Introduction
Lung cancer is the most common cause of
cancer-associated mortality globally (1). Despite improvements in early diagnosis
and novel therapies, the 5-year survival rate for patients with
lung cancer is still <15%. The major cause of the low survival
rate of patients with lung cancer is distant metastasis (2). Previous studies have demonstrated that
epithelial-to-mesenchymal transition (EMT), which is characterized
by the loss of epithelial markers and the gain of a mesenchyme-like
phenotype, serves a key function in the early process of cancer
cell metastasis (3,4). During the transition, cancer cells lose
the expression of genes that promote cell-cell contact, including
epithelial cadherin (E-cadherin), and gain the expression of
mesenchymal markers, including vimentin, fibronectin and neural
cadherin (N-cadherin). Transited tumor cells are thought to have
increased potential for motility, invasion and metastasis, as well
as increased resistance to chemotherapy (5–7). A group
of transcription factors has been confirmed to be capable of
orchestrating EMT programs in cancer progression. These include
direct transcriptional repressors of E-cadherin, including Snail,
Slug, zinc finger E-box binding homeobox (ZEB)1, ZEB2 and others,
including Twist-related protein 1 (Twist1) and forkhead box C2
(8).
MicroRNAs (miRNAs/miRs) represent a group of small
non-coding RNAs (18–25 nucleotides) that induce translational
repression or target degradation by binding to the 3′-untranslated
regions of target mRNAs (9). miRNAs
are involved in almost every biological process, including the cell
cycle, growth, apoptosis, differentiation and stress responses, and
the regulation of gene expression (10). Furthermore, evidence indicates that
certain miRNAs may function as tumor suppressors or oncogenes,
potentially contributing to tumorigenesis by binding to their
targets (9,11,12).
miR-126 has frequently been reported to be downregulated in human
cancers, including osteosarcoma, breast cancer, pancreatic cancer
and colorectal cancer (13–15). In these types of cancer, miR-126
inhibits cancer cell growth by binding to targets including insulin
receptor substrate 1, phosphoinositide 3-kinase regulatory subunit
2, ADAM metallopeptidase domain 9, vascular endothelial growth
factor and certain other molecules (16–18).
Furthermore, certain studies have demonstrated that miR-126 acts as
a negative regulator of tumor invasion and metastasis in breast
cancer (18,19). In lung cancer cells, miR-126 also
inhibits cell proliferation and invasion (20–23), but
the function of miR-126 in lung cancer metastasis, its role in
patients with lung cancer and in murine lung cancer models, as well
as the mechanisms by which miR-126 performs its functions and
modulates the malignant phenotypes of lung cancer cells, remain to
be fully understood.
The present study demonstrated that overexpression
of miR-126 inhibited transforming growth factor-β1 (TGF-β1)-induced
EMT. Furthermore, stable ectopic expression of miR-126 inhibited
the metastasis of lung cancer cells in a mouse xenograft model,
suggesting that EMT inhibition may be a central mechanism that
suppresses cancer invasion and metastasis. Furthermore, the present
study identified that the inhibitory function of miR-126 may be
achieved by targeting the phosphoinositide 3-kinase (PI3K)/protein
kinase B (AKT)/Snail signaling pathway. Thus, the results of the
present study provide valuable insights toward developing more
effective clinical therapies for lung cancer in the future.
Materials and methods
Cell culture and transfections
The SPC-A1 and LLC cell lines were purchased from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), and maintained at 37°C
in a humidified environment containing 5% CO2. SPC-A1
cells were seeded into 6-well plates with 2.5×104
cells/well. Cells were grown to 70% confluence, serum-starved for
24 h, and then treated with human recombinant TGF-β1 (5 ng/ml; cat.
no. 100-21; PeproTech. RockyHill, NJ, USA) or equal amounts of
dimethyl sulfoxide (DMSO) for 48 h at 37°C. For LY294002 treatment,
24 h after seeding, the medium was removed and replaced with
LY294002 (50 µmol/l; cat. no. L9908; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) or equal amounts of DMSO and incubated for 48 h
at 37°C. miR-126 mimics (5′-UCGUACCGUGAGUAAUAAUGCG-3′) and its
scramble control (5′-UAGUCAACGAGUCUAUGAGUCG-3′) were designed and
chemically synthesized by RiboBio Co., Ltd (Guangzhou, China). For
transfection, the cells were plated into 6-well plates with
2.5×104 cells/well. Once the cells were 30–50%
confluent, miR-126 mimics or NC mimics were transfected into the
SPC-A1 cells using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from SPC-A1 cells with
TRIzol® (Invitrogen; Thermo Fisher Scientific Inc.). A
total of 1 µg RNA was transcribed into cDNA using 1st Strand cDNA
Synthesis Kit (Vazyme Biotech Co., Ltd., Nanjing, China) for mRNA
and an RT-qPCR kit (Takara Bio Inc., Otsu, Japan) for miRNA
according to the manufacturer's protocol. The expression levels of
the genes were detected by qPCR. The following primers were used:
Human (h-) miR-126 forward, 5′-GTCGTATCCAGTGCAGGGTCCGAG-3′ and
reverse, 5′-GTATTCGCACTGGATACGAC-3′; h-U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
h-Snail forward, 5′-TCGGAAGCCTAACTACAGCGA-3′ and reverse,
5′-AGATGAGCATTGGCAGCGAG-3′; h-Twist1 forward,
5′-TGCAGACGCAGCGGGTCATG-3′ and reverse, 5′-GGACCGGCGGTCGAACTCCC-3′;
h-ZEB1 forward, 5′-GATGATGAATGCGAGTCAGATGC-3′ and reverse,
5′-ACAGCAGTGTCTTGTTGTTGT-3′; h-ZEB2 forward,
5′-CAAGAGGCGCAAACAAGCC-3′ and reverse, 5′-GGTTGGCAATACCGTCATCC-3′;
E-cadherin forward, 5′-CAGCCTGTCGAAGCAGGATTGC-3′ and reverse,
5′-GAGCTCAGACTAGCAGCTTCGG-3′. qPCR was performed using the SYBR
Green (Takara Biotechnology Co. Ltd, Dalian, China) dye detection
method on the ABI PrismStep-One Plus instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 95°C for 30 sec; followed by 40 cycles
of 95°C for 5 sec; and 60°C for 30 sec. The comparative Cq method
was used for quantification of the transcripts (24).
Immunofluorescent (IF) staining
For IF staining, the SPC-A1 cells grown on the
slides were fixed with 4% paraformaldehyde for 30 min at 4°C, then
blocked with 5% bovine serum album in for 1 h at room temperature
and incubated with primary antibodies overnight at 4°C. The next
day, the slides were washed with PBS, and incubated with Cy3-goat
anti-rabbit secondary antibodies (1:200; cat. no. 111-165-003;
Jackson Immuno Research Laboratories, West Grove, PA, USA) and DAPI
(1 mg/ml; cat. no. D6584; Sangon Biotech Co., Ltd., Shanghai,
China) for 2 h at room temperature. The following primary
antibodies were used: E-cadherin (1:200; cat. no. ab1416; Abcam,
Cambridge, UK), Fibronectin (1:200; cat. no. ab2413; Abcam), Ki-67
(1:100; cat. no. GA626; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Fluorescence microscopy images were obtained with
a research fluorescence microscope (Olympus Corporation, Tokyo,
Japan) equipped with a digital camera (at least 5 fields of view at
×400 magnification).
Western blotting
Cells or tumors were lysed in RIPA buffer
supplemented with complete Protease Inhibitor Cocktail tablets
(Roche, Inc., Basel, Switzerland) for 30 min on ice. Protein
lysates (30 µg) were subjected to 10% SDS-PAGE (Pierce; Thermo
Fisher Scientific, Inc.) and transferred to PVDF membrane.
Subsequent to blocking with 5% non-fat milk in 0.05% TBS-Tween-20
(v/v) for 1 h at room temperature, the membranes were incubated
with the appropriate primary antibodies overnight at 4°C. Primary
antibodies used were as follows: Anti-E-cadherin (1:1,000; cat. no.
3195; Cell Signaling Technology, Inc.), N-cadherin (1:2,000; cat.
no. 4061; Cell Signaling Technology, Inc.), vimentin (1:1,000; cat.
no. 3932; Cell Signaling Technology, Inc.), phosphorylated (p-)AKT
(ser-473; 1:2,000; cat. no. 2118-1; Epitomics; Abcam), p-PDK1
(ser-241; 1:500; cat. no. ab131067; Abcam) and β-actin (1:5,000;
cat. no. MS-1295-P; Pierce; Thermo Fisher Scientific, Inc.). The
secondary antibodies were horseradish peroxidase (HRP)-conjugated
goat anti-mouse IgG (cat. no. AP308P; heavy + light; 1:3,000;
Sigma-Aldrich; Merck KGaA) and HRP-conjugated goat anti-rabbit IgG
(cat. no. AP307P; heavy + light; 1:3,000; Sigma-Aldrich; Merck
KGaA). The secondary antibodies were incubated for 1 h at room
temperature. Protein detection was performed using an enhanced
chemiluminescence substrate (Thermo Fisher Scientific, Inc.) prior
to exposure to film.
Stable overexpression of miR-126 in
lung cancer cells and mouse xenograft assay
The oligonucleotide sequence of miR-126
(5′-UCGUACCGUGAGUAAUAAUGCG-3′) and scramble control
(5′-UAGUCAACGAGUCUAUGAGUCG-3′) were synthesized and cloned
intopGFP-C-shLentia lentiviral vector (cat. no. TR30023; OriGene
Technologies, Inc., Rockville; MD, USA) as previously reported
(25). The following primers were
used: miR-126 forward, 5′-TATCTTGTGGAAAGGACGCG-3′ and reverse,
5′-AGACGTTCCAAAAAATCGTACCGTGAGTAATAATGCGTCAAGAGCGCATTATTACTCACGGTACGAGTCTTCTGACGCTGCTGCCTG-3′;
scramble control forward, 5′-TATCTTGTGGAAAGGACGCG-3′ and reverse,
5′-AGACGTTCCAAAAAATAGTCAACGAGTCTATGAGTCGTCAAGAGCGACTCATAGACTCGTAGTCTAGTCTTCTGACGCTGCTGCCTG-3′;
Advantage GC 2 polymerase mix (cat. no. 639114; Clontech
Laboratories, Inc., Mountainview, CA, USA) was used for
amplification. For lentiviral infection, non-transfected LLC cells
were eliminated by 2 week spuromycin incubation at 37°C. The
overexpression of miR-126 in puromycin-resistant LLC cells was
verified by RT-qPCR as previously mentioned.
Female C57BL/6 mice were (n=8) purchased from the
Model Animal Research Center of Nanjing University (Nanjing, China;
age, 4–6 weeks; weight, 15–18 g). Mice were bred in an SPF room of
the core animal facility of the Model Animal Research Center of
Nanjing University (Nanjing, China). The room temperature was 25°C
and the light-cycle is automatically controlled (12 h light and 12
h dark). Mice had free access to food and water. LLC cells
(1×106) stably overexpressing miR-126 or NC were
suspended with 100 µl 50% Matrigel and injected subcutaneously into
one side of the flanks of the mice. Mice were anesthetized by
intraperitoneal injection with fresh 2,2,2-tribromoethanol
(Avertin; cat. no. B65586; Sigma-Aldrich; Merck KGaA) in sterile
PBS at a dose of 0.4 mg/g. Primary tumors were surgically removed 1
week following transplantation or when reaching a volume of 800
mm3 to allow further growth of metastatic nodules.
Following 3 weeks of tumor growth, the mice were sacrificed and
metastasis to the lung was determined by counting individual
metastatic nodules. The results are presented as the mean ±
standard error of the mean (SEM).
Hematoxylin-eosin (H&E)
staining
Mouse lungs were dissected and washed with cold PBS
and fixed in 4% paraformaldehyde overnight at 4°C. The samples were
processed successively by: i) a 30 min wash in PBS at 4°C; ii) an 1
h incubation in 70, 80 and 95% ethanol and a 1 h incubation in 100%
ethanol at room temperature; iii) a 20 min incubation in xylene at
room temperature; iv) a 1 h incubation in paraffin/xylene (1:1) at
65°C; and v) a 1 h incubation in fresh paraffin at 65°C. The
processed samples were then embedded in paraffin and sectioned (8
µm thick). The sections were applied for H&E staining: Briefly,
sections were washed in distilled water and incubated with 0.2%
hematoxylin solution (w/v) for 5 min. Differentiation followed for
30 sec in 1% acid alcohol (1% HCl in 70% Ethanol). Subsequently,
the sections were stained with 0.25% eosin (w/v) for 15 sec and
dehydrated with an alcohol series (70, 80, 90 and 100%) for 2 min
each. The sections were visualized under a light microscope
(magnification, ×6).
Statistical analysis
Each value in the present study was obtained from at
least three independent experiments. Data were presented as the
mean ± SEM. Since two groups were being compared in the present
study, statistical significance was determined using the unpaired
two-tailed Student's t-test. Statistical analysis was performed
using GraphPad Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-126 inhibits the EMT of lung
cancer cells in vitro
EMT is an important process involved in the invasion
and metastasis of tumor cells. To assess whether miR-126 affects
the EMT of lung cancer cells, miR-126 mimics were used to
overexpress miR-126 in the human lung cancer SPC-A1 cell line. The
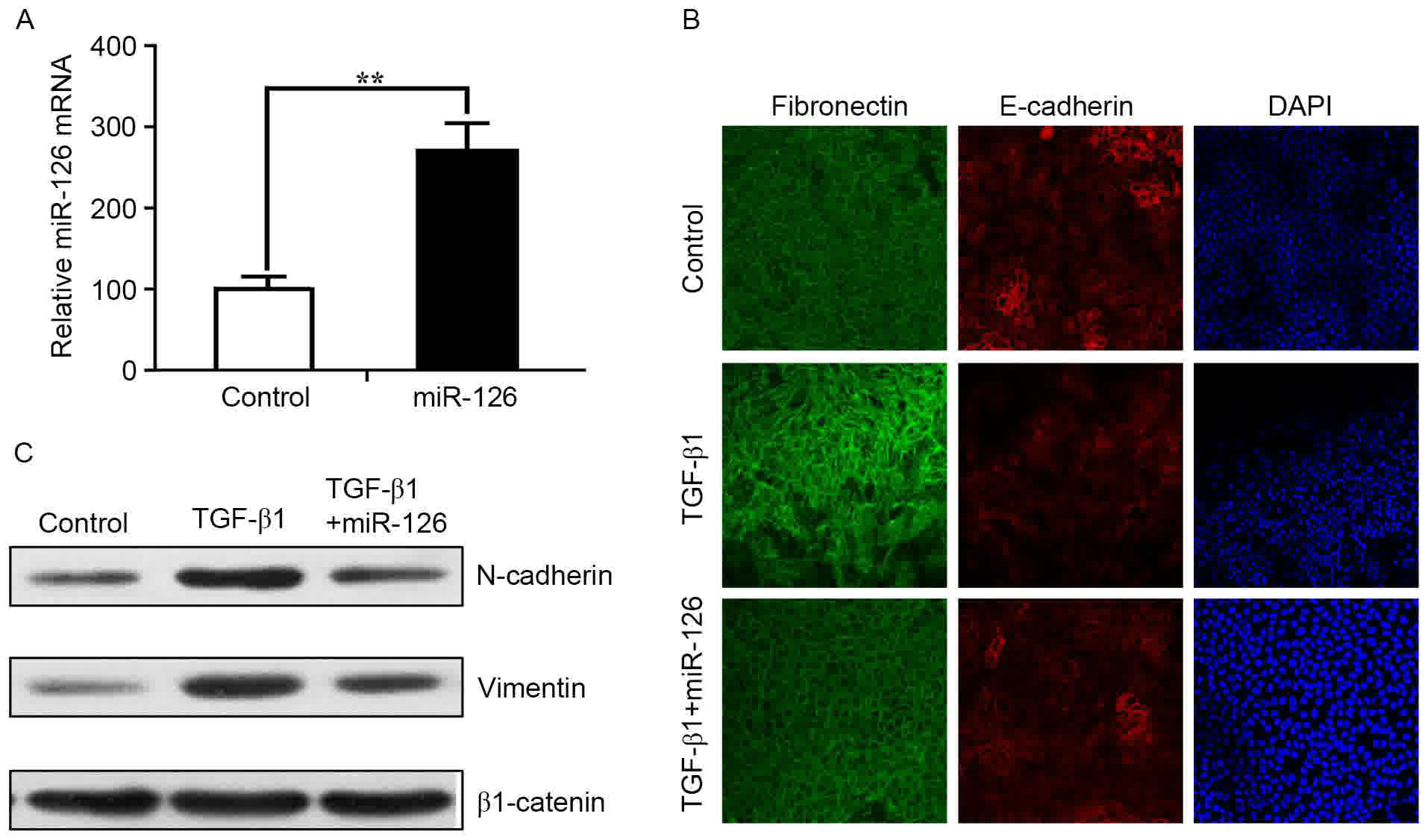
expression of miR-126 was verified by RT-qPCR (Fig. 1A). Cells were treated with TGF-β1 (5
ng/ml) 2 h following transfection with miR-126 mimics and NC.
TGF-β1 is widely used to induce EMT in epithelial cells (26). Immunofluorescent staining of
fibronectin and E-cadherin revealed that TGF-β1-treated control
cells exhibited loss of tight junctions and epithelial phenotypes
while acquiring spindle-like, fibroblastic morphology (Fig. 1B). However, miR-126 overexpression
inhibited EMT-associated changes (Fig.
1B). The relative protein expression of EMT markers was also
measured by western blot analysis. Upregulation of miR-126
expression in SPC-A1 cells resulted in a visible increase in
E-cadherin expression and a decrease in N-cadherin and vimentin
expression compared with control cells (Fig. 1C). These results indicated that
ectopic expression of miR-126 inhibited the EMT phenotype in SPC-A1
cells.
miR-126 inhibits metastasis of lung
cancer cells in vivo
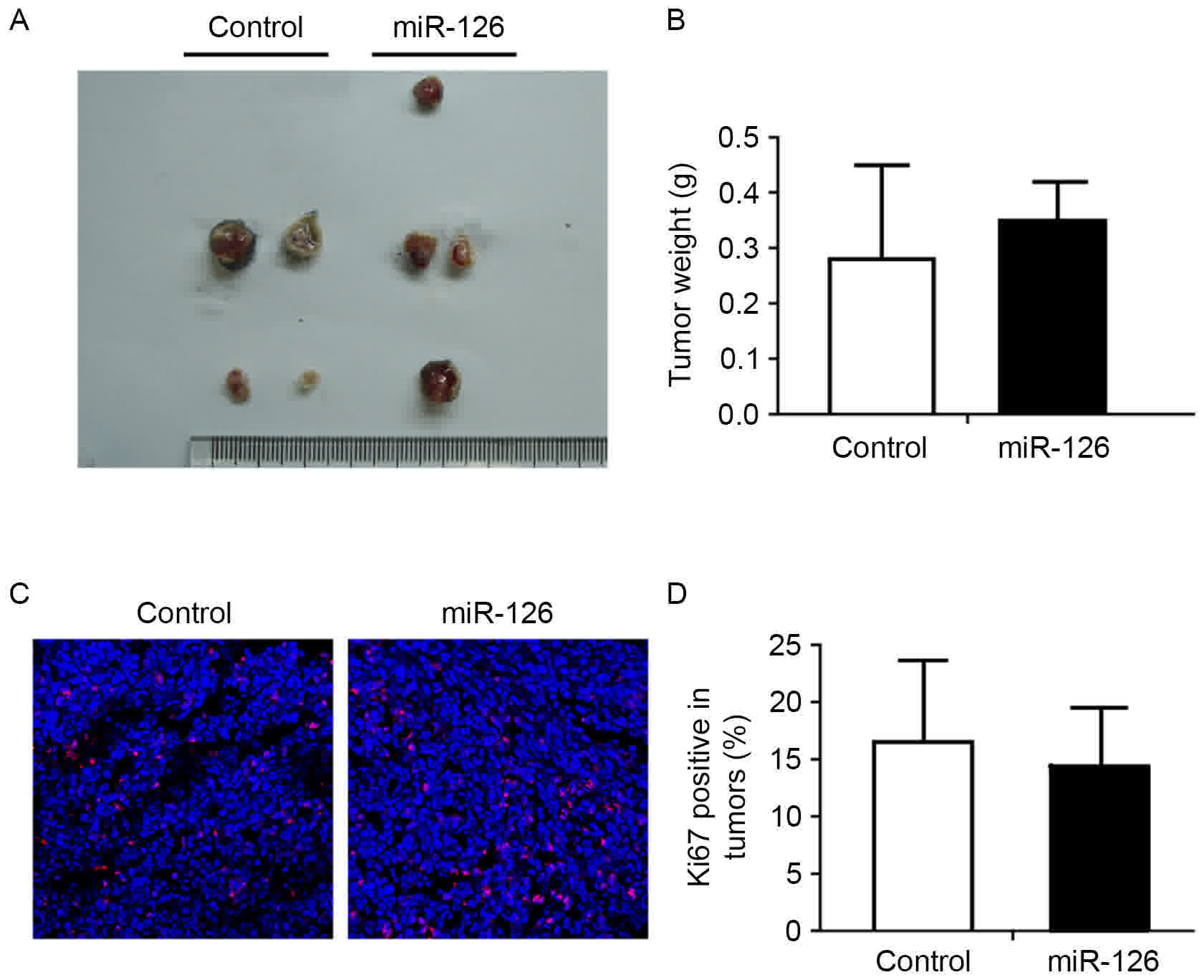
To directly evaluate the function of miR-126 in
tumor metastasis in vivo, a xenograft LLC model in C57BL/6
mice was used. LLC cells transfected with miR-126 or scramble
control lentivirus were injected subcutaneously into each flank of
the mice. Primary tumors were surgically removed ~1 week following
injection. There was no significant difference in the size and
weight of primary tumors between these two groups (Fig. 2A and B). Immunofluorescent staining of
the Ki-67 proliferation marker also confirmed these results
(Fig. 2C and D). To examine whether
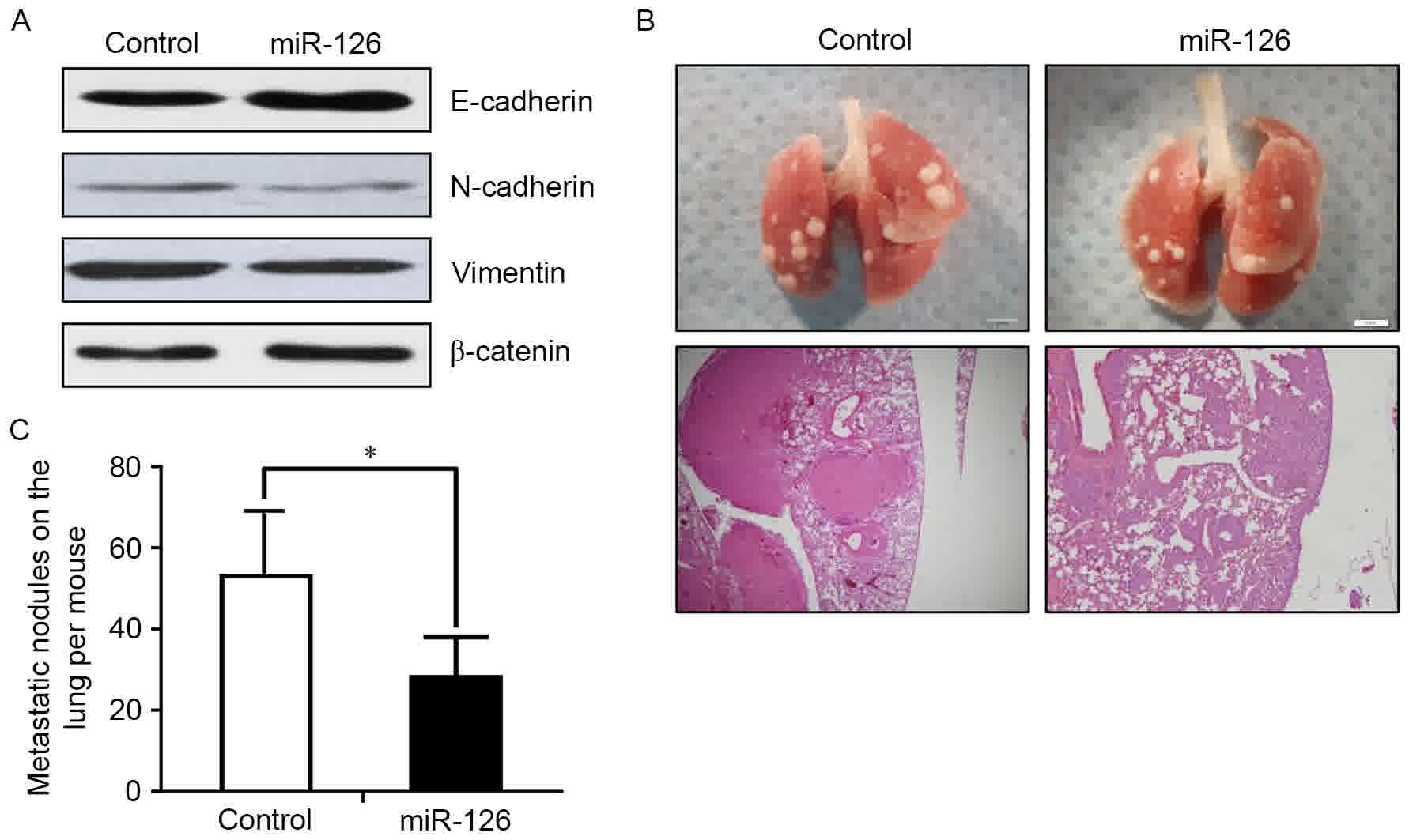
miR-126 inhibited the EMT of lung cancer cells in vivo, the
expression of EMT markers was examined by western blot analysis.
Consistent with the in vitro results, increased expression
of E-cadherin and decreased expression of N-cadherin and vimentin
were observed in the miR-126 overexpression group (Fig. 3A). Stable ectopic miR-126 expression
significantly suppressed the formation of lung metastases 3 weeks
following the surgical removal of the primary tumors (Fig. 3B and C). These results indicated that
miR-126 maybe capable of repressing lung cancer metastasis by
preventing the EMT process in vivo.
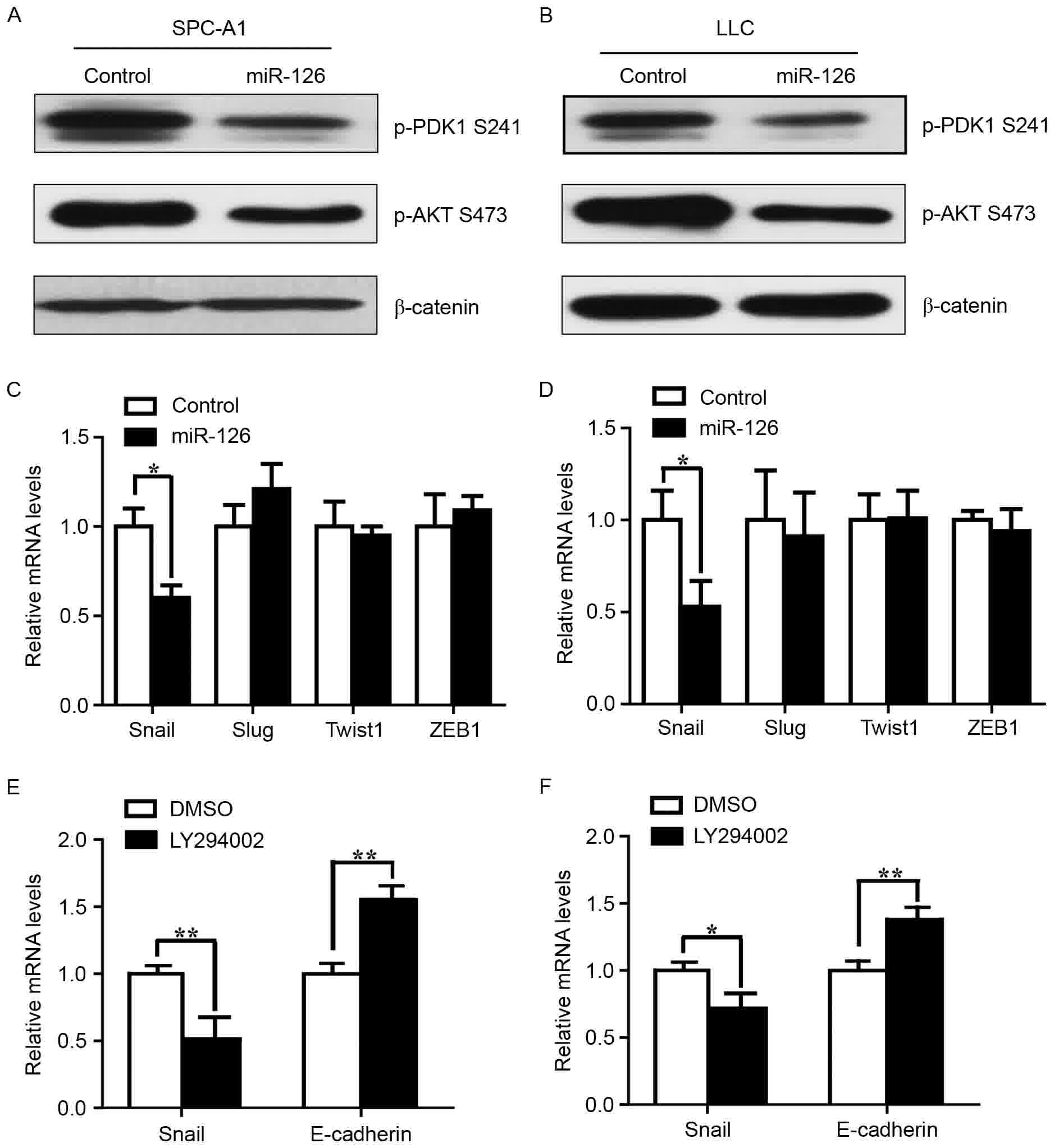
The PI3K/AKT/Snail pathway is a direct
target of miR-126 in lung cancer cells
Previous studies have demonstrated that multiple
predicted and validated miR-126 targets are signaling inputs for
AKT activity (27,28). To investigate whether the inhibitory
function of miR-126 on the metastasis of lung cancer cells is
mediated by the PI3K/AKT pathway, the activity of this pathway was
examined following miR-126 overexpression. p-PDK1 and p-AKT were
significantly downregulated in SPC-A1 and LLC cells overexpressing
miR-126 (Fig. 4A and B). Expression
of the transcription factor Snail was reduced in the two miR-126
transfected cell lines compared with the NC cells, but Slug, Twist1
and ZEB1 were not affected (Fig. 4C and
D). Constitutively active AKT induces Snail expression
(29). To examine whether reduced
Snail expression accounted for the reduced PI3K/AKT activity,
LY294002 was used to treat SPC-A1 and the LLC cells. Snail mRNA
expression was reduced following treatment with LY294002, compared
with the control (Fig. 4E and F).
Snail represses E-cadherin expression by directly targeting the
promoter of the latter (30).
Expression of E-cadherin was also reduced following the addition of
LY294002 to the SPC-A1 and the LLC cells, compared with control
cells (Fig. 4E and F). These results
revealed that miR-126 may inhibit the EMT and metastasis of lung
cancer cells via the PI3K/AKT/Snail signaling pathway.
Discussion
EMT serves a crucial function not only in the
development of cancer, but also in cancer progression (31). Cancer cells undergoing EMT are endowed
with more aggressive phenotypes than cells that have not undergone
EMT, including mesenchymal and stem cell-like features which result
in the acquisition of malignant properties, including invasion,
metastasis, recurrence and drug resistance. Understanding the
molecular mechanisms that regulate EMT is necessary to improve lung
cancer treatment. The present study demonstrated that
overexpression of miR-126 inhibited TGF-β1-induced EMT in lung
cancer cells. The present study also demonstrated that regulation
of the PI3K/AKT/Snail signaling pathway is involved
inmiR-126-mediated EMT and metastasis inhibition in lung cancer
cells.
Previous studies have demonstrated that miR-126
inhibits the invasion and migration of lung cancer cells (23). EMT, which is characterized by a loss
of cell junctions and the gain of migratory functions, serves a key
role in the early process of metastasis of cancer cells. The
present study revealed that overexpression of miR-126 inhibited
TGF-β1-induced EMT. EMT inhibition may be the main mechanism to
account for miR-126-mediated inhibition of lung cancer.
Previous studies have reported that miR-126
inhibited the proliferation of lung cancer cells (20,22).
However, in the present study there was no significant inhibitory
effect on the proliferation of LLC cells in vivo. This may
be due to distinct regulatory networks in different types of cancer
cell or the complicated microenvironment in vivo. Further
studies are needed to address this. Zhang et al (19) reported that miR-126 inhibited breast
cancer metastasis by repressing the recruitment of mesenchymal stem
cells and inflammatory monocytes. However, in the present study
there was no difference in the number or size of metastatic lung
cancer tumor nodules between the control and miR-126 overexpression
groups. Zhang et al (19)
adapted the tail-vein injection assay, which excludes the influence
of miR-126 on the cancer cells at the primary tumor site prior to
intravasation into the circulation. The present study demonstrated
that miR-126 inhibited the mesenchymal-like changes of primary
tumor cells, thereby inhibiting the initial step necessary for
metastasis.
It has become evident that EMT is one of the
numerous cellular processes subject to AKT kinase regulation
(28). In addition, TGF-β1 induces
EMT in human lung cancer cells via the PI3K/AKT and
mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase 1/2 signaling pathways (32). EMT is driven by activating AKT and
involves upregulation of the mesenchymal cell-specific protein
Snail and downregulation of numerous epithelial cell-specific
proteins, including E-cadherin (29).
SNAIL represses E-cadherin expression by directly targeting the
promoter of the latter (30).
Previous studies and the present study have demonstrated that
miR-126 significantly inhibits the AKT pathway as revealed by
reduced p-PDK1 and p-AKT protein. AKT pathway inhibition resulted
in reduced Snail expression. Thus, the PI3K/AKT/Snail pathway may
be an important means through which miR-126 inhibits the EMT and
metastasis of lung cancer cells.
In conclusion, the present study demonstrated that
overexpression of miR-126 suppressed the EMT and metastasis of lung
cancer cells by targeting the PI3K/AKT/Snail pathway. To the best
of our knowledge, this is the first study to demonstrate that
miR-126 regulates the EMT of lung cancer cells and also the first
to demonstrate that miR-126 inhibits lung cancer metastasis in
vivo. However, further studies are required to identify and
understand the clinical therapeutic significance of miR-126.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81541059) and
Nanjing Health Bureau (grant no. YKK15065).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ, YZ, and QX conducted the experiments; WG
designed the experiments and wrote the paper; AG helped analyze the
data.
Ethics approval and consent to
participate
In the present study, all methods were subject to
approval by the Model Animal Research Center and Ethical Committee
of Nanjing University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: Molecular histology of lung cancer: From targets to
treatments. Cancer Treat Rev. 41:361–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tse JC and Kalluri R: Mechanisms of
metastasis: Epithelial-to-mesenchymal transition and contribution
of tumor microenvironment. J Cell Biochem. 101:816–829. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim AY, Kwak JH, Je NK, Lee YH and Jung
YS: Epithelial-mesenchymal transition is associated with acquired
resistance to 5-fluorocuracil in HT-29 colon cancer cells. Toxicol
Res. 31:151–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, He A, Zhang Q and Tao C: miR-126
inhibits cell growth, invasion, and migration of osteosarcoma cells
by downregulating ADAM-9. Tumour Biol. 35:12645–12654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu
T, Bai Y, Shen Y, Yuan W, Jing Q and Qin Y: Endothelial-specific
intron-derived miR-126 is down-regulated in human breast cancer and
targets both VEGFA and PIK3R2. Mol Cell Biochem. 351:157–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XM, Wang AM, Zhang J and Yi H:
Down-regulation of miR-126 expression in colorectal cancer and its
clinical significance. Med Oncol. 28:1054–1057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo C, Sah JF, Beard L, Willson JK,
Markowitz SD and Guda K: The noncoding RNA, miR-126, suppresses the
growth of neoplastic cells by targeting phosphatidylinositol
3-kinase signaling and is frequently lost in colon cancers. Genes
Chromosomes Cancer. 47:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, mir-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamada S, Satoh K, Fujibuchi W, Hirota M,
Kanno A, Unno J, Masamune A, Kikuta K, Kume K and Shimosegawa T:
MiR-126 acts as a tumor suppressor in pancreatic cancer cells via
the regulation of ADAM9. Mol Cancer Res. 10:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: miR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Lan H, Huang X, Liu B and Tong Y:
MicroRNA-126 inhibits tumor cell growth and its expression level
correlates with poor survival in non-small cell lung cancer
patients. PLoS One. 7:e429782012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Chen BB, Zhang MH and Wang XR:
MicroRNA-126 inhibits the proliferation of lung cancer cell line
A549. Asian Pac J Trop Med. 8:239–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crawford M, Brawner E, Batte K, Yu L,
Hunter MG, Otterson GA, Nuovo G, Marsh CB and Nana-Sinkam SP:
MicroRNA-126 inhibits invasion in non-small cell lung carcinoma
cell lines. Biochem Biophys Res Commun. 373:607–612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiscornia G, Singer O and Verma IM: Design
and cloning of an shRNA into a lentiviral silencing vector: Version
A. CSH Protoc. 2008:pdb.prot50092008.PubMed/NCBI
|
|
26
|
Zhang A, Dong Z and Yang T: Prostaglandin
D2 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition
in MDCK cells. Am J Physiol Renal Physiol. 291:F1332–F1342. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martelli AM, Evangelisti C, Chiarini F and
McCubrey JA: The phosphatidylinositol 3-kinase/Akt/mTOR signaling
network as a therapeutic target in acute myelogenous leukemia
patients. Oncotarget. 1:89–103. 2010.PubMed/NCBI
|
|
28
|
Lechman ER, Gentner B, Ng SW, Schoof EM,
van Galen P, Kennedy JA, Nucera S, Ciceri F, Kaufmann KB, Takayama
N, et al: miR-126 regulates distinct self-renewal outcomes in
normal and malignant hematopoietic stem cells. Cancer Cell.
29:214–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
30
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/Akt pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou
WY, Zhang H, Zhao MC, Su JM, Gao W, Zhang L, et al: Transforming
growth factor-β1 induces epithelial-to-mesenchymal transition in
human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling
pathways. Mol Biol Rep. 39:3549–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|