Introduction
Allograft transplant patients exhibit an increased
risk of developing polyclonal or monoclonal lymphoproliferative
disorders, compared with the general population, due to the
persistent intake of an immunosuppressant, and incidence increases
with time following transplantation (1). The majority of these clonal lesions
retain certain histological vestiges of a reactive condition, which
suggests that the lesions may represent an early form of developed
plasma cell malignancies (2).
Post-transplant lymphoproliferative disorders are infected de
novo by Epstein-Barr virus (EBV) through the transplanted
kidney which may present early or late, be nodal or extranodal, be
polymorphic or monomorphic, and run an indolent or aggressive
clinical course (3). In contrast with
the classical post-transplant lymphoproliferative disorders, plasma
cell neoplasms (PCNs; comprising multiple myeloma and
plasmacytoma), which occur following solid organ transplantation,
have received relatively limited attention. Multiple myeloma (MM)
represents ≤4% of all post-transplantation lymphoproliferative
disorders (4), and is associated with
a poor response to discontinuation of immunosuppression and
conventional therapy, and a short median survival (5). MM or extramedullary plasmacytomas have
been identified in post-renal transplant recipients (6) and, typically, the presence of plasma
cells in the liver is associated with a more aggressive form of MM
(7,8).
The present case report focuses on a patient with MM who
recurrently developed post-transplant EBV-associated multiple
extramedullary plasmacytomas (including left scapula, liver,
vertebrae, breast, skin, muscle and soft tissues) 11 years after
renal transplantation. The patient was successfully treated by, and
well-tolerated, a combined lenalidomide regimen followed by an
autologous stem cell transplantation (ASCT).
Case report
A 45-year-old female was admitted to The First
People's Hospital of Changzhou (Changzhou, China) with left
shoulder pain in February 2012. Early in November 2001, the patient
received a cadaveric kidney transplantation for uremia and was
administered the immunosuppressives cyclosporine, azathioprine and
prednisone, in a dose-tapering manner. After 2 years, the patient
received cyclosporine only. In March 2008, the patient developed
extramedullary plasmacytoma localized in the left nasal cavity and
immunohistochemical examination of biopsy tissue was performed.
Immunohistochemical staining was performed using the EnVision
method (9). Primary antibodies for
AE1/AE 3 (cat. no. 170308049b), CD79α (cat. no. 161122552E, clone
sp18), EMA (cat. no. 16391310, clone E29), CD20 (cat. no.
170124020a, clone MX003), CD3 (cat. no. 160907543f, clone sp7),
CD45RO (cat. no. 161207593h, clone uchl-1), CD138 (cat. no.
161014200E, clone MI15) and Ki-67 (cat. no. 17A11203; clone MX006)
[all Fuzhou Maxim (Maixin) Biotech Co., Ltd., Fuzhou, China]
diluted with antibody diluent (cat. no. ab64211; Abcam, Cambridge,
MA, USA) at the title of 1:50 were used in the present study.
Diaminobenzidinetetrahydrochloride (cat. no. DAB-0031) solution
were provided by Fuzhou Maxim (Maixin) Biotech Co., Ltd. All
samples were fixed in formalin solution and embedded in paraffin.
Sections (3–4 µm) were de-paraffinized in xylene, dehydrated in
ethanol, and incubated in 3% H2O2 for 15 min
to destroy the activity of endogenous peroxidase. Following
incubation at 4°C in 10% normal bovine serum (Hangzhou Bori
Technology Co., Ltd., Hangzhou, China) for 10 min to block
membranes, each slide was incubated with the primary antibodies at
4°C overnight. Biotin-labeled mouse anti-rabbit immunoglobulin
(cat. no. BM2004; Boster Biological Technology, Pleasanton, CA,
USA) was chosen as the secondary antibody. The positive and
negative controls were provided by the manufacturer.
Immunohistochemical analysis by two pathologists using a light
microscope (Olympus Corporation, Tokyo, Japan) revealed the
following: Cluster of differentiation (CD)79α+,
vimentin+, 50% of Ki-67+, epithelial membrane
antigen (EMA)−, antibodies AE1/AE3−,
CD20−, CD3−, CD45RO− and CD138
partial+. Usually, 10 randomly selected microscopic
fields (magnification, ×100) were observed, and images were
captured and analyzed using Image-Pro Plus software version 14
(Media Cybernetics, Inc., Rockville, MD, USA), using the following
formula: Mean OD = sum of total integrity OD/total area. Bone
marrow examination identified no abnormal plasmocytes and the X-ray
revealed no bone lesion. The patient was diagnosed with isolated
plasmacytoma, thus cyclosporine was discontinued and the patient
was administered rapamycin (2 mg daily) and mycophenolatemofetil (1
mg twice daily), to prevent renal rejection. Between 26 March and
14 May 2008, the patient received local radiotherapy in the
bilateral nasal cavity, ethmoid sinus and maxillary sinus with a
total dose of 48 Gy. The following periodical examination
identified normal renal function.
In February 2012, the patient began to experience
left shoulder pain of an unknown cause and the following computed
tomography (CT) scan revealed bone destruction of the left scapula,
reactive bone formation at the 10th rib on the right side. A
subsequent positron emission tomography (PET)-CT scan revealed
increased metabolism of 18-fluorodeoxyglucoseat a standardized
uptake value (SUV) of 5.5 at the left scapula with bone
destruction. Subsequently, the vicent underwent left scapula mass
biopsy and postoperative pathological examination suggested plasma
cell myeloma with immunohistochemistry results of CD20−,
part of CD79α+, CD3−, CD38+,
CD138+, EMA−, multiple myeloma oncogene 1
(MUM-1)−, EBV-encoded small RNAs (EBERs)+ and
<5% of Ki-67+. A bone marrow smear revealed 2% of
mature plasma cells with a normal female chromosome karyotype.
Interphase fluorescence chromosomal in situ hybridization
(FISH) (10) of bone marrow cells
identified 1q21, retinoblastoma protein 1, p53, D13S319 and
immunoglobulin (Ig) H gene abnormalities. Serum IgG concentration
was 43.8 g/l and λ-light chain was 4,930 mg/dl, and serumprotein
electrophoresis identified a monoclonal spike in the γ-globulin
region. Urine electrophoresis revealed no monoclonal spike. Serum
immunofixation electrophoresis confirmed an IgG-λ chain monoclonal
M component. The hemoglobin level, serum albumin, calcium,
β2-microglobulin, lactate dehydrogenase and renal
function were all normal. No marked change in antibody titers
(anti-cytomegalovirus, anti-EBV or anti-hepatitis B virus surface
antigen) was determined. Retrospective analysis of nasal
plasmacytoma in 2008 identified EBER+. Thus, the patient
was diagnosed with secondary multiple myeloma IgG-λ chain type,
stage I and group A (International Staging System), post-renal
transplantation. The patient refused the novel agent bortezomib and
received a modified VADT (vincristine at 0.4 mg on days 1–4,
doxorubicin at 10 mg on days 1–4, dexamethasone at 40 mg on days
1–4 and thalidomide at 50–150 mg on days 1–28, every 28 days)
regimen for 3 cycles.
In June 2012, the patient was diagnosed as being in
complete remission with negative serum immunofixation
electrophoresis characterized by no monoclonal protein, as
described previously (11), only 2%
of mature plasma cells in a bone marrow smear and no hypercalcemia,
renal failure or anemia. Subsequently, the patient received a
fourth cycle of VADT regimen, followed by 3 cycles of the TD
regimen (thalidomide at 100 mg on days 1–28 and dexamethasone at 40
mg on days 1–4, with 28 days/cycle) for maintenance therapy. During
the period of chemotherapy, the patient was administered a
decreased dose of immunosuppressive therapy (rapamycin at 2 mg
daily and mycophenolate mofetil at 1 g twice daily, tapered to 0.5
g twice daily), and followed by monthly urinalysis and analysis of
blood urea nitrogen, creatinine, serum IgG and light chains.
In March 2013, on routine follow-up, the patient was
observed to have an increased serum IgG level (28 g/l), confirmed
with M protein using serum immunofixation electrophoresis (11), suggesting an asymptomatic relapse of
MM. The patient refused to receive novel agents, including
bortezomib or lenalidomide, but accepted VADT regimen, as
aforementioned, for 3 cycles. However, the patient's condition
deteriorated gradually and, in June 2013, serum IgG increased to 57
g/l with positive serum immunofixation electrophoresis, indicating
that the VADT regimen was no longer effective. Re-examination of
plasma EBVDNA remained negative, suggesting no EBV viremia.
Therefore, novel agents were administered for rescue therapy.
On 26 July, 23 August, 19 September and 25 October
2013, the patient received the CyB or DT regimen (cyclophosphamide
at 300 mg/m2 on days 1 and 8, bortezomib at 1.3
mg/m2 on days 1, 4, 8 and 11, dexamethasone at 20 mg on
days 1, 2, 4, 5, 8, 9, 11 and 12, and thalidomide at 100 mg on days
1–21) for 4 cycles. The subsequent evaluation revealed negative
serum immunofixation electrophoresis with normal IgG levels and no
abnormal plasma cells in bone marrow smear so the patient achieved
a second clinical complete remission. The patient selected the TD
regimen, rather thanbortezomib, for maintenance therapy and
received monthly routine laboratory investigations, including
ultrasonography of the abdomen.
However, 3 months later, in early February 2014, the
patient began to experience a fever (temperature, 38.7°C) with the
onset of fatigue, progressive lumbago and a non-tender skin mass of
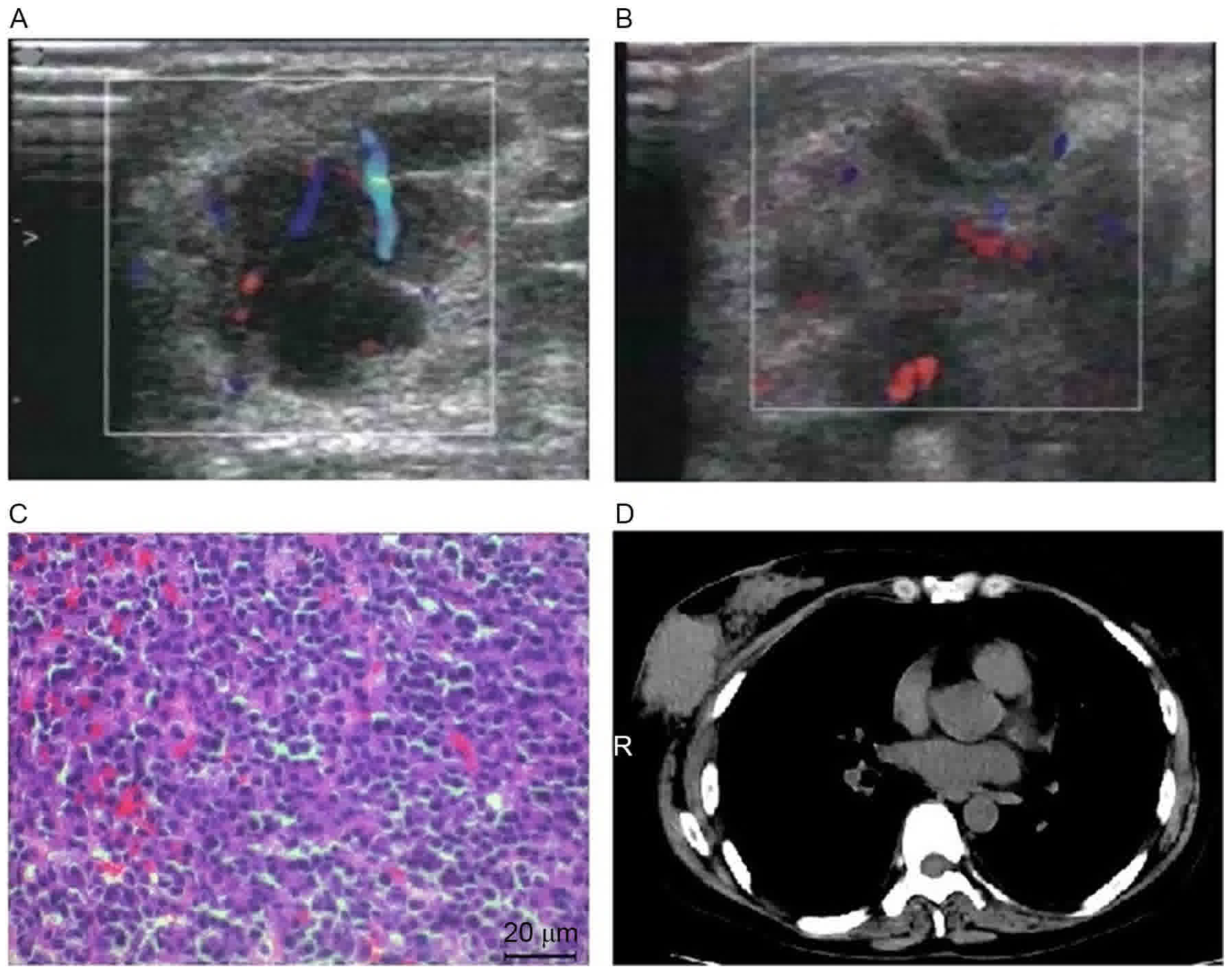
the submaxilla. The following abdominal ultrasound identified a
diffused hypoechoic lesion (maximum diameter, 8.0×6.5 cm) in the
liver. A full blood count revealed: Hemoglobin, 84 g/l; hematocrit,
35.6%; mean corpuscular volume, 86 fl; white blood cells,
8.79×109 cells/l; and platelets, 134×109
cells/l. Liver function tests were in the normal range. The total
protein serum level was 28 g/l and the protein electrophoresis
revealed the presence of a monoclonal band in the gamma region,
which was identified as IgG-λ using immunofixation. Viral markers
for hepatitis C and hepatitis B virus were negative. Neither serum
anti-EBV antibody nor plasma EBV-DNA was detectable. An upper
abdominal CT scan revealed that multiple round-like and
well-defined lesions, of various sizes, with low attenuations were
present in hepatic parenchyma. Following intravenous injection of
meglumine diatrizoate contrast media, the enhancements of lesions
were mild and heterogeneous. These results were considered to be
caused by myeloma (Fig. 1). Magnetic
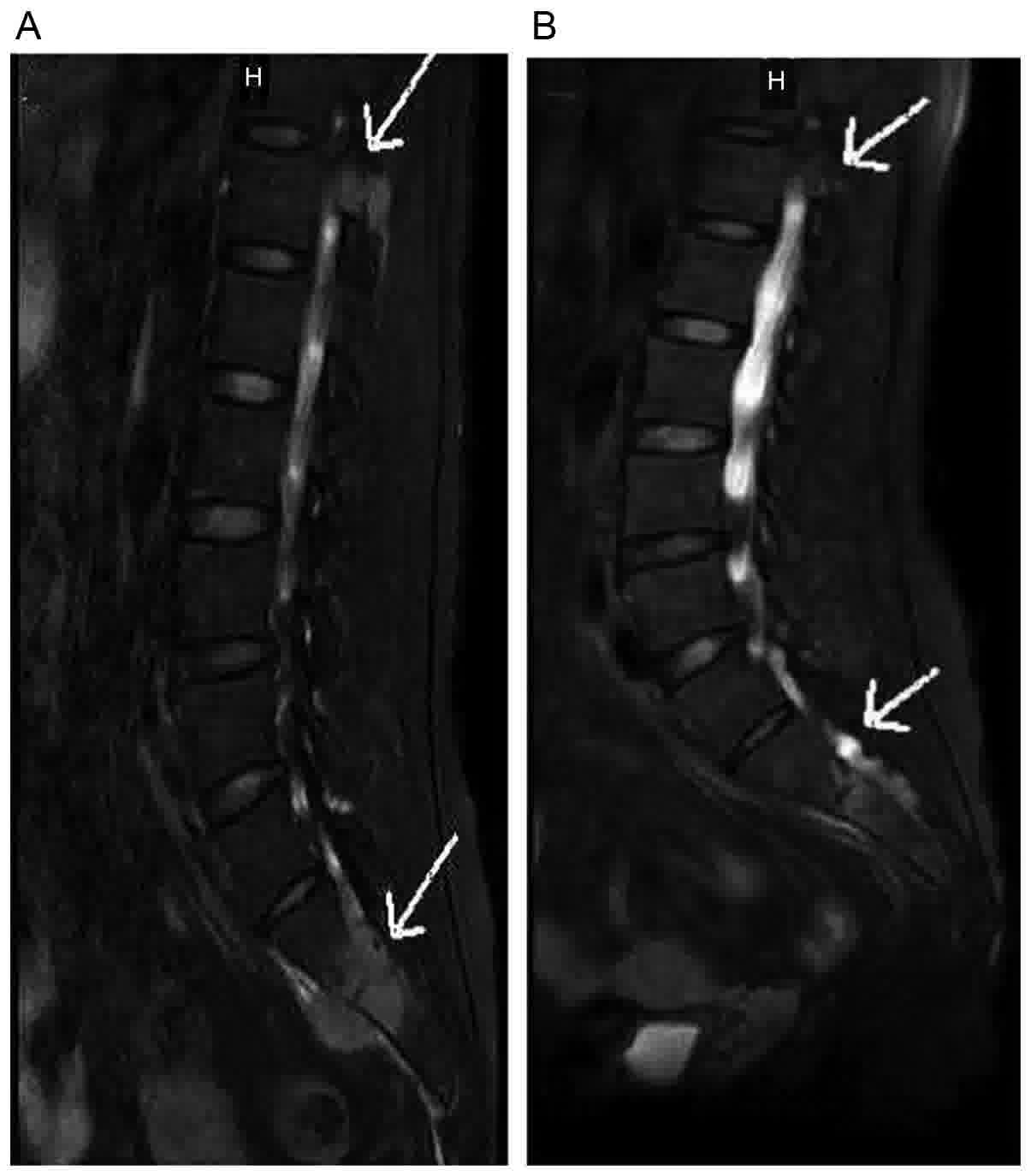
resonance imaging (MRI) fat suppression T2WI (12) identified soft mass and multiple patchy
lesions with high attenuations in T12 thoracic and L1-L4 lumbar
vertebrae. The soft tissue surrounding S1 and S2 was swollen
(Fig. 2). Therefore, a CT-guided
percutaneous needle biopsy of the hepatic lesion was performed and
the subsequent histological study (hematoxylin and eosin stain)
revealed diffused viability of plasma cells. The immunochemical
examination revealed CD20−, CD3−,
CD38+, CD138+,
IgG-κ+<IgG-λ+, MUM-1+, between
30 and 40% Ki67+, and EBV-encoded RNA+,
indicating extramedullary liver plasmacytoma (Fig. 3). Bone marrow aspirate identified 6%
plasma cell infiltration and CD138 sorting interphase FISH of bone
marrow cells revealed 1q21 amplification. Serum IgG was 62 g/l and
was confirmed with M protein using serum immunofixation
electrophoresis.
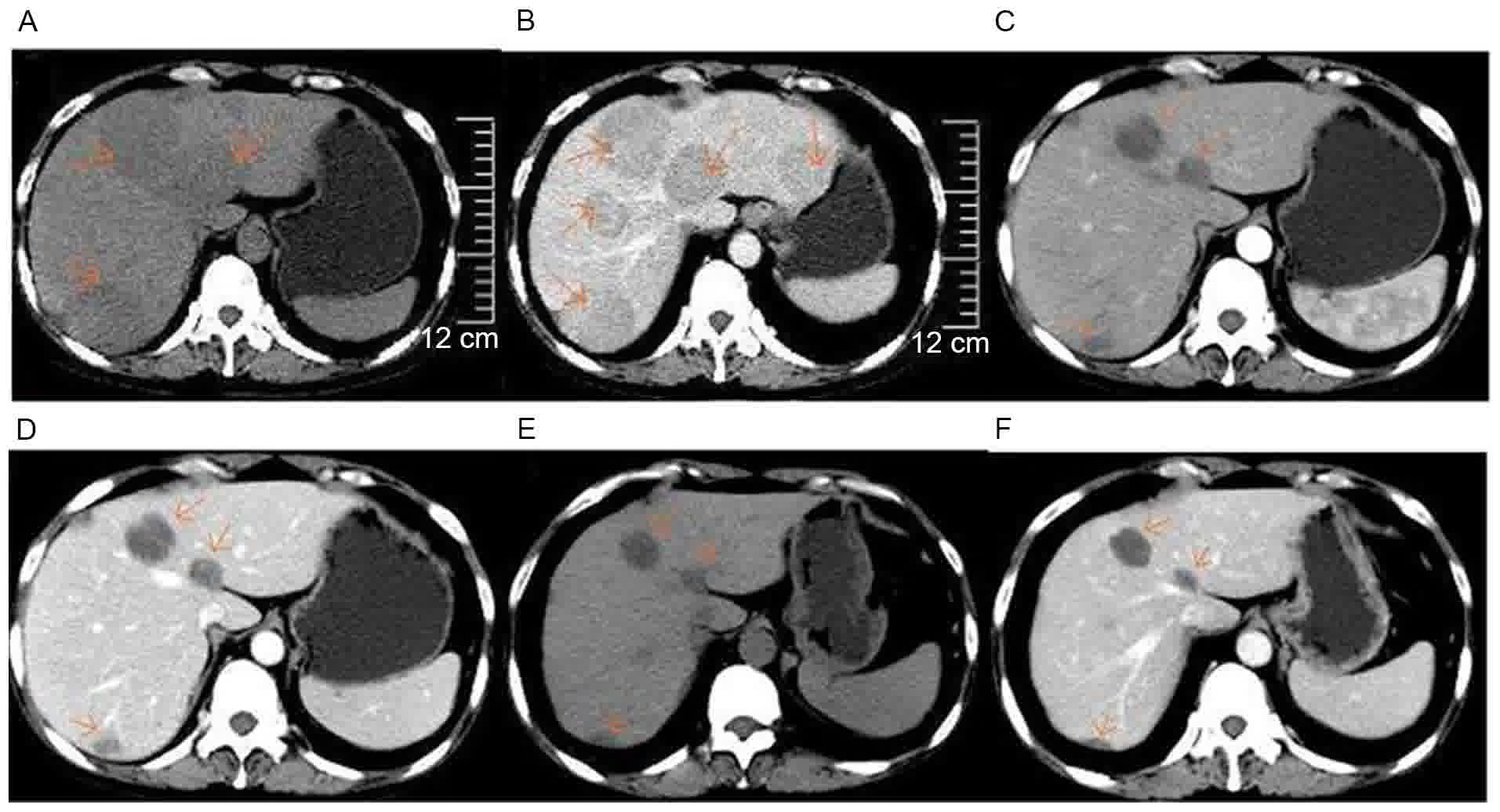 | Figure 1.Upper abdominal computed tomography
scan images prior to and following lenalidomide therapy. (A)
Non-enhanced; (B) enhanced, prior to lenalidomide therapy (25
February 2014). Multiple round-like and well-defined lesions of
distinct sizes with low attenuations were present in hepatic
parenchyma. Following administration of contrast materials, the
enhancements of lesions were mild and heterogeneous. (C)
Non-enhanced; (D) enhanced, following four cycles of RCd regimen
therapy (1 July 2014). Decreased size of round-like lesions were
observed in hepatic parenchyma, compared with (A and B). (E)
Non-enhanced; (F) enhanced, following five cycles of RCd regimen
(25 August 2014). Further decreased size of round-like lesions were
present in hepatic parenchyma, compared with (C and D). RCd
regimen, lenalidomideat 25 mg on days 1–21, cyclophosphamide at 50
mg on days 1–21 and dexamethasone at 20 mg on days 1, 8, 15 and 22,
with 28 days/cycle. The arrow in each of the images point to the
plasmacytoma in the liver. |
The patient experienced rapid relapse 3 months after
the CyB or D regimen and therefore accepted the RCd regimen
(lenalidomide at 25 mg on days 1–21, cyclophosphamide at 50 mg on
days 1–21, dexamethasone at 20 mg on days 1, 8, 15 and 22, with 28
days/cycle) and discontinued the immunosuppressant. Re-evaluation
demonstrated that serum IgG decreased to 27 g/l and ultrasonography
revealed that the hepatic mass decreased in size to 6.8×5.5 cm,
indicating that extramedullary liver plasmacytoma was sensitive to
the RCd regimen.
The patient continued the third and fourth cycle of
the RCd regimen and the following upper abdominal CT scan revealed
that the extramedullary liver plasmacytoma decreased (1 July 2014;
Fig. 1C and D). The hemoglobin level
was 114 g/l and serum IgG was 13.6 g/l. During the fourth cycle of
the RCd regimen, the patient experienced transient agranulocytosis
with pulmonary infection. The patient exhibited no fever or lumbago
following treatment for the infection and recovery of
myelosuppression, indicating that the patient exhibited good
partial remission, following four cycles of the RCd regimen. The
fifth cycle was subsequently administered. The following abdominal
CT (25 August 2014) demonstrated further decreased size of
round-like lesions in hepatic parenchyma (Fig. 1E and F) and a lumbar vertebral MRI (26
August 2014) identified a decrease in soft tissue mass in T12 and
alleviated swelling of soft tissue surrounding S1 and S2 (Fig. 2B). The patient was under outpatient
follow-up and accepted the RCd regimen for maintenance therapy from
September 2014, exhibiting a good response and stable disease, with
the exception of controllable myelosuppression. However, the
patient's condition deteriorated from 4 December 2014 with the
manifestation of progressively enlarged, hard right breast lump
(diameter between 7.7×8.5 and 9.6×10.2 cm), detected using
ultrasonography (Fig. 4A and B). The
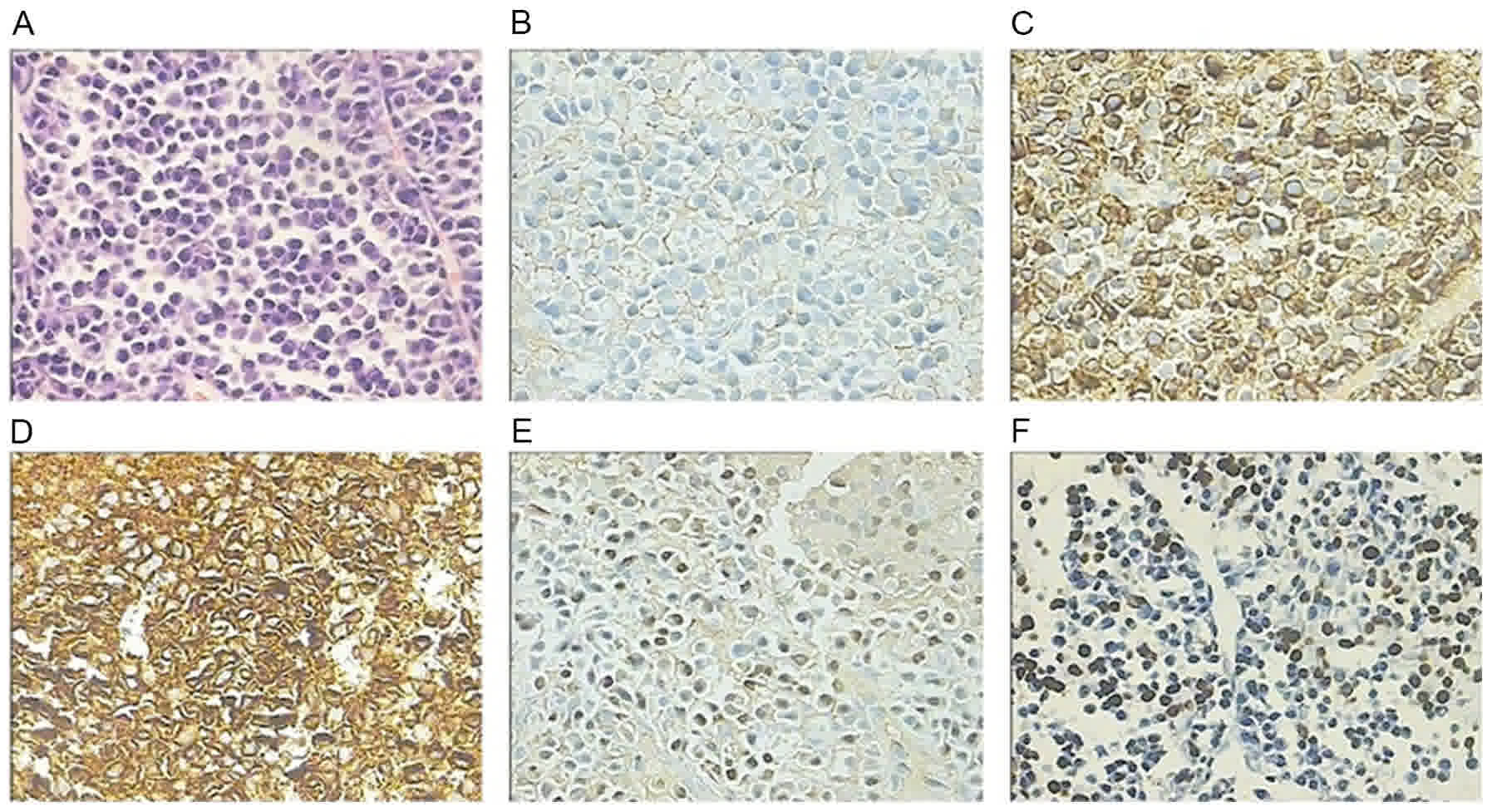
following biopsy revealed plasmacytoma with similar immunochemistry
results: CD38+, CD138+,
IgG-κ+<IgG-λ+, MUM-1+, with the
exception of 80% Ki-67+, which was increased compared
with liver plasmacytoma (Fig. 4C). At
this time, the patient had received 6.5 courses of the RCd regimen
and bone marrow examination revealed no evidence of bone marrow
infiltration. Thus, ASCT was subsequently carried out. On 19
December 2014, when absolute neutrophil count
<0.5×109 cells/l, the patient was mobilized with
etoposide (1.6 g/m2) intravenously, followed by
granulocyte colony-stimulating factor (G-CSF; 10 µg/kg/day;
Jilifen, China) and this continued until leukapheresis was
completed. The total number of mononuclear cells collected was
11.33×108 cells/kg and a total of 12.68×106
cells/kg CD34+ cells were collected. However, a CT scan
revealed that the right breast plasmacytoma remained the same size
which suggested that it was resistant to a high dose of etoposide
chemotherapy (Fig. 4D). An abdominal
CT scan identified a mild lesion in the liver.
Between 19 January and 2 March 2015, the patient
received radiotherapy of planning target volume (PTV) at 5,
035.8cGy/25 fractions (fx) for right breast plasmacytoma and 95%
PTV at 4,500.8cGy/25fx for right breast. During radiotherapy, the
patient experienced IV grade myelosuppression which resulted in
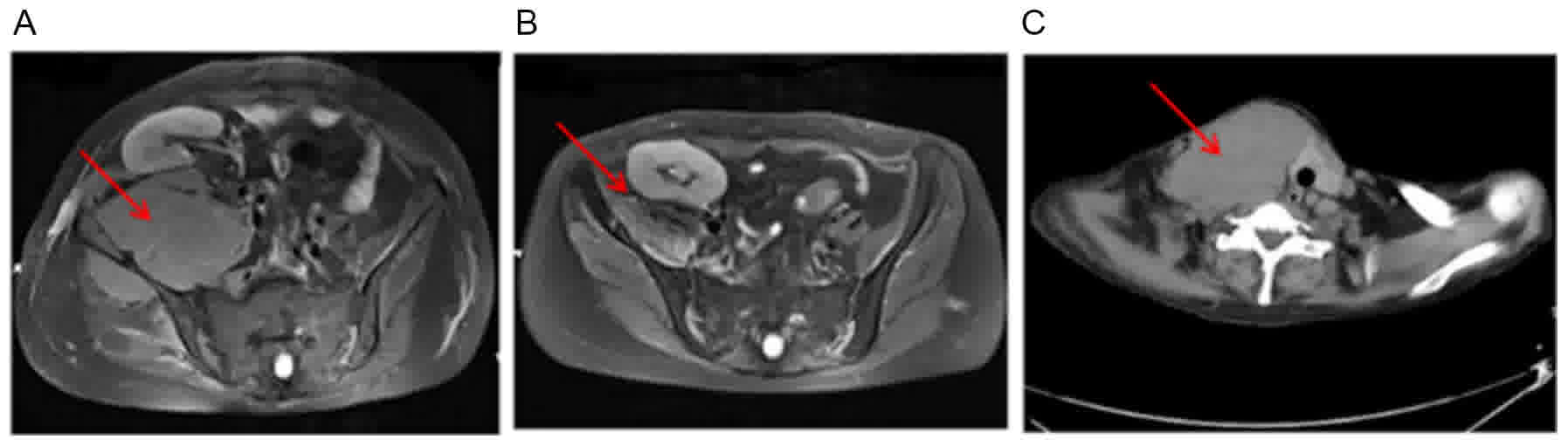
delayed radiotherapy and gradually worsening right hip pain. An MRI
scan (28 February 2015) of the hip revealed an abnormal signal of
bilateral femur neck, middle-upper part of the femur, iliac bone
and right internal iliac muscle, indicating myeloma-associated bone
disease and extramedullary plasmacytoma. The patient received
palliative supportive therapy until mid-May 2015 due to severe
myelosuppression with a progressively increased IgG level,
aggravated right hip pain and enlarged internal iliac muscle lump.
The IgG level increased to 38.9 g/l, the right internal iliac
muscle lump enlarged to 12 cm in diameter (Fig. 5A). Plasma EBVDNA remained
undetectable, indicating no EBV viremia. The patient experienced
unbearable pain and was unable to lie down. The patient elected to
receive ASCT as the final salvage therapy. Following provision of
written informed consent, stem cells were infused on day 0 and the
patient was administered a conditioning regimen of a high dose of
melphalan (200 mg/m2) on day 2. The patient received
subcutaneous G-CSF (10 µg/kg) from day 3 of ASCT for 3 consecutive
days, until the neutrophil counts were >1.0×109
cells/l. Prophylaxis for opportunistic infections and antimicrobial
therapy in cases of febrile episodes were administered, according
to the Chinese guideline for the clinical application of
antibacterial drugs for agranulocytosis with fever (13). The patient reached an absolute
neutrophil count of >0.5×109 cells/l on day 13
following infusion of stem cells, however, the platelet count was
persistently <20×109 cells with intermittent platelet
transfusion twice a week for ~2 months. During the transplantation,
dynamic detection of the liver and renal function was normal,
despite stopping immunosuppressants, the hip pain relieved from day
1 and the enlarged lump of the right hip was non-palpable. After 2
months, an MRI revealed improvement of the right hip, compared with
those of the pre-transplantation (Fig.
5B). The patient received MPR (melphalan, prednisone and
lenalidmide) as maintained therapy and periodical platelet
transfusion if platelet counts were <20×109
cells/l.
The patient remained relatively stable until October
2015. The patient experienced a rapid relapse of right neck soft
tissue plasmacytoma which progressively enlarged from mid-October
2015 (Fig. 5C). Therefore, the
patient was administered decitabine (20 mg/m2 for 5
days) as an experimental treatment. However, the condition
deteriorated during myelosuppression and the patient succumbed to
pulmonary infection in mid-November 2015, following a prolonged
survival of 6 months from salvage ASCT.
Discussion
The number of solid organ transplant recipients
continues to increase, therefore it is hypothesized that the cases
of post-transplant lymphoproliferative disorder (PTLD) will also
increase. PTLD represents a heterogeneous group of
lymphoproliferative processes associated with the immunosuppression
in transplant recipients (4,14). It is a potentially life-threatening
complication of organ transplantation which affects between 3 and
20% of solid organ transplant recipients (4,15) and the
incidence of PTLD in transplant recipients is increased between 30-
and 50-fold, compared with that of the general population (16). Risk factors for PTLD include degree
and length of immunosuppression, recipient's EBV infection status
and type of organ transplant (4,17). The
median time for PTLD diagnosis was between 5 and 7 years following
transplantation (18). According to
the World Health Organization Classification, PTLD is characterized
into four groups as follows: Early lesions, polymorphic PTLD,
monomorphic PTLD and classical non-Hodgkin's lymphoma type PTLD
(19).
Plasmacytoid PTLD is a rare type of PTLD, with an
incidence of <4% of PTLD cases (20), and there are limited studies on the
clinical presentation and outcome of this disease subtype. Unlike
traditional multiple myeloma, which commonly involves the bone
marrow only and may present with lytic boney lesions, plasmacytoid
PTLD uniformly presented with plasmacytomas, which behave in a
manner similar to a lymphoma with mass lesions (21). The possible contributing factors
include older age recipients, a donor who was brain dead at the
point of donation, onset of EBV infection post transplantation
(4), hepatitis C virus infection in
recipients and administration of antithymocyte/antilymphocyte
globulin following solid organ transplantation (4). Post-transplant plasmacytomas have been
described in the allograft, skin, peritoneum, gastrointestinal
tract, gingiva and, occasionally, at other sites (21–25). To
the best of our knowledge, extramedullary plasmacytoma of liver is
associated with more aggressive forms of multiple myeloma and its
presumptive ante mortem diagnosis is often based on clinical
findings, including hepatomegaly and laboratory alterations of
liver function tests (25). In
patients with plasmacytoid PTLD, the predominant transplanted
organs were kidneys or the heart (26). Of kidney transplant recipients,
between 1 and 4% developed PTLD (27)
and limited cases of extramedullary plasmacytomas have been
examined in kidney transplant recipients (23). The incidence of multiple myeloma is
rare in renal graft recipients, despite monoclonal gammopathies
presenting in these patients post-transplantation (28). The present case report describes the
identification of a rare clinical case, which met the required
clinical and laboratory criteria for plasmacytoid PTLD, following
renal transplantation. The patient in the present case report
developed EBV-associated nasal plasmacytoma 7 years post-renal
transplantation with presentation of multiple myeloma.
Subsequently, after 11 years, the patient relapsed with
extramedullary plasmacytoma of the liver, vertebrae, breast,
muscle, skin and soft tissues. Additionally, no high frequency of
abnormal plasma cells was present in bone marrow cells, which was
consistent with literature reports. For the patient, deceased-donor
and EBV infection were the two major contributing factors for
plasmacytoid PTLD.
The mean time between PTLD diagnosis and
transplantation was 129 months which suggests that long-term
immunosuppression or antigen stimulation, following
transplantation, may serve a role in the pathogenesis of PTLD
(19). B-cell viability is considered
to be induced by EBV infection in a number of PTLD cases (29), which arises in the setting of
pharmacological immunosuppression, leading to impaired T-cell
function and loss of control over EBV-infected cells. EBV-naive
patients who receive a donor organ from an EBV-infected donor are
at the highest risk of developing PTLD (30). The presence of EBV within the tumor
cells of EBV-naive patients who received a donor organ from an
EBV-infected donor distinguishes between plasmacytic PTLD and
classical plasma cell myeloma observed in non-transplant patients
where EBV is rarely present (5). Of
the patients with PTLD which developed following kidney transplant,
~90% was of B-cell origin and between 90 and 95% contained EBV as a
consequence of either reactivation of the dormant virus
post-transplantation or acquisition from the kidney donor (20,23). In
the present case report, EBV-associated multiple extramedullary
plasmacytomas of the nasal cavity, left scapula, liver, vertebrate,
breast, skin, muscle and soft tissues in a renal allograft
recipient were examined. In this patient, nasal, scapula, liver and
breast plasmacytomas were confirmed by postoperative
histopathological examination or CT-guided percutaneous needle
biopsy. In situ hybridization analysis of EBV-encoded RNA
(EBER) was positive in the nasal cavity, scapula, liver and breast
lesion, indicating that the plasmacytoid PTLD of this patient was
associated with EBV infection.
There is no consensus on the optimum approach to the
treatment of PTLD. The initial treatment involves immunosuppression
modification (31,32), which is adequate in a number of
patients; however, additional treatment including chemotherapy and
antiviral therapy are required in patients with aggressive tumors
(22,33). There are currently no clear guidelines
for the solitary plasmacytoma therapy in renal transplant
recipients, due to its rarity, and it is hypothesized that the
treatment for these patients should be individualized.
For patients with eligible multiple myeloma, the
treatment of choice includes induction therapy (usually involving
novel biological agents) followed by ASCT (34). The CyB or D regimen is effective in
multiple myeloma and produces rapid and complete hematological
responses in the majority of patients (35–38).
Previous case reports suggest that lenalidomide is an effective
agent in combination with dexamethasone (38,39).
However, this treatment is generally not considered to be curative
and relapses occur. The CyB or D regimen was administered to the
patient in the present case report and a good clinical response was
observed, which was consistent with previous studies (35–37).
Following the patient's third relapse with
extramedullary liver plasmacytoma within 3 months, the patient was
administered the RCd regimen (avoiding bortezomib resistance)
combined with intermittent ganciclovir (for EBV infection) and a
good partial remission was observed, suggesting that the novel
immunomodulatory lenalidomide is effective for the treatment of
extramedullary liver plasmacytoma. Following the patient's fourth
relapse with breast, hip muscle, femur neck, skin and soft tissue
plasmacytomas, local radiotherapy and subsequent ASCT, using a
conditioning regimen of a high dose of melphalan, was administered
and a good response was observed. This indicated that radiotherapy
is an effective treatment of breast plasmacytoma and melphalan is
effective at treating soft tissue plasmacytoma. Notably, the
patient's liver plasmacytoma was stable following lenalidomide
treatment, suggesting that distinct plasmacytoma may exhibit
distinct biological features; however, further gene expression
profile screening is required for validation.
The aforementioned treatments are typically not
considered curative and relapses occur. It is hypothesized that an
allo- or non-myeloablative hematopoietic stem cell transplantation
or novel agents may be considered for patients with plasmacytoid
PTLD. Additional studies are required to identify correct
management of this condition.
The present case report demonstrates an unusual form
of plasmacytoid PTLD, distinguished by its involvement of multiple
extramedullary sites, stable liver plasmacytoma situation and
multiple times of disease relapse, and novel curative regimens,
including newer biological agents and ASCT. The present case report
identifies areas of required investigating including the
pathogenesis, the clinical behavior and the appropriate treatment
of post-transplant extramedullary plasmacytoma. Notably, the
present case report identifies ASCT as a valuable, feasible and
well-tolerated treatment for post-renal transplant patients with
refractory multiple myeloma. Additional clinical studies are
required to further elucidate and validate these hypotheses.
Acknowledgements
The authors thank Dr Changqing Lu and Dr Tongbin
Chen (The First People's Hospital of Changzhou, Changzhou, China)
for their assistance with the pathological analyses, and Dr Jun Sun
(The First People's Hospital of Changzhou) for his assistance with
radiological analyses.
Funding
The present case report was supported by the Fund of
Science and Technology Bureau of Changzhou, Jiangsu, China (grant
nos. CJ20130035 and CJ20150418), the Changzhou High-Level Medical
Talents Training Project (grant no. 2016ZCLJ024) and the Fund of
333 Project of Jiangsu Province, China (grant no. BRA2015088).
Availability of data and materials
All datasets generated and analysed in the present
study are included in this published article.
Authors' contributions
XX, WG conceived and designed the study. YL, YC, WD,
WW, YZ, DL, HL and WG performed all the clinical diagnosis and
treatment. QL performed the pathological detection. YL and YC
analyzed the data. XX and YL wrote the manuscript. WG revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics and consent to participate
This study was approved by the Ethic Committee of
the First People's Hospital of Changzhou, and the written informed
consent was gained from all participants.
Consent for publication
All the study participants provided consent for the
data to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Andrés A: Cancer incidence after
immunosuppressive treatment following kidney transplantation. Crit
Rev Oncol Hematol. 56:71–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nalesnik MA: Plasma cell tumors in
transplant patients. Blood. 121:1247–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caillard S, Dharnidharka V, Agodoa L,
Bohen E and Abbott K: Posttransplant lymphoproliferative disorders
after renal transplantation in the United States in era of modern
immunosuppression. Transplantation. 80:1233–1243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engels EA, Clarke CA, Pfeiffer RM, Lynch
CF, Weisenburger DD, Gibson TM, Landgren O and Morton LM: Plasma
cell neoplasms in US solid organ transplant recipients. Am J
Transplant. 13:1523–1532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karuturi M, Shah N, Frank D, Fasan O,
Reshef R, Ahya VN, Bromberg M, Faust T, Goral S, Schuster SJ, et
al: Plasmacytic post-transplant lymphoproliferative disorder: A
case series of nine patients. Transpl Int. 26:616–622. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joseph G, Barker RL, Yuan B, Martin A,
Medeiros J and Peiper SC: Posttransplantation plasma cell
dyscrasias. Cancer. 74:1959–1964. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda K, Matsui H, Watanabe T, Seki J,
Ichinohe T, Tsuji Y, Matsumura K, Sawai Y, Ida H, Ueda Y and Chiba
T: Spontaneous rupture of liver plasmacytoma mimicking
hepatocellular carcinoma. Intern Med. 49:653–657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El Maaroufi H, Doghmi K, Rharrassi I and
Mikdame M: Extramedullary plasmacytoma of the liver. Hematol Oncol
Stem Cell Ther. 5:172–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tao K, Zhu X, Xu W, Chen Z and Lu H: A
clinicopathologic and immunohistochemical study of diffuse large
B-cell lymphoma. Zhonghua Bing Li Xue Za Zhi. 31:112–115.
2002.PubMed/NCBI
|
|
10
|
Hu Y, Chen L, Sun CY, She XM, Ai LS and
Qin Y: Clinical significance of chromosomal abnormalities detected
by interphase fluorescence in situ hybridization in newly diagnosed
multiple myeloma patients. Chin Med J (Engl). 124:2981–2985.
2011.PubMed/NCBI
|
|
11
|
Nau KC and Lewis WD: Multiple myeloma:
Diagnosis and treatment. Am Fam Physician. 78:853–859.
2008.PubMed/NCBI
|
|
12
|
Rahmouni A, Divine M, Mathieu D, Golli M,
Dao TH, Jazaerli N, Anglade MC, Reyes F and Vasile N: Detection of
multiple myeloma involving the spine: Efficacy of fat-suppression
and contrast-enhanced MR imaging. AJR Am J Roentgenol.
160:1049–1052. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chinese guidelines for the clinical
application of antibacterial drugs for agranulocytosis with fever.
Zhonghua Xue Ye Xue Za Zhi. 33:693–696. 2012.(In Chinese).
PubMed/NCBI
|
|
14
|
Choi JH, Park BB, Suh C, Won JH, Lee WS
and Shin HJ: Clinical characteristics of monomorphic
post-transplant lymphoproliferative disorders. J Korean Med Sci.
25:523–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Opelz G and Döhler B: Lymphomas after
solid organ transplantation: A collaborative transplant study
report. Am J Transplant. 4:222–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salama S, Todd S, Cina DP and Margetts P:
Cutaneous presentation of post-renal transplant lymphoproliferative
disorder: A series of four cases. J Cutan Pathol. 37:641–653. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nalesnik MA: Clinicopathologic
characteristics of post-transplant lymphoproliferative disorders.
Recent Results Cancer Res. 159:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Richendollar BG, Hsi ED and Cook JR:
Extramedullary plasmacytoma-like posttransplantation
lymphoproliferative disorders: Clinical and pathologic features. Am
J Clin Pathol. 132:581–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuppachi S, Naina HV, Self S and Fenning
R: Plasmacytoma-like post-transplantation lymphoproliferative
disorder confined to the renal allograft: A case report. Transplant
Proc. 45:2791–2794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamdar KY, Rooney CM and Heslop HE:
Posttransplant lymphoproliferative disease following liver
transplantation. Curr Opin Organ Transplant. 16:274–280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trappe R, Zimmermann H, Fink S, Reinke P,
Dreyling M, Pascher A, Lehmkuhl H, Gärtner B, Anagnostopoulos I and
Riess H: Plasmacytoma-like post-transplant lymphoproliferative
disorder, a rare subtype of monomorphic B-cell post-transplant
lymphoproliferation, is associated with a favorable outcome in
localized as well as in advanced disease: A prospective analysis of
8 cases. Haematologica. 96:1067–1071. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McFarlane R, Hurst S, Sabath D, George E
and Argenyi Z: A rare case of plasmacytoma-like post-transplant
lymphoproliferative disorder presenting in the skin of a lung
transplant patient. J Cutan Pathol. 35:599–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bidros M, Bauer F, Codreanu I and Dasanu
CA: High-grade solitary extramedullary plasmacytoma arising in
skeletal muscle of a kidney transplant recipient. Leuk Res.
35:e181–e183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galieni P, Cavo M, Avvisati G, Pulsoni A,
Falbo R, Bonelli MA, Russo D, Petrucci MT, Bucalossi A and Tura S:
Solitary plasmacytoma of bone and extramedullary plasmacytoma: Two
different entities? Ann Oncol. 6:687–691. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petrucci MT, Tirindelli MC, De Muro M,
Martini V, Levi A and Mandelli F: Extramedullary liver plasmacytoma
a rare presentation. Leuk Lymphoma. 44:1075–1076. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plant AS, Venick RS, Farmer DG, Upadhyay
S, Said J and Kempert P: Plasmacytoma-like post-transplant
lymphoproliferative disorder seen in pediatric combined liver and
intestinal transplant recipients. Pediatr Blood Cancer.
60:E137–E139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanto DW: Classification of Epstein-Barr
virus-associated posttransplant lymphoproliferative diseases:
Implications for understanding their pathogenesis and developing
rational treatment strategies. Annu Rev Med. 46:381–394. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Renoult E, Bertrand F and Kessler M:
Monoclonal gammopathies in HBsAg-positive patients with renal
transplants. N Engl J Med. 318:12051988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holmes RD and Sokol RJ: Epstein-Barr virus
and post-transplant lymphoproliferative disease. Pediatr
Transplant. 6:456–464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knight JS, Tsodikov A, Cibrik DM, Ross CW,
Kaminski MS and Blayney DW: Lymphoma after solid organ
transplantation: Risk, response to therapy, and survival at a
transplantation center. J Clin Oncol. 27:3354–3362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taylor AL, Marcus R and Bradley JA:
Post-transplant lymphoproliferative disorders (PTLD) after solid
organ transplantation. Crit Rev Oncol Hematol. 56:155–167. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Samolitis NJ, Bharadwaj JS, Weis JR and
Harris RM: Post-transplant lymphoproliferative disorder limited to
the skin. J Cutan Pathol. 31:453–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mikhael JR, Schuster SR, Jimenez-Zepeda
VH, Bello N, Spong J, Reeder CB, Stewart AK, Bergsagel PL and
Fonseca R: Cyclophosphamide-bortezomib-dexamethasone (CyBorD)
produces rapid and complete hematologic response in patients with
AL amyloidosis. Blood. 119:4391–4394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rajkumar SV and Kumar S: Multiple myeloma:
Diagnosis and treatment. Mayo Clin Proc. 91:101–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reeder CB, Reece DE, Kukreti V, Mikhael
JR, Chen C, Trudel S, Laumann K, Vohra H, Fonseca R, Bergsagel PL,
et al: Long-term survival with cyclophosphamide, bortezomib and
dexamethasone induction therapy in patients with newly diagnosed
multiple myeloma. Br J Haematol. 167:563–565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leiba M, Kedmi M, Duek A, Freidman T,
Weiss M, Leiba R, Nagler A and Avigdor A:
Bortezomib-cyclophosphamide-dexamethasone (VCD) versus
bortezomib-thalidomide-dexamethasone (VTD)-based regimens as
induction therapies in newly diagnosed transplant eligible patients
with multiple myeloma: A meta-analysis. Br J Haematol. 166:702–710.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reeder CB, Reece DE, Kukreti V, Chen C,
Trudel S, Hentz J, Noble B, Pirooz NA, Spong JE, Piza JG, et al:
Cyclophosphamide, bortezomib and dexamethasone induction for newly
diagnosed multiple myeloma: High response rates in a phase II
clinical trial. Leukemia. 23:1337–1341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saboo SS, Fennessy F, Benajiba L, Laubach
J, Anderson KC and Richardson PG: Imaging features of
extramedullary, relapsed, and refractory multiple myeloma involving
the liver across treatment with cyclophosphamide, lenalidomide,
bortezomib, and dexamethasone. J Clin Oncol. 30:e175–e179. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Calvo-Villas JM, Alegre A, Calle C,
Hernández MT, Garcia-Sánchez R and Ramirez G: GEM-PETHEMA/Spanish
MyelomaGroup, Spain: Lenalidomide is effective for extramedullary
disease in relapsed or refractory multiple myeloma. Eur J Haematol.
87:281–284. 2011. View Article : Google Scholar : PubMed/NCBI
|