature. Eur Urol. 68:238–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martens-Uzunova ES, Bottcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pandey GK, Mitra S, Subhash S, Hertwig F,
Kanduri M, Mishra K, Fransson S, Ganeshram A, Mondal T, Bandaru S,
et al: The risk-associated long noncoding RNA NBAT-1 controls
neuroblastoma progression by regulating cell proliferation and
neuronal differentiation. Cancer Cell. 26:722–737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoon JH, Abdelmohsen K and Gorospe M:
Posttranscriptional gene regulation by long noncoding RNA. J Mol
Biol. 425:3723–3730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCleland ML, Mesh K, Lorenzana E, Chopra
VS, Segal E, Watanabe C, Haley B, Mayba O, Yaylaoglu M, Gnad F and
Firestein R: CCAT1 is an enhancer-templated RNA that predicts BET
sensitivity in colorectal cancer. J Clin Invest. 126:639–652. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Chen Z, Tian L, Zhou C, He MY, Gao
Y, Wang S, Zhou F, Shi S, Feng X, et al: LncRNA profile study
reveals a three-lncRNA signature associated with the survival of
patients with oesophageal squamous cell carcinoma. Gut.
63:1700–1710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Wang J, Qian J, Kong X, Tang J, Wang
Y, Chen H, Hong J, Zou W, Chen Y, et al: Long noncoding RNA GAPLINC
regulates CD44-dependent cell invasiveness and associates with poor
prognosis of gastric cancer. Cancer Res. 74:6890–6902. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang P, Chen T, Xu Z, Zhu H, Wang J and He
Z: Long noncoding RNA GAPLINC promotes invasion in colorectal
cancer by targeting SNAI2 through binding with PSF and NONO.
Oncotarget. 7:42183–42194. 2016.PubMed/NCBI
|
|
15
|
Paner GP, Stadler WM, Hansel DE, Montironi
R, Lin DW and Amin MB: Updates in the eighth edition of the
tumor-node-metastasis staging classification for urologic cancers.
Eur Urol. 8:31064–31073. 2018.
|
|
16
|
Li J, Han L, Roebuck P, Diao L, Liu L,
Yuan Y, Weinstein JN and Liang H: TANRIC: An interactive open
platform to explore the function of lncRNAs in cancer. Cancer Res.
75:3728–3737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang JF, Guo YJ, Zhao CX, Yuan SX, Wang
Y, Tang GN, Zhou WP and Sun SH: Hepatitis B virus X protein
(HBx)-related long noncoding RNA (lncRNA) down-regulated expression
by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by
targeting the intermediate filament protein vimentin. Hepatology.
57:1882–1892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mehra R, Udager AM, Ahearn TU, Cao X, Feng
FY, Loda M, Petimar JS, Kantoff P, Mucci LA and Chinnaiyan AM:
Overexpression of the long non-coding RNA SChLAP1 independently
predicts lethal prostate cancer. Eur Urol. 70:549–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Zhao X, Zou H, Bai R, Yang K and
Tian Z: Hypoxia promotes gastric cancer malignancy partly through
the HIF-1α dependent transcriptional activation of the long
non-coding RNA GAPLINC. Front Physiol. 7:4202016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feitelson MA, Arzumanyan A, Kulathinal RJ,
Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML,
Nawroth R, Sanchez-Garcia I, et al: Sustained proliferation in
cancer: Mechanisms and novel therapeutic targets. Semin Cancer
Biol. 35 Suppl:S25–S54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olsson AY, Feber A, Edwards S, Poele Te R,
Giddings I, Merson S and Cooper CS: Role of E2F3 expression in
modulating cellular proliferation rate in human bladder and
prostate cancer cells. Oncogene. 26:1028–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jirawatnotai S, Sharma S, Michowski W,
Suktitipat B, Geng Y, Quackenbush J, Elias JE, Gygi SP, Wang YE and
Sicinski P: The cyclin D1-CDK4 oncogenic interactome enables
identification of potential novel oncogenes and clinical prognosis.
Cell Cycle. 13:2889–2900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pei XH, Bai F, Tsutsui T, Kiyokawa H and
Xiong Y: Genetic evidence for functional dependency of p18Ink4c on
Cdk4. Mol Cell Biol. 24:6653–6664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gontero P, Banisadr S, Frea B and Brausi
M: Metastasis markers in bladder cancer: A review of the literature
and clinical considerations. Eur Urol. 46:296–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jacobs BL, Lee CT and Montie JE: Bladder
cancer in 2010: How far have we come? CA Cancer J Clin. 60:244–272.
2010. View Article : Google Scholar : PubMed/NCBI
|