Introduction
Gastric cancer is the third leading cause of
cancer-associated mortality worldwide, accounting for 723,000
mortalities in 2012 (1). Advanced
gastric cancer continues to result in a high mortality rate despite
progress in surgical techniques, diagnostic procedures and
chemotherapy. The standard therapy for stage II/III gastric cancer
is surgical resection followed by adjuvant chemotherapy (2). Gastrectomy with D2-lymph-node
dissection, followed by chemotherapy with S-1 for 1 year, was
demonstrated to significantly improve survival in the Adjuvant
Chemotherapy Trial of S-1 for Gastric Cancer (2,3). However,
cancer recurs in a large number of patients even in those who have
received therapy. Therefore, novel diagnostic and treatments
approaches, including those based on personalized medicine and
individual biomarker analysis, are required.
Secreted protein acidic and rich in cysteine
(SPARC), also known as osteonectin, is a bone-specific
protein that binds selectively to both hydroxyapatite and collagen
(4). Basement membrane protein 40,
obtained from the basement membrane of a tumor, is also identical
to SPARC (5). SPARC is
expressed by a number of cell types, and its expression contributes
to the production and activity of matrix metalloproteinases, which
are important for embryogenesis, adult bone organization, wound
healing and tissue remodeling (6,7).
SPARC also regulates other biological functions, including
cell proliferation, migration, de-adhesion, differentiation and
angiogenesis (8). SPARC
expression is upregulated in many types of cancer (9), including pancreatic cancer, breast
cancer, prostate cancer, colorectal cancer, gastric cancer and
gliomas (7,10–15).
SPARC expression is also associated with patient outcomes
and clinicopathological features, including the depth of cancer
cell invasion and metastasis (16,17).
The present study measured mRNA expression levels of
SPARC in gastric cancer tissues and adjacent normal mucosa
obtained from 134 patients with stage II/III gastric cancer. The
objective of the present study was to evaluate the clinical
significance of SPARC gene expression in patients with stage
II/III gastric cancer after curative resection and adjuvant
chemotherapy with S-1.
Materials and methods
Patients and tissue samples
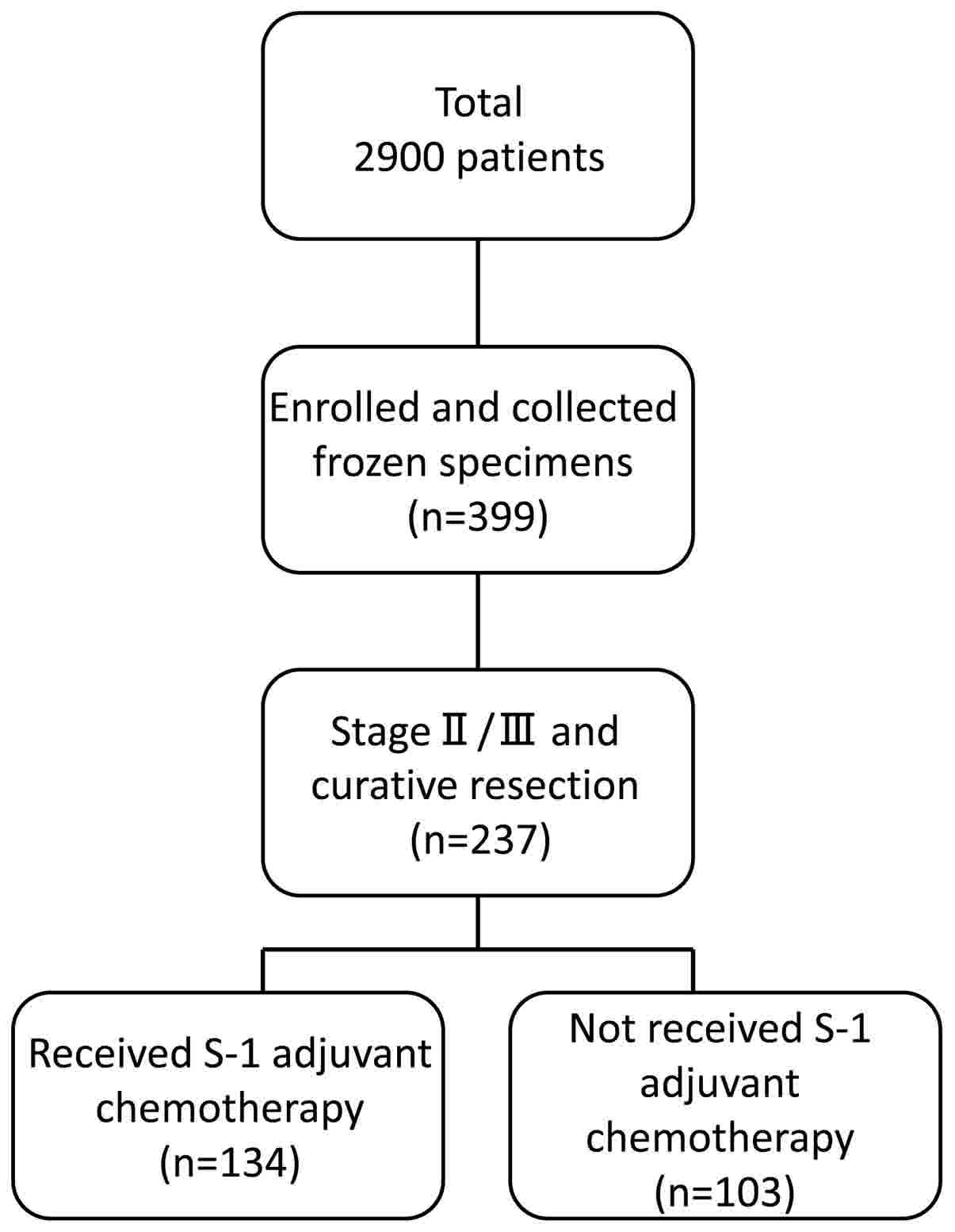
A total of 2,900 patients with histologically
confirmed gastric adenocarcinoma underwent gastrectomy between June
2002 and May 2010 at the following institutions: The Department of
Surgery at Yokohama City University (Yokohama, Japan), the
Gastroenterological Center at Yokohama City Medical Center
(Yokohama, Japan) and the Department of Gastrointestinal Surgery at
Kanagawa Cancer Center (Yokohama, Japan). Among these 2,900
patients, 399 agreed to participate in the present study by
donating samples of gastric tissue. Among these 399 patients, 237
were diagnosed with stage II/III cancer and underwent surgical
resection as part of their primary treatment. Tissue specimens of
cancer tissue and adjacent normal mucosa were obtained during
curative resection from 134 patients with stage II/III gastric
cancer who had received adjuvant chemotherapy with S-1 between June
2002 and May 2010. The patient's age ranged from 42–82 years old
(average, 65.3 years old), and the sex is 42 males and 92 females.
Eligible criteria include PS 0–1 cases and cases where functions of
major organs are preserved. As a reference group, the remaining 103
patients diagnosed with stage II/III gastric cancer, who had
undergone surgical resection but had not received adjuvant S-1
chemotherapy, were also included in the present study (Fig. 1). All tissue samples were embedded in
Optimal Cutting Temperature compound (Sakura Finetek USA, Inc.,
Torrance, CA, USA) and immediately stored at −80°C until further
use. Tissue specimens were stained with hematoxylin and eosin and
were examined histologically. Sections that consisted of >80%
cancer cells were subsequently used to prepare total RNA. Tumors
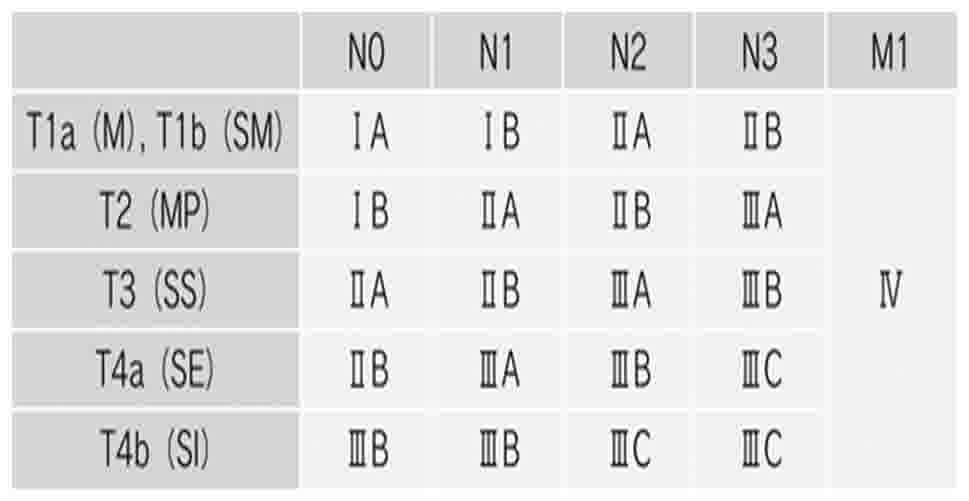
were staged according to the seventh edition of the Union for
International Cancer Control Tumor-Node-Metastasis (TNM)
classification of malignant tumors (18) (Fig. 2).
Written informed consent was obtained from each patient, and study
protocols were approved by the Ethics Committees of Yokohama City
University Medical Center, Yokohama City University (approval
number: 18-7A-4) and Kanagawa Cancer Center (approval number:
epidemiological study-29) prior to the initiation of the present
study. No other malignancies were identified in patients enrolled
in the present study.
Cell lines
Human gastric cancer cell lines (MKN1, MKN7, MKN45,
MKN74, NUGC-3, NUGC-4 and KATO III) were provided by the Japanese
Cancer Research Bank (Tokyo, Japan). Cell lines were maintained in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% fetal bovine serum
(Equitech-Bio, Inc., Kerrville, TX, USA), and 100 U/ml penicillin G
and streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.).
Cells were incubated in 5% CO2 at 37°C and passaged
every 3–4 days, except for the MKN7 cells, which were passaged
every 7 days because the passage time was different from other cell
lines.
RNA extraction and cDNA synthesis
Total RNA was extracted from gastric cancer tissues
and adjacent normal mucosa using TRIzol reagent (Gibco; Thermo
Fisher Scientific, Inc.). cDNA was synthesized from 2 µg total RNA
using an iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), prior to being diluted with water to 2 µg/µl
and stored at −20°C until use.
Reverse-transcription polymerase chain
reaction (RT-PCR)
RT-PCR was performed using SPARC gene-specific
oligonucleotide primers (Table I).
SPARC was amplified using the following thermocycling conditions:
40 cycles of denaturation at 95°C for 1 min, annealing at 55°C for
1 min and primer extension at 72°C for 1 min. β-actin was used as
an internal loading control. β-actin was amplified using the
following thermocycling conditions: 40 cycles of denaturation at
95°C for 1 min, annealing at 60°C for 1 min and primer extension at
72°C for 1 min. PCR products were separated by gel electrophoresis
on a 3% agarose gel, stained with ethidium bromide and visualized
under UV illumination.
 | Table I.Polymerase chain reaction primers and
conditions. |
Table I.
Polymerase chain reaction primers and
conditions.
| Gene | Primer | Annealing
temperature, °C | Product size, base
pairs |
|---|
| Secreted protein,
acidic and cysteine-rich |
|
|
|
| Sense
primer |
5′-GCTGGATGAGAACAACAC-3′ | 55.0 | 126 |
|
Anti-sense primer |
5′-AAGAAGTGGCAGGAAGAG-3′ |
|
|
| β-actin |
|
|
|
| Sense
Primer | 5′-
AGTTGCGTTACACCCTTTCTTGAC-3′ | 60.0 | 171 |
|
Anti-sense primer | 5′-
GCTCGCTCCAACCGACTGC-3′ |
|
|
RT-qPCR
RT-qPCR was performed using iQ SYBR-Green Supermix
(Bio-Rad Laboratories, Inc.). PCR reactions were performed in a
total volume of 15 µl, containing cDNA prepared from 0.2 µg total
RNA, 0.4 µM each gene-specific primer, 7.5 µl iQ SYBR-Green
Supermix (which contained dATP, dCTP, dGTP and dTTP, each at
concentrations of 400 µM) and 50 U/ml iTag DNA polymerase. The
following thermocycling conditions were used: Initial denaturation
at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C
for 15 sec, annealing at 55°C or 60°C for 15 sec for SPARC or
β-actin, respectively, and primer extension at 72°C for 30 sec,
followed by a final extension at 72°C for 10 min. To distinguish
specific from non-specific products and primer dimers, melting
curve analyses were performed. To evaluate specific mRNA expression
in the samples, a standard curve was created for each run, based on
three points from human control cDNA (Clontech Laboratories, Inc.,
CA, USA). The concentrations of each sample were calculated by
relating their crossing point to the standard curve. The number of
experimental repeats was three times, and the method used for
quantitation was relative quantities (19) (iQ5 software version 2.0; Bio-Rad
Laboratories, Inc.). β-actin was used as an internal loading
control. PCR primer sequences for amplifying SPARC and β-actin are
presented in Table I.
Immunohistochemistry
Immunohistochemical studies were performed using
formalin-fixed, paraffin-embedded tissue specimens obtained from
patients with stage II/III gastric cancer. The tissues were fixed
with 10% formalin at room temperature for 48 h. The thickness of
the sections was 4 µm. Tissue sections were deparaffinized with
xylene and descending alcohol series (100% ethanol twice, 95%
ethanol once and finally 70% ethanol once) and soaked in 10 mM
sodium citrate buffer (pH 9.0) at 121°C for 15 min for antigen
retrieval. Sections were subsequently incubated at 4°C for 20 h to
allow antigen-antibody binding. Primary polyclonal antibodies
against SPARC (dilution, 1:50; cat. no. sc-25574; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). A peroxidase-labeled polymer
(undiluted; EnVision+ anti-rabbit immunoglobulin/goat
polyclonal antibody; cat. no. K4002; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) was used to detect signals of the
antigen-antibody reaction at room temperature for 30 min, and the
internal control using a rabbit immunoglobulin antibody (dilution,
1:5,000; cat. no. X0903; Dako; Agilent Technologies, Inc.) Blocking
reagent was 3% hydrogen peroxide at room temperature for 5 min. All
sections were counterstained at room temperature for 50 sec with
hematoxylin. Immunohistochemistry was viewed using a light
microscope at a magnification of ×200.
Statistical analysis
SPARC gene expression levels in gastric cancer
tissues were compared with those in adjacent normal mucosa using
the Wilcoxon signed-rank test. A univariate Cox proportional
hazards model was used to evaluate the degree of association
between overall survival rates and SPARC gene expression levels and
other potential prognostic factors, including age, sex,
histological type, tumor size, depth of invasion, lymph-node
metastasis, number of lymph-node metastases, lymphatic invasion,
venous invasion and TNM stage. Cut-off values of SPARC gene
expression levels were evaluated using a multivariate Cox
proportional hazards model comprising prognostic factors that were
significantly associated with overall survival rates in univariate
analysis. The optimal cut-off value was selected by the minimum
p-value method, and the internal validity of the cut-off value was
evaluated using a 2-fold cross-validation approach (20). The association between gene expression
levels and potential prognostic factors was evaluated using the
χ2 test. The postoperative survival rate was evaluated
using the Kaplan-Meier method, and differences in survival rates
were assessed with the log-rank test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were conducted using SPSS software, version 22 for Windows
(IBM Corp., Armonk, NY, USA), and SAS, version 9.3 (SAS Institute,
Inc., Cary, NC, USA).
Results
Immunohistochemistry of SPARC
expression
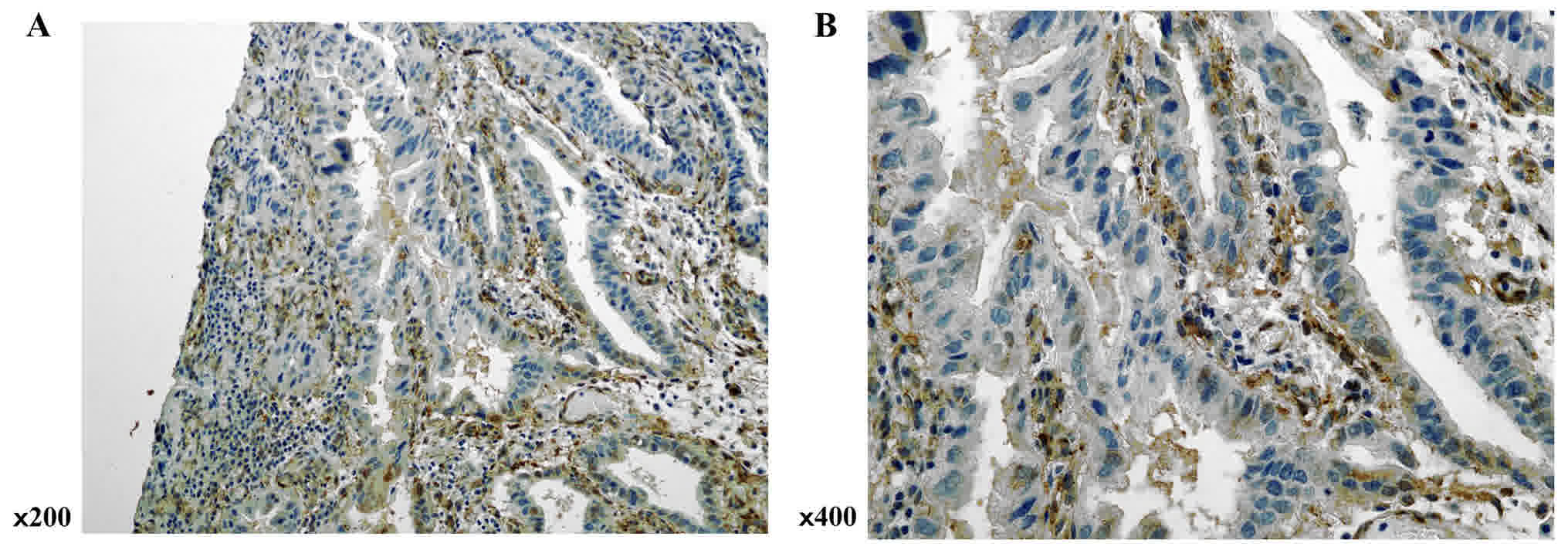
SPARC expression was evaluated in gastric cancer
tissues by immunohistochemical analysis. Although SPARC
immunopositive staining was observed in both stromal and cancer
cells, expression was higher in the former (Fig. 3).
SPARC mRNA expression in gastric
cancer cell lines and patient tissue samples
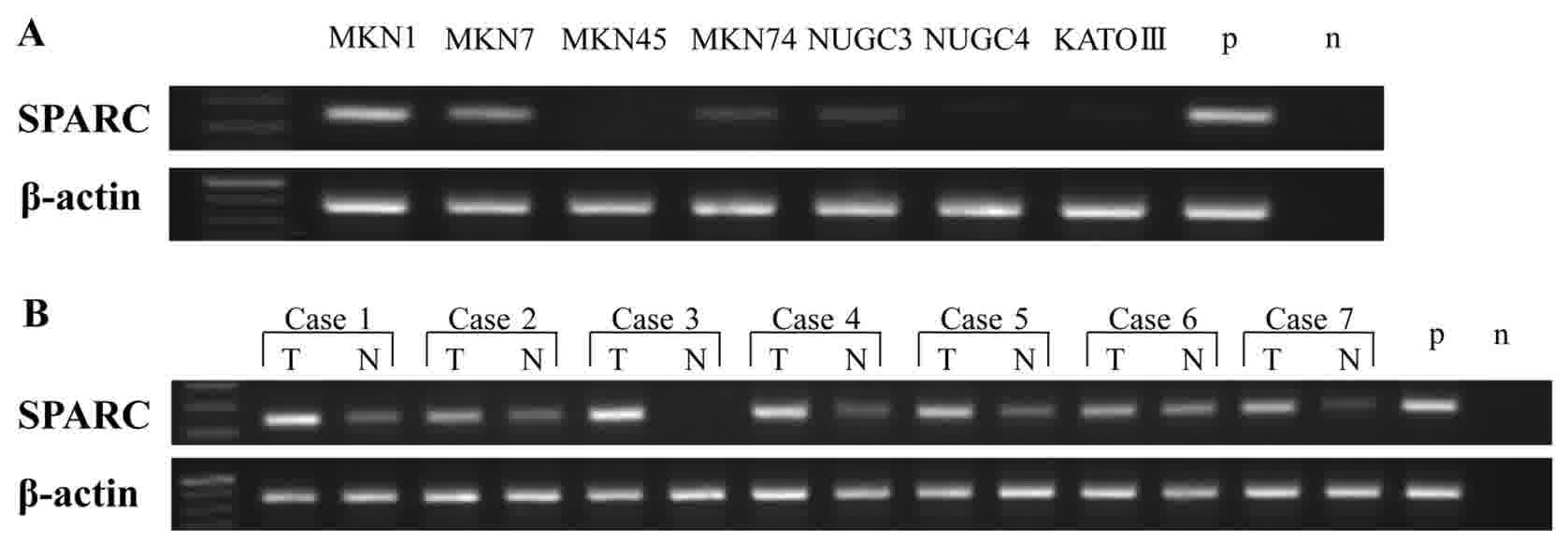
Expression of SPARC mRNA in human gastric cancer
cell lines and patient tissue samples was analyzed by RT-PCR. SPARC
mRNA was expressed in human gastric cancer MKN1, MKN7, MKN74 and
NUGC-3 cell lines (Fig. 4A), but its
expression varied from low to high depending on the cell line. MKN1
and MKN7 were highly expressed, but MKN74 and NUGC-3 had a low
expression. RT-PCR analysis of SPARC mRNA expression in gastric
cancer tissue samples and adjacent normal mucosa (n=7) revealed
that SPARC mRNA was expressed in both tissue types, but that
expression was higher in gastric cancer tissues than in adjacent
normal mucosa (Fig. 4B).
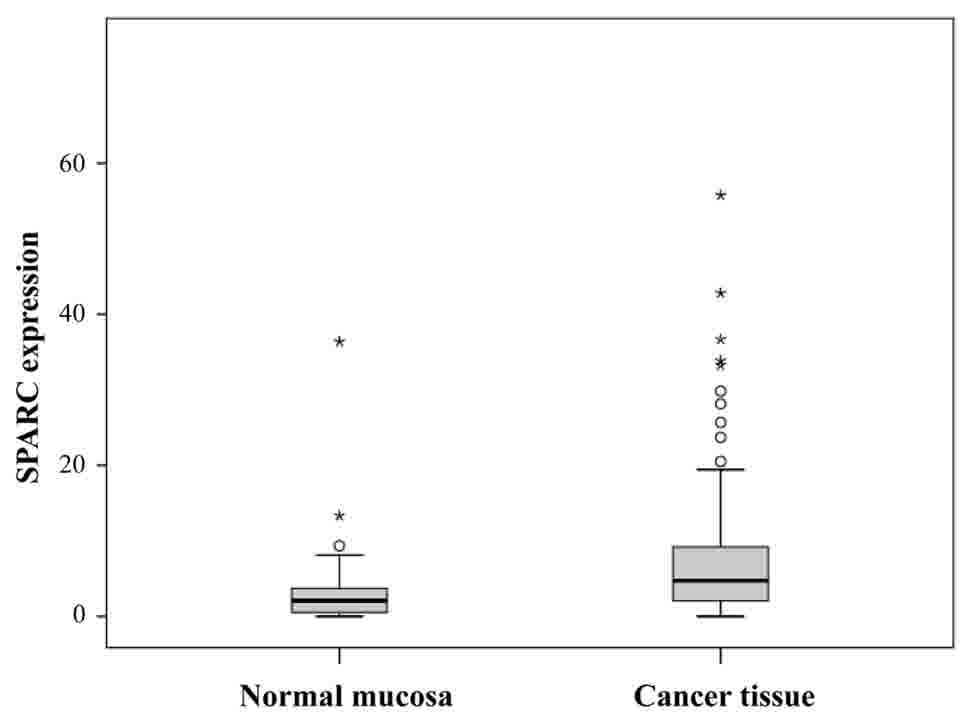
SPARC mRNA levels are higher in
gastric cancer tissues than in adjacent normal mucosa
SPARC mRNA expression in patient tissue samples by
was confirmed by RT-qPCR. SPARC mRNA levels were significantly
higher in cancer tissues than in normal adjacent mucosa (P=0.0012;
Fig. 5).
Univariate and multivariate analyses
of potential prognostic variables, SPARC gene expression and
postoperative patient outcomes
SPARC mRNA expression levels (P=0.0021) and TNM
stage (P=0.041) were associated with patient survival in univariate
analysis using a Cox proportional hazards model. Other variables,
including age, sex, tumor size, histological type, T factor, number
of lymph-node metastases, lymphatic invasion and venous invasion
were not statistically significant predictors of patient survival
(Table II). SPARC expression levels
were then categorized as low or high in multivariate analysis,
using the prognostic factors identified in univariate analysis,
with a Cox proportional hazards model (cut-off point, 7.101). A
2-fold cross-validation approach confirmed that high SPARC gene
expression was a significant predictor of poor survival in patients
with stage II/III gastric cancer (HR, 5.347; 95% CI 2.493–11.468;
P<0.0001; Table III).
 | Table II.Univariate analysis of potential
prognostic variables for overall survival. |
Table II.
Univariate analysis of potential
prognostic variables for overall survival.
|
| Univariate
analysis |
|
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
≥65 | 0.685 | 0.356–1.319 | 0.258 |
|
<64 |
|
|
|
| Sex |
|
|
|
|
Male | 0.676 | 0.318–1.438 | 0.310 |
|
Female |
|
|
|
| Histological
type |
|
|
|
|
Differentiated | 0.973 | 0.497–1.907 | 0.973 |
|
Undifferentiated |
|
|
|
| Tumor size, mm |
|
|
|
|
≥60 | 1.067 | 0.553–2.060 | 0.847 |
|
<60 |
|
|
|
| Depth of
invasion |
|
|
|
|
T1-T3 | 1.532 | 0.753–3.118 | 0.239 |
| T4 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
Absent | 2.726 | 0.655–11.356 | 0.168 |
|
Present |
|
|
|
| Number of lymph
node metastasis |
|
|
|
|
0–6 | 0.764 | 0.492–1.185 | 0.229 |
| ≥7 |
|
|
|
| Lymphatic
invasion |
|
|
|
|
Absent | 1.002 | 0.456–2.201 | 0.997 |
|
Present |
|
|
|
| Venous
invasion |
|
|
|
|
Absent | 1.566 | 0.685–3.580 | 0.288 |
|
Present |
|
|
|
| TNM stage |
|
|
|
| II | 2.501 | 1.039–6.019 | 0.041 |
|
III |
|
|
|
| SPARC
expression |
|
|
|
|
Continuous | 1.017 | 1.006–1.027 | 0.0021 |
| SPARC
expression |
|
|
|
|
High | 4.876 | 2.286–10.402 | <0.0001 |
|
Low |
|
|
|
 | Table III.Multivariate analysis of potential
prognostic variables for overall survival. |
Table III.
Multivariate analysis of potential
prognostic variables for overall survival.
|
| Multivariate
analysis |
|
|---|
|
|
|
|
|---|
| Variable | HR | 95%CI | P-value |
|---|
| TNM stage |
|
|
|
| II | 1.229 | 0.378–3.995 | 0.732 |
|
III |
|
|
|
| SPARC
expression |
|
|
|
|
High | 5.347 | 2.493–11.468 | <0.0001 |
|
Low |
|
|
|
Association between SPARC gene
expression and potential prognostic variables
Patient tissue samples were divided into two groups
[low expression group (n=73) and high expression group (n=61)]
according to their SPARC mRNA expression levels (cut-point, 7.101).
SPARC gene expression levels were not associated with any of the
potential prognostic variables analyzed in the present study
(Table IV).
 | Table IV.Association between SPARC gene
expression and potential prognostic variables. |
Table IV.
Association between SPARC gene
expression and potential prognostic variables.
|
| SPARC
expression |
|
|---|
|
|
|
|
|---|
| Variable | Low (n=73) | High (n=61) | P-value |
|---|
| Age, years |
|
|
|
|
≥65 | 43 | 35 | 0.499 |
|
<64 | 30 | 26 |
|
| Sex |
|
|
|
|
Male | 48 | 44 | 0.273 |
|
Female | 25 | 17 |
|
| Histological
type |
|
|
|
|
Differentiated | 28 | 25 | 0.447 |
|
Undifferentiated | 45 | 36 |
|
| Tumor size |
|
|
|
| ≥60
mm | 44 | 30 | 0.133 |
| <60
mm | 29 | 31 |
|
| Depth of
invasion |
|
|
|
|
T1-T3 | 29 | 24 | 0.553 |
| T4 | 44 | 37 |
|
| Lymph node
metastasis |
|
|
|
|
Absent | 8 | 8 | 0.451 |
|
Present | 65 | 53 |
|
| Number of lymph
node metastasis |
|
|
|
|
0–6 | 46 | 35 | 0.313 |
| ≥7 | 27 | 26 |
|
| Lymphatic
invasion |
|
|
|
|
Absent | 17 | 13 | 0.475 |
|
Present | 56 | 48 |
|
| Venous
invasion |
|
|
|
|
Absent | 22 | 11 | 0.077 |
|
Present | 51 | 50 |
|
| TNM stage |
|
|
|
| II | 22 | 18 | 0.545 |
|
III | 51 | 43 |
|
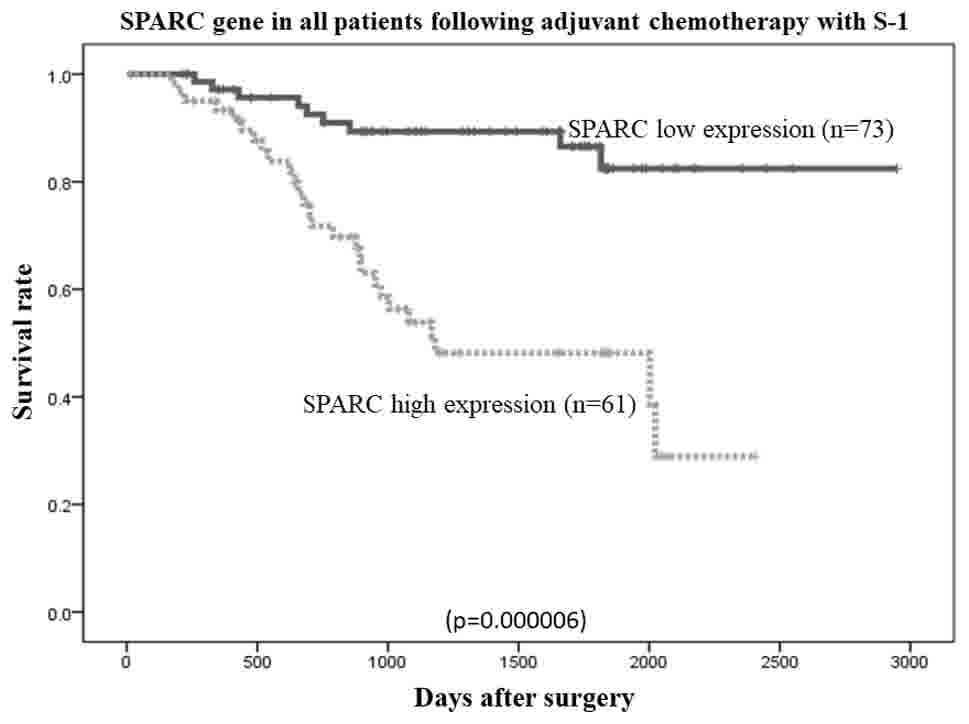
Survival curves of patients, ranked by
SPARC mRNA expression levels
The overall survival rates of patients were plotted
relative to the measured SPARC mRNA expression levels using the
Kaplan-Meier method. The median follow-up was 1,107 days. In the
study group (n=134 patients), the overall survival rate was lower
in patients with high SPARC mRNA expression than in those with low
SPARC mRNA expression (P=0.000006; Fig.
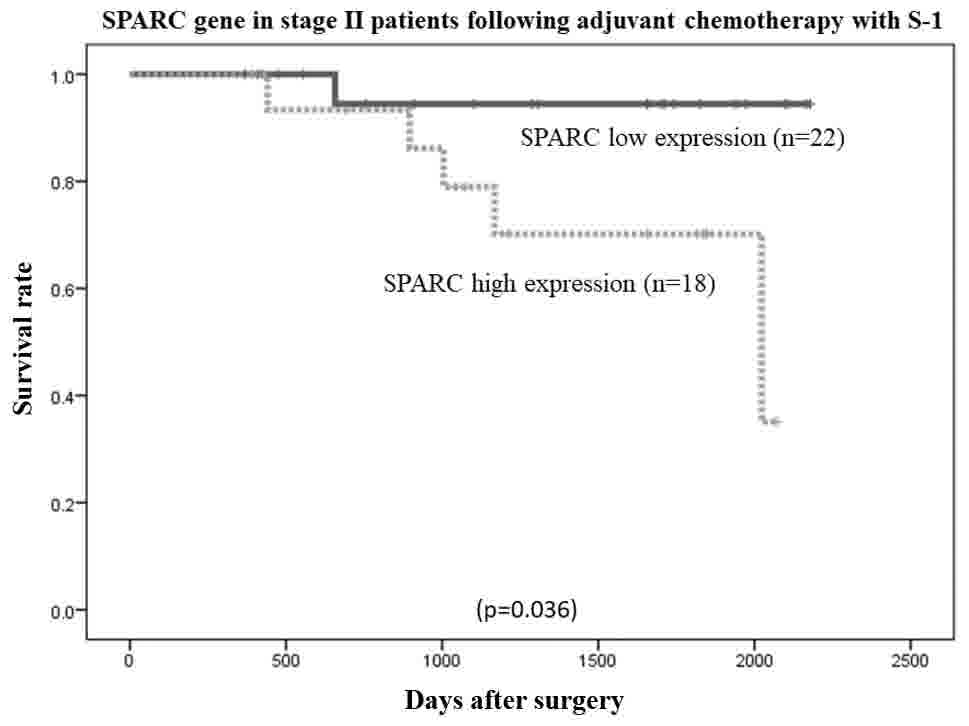
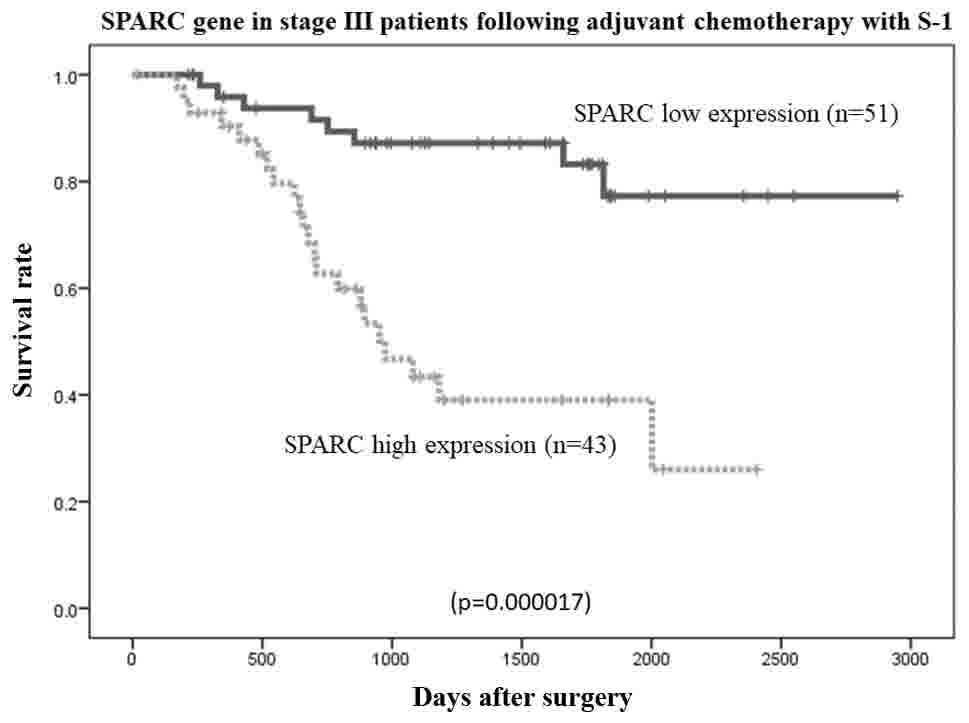
6). Among patients with stage II (n=40) and stage III (n=94)
cancer, the overall survival rate was lower in patients with high
SPARC mRNA expression than in those with low SPARC mRNA expression
(P=0.036 and P=0.0000017, respectively; Figs. 7 and 8,
respectively). However, no statistically significant differences in
the survival rates of patients in the reference group who were
diagnosed with stage II/III gastric cancer and had undergone
surgical resection, but had not received adjuvant chemotherapy with
S-1 (n=103), were observed between the low or high SPARC expression
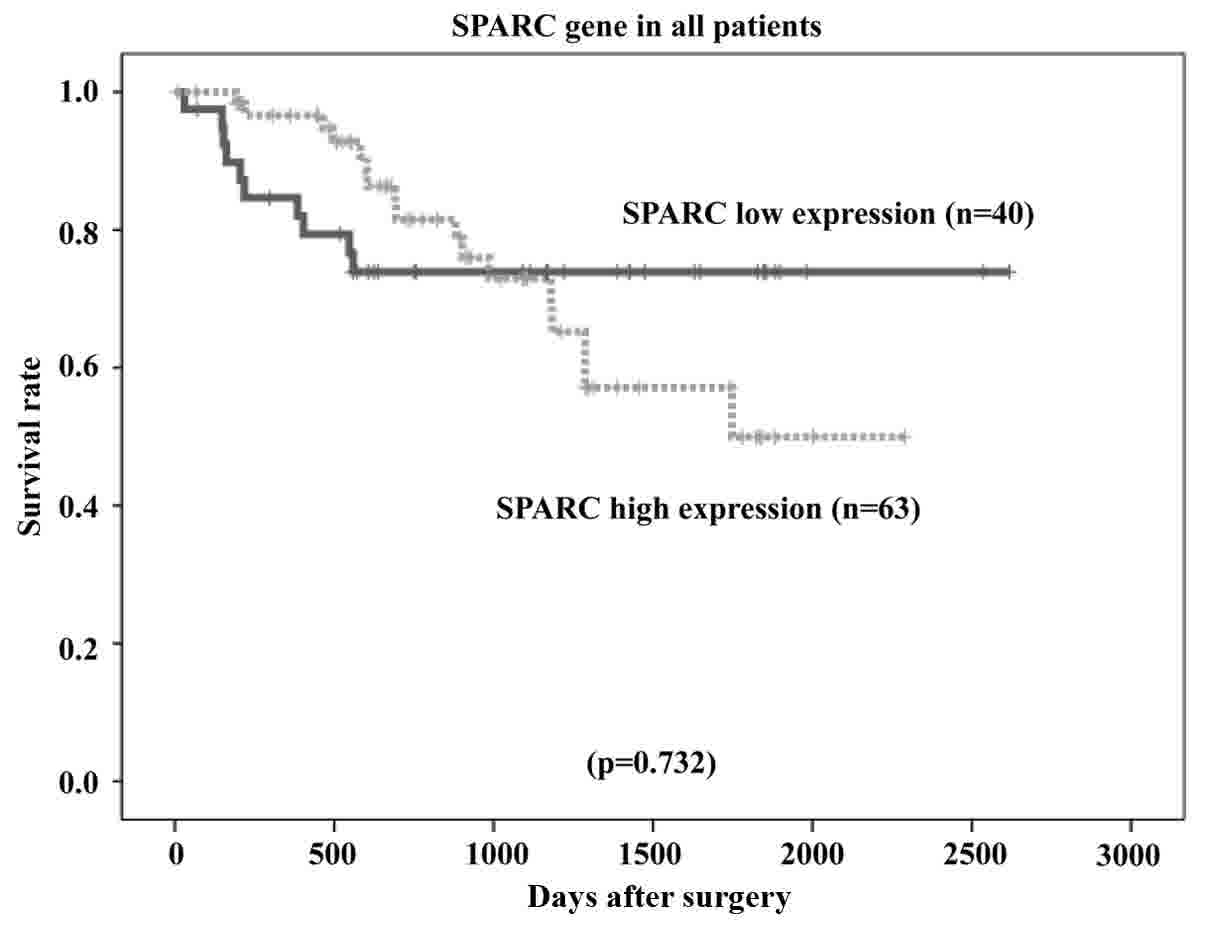
groups (P=0.732; Fig. 9).
Discussion
The results of the present study demonstrated that
SPARC mRNA expression levels were higher in gastric cancer
tissues than in adjacent normal mucosa, which is in line with the
results of previous studies (17,21).
In addition, through univariate and multivariate
analyses of potential prognostic factors using Cox proportional
hazards models, high SPARC expression was revealed to be a
significant predictor of poor survival in patients with stage
II/III gastric cancer, who had undergone curative surgical
resection and adjuvant chemotherapy with S-1. However, no
statistical differences in the overall survival rate of patients
with stage II/III cancer who had undergone curative resection but
had not received adjuvant chemotherapy with S-1 were observed
between patients with low or high SPARC expression. The
results of the present study are consistent with those of previous
studies reporting an association between high SPARC gene
expression and poor patient outcomes. Koukourakis et al
(22) reported that high SPARC
expression was associated with poor outcomes in patients with stage
I/II non-small cell lung cancer who had undergone surgical
resection. Infante et al (23)
reported that patients whose pancreatic cancer stroma expressed
SPARC exhibited a significantly poorer outcome than patients
whose tumor stroma did not express SPARC. Zhao et al
(16) also reported that high
SPARC expression was significantly associated with poorer
5-year survival rates for patients with all stages of gastric
cancer. Furthermore, Jeung et al (24) reported that high SPARC
expression was associated with early progressive disease (PD) and
poor survival in patients with unresectable gastric cancer who had
received combinatorial S-1 plus docetaxel chemotherapy.
Analysis of the potential association between
SPARC gene expression and various clinicopathological
features revealed no significant associations in patients with
stage II/III gastric cancer who had undergone surgical resection
followed by adjuvant chemotherapy with S-1. However, previous
studies have reported that high SPARC gene expression is
associated with the depth of tumor invasion of stage I–IV gastric
cancer (25), lymph node metastasis
in esophageal cancer (26), tumor
size, degree of differentiation, depth of invasion, vascular
invasion, lymph-node metastases, distant metastases and TNM stage
in gastric cancer (16,17).
The molecular mechanisms underlying the association
between SPARC gene expression and the outcomes of patients
with gastric cancer remain to be fully elucidated. McClung et
al (27) reported that
SPARC upregulated MT1-MMP expression and MMP2 activity in
SPARC-transfected clones of glioma cells. These MMPs induce
degradation of the extracellular matrix and promote cancer cell
invasion and metastasis, leading to poorer outcomes (28). In vitro, SPARC inhibits
apoptosis by interacting with integrin β1 heterodimers that enhance
integrin-linked kinase activation (29). Chemoresistance due to anti-apoptotic
activity of SPARC was suggested to be associated with poor
treatment prognosis in patients with unresectable gastric cancer.
Further studies are required to clarify whether high SPARC
levels may result in nonspecific chemoresistance.
Using coimmunoprecipitation experiments, Huynh et
al (30) demonstrated an
association between SPARC expression and tubulin in
Xenopus embryonic cell lysates, indicating a role for
SPARC in mitosis. Recently, SPARC has been suggested
to participate in the tumor response to taxanes, which stabilize
microtubules, thereby preventing tumor cell division. In previous
studies on patients with breast cancer, SPARC was selected
as a candidate biomarker of the response to docetaxel (24) and was suggested as a useful biomarker
of the effectiveness of nab-paclitaxel therapy (31,32).
The standard treatment for stage II/III gastric
cancer is curative resection and follow-up chemotherapy with
fluoropyrimidine anticancer agents, including S-1. According to
Japanese gastric cancer treatment guidelines (2014), second-line
chemotherapies, including paclitaxel and ramucirumab, are
recommended for the treatment of patients with recurring gastric
cancer (33). Although high
SPARC expression increases the probability of recurrence
following first-line treatment, it may explain the high therapeutic
effect of paclitaxel for the aforementioned reasons. Therefore,
SPARC may represent an important biomarker in designing a
treatment strategy for patients with recurring stage II/III gastric
cancer.
High SPARC gene expression is a significant
prognostic indicator of the outcomes of patients with stage II/III
gastric cancer following curative resection and adjuvant
chemotherapy with S-1.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YS and TO were responsible for the conception and
design of the study. TO and KY were responsible for the development
of methodology. TA, HC, MS, TY and YR performed the collection of
specimens, and YS, TO, KS, TI and MM performed analysis and
interpretation of the data.
Ethics approval and consent to
participate
Study protocols were approved by the Ethics
Committees of Yokohama City University Medical Center, Yokohama
City University (approval number: 18-7A-4) and Kanagawa Cancer
Center (approval number: epidemiological study-29) prior to the
initiation of the present study. Written informed consent was
obtained from each patient.
Consent for publication
Written informed consent was obtained from each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Termine JD, Kleinman HK, Whitson SW, Conn
KM, McGarvey ML and Martin GR: Osteonectin, a bone-specific protein
linking mineral to collagen. Cell. 26:99–105. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mann K, Deutzmann R, Paulsson M and Timpl
R: Solubilization of protein BM-40 from a basement membrane tumor
with chelating agents and evidence for its identity with
osteonectin and SPARC. FEBS Lett. 218:167–172. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagaraju GP and Sharma D: Anti-cancer role
of SPARC, an inhibitor of adipogenesis. Cancer Treat Rev.
37:559–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guweidhi A, Kleeff J, Adwan H, Giese NA,
Wente MN, Giese T, Büchler MW, Berger MR and Friess H: Osteonectin
influences growth and invasion of pancreatic cancer cells. Ann
Surg. 242:224–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bornstein P and Sage EH: Matricellular
proteins: Extracellular modulators of cell function. Curr Opin Cell
Biol. 14:608–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Podhajcer OL, Benedetti LG, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsiao YH, Lien HC, Hwa HL, Kuo WH, Chang
KJ and Hsieh FJ: SPARC (osteonectin) in breast tumors of different
histologic types and its role in the outcome of invasive ductal
carcinoma. Breast J. 16:305–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin/SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
12
|
Chan SK, Griffith OL, Tai IT and Jones SJ:
Meta-analysis of colorectal cancer gene expression profiling
studies identifies consistently reported candidate biomarkers.
Cancer Epidemiol Biomarkers Prev. 17:543–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franke K, Carl-McGrath S, Röhl FW,
Lendeckel U, Ebert MP, Tänzer M, Pross M and Röcken C: Differential
expression of SPARC in intestinal-type gastric cancer correlates
with tumor progression and nodal spread. Transl Oncol. 2:310–320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ledda F, Bravo AI, Adris S, Bover L,
Mordoh J and Podhajcer OL: The expression of the secreted protein
acidic and rich in cysteine (SPARC) is associated with the
neoplastic progression of human melanoma. J Invest Dermatol.
108:210–214. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rempel SA, Golembieski WA, Ge S, Lemke N,
Elisevich K, Mikkelsen T and Gutiérrez JA: SPARC: A signal of
astrocytic neoplastic transformation and reactive response in human
primary and xenograft gliomas. J Neuropathol Exp Neurol.
57:1112–1121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Yang M, Shan L, Qi L, Chai C, Zhou
Q, Yao K, Wu H and Sun W: The role of SPARC protein expression in
the progress of gastric cancer. Pathol Oncol Res. 18:697–702. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobin LH, Gospodarowicz MK and Wittekind
C: UICC International Union Against Cancer: TNM Classification of
Malignant Tumours. 7th edition. Wiley-Blackwell; Chichester, West
Sussex, UK: 2009
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazumdar M, Smith A and Bacik J: Methods
for categorizing a prognostic variable in a multivariable setting.
Stat Med. 22:559–571. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maeng HY, Song SB, Choi DK, Kim KE, Jeong
HY, Sakaki Y and Furihata C: Osteonectin-expressing cells in human
stomach cancer and their possible clinical significance. Cancer
Lett. 184:117–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koukourakis MI, Giatromanolaki A, Brekken
RA, Sivridis E, Gatter KC, Harris AL and Sage EH: Enhanced
expression of SPARC/osteonectin in the tumor-associated stroma of
non-small cell lung cancer is correlated with markers of
hypoxia/acidity and with poor prognosis of patients. Cancer Res.
63:5376–5380. 2003.PubMed/NCBI
|
|
23
|
Infante JR, Matsubayashi H, Sato N,
Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C and
Goggins M: Peritumoral fibroblast SPARC expression and patient
outcome with resectable pancreatic adenocarcinoma. J Clin Oncol.
25:319–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeung HC, Rha SY, Im CK, Shin SJ, Ahn JB,
Yang WI, Roh JK, Noh SH and Chung HC: A randomized phase 2 study of
docetaxel and S-1 versus docetaxel and cisplatin in advanced
gastric cancer with an evaluation of SPARC expression for
personalized therapy. Cancer. 117:2050–2057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato T, Oshima T, Yamamoto N, Yamada T,
Hasegawa S, Yukawa N, Numata K, Kunisaki C, Tanaka K, Shiozawa M,
et al: Clinical significance of SPARC gene expression in patients
with gastric cancer. J Surg Oncol. 108:364–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamashita K, Upadhay S, Mimori K, Inoue H
and Mori M: Clinical significance of secreted protein acidic and
rich in cystein in esophageal carcinoma and its relation to
carcinoma progression. Cancer. 97:2412–2419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McClung HM, Thomas SL, Osenkowski P, Toth
M, Menon P, Raz A, Fridman R and Rempel SA: SPARC upregulates
MT1-MMP expression, MMP-2 activation, and the secretion and
cleavage of galectin-3 in U87MG glioma cells. Neurosci Lett.
419:172–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weaver MS, Workman G and Sage EH: The
copper binding domain of SPARC mediates cell survival in vitro via
interaction with integrin beta1 and activation of integrin-linked
kinase. J Biol Chem. 283:22826–22837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huynh MH, Sodek K, Lee H and Ringuette M:
Interaction between SPARC and tubulin in Xenopus. Cell Tissue Res.
317:313–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desai NP, Trieu V, Hwang LY, Wu R,
Soon-Shiong P and Gradishar WJ: Improved effectiveness of
nanoparticle albumin-bound (nab) paclitaxel versus
polysorbate-based docetaxel in multiple xenografts as a function of
HER2 and SPARC status. Anticancer Drugs. 19:899–909. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gradishar WJ: Albumin-bound paclitaxel: A
next-generation taxane. Expert Opin Pharmacother. 7:1041–1053.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|