Introduction
Gastric cancer (GC) represents a major public health
threat globally. GC develops due to various molecular
abnormalities, including the activation of a number of oncogenes,
the inactivation of several cancer suppressor genes and the
abnormal regulation of various growth factors and their receptors
(1,2).
Inhibitor of apoptosis-stimulating protein of p53
(iASPP) is a member of the ASPP family that acts as a negative
regulator of the wild-type p53 (3,4). Wild-type
p53 is a well-known tumor suppressor that regulates the cell cycle
and apoptosis (5,6). iASPP directly binds to the DNA-binding
domains of p53 and inhibits its function, leading to abnormal cell
proliferation and malignant transformation (7). iASPP may serve as a pro-oncogene since
abnormal overexpression of iASPP has been detected in several types
of human cancer, including breast carcinoma (8), acute leukaemia (9), lung cancer (10) and hepatocellular carcinoma (11). Previous studies demonstrated that the
expression of iASPP was significantly increased in GC tissues
compared with that in the corresponding normal tissues (12). Additionally, wild-type p53 was
detected in ~2/3 of GC cases (12,13). This
evidence indicated that iASPP may act as an oncogene in GC. It may
be possible to inhibit wild-type p53 to promote the development of
GC. To the best of our knowledge, few studies have investigated the
function of iASPP in human GC cells (12). Previous studies have focused on the
downstream activities of iASPP targets (14,15).
Therefore, the upstream molecular mechanism of iASPP remains
unclear.
Kruppel-like factor 4 (KLF4) is a zinc-finger
transcription factor that contains Cys2-His2 motifs and is highly
expressed in the gastrointestinal tract (16). It has been demonstrated that KLF4
exhibits dual functions in carcinogenesis since it may act as a
tumor suppressor gene and as an oncogene (17). KLF4 has been demonstrated to function
as an oncogene in several types of tumor, including breast cancer,
head and neck squamous cell cancer, and oral squamous cancer, since
its expression was upregulated and thus promoted the development
and progression of these types of cancer (17–19).
However, KLF4 may also function as a tumor suppressor since
downregulation of KLF4 has been detected in colorectal, gastric,
bladder, oesophageal and cervical cancer (17,20–24).
Previous studies demonstrated that knockdown of KLF4 in the stomach
increased cell proliferation and triggered the formation of
pre-cancerous lesions. Additionally, progressive downregulation of
KLF4 was associated with advanced tumor stages and increased
metastases in patients with GC. However, increased expression of
KLF4 in patients with GC was associated with increased survival
rate (25).
KLF4 may regulate cellular proliferation, apoptosis
and differentiation by binding to the CACCC or GC-rich DNA
sequences (26,27). Previous studies have indicated an
interaction between KLF4 and p53. KLF4 may regulate the
transcription of the p53 promoter, whereas p53 may regulate the
expression of KLF4 (28,29). The feedback loop between KLF4 and p53
may regulate cell apoptosis. Since iASPP is an evolutionarily
conserved inhibitor of p53, the aim of the present study was to
investigate whether there is a functional association between KLF4,
iASPP and p53.
The present study examined whether the expression of
the transcription factor KLF4 regulated iASPP in the gastric
mucosa. Additionally, since KLF4 and iASPP interact with p53, it
was hypothesized that KLF4 may exhibit an anticancer effect through
regulating the expression of iASPP in GC. Additionally, the effects
of downregulating the expression of KLF4 in cellular proliferation
and differentiation and in regulating the expression of iASPP in GC
were investigated.
The precise molecular mechanism underlying the
effects of KLF4 and iASPP remains unclear and future studies are
required to unravel the effects of this signaling pathway in GC.
The present study examined the expression of KLF4 and iASPP in GC
cells and investigated their underlying molecular mechanism in the
proliferation and apoptosis of GC cells.
Materials and methods
Cell lines
The normal gastric mucosa GES1 cell line was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). GES1 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% foetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) without antibiotics at 37°C in a
humidified atmosphere containing 5% CO2. GC cell lines
(MKN45, BGC823 and SGC7901) were obtained from the American Type
Culture Collection (Manassas, VA, USA). MKN45 is a cell line
expressing wild-type p53 (30),
SGC7901 is a cell line expressing mutant-type p53 (31) and BGC823 is a cell line that can
express either wild-type p53 or mutant-type p53 (32,33). The
GC cells were routinely cultured in RPMI-1640 (Thermo Fisher
Scientific, Inc.) medium supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) without antibiotics at 37°C in a humidified
atmosphere containing 5% CO2.
Lentiviral transfection
To upregulate the KLF4 and iASPP expression in MKN45
cells, KLF4-overexpression-shRNA, iASPP-overexpression-shRNA and
negative control-shRNA lentiviral were transfected. To downregulate
the iASPP expression in MKN45 cells, the iASPP-inhibition-shRNA and
negative control-shRNA lentiviral were transfected with
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific Inc.,
Waltham, MA, USA) at a final concentration of 100–200 nM, according
to the manufacturer's instructions. All lentiviral suspensions were
purchased from Shanghai Genepharma Co., Ltd. (Shanghai, China). To
perform the lentiviral transfection, the target GC cells were grown
to 20–30% confluence in a 6-well plate (~5×104
cells/well) and incubated for 12 h prior to transfection. During
the transfection, the medium was replaced with a supernatant fluid
that contained an appropriate titre of the virus (multiplicity of
infection=virus number/cell number=20) and supplemented with 5
µg/ml polybrene (Sigma-Aldrich; Merck KGaA). Following incubation
for 12 h at 37°C, the supernatant containing lentivirus particles
was replaced with fresh media. At 48 h post-transfection, cells
were selected using puromycin (2 mg/ml; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) to establish the stable
clone cell lines. A total of 72 h later, the transfection
efficiency of cells was observed using fluorescence microscopy
(Leica Microsystems GmbH, Wetzlar, Germany). Cells were harvested
96 h after transfection, and the KLF4 or iASPP expression was
evaluated using western blot analysis. Once the detections above
were performed, the cells were used for further analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from GES1, MKN45, BGC823 and
SGC7901 cells using TRIzol reagent (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocol. cDNA was amplified using
the PrimeScript First Strand cDNA Synthesis kit (Takara Bio, Inc.).
qPCR was performed using the SYBRR Premix Ex Taq™ II system (Takara
Bio, Inc.) and the Bio-Rad CFX96™ Real-time system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The following primers were
used: iASPP, 5′-GCGGTGAAGGAGATGAACGA-3′ (forward) and
5′-GCGGTGAAGGAGATGAACGA-3′ (reverse); KLF4,
5′-CCCGGATCCATGGCTGTCAGCGACGCGCTG-3′ (forward) and
5′-CCCGAATTCTTAAAATGCCTCTTCATGTGTAAG-3′ (reverse); and β-actin,
5′-CCACGAAACTACCTTCAACTCC-3′ (forward) and
5′-GTGATCTCCTTCTGCATCCTGT-3′ (reverse). β-actin was used as an
internal control. The thermocycling conditions were as follows:
Initial denaturation of 94°C for 60 sec, followed by 40 cycles of
94°C for 40 sec and 60°C for 40 sec, and final extension of 72°C
for 6 min. Relative expression levels of the genes were determined
using 2−ΔΔCq analysis (34).
Western blot analysis
MKN45 cells were washed with ice-cold PBS and lysed
using a radioimmunoprecipitation assay buffer (Beyotime Institute
of Biotechnology, Shanghai, China) for 30 min on dry ice. The
lysates were collected with a rubber scraper, sonicated and
centrifuged at 12,000 × g (4°C for 20 min). The concentrations of
the total proteins were determined using a bicinchoninic acid assay
(Thermo Fisher Scientific, Inc.). Each sample was adjusted to 2
µg/µl and a 20 µl volume was mixed with 2 × SDS sample buffer (100
mM Tris-HCl, pH 6.8, 10 mM EDTA, 4% SDS, 10% glycine) and separated
using 10% SDS-PAGE (Bio-Rad Laboratories, Inc.). Electrophoresed
proteins were transferred onto polyvinylidene fluoride membranes
(Merck KGaA, Darmstadt, Germany). The membranes were blocked with
5% skimmed milk in PBS for 1 h at room temperature and were
incubated with primary antibodies against iASPP (cat no. ab34898;
1:5,000; Abcam, Cambridge, MA, USA), KLF4 (cat no. ab106629;
1:5,000; Abcam) and β-actin (cat no. MAB1501; 1:5,000, Merck KGaA)
overnight at 4°C. Membranes were washed three times with
Tris-buffered saline with 0.1% Tween-20 (TBST) for 5 min and
incubated with horseradish peroxidase-conjugated goat anti-mouse or
anti-rabbit immunoglobulin (cat no. sc2004; 1:5,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature,
and then washed three times for 5 min with TBST. Protein bands were
visualized using an enhanced chemiluminescence reagent (EMD
Millipore, Billerica, MA, USA) and quantified by densitometry using
Quantity One Image Analysis Software (Version 4.6.7; Bio-Rad
Laboratories, Inc.).
MTT assay
Cell proliferation levels were evaluated using an
MTT assay. In brief, MKN45 cells were seeded in a 96-well plate at
2×103 cells/well for 24, 48 and 72 h. MTT solution (20
µl of 5 mg/ml MTT) was added to the cells (for a total culture
volume of 200 ml) prior to incubation at 37°C for an additional 4
h. The formazan crystals that formed were dissolved in 100 µl
dimethylsulfoxide. The absorbance values were read at 560 nm.
Colony formation assay
The colony forming ability of the MKN45 cells was
detected using a colony formation assay. The target cells were
seeded at a low density (500 cells/plate) in a 6-well plate and
cultured until visible colonies appeared (~2 weeks). The cell
colonies were then stained with Giemsa solution for 15 min at room
temperature. The colonies containing >50 cells were counted as
positive.
Flow cytometric analysis of
apoptosis
MKN45 cells were collected and washed twice with
ice-cold PBS. The apoptosis rate of the cells was detected with
Annexin V-fluorescein and propidium iodide double staining
(Apoptosis kit; eBioscience; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Flow cytometric analysis
was performed and data were analysed using a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Flow cytometry
analysis was repeated at least 3 times using Windows Multiple
Document Interface Flow Cytometry Application (WinMDI version 2.9;
Microsoft, Redmond, WA, USA).
Xenograft experiments
A total of 36 athymic nude mice (male, 6- to
8-week-old, 20–30 g in body weight), were obtained from the Animal
Experimental Centre of Chongqing Medical University (Chongqing,
China). The mice were acclimated for 2 weeks with ad libitum
access to standard food and water. They were maintained in an
isolated pathogen-free ventilation chamber with a 12 h light-dark
cycle at a temperature of 22±2°C and 40–50% relative humidity. To
establish the gastric cancer model, equal numbers of MKN45 cells
(1×106) were injected subcutaneously into the right rear
flank of each mouse. The mice were divided into four groups (3
mice/group): A negative-control group (injected with
control-transfected MKN45 cells), KLF4-overexpression group
(injected with KLF4-overexpression-shRNA transfected MKN45 cells),
iASPP-downregulation group (injected with iASPP-inhibition-shRNA
transfected MKN45 cells) and combined KLF4/iASPP overexpression
group (injected with MKN45 cells overexpressed KLF4 and iASPP).
Tumor growth was observed daily in each group and tumor diameter
was measured once a week using callipers. Tumor volume was
calculated with the following formula: Tumor volume = (L ×
S2)/2, where L is the longest tumor axis and S is the
shortest tumor axis. At 4 weeks post-injection, the mice were
sacrificed, or when the maximum tumor diameter reached 2.0 cm and
the tumors were used for further analysis. This study was conducted
in accordance with the recommendations of the Guide for the Care
and Use of Laboratory Animals of Chongqing Medical University. All
animal experiments were approved by the Committee on the Ethics of
Animal Experiments of Chongqing Medical University and Chongqing
Cancer Hospital. All surgeries were performed under sodium
pentobarbital anesthesia and all efforts were made to minimize
suffering.
Statistical analysis
Data were analyzed using SPSS software (version
18.0; SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean
± standard deviation. Results were analyzed using one-way analysis
of variance with a least significant difference test for post hoc
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of KLF4 and iASPP in GC
cells
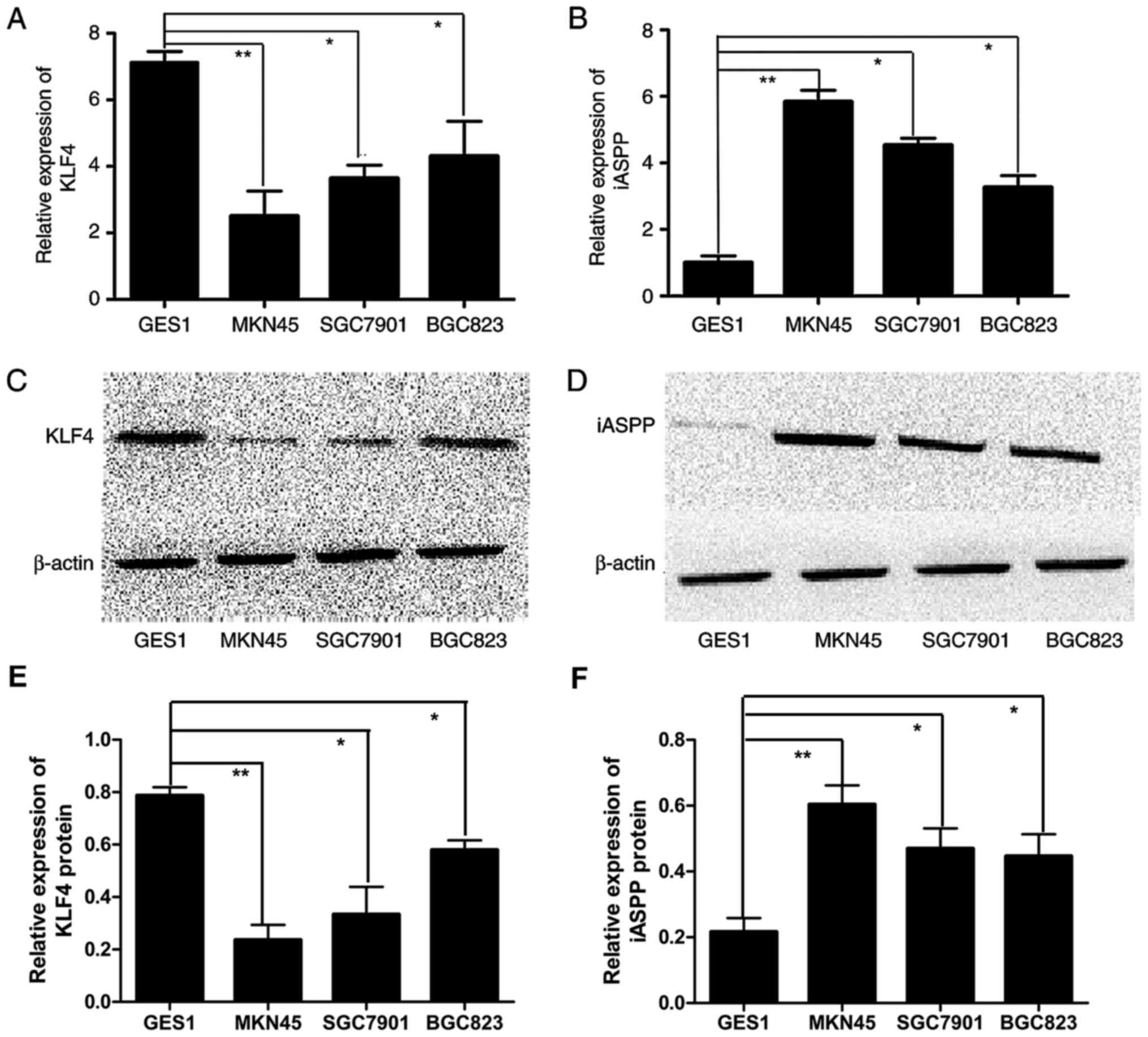
The expression of KLF4 and iASPP was detected in
normal (GES1) and GC cell lines (MKN45, BGC823 and SGC7901) using
RT-PCR and western blot analysis. The results demonstrated that the
mRNA and protein expression of KLF4 was significantly downregulated
in GC cells compared with that in GES1 cells (Fig. 1A and C/E). However, the mRNA and
protein expression of iASPP was upregulated in GC cells compared
with that in GES1 cells (Fig. 1B and
D/F). Additionally, as there was a negative association between
the expression of KLF4 and iASPP particularly in MKN45 cells, which
is why the MKN45 cell line was selected for subsequent experiments
(Fig. 1A-D).
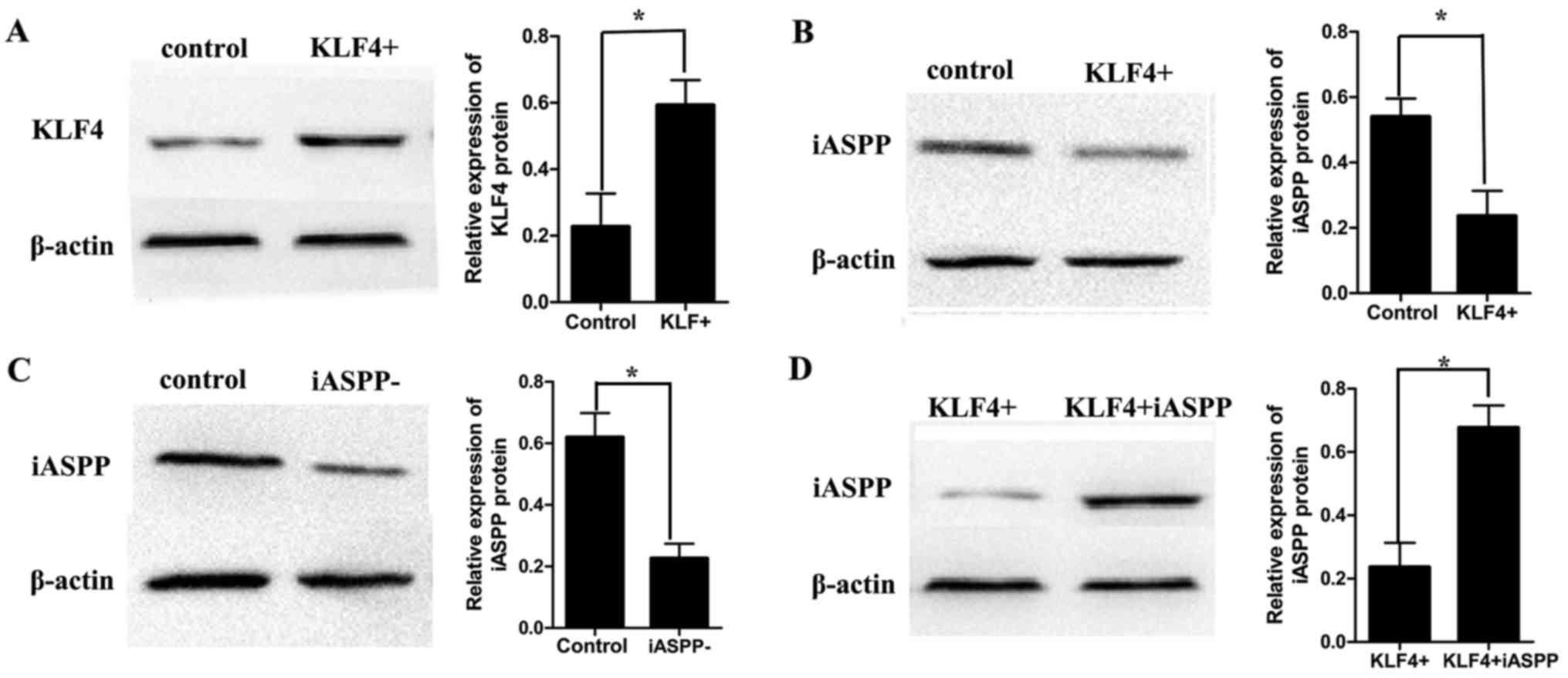
Expression of KLF4 and iASPP in MKN45
cells following lentiviral transfection
In order to overexpress KLF4 in MKN45 cells, cells
were transfected with KLF4-overexpression-shRNA lentivirus.
Following transfection and selection, expression of KLF4 and iASPP
was evaluated in MKN45 cells using western blot analysis. The
results demonstrated that the expression of KLF4 was significantly
upregulated following KLF4 overexpression via lentiviral delivery
(Fig. 2A). Additionally, expression
of iASPP was downregulated following overexpression of KLF4
(Fig. 2B). Next, MKN45 cells were
transfected with iASPP-inhibition-shRNA lentivirus and expression
of iASPP was evaluated using western blot analysis. The results
demonstrated that the expression of iASPP was significantly
downregulated following lentiviral transfection (Fig. 2C). Next, an iASPP-overexpression-shRNA
lentivirus was transfected into the KLF4-overexpressing MKN45
cells. Following transfection, iASPP expression was evaluated using
western blot analysis. The results demonstrated that iASPP was
upregulated in iASPP-overexpressing MKN45 cells compared with that
in the non-transfected cells which overexpressed KLF4 but not iASPP
(Fig. 2D).
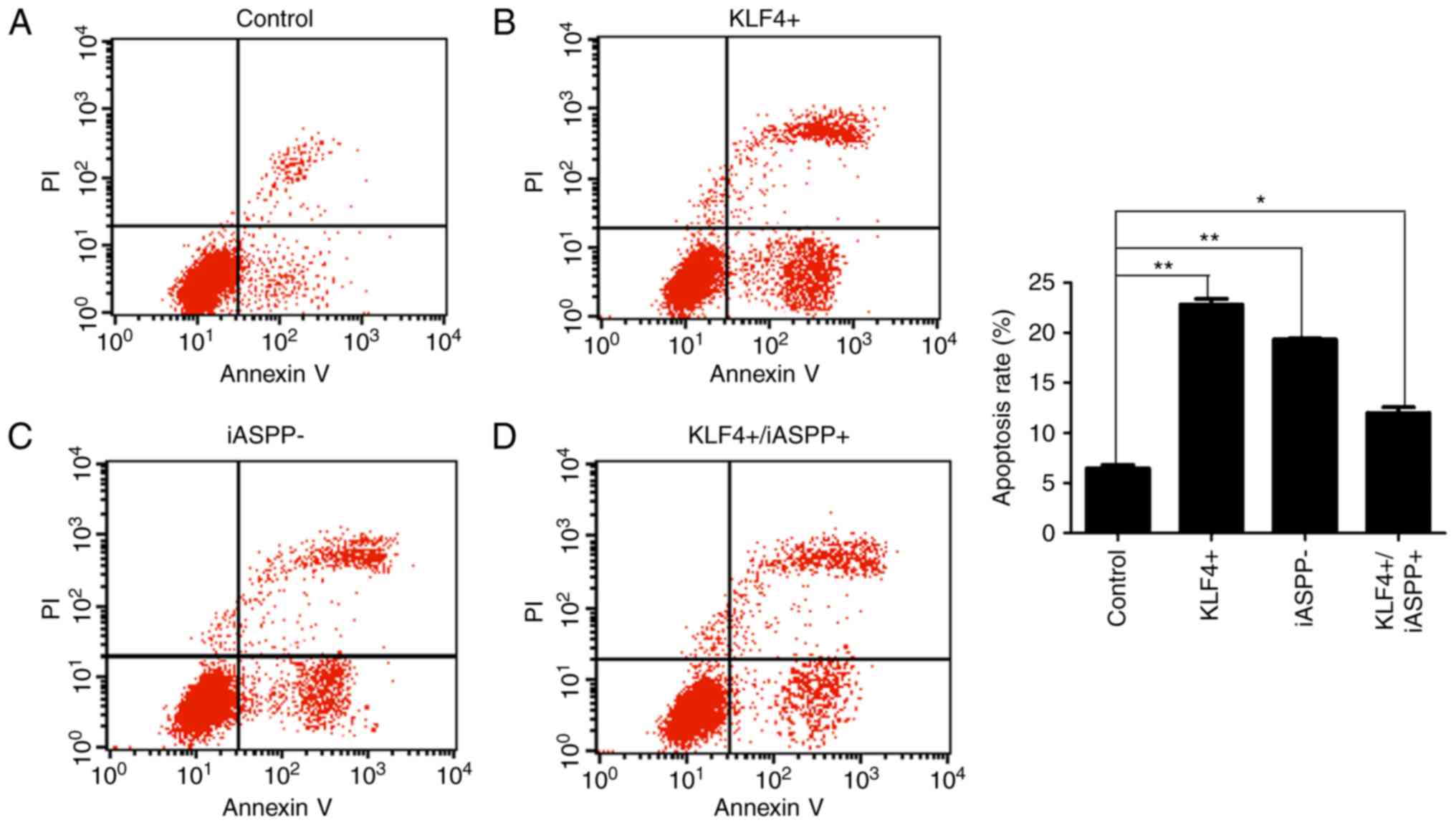
Apoptosis of MKN45 cells following
lentiviral transfection
Flow cytometric analysis of apoptosis was applied in
order to determine the function of KLF4 in regulating iASPP in
MKN45 cells. The results demonstrated that overexpression of KLF4
and downregulation of iASPP led to an increased rate of apoptosis
compared with that in the control MKN45 cells (Fig. 3A-C). The apoptosis rate was partially
restored by overexpression of iASPP, which inhibited apoptosis in
the MKN45 cells (Fig. 3D).
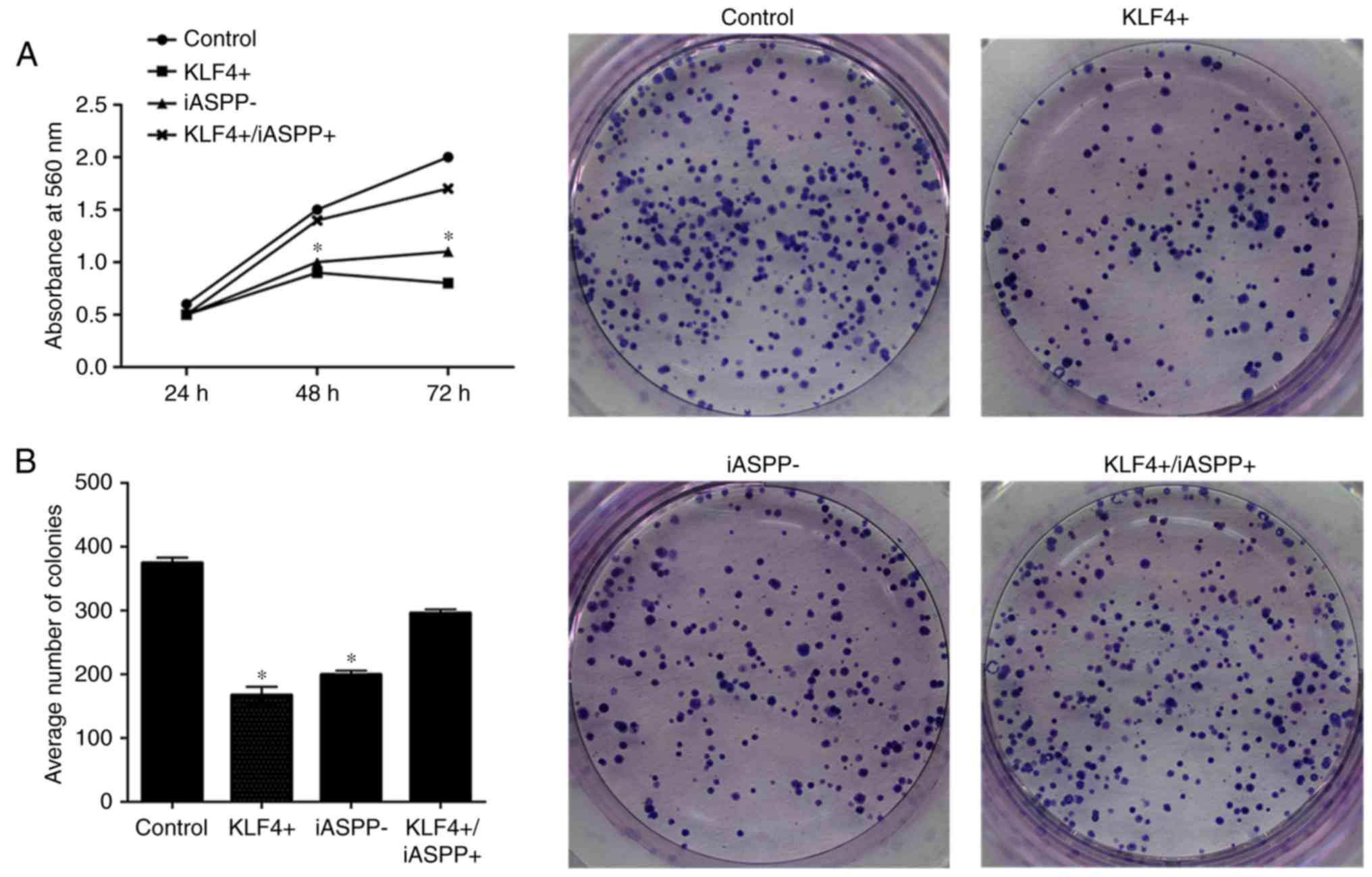
Proliferation and colony-forming
ability of MKN45 cells following lentiviral transfection
MTT and colony formation assays were employed to
evaluate the viability and colony-forming ability of MKN45 cells,
respectively. As presented in Fig. 4,
overexpression of KLF4 or downregulation of iASPP inhibited the
proliferation and colony-forming ability of the MKN45 cells,
whereas combined KLF4 and iASPP overexpression significantly
promoted the proliferation and colony-forming ability of the cells
compared with KLF4 overexpressing alone (P<0.05; Fig. 4).
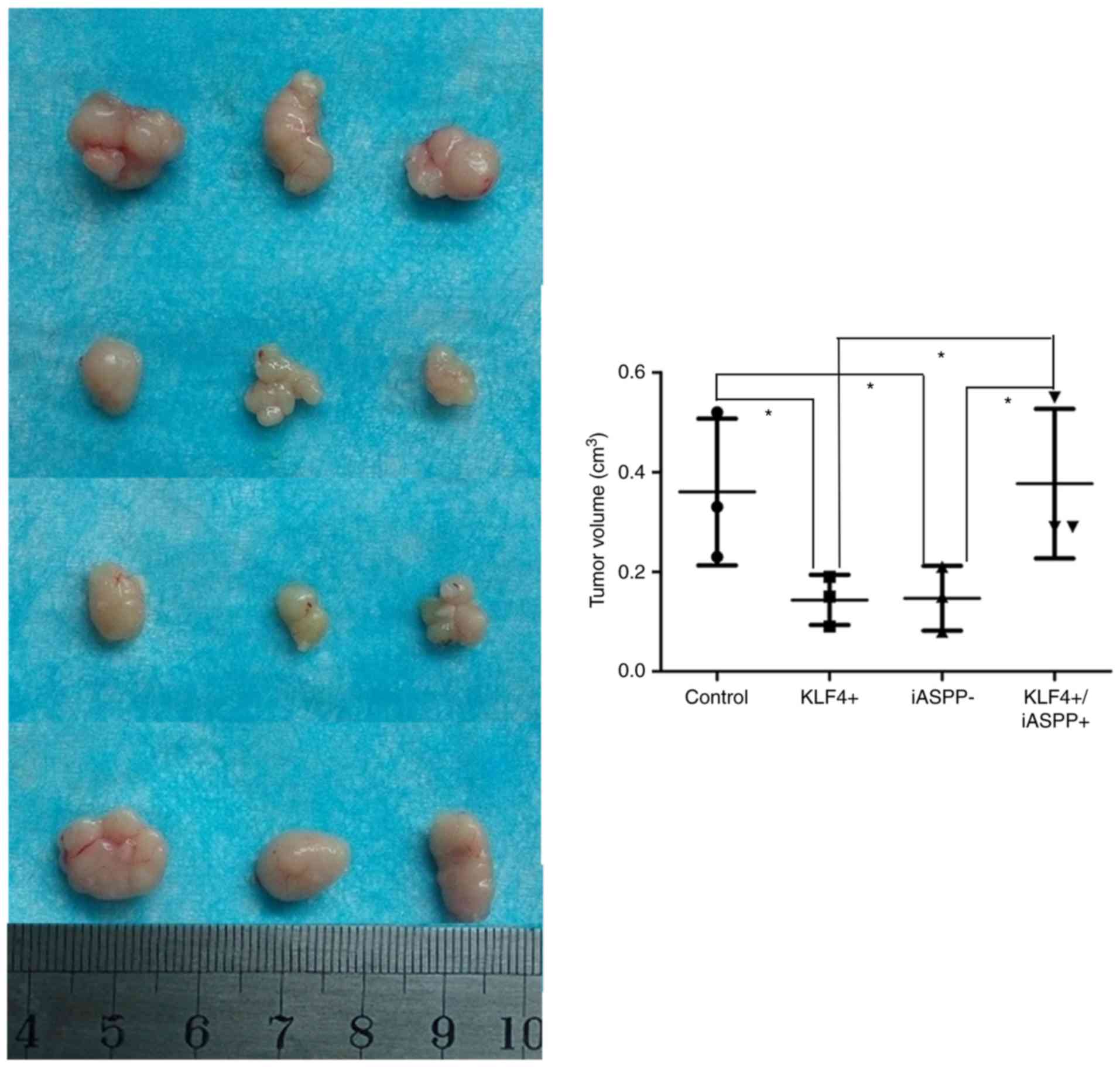
In vivo tumor growth following
lentiviral transfection
To investigate the function of KLF4 and iASPP on
tumor growth in vivo, equal numbers of MKN45 cells
(106 cells/mouse) were subcutaneously injected into nude
mice. Tumors were evident in all mice. As presented in Fig. 5, overexpression of KLF4 and
downregulation of iASPP significantly inhibited tumor growth in
vivo, whereas combined KLF4 and iASPP overexpression promoted
the growth of tumors in vivo (Fig.
5).
Discussion
Wild-type p53 may act as a tumor suppressor gene and
inhibit the malignant transformation of cells, whereas the
mutant-type p53 may significantly contribute to the development of
cancer. A previous study confirmed that mutations in the p53 gene
are not a predominant event in gastric carcinogenesis (12,13). The
molecular mechanism behind the occurrence of gastric cancer
expressing wild-type p53 is therefore important. In the present
study, the expression levels of KLF4 and iASPP were evaluated in GC
cell lines with wild-or mutant-type of p53 (MKN45, BGC823 and
SGC7901 cells) and in normal gastric mucosa GES1 cells. It was
demonstrated that expression of KLF4 was significantly decreased
and expression of iASPP was increased in GC cells compared with
that in GES1 cells, but particularly in the wild-type
p53-expressing cell line MKN45. Previous studies have demonstrated
that KLF4 acts as a tumor suppressor and inhibits the proliferation
of GC cells (17,22). The results of the present study
demonstrated that proliferation of the MKN45 cells was
significantly inhibited, whereas apoptosis was promoted following
overexpression of KLF4, which is consistent with previous
findings.
iASPP is an evolutionarily conserved inhibitor of
wild-type p53 (4) and overexpression
of iASPP has been observed in several types of human cancer
(8–11). The results of the present study
demonstrated that the expression levels of iASPP were increased in
GC cells compared with those in GES1 cells. These results suggest
that iASPP and KLF4 may serve as independent prognostic markers for
tumor cell proliferation in GC. Previous studies have confirmed
that the knockdown of iASPP may significantly inhibit tumor growth
and cell proliferation in various types of cancer, including
glioblastoma (35), prostate cancer
(36) and lung cancer (37). The present study demonstrated that
changes in the expression of iASPP were associated with
proliferation and apoptosis rates of MKN45 cells. The imbalance
between proliferation and apoptosis contributes to the formation
and development of human tumors (38). The downregulation of iASPP decreased
the proliferation and colony-forming ability of the MKN45 cells.
Additionally, downregulation of iASPP promoted cell apoptosis.
These results suggest that deregulated expression of iASPP may
contribute to tumorigenesis in GC cells that express wild-type p53.
These results are consistent with previous studies on leukaemia and
non-small cell lung cancer in which iASPP is highly expressed
(39,40).
The iASPP-mediated signaling pathway that regulates
cancer cell proliferation remains unclear. The present study
demonstrated that the expression of KLF4 was significantly
decreased and that the expression of iASPP was significantly
increased in GC cells, particularly MKN45 cells. It has been
indicated that KLF4 and p53 may interact with each other, whereas
iASPP is an evolutionarily conserved inhibitor of p53 (7,28,29). Since there may be a functional
association between KLF4, iASPP and p53, the aim of the present
study was to investigate the association between KLF4 and iASPP in
GC. In the present study, the expression of iASPP was evaluated in
response to the overexpression of KLF4 (achieved using lentiviral
delivery). The results demonstrated that overexpression of KLF4 led
to downregulation of iASPP, suggesting that KLF4 may regulate the
expression of iASPP. Additionally, overexpression of KLF4 inhibited
the proliferation and colony formation ability of the MKN45 cells.
Since the decrease in KLF4 expression led to increased expression
iASPP in GC, the present study investigated whether low levels of
KLF4 and increased levels of iASPP may promote cellular
proliferation in GC. Next, iASPP was overexpressed following KLF4
expression by using specific shRNAs in order to further validate
the association between KLF4 and iASPP. The results demonstrated
that overexpression of iASPP inhibited the effects of KLF4 in MKN45
cells. Increased expression levels of KLF4 inhibited the
proliferation and promoted the apoptosis of MKN45 cells, whereas
KLF4-mediated effects were reversed by overexpression of iASPP.
These results suggest that KLF4 may act as a potential tumor
suppressor by inhibiting the expression of iASPP in GC.
Additionally, tumor growth was significantly inhibited in response
to the downregulation of iASPP or upregulation KLF4 in vivo.
However, in vivo KLF4-mediated effects were reversed by
iASPP overexpression. These results suggest that this decrease in
tumor growth may be due to the decrease in the expression of iASPP,
which was caused by KLF4 overexpression. Previous studies have
indicated a novel molecular mechanism of action for KLF4 and iASPP
in GC (41). However, in the present
study, the association between KLF4, iASPP and p53 was not further
investigated. Therefore, future studies are required to investigate
the function of this signaling pathway in the development of GC,
employing GC cells that express the wild-type p53. Additionally,
since the ASPP family contains three members (iASPP, ASPP1 and
ASPP2), which share similar sequences in their C-terminus domain
(42), there may be also an
association between KLF4 and ASPP1 or ASPP2.
KLF4 may act as a tumor suppressor that may inhibit
cellular proliferation, whereas iASPP may act as an oncogene that
promotes the proliferation of GC cells that express the wild-type
p53. In the present study, it was demonstrated that KLF4 inhibited
cell proliferation and colony formation ability partly by targeting
iASPP. Therefore, the KLF4/iASPP axis may serve a vital function in
regulating the proliferation of GC cell, and provide a potential
diagnostic and therapeutic target for GC.
Acknowledgements
The authors would like to thank Professor Dong-Lin
Wang (Chongqing Cancer Hospital, Chongqing, China), for providing
mentor support, incisive comments and useful suggestions.
Funding
The present study was supported by the special fund
for Postdoctoral Scientific Research Projects (grant no.
Xm2017091).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
LLW contributed to the study design, experimental
work, data analysis and preparation of the manuscript. DLW, YL,
LCL, ZJW and HWM participated in the design of the study and
experimental work. YZW, HQY and DY performed the statistical
analysis. DLW participated in the study design and coordination,
and contributed to the drafting of the manuscript. All authors read
and approved the final manuscript.
Ethics and consent to participate
The study was approved by the Ethics Committee of
Chongqing Cancer Hospital and Chongqing Medical University, and all
patients provided informed consent for participation in the present
study.
Consent for publication
The study participants provided consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong P, Ihira K, Hamada J, Watari H,
Yamada T, Hosaka M, Hanley SJ, Kudo M and Sakuragi N: Reactivating
p53 functions by suppressing its novel inhibitor iASPP: A potential
therapeutic opportunity in p53 wild-type tumors. Oncotarget.
6:19968–19975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duffy MJ, Synnott NC, McGowan PM, Crown J,
O'Connor D and Gallagher WM: p53 as a target for the treatment of
cancer. Cancer Treat Rev. 40:1153–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meek DW: Regulation of the p53 response
and its relationship to cancer. Biochem J. 469:325–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laska MJ, Vogel UB, Jensen UB and Nexø BA:
p53 and PPP1R13L (alias iASPP or RAI) form a feedback loop to
regulate genotoxic stress responses. Biochim Biophys Acta 1800:.
1231:12402010.
|
|
8
|
Wang C, Gao C, Chen Y, Yin J, Wang P and
Lv X: Expression pattern of the apoptosis-stimulating protein of
p53 family in p53+ human breast cancer cell lines. Cancer Cell Int.
13:1162013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Wang M, Diao S, Rao Q, Zhang X,
Xing H and Wang J: siRNA-mediated down-regulation of iASPP promotes
apoptosis induced by etoposide and daunorubicin in leukemia cells
expressing wild-type p53. Leuk Res. 33:1243–1248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin BL, Xie DY, Xie SB, Xie JQ, Zhang XH,
Zhang YF and Gao ZL: Down-regulation of iASPP in human
hepatocellular carcinoma cells inhibits cell proliferation and
tumor growth. Neoplasma. 58:205–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng WD, Chu RX, Wang BZ, Wang LP, Ma LL
and Wang LX: Helicobacter pylori infection and expressions of
apoptosis-related proteins p53, ASPP2 and iASPP in gastric cancer
and precancerous lesions. Pathol Biol. 61:199–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berloco P, Russo F, Cariola F, Gentile M,
Giorgio P, Caruso ML, Valentini AM, Di Matteo G and Di Leo A: Low
presence of p53 abnormalities in H. pylori-infected gastric mucosa
and in gastric adenocarcinoma. J Gastroenterol. 38:28–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai Y, Qiu S, Gao X, Gu SZ and Liu ZJ:
iASPP inhibits p53-independent apoptosis by inhibiting
transcriptional activity of p63/p73 on promoters of proapoptotic
genes. Apoptosis. 17:777–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gillotin S and Lu X: The ASPP proteins
complex and cooperate with p300 to modulate the transcriptional
activity of p53. FEBS Lett. 585:1778–1782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee HY, Ahn JB, Rha SY, Chung HC, Park KH,
Kim TS, Kim NK and Shin SJ: High KLF4 level in normal tissue
predicts poor survival in colorectal cancer patients. World J Surg
Oncol. 12:2322014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghaleb AM and Yang VW: Krüppel-like factor
4 (KLF4): What we currently know. Gene. 611:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Foster KW, Ren S, Louro ID, Lobo-Ruppert
SM, McKie-Bell P, Grizzle W, Hayes MR, Broker TR, Chow LT and
Ruppert JM: Oncogene expression cloning by retroviral transduction
of adenovirus E1A-immortalized rat kidney RK3E cells:
Transformation of a host with epithelial features by c-MYC and the
zinc finger protein GKLF. Cell Growth Differ. 10:423–434.
1999.PubMed/NCBI
|
|
19
|
Foster KW, Frost AR, McKie-Bell P, Lin CY,
Engler JA, Grizzle WE and Ruppert JM: Increase of GKLF messenger
RNA and protein expression during progression of breast cancer.
Cancer Res. 60:6488–6495. 2000.PubMed/NCBI
|
|
20
|
Ton-That H, Kaestner KH, Shields JM,
Mahatanankoon CS and Yang VW: Expression of the gut-enriched
Krüppel-like factor gene during development and intestinal
tumorigenesis. FEBS Lett. 419:239–243. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Krüppel-like factor 4 as
a potential tumor suppressor gene in colorectal cancer. Oncogene.
23:395–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu LS, Chan CP, Chen CJ, Lin SH, Lai MT,
Hsu JD, Yeh KT and Soon MS: Decreased s-like factor 4 (KLF4)
expression may correlate with poor survival in gastric
adenocarcinoma. Med Oncol. 30:6322013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohnishi S, Ohnami S, Laub F, Aoki K,
Suzuki K, Kanai Y, Haga K, Asaka M, Ramirez F and Yoshida T:
Downregulation and growth inhibitory effect of epithelial-type
Krüppel-like transcription factor KLF4, but not KLF5, in bladder
cancer. Biochem Biophys Res Commun. 308:251–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang WT and Zheng PS: Kruppel-like factor
4 function as a tumor suppressor in cervical carcinoma. Cancer.
118:3691–3702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krstic M, Stojnev S, Jovanovic L and
Marjanovic G: KLF4 expression and apoptosis-related markers in
gastric cancer. J BUON. 18:695–702. 2013.PubMed/NCBI
|
|
26
|
Kaczynski J, Cook T and Urrutia R: Sp1-
and Krüppel-like transcription factors. Genome Biol. 4:2062003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elemento O: KLF4: A new player in plasma
cell development. Cell Cycle. 15:2691–2692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brosh R, Assia-Alroy Y, Molchadsky A,
Bornstein C, Dekel E, Madar S, Shetzer Y, Rivlin N, Goldfinger N,
Sarig R and Rotter V: p53 counteracts reprogramming by inhibiting
mesenchymal-to-epithelial transition. Cell Death Differ.
20:312–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokozaki H: Molecular characteristics of
eight gastric cancer cell lines established in Japan. Pathol Int.
50:767–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sigal A and Rotter V: Oncogenic mutations
of the p53 tumor suppressor: The demons of the guardian of the
genome. Cancer Res. 60:6788–6793. 2000.PubMed/NCBI
|
|
32
|
Zhang SW, Xiao SW and Lü YY:
Adenovirus-mediated P53 cancer transfer increases the
thermosensitivity of human gastric carcinoma cell lines in vitro
and in vivo. Chin J Cancer Res. 15:107–111. 2003. View Article : Google Scholar
|
|
33
|
Liu J, Zhang Y, Xie YS, Wang FL, Zhang LJ,
Deng T and Luo HS: 5-Aza-2′-deoxycytidine induces cytotoxicity in
BGC-823 cells via DNA methyltransferase 1 and 3a independent of p53
status. Oncol Rep. 28:545–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li G, Wang R, Gao J, Deng K, Wei J and Wei
Y: RNA interference-mediated silencing of iASPP induces cell
proliferation inhibition and G0/G1 cell cycle arrest in U251 human
glioblastoma cells. Mol Cell Biochem. 350:193–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Xiao H, Huang Z, Hu Z, Qi T, Zhang
B and Liu SH: MicroRNA124 regulate cell growth of prostate cancer
cells by targeting iASPP. Int J Clin Exp Pathol. 7:2283–2290.
2014.PubMed/NCBI
|
|
37
|
Li S, Shi G, Yuan H, Zhou T, Zhang Q, Zhu
H and Wang X: Abnormal expression pattern of the ASPP family of
proteins in human non-small cell lung cancer and regulatory
functions on apoptosis through p53 by iASPP. Oncol Rep. 28:133–140.
2012.PubMed/NCBI
|
|
38
|
Grabenbauer GG, Suckorada O, Niedobitek G,
Rödel F, Iro H, Sauer R, Rödel C, Schultze-Mosgau S and Distel L:
Imbalance between proliferation and apoptosis may be responsible
for treatment failure after postoperative radiotherapy in squamous
cell carcinoma of the oropharynx. Oral Oncol. 39:459–469. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Wang M, Diao S, Rao Q, Zhang X,
Xing H and Wang J: siRNA-mediated down-regulation of iASPP promotes
apoptosis induced by etoposide and daunorubicin in leukemia cells
expressing wild-type p53. Leuk Res. 33:1243–1248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang LL, Xu Z, Peng Y, Li LC and Wu XL:
Downregulation of inhibitor of apoptosis-stimulating protein of p53
inhibits proliferation and promotes apoptosis of gastric cancer
cells. Mol Med Rep. 12:1653–1658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sullivan A and Lu X: ASPP: A new family of
oncogenes and tumour suppressor genes. Br J Cancer. 96:196–200.
2007. View Article : Google Scholar : PubMed/NCBI
|