Introduction
Liposarcoma (LS) is the most common tumor among
sarcomas of the soft tissue (−20% of the tumors in adults)
(1). This neoplasm was first
described by Virchow (2) in 1857 and
has been well documented thereafter (3,4). LS is
categorized into four subgroups: atypical lipomatous tumor
(ALT)/well-differentiated liposarcoma (WDLS), myxoid liposarcoma,
pleomorphic liposarcoma, and dedifferentiated liposarcoma (DDLS)
(5). Among these, DDLS is defined as
a subtype of ALT/WDLS with non-lipogenic lesions (heterogenous
lesions in one tumor) (5). DDLS has a
high degree of malignancy; hence, its recurrence and metastasis
rates are higher than those of other types of LS (6,7). DDLS can
develop anywhere in the body; however, the head and neck (H&N)
is a relatively rare site of occurrence of this lesion (7,8). The
pathological features of DDLS are well defined (5,9). Here we
report the case of a 69-year-old male patient with DDLS of the oral
floor. It was difficult to determine the diagnosis clinically.
Furthermore, to date, no definite protocol has been established for
the diagnosis and treatment of H&N DDLS.
Case study
Written informed consent was obtained from the
patient for the publication of this case report and accompanying
images. The report was submitted for ethical review to the Ethics
Committee of the University of the Ryukyus (Okinawa, Japan), which
waived the requirement for review per institutional protocol
because the study does not contain content that requires ethical
approval. The Ethics Committee approved the submission and
publication of the manuscript.
A 69-year-old man presented to the Department of
Oral and Maxillofacial Surgery at Ryukyu University Hospital. He
had noticed a slow-growing mass in his mouth and experienced
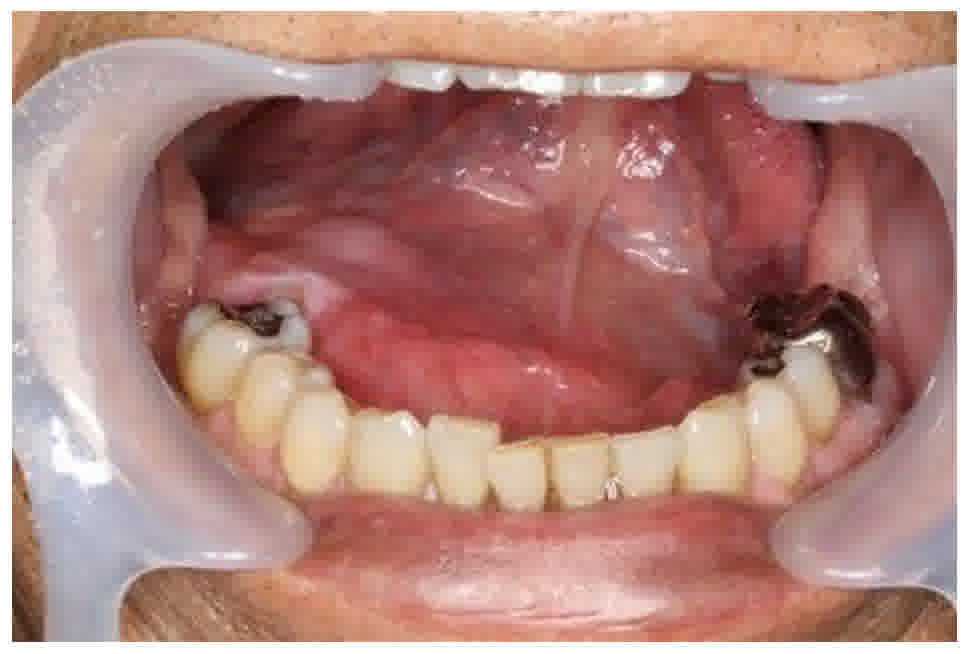
difficulty in talking for approximately 1 year. Physical
examination revealed a painless, smooth, and non-tender (firm) mass
at the floor of the mouth (Fig. 1).
The mass was covered by an intact mucosa. The Wharton duct was not
involved by the mass, and clear saliva could be expressed from the
sublingual gland duct. The patient's facial appearance was
symmetrical, and there was no cervical lymphadenopathy. He had a
history of alcohol consumption and was a current smoker, with no
history of malignancy. The patient was being treated for diabetes
mellitus. His brother had a history of colorectal cancer.
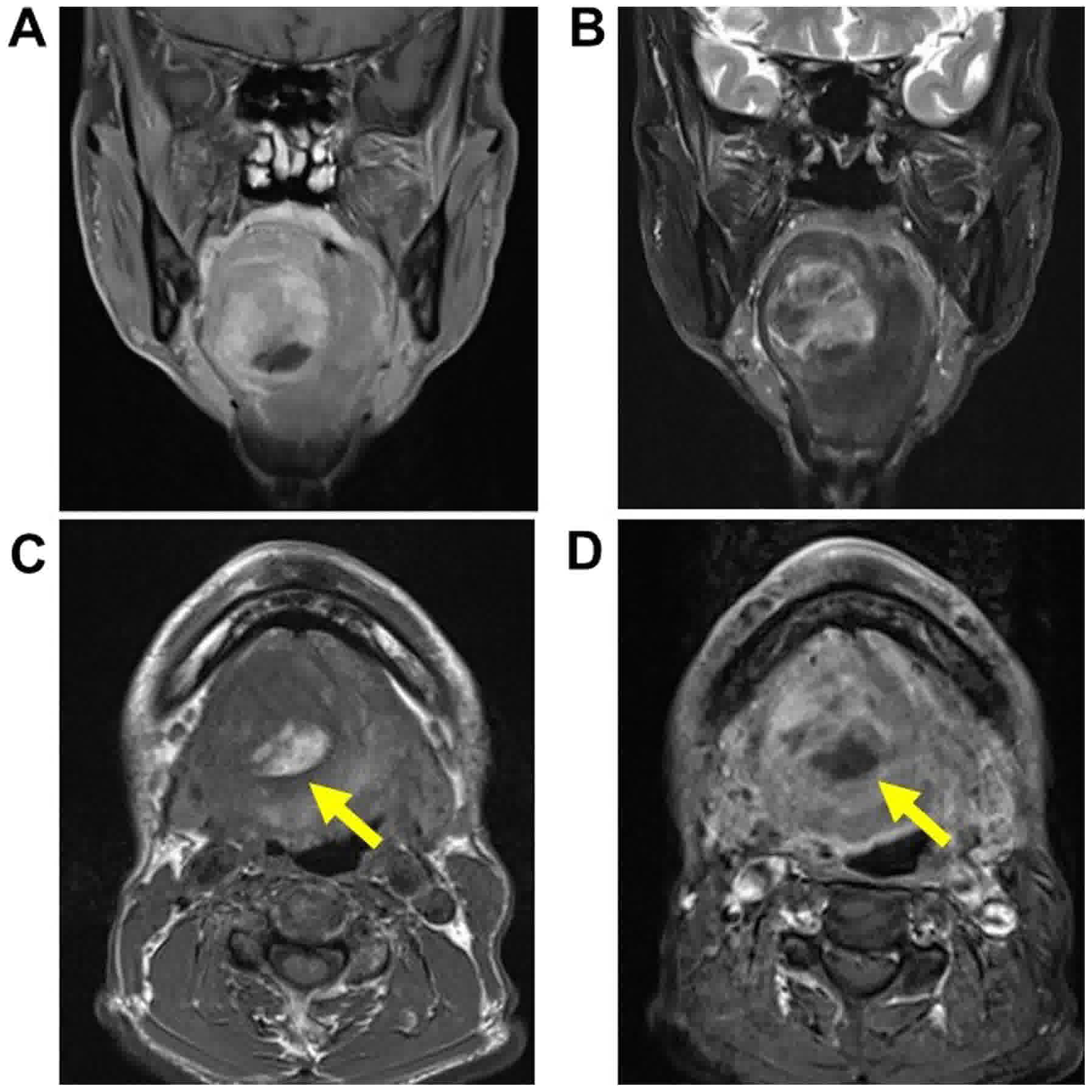
Contrast-enhanced computed tomography (CT) demonstrated a large
heterogenous mass under the tongue that seemed to push the
hyoglossus muscles, but no invasive lesion was present. The margins
of the lesion were well defined. The adipose-like section of the
mass was partially suspected. No other lesions were detected in the
H&N, bones, and lungs. Contrast-enhanced magnetic resonance
imaging (MRI) demonstrated a 50×39×43 mm lesion that pushed the
hyoglossus muscle into the sublingual space and seemed to contain
heterogeneous components (Fig. 2).
Most of the mass revealed low-signals in T1-weighted image and
high-signals in T2-image. On the other hand, at the bottom of the
mass, fat signals were partially detected. No other lesion was
present. Based on the findings, the oral floor lesion was
considered a tumor or cyst; however, an apparent clinical diagnosis
could not be made. Moreover, performing biopsy for an oral floor is
difficult (10). Therefore, we
planned for surgical resection and accurate pathological
examination.
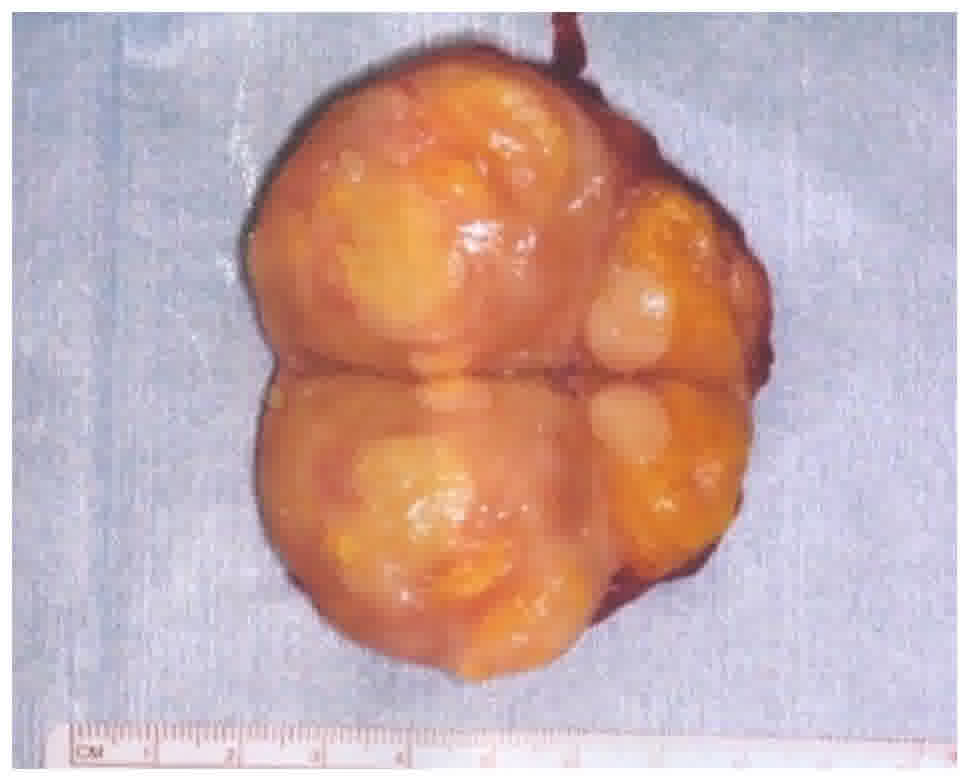
The patient underwent surgical resection of the mass
under general anesthesia. The mass had no adhesions to the
surrounding tissue. The excised specimen was a 60×45×45 mm
capsulated mass. The resected mass showed two areas: A pale yellow
(fatty) area and milky-white (non-fatty) area; however, no cystic
lesion was found (Fig. 3).
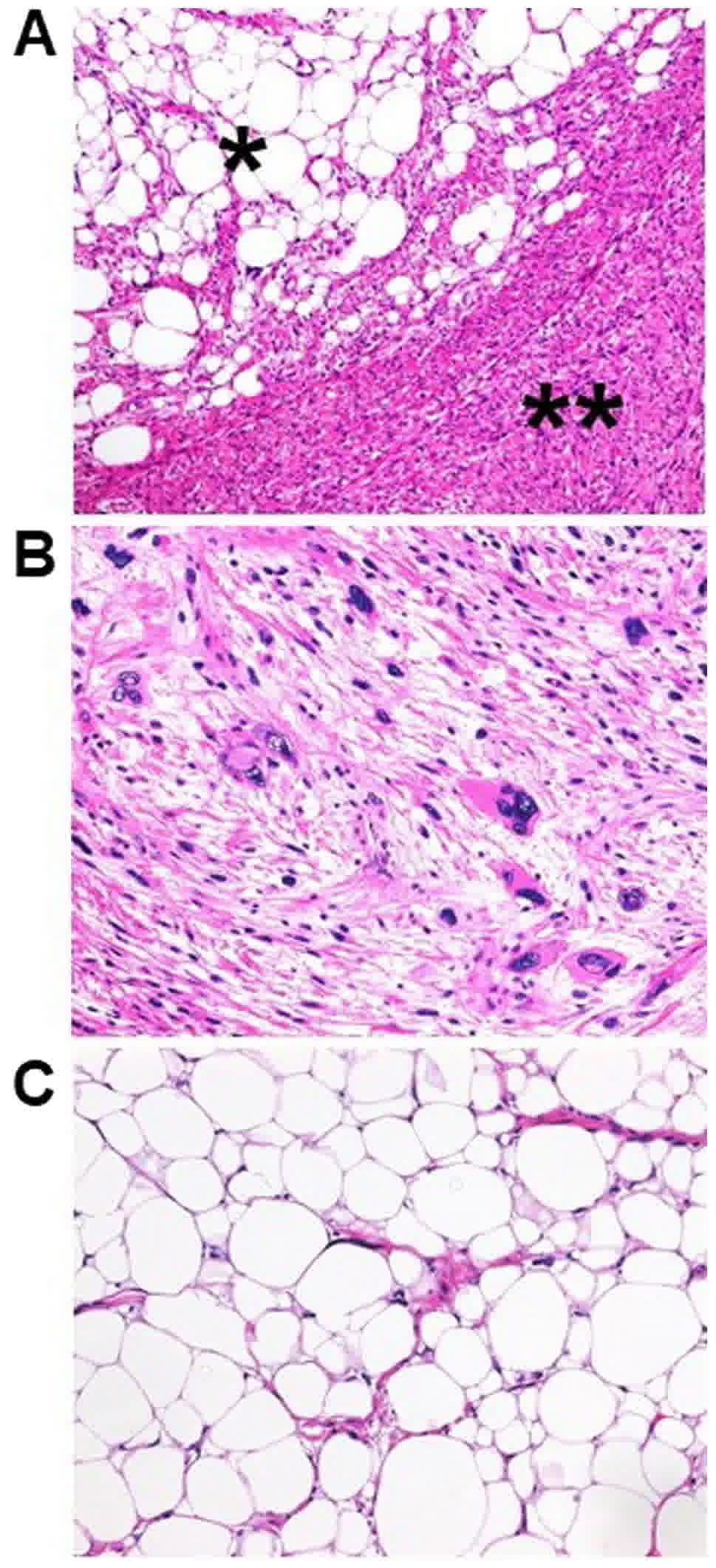
Histopathological examination also revealed two distinct areas, but
the findings were contrasting (Fig.
4A): i) The milky-white area contained a dedifferentiated area
which was composed of spindle cell and pleomorphic cell with patchy
necrosis. Spindle cell showed a fascicular architecture with
hyperchromatic plump nuclei and eosinophilic cytoplasm. Bizarre
multinucleate giant cells were occasionally seen (Fig. 4B); ii) the yellow area was a
well-differentiated area, which demonstrated adipocytic
proliferation with hyperchromatic stromal cells (Fig. 4C). The two areas mostly transitioned
abruptly, and partly transitioned gradually. Immunohistochemical
examination revealed positive results for S-100 in the adipocytic
cells, whereas it revealed partial positive results for SMA, desmin
and CDK4, but negative for caldesmon or MDM2 in the
dedifferentiated component. Based on the findings, DDLS (FNCLCC
system grade 2) was diagnosed. The tumor was clinically resected;
however, histological surgical margin was positive. Therefore,
postoperative radiotherapy (RT) (total 60 Gy) was performed to
treat the residual tumor and to prevent the recurrence or
metastasis of the disease (3,11). At 5 years 8 months postoperatively, no
sign of local recurrence or distant metastasis of DDLS had been
found, until the time of writing this report. However, pleomorphic
LS of the chest wall was detected after 5 years 2 months
postoperatively. The patient was treated and followed up at another
hospital (Fukuoka University Hospital, Fukuoka, Japan) to this
writing. Histologically, atypical spindle-shaped cells, bizarre
giant cells, and lipoblast-like cells were revealed in the chest
wall tumor. These cells were negative for MDM2 or CDK4. Further, no
DDLS component was observed. Therefore, the chest wall tumor was
considered a second primary tumor rather than a metastasis of
DDLS.
Discussion
To the best of our knowledge, this is the first
documented case of oral floor DDLS. Furthermore, our case is the
first to exhibit the development of a second primary malignancy
(SPM) after the treatment of H&N DDLS. We searched the English
literature for H&N DDLS cases that occurred between 1979 and
2017, using Pubmed and Google Scholar. The exclusion criteria were
i) cases from non-English literature, ii) cases in which DDLS
metastasis from non-H&N regions was apparent (12,13) and
iii) a case in which it could not be determined whether the lesion
was a DDLS or WDLS (14). We
identified 50 cases [excluding the cases in Tirumani et al
(12) study, where the number of
cases was stated as ‘not applicable’ (NA)], which are listed in
Table I (7,11,12,15–42).
However, no patient had oral floor DDLS. This list includes 2 cases
of tongue DDLS (19,22), but clinical information regarding
these cases was sparse. Therefore, we could not confirm whether the
DDLSs involved the oral floor in these cases. As described in
Table I, DDLS has been reported to
develop at various sites in the H&N region (7,11,12,15–42). Among
these, the most common site was the larynx (6 patients), followed
by the cheek (5 patients), neck (3 patients), orbit (3 patients),
pyriform sinus (3 patients), buccal area (2 patients), tongue (2
patients), parotid gland, pharyngeal space, posterior neck,
paralaryngeal area, nose, maxillary gingiva, and oral floor
(current case), i.e., anywhere in the H&N region (Table I). The mean age was 58.78±17.27 years
(range, 20–86 years), with a male/female ratio of 1.8:1. Most of
the patients (except for the NA case) underwent contrast-enhanced
CT or MRI for initial staging; however, no patient underwent
positron emission tomography (PET) for initial staging (date not
shown in Table I). Some patients
underwent PET as an additional detection test after the first
surgery (31) or as follow-up of
radical surgery (37,42). For H&N DDLS, the outcomes are
reportedly good with wide surgical excision (11). No patients underwent preoperative
therapy, but 12 patients (including our case) underwent
postoperative RT. No patient underwent postoperative chemotherapy,
but one patient underwent therapy for the recurrence of the tumor
(15). Given the sparse clinical
details, the present literature review was unable to report any
conclusions regarding treatment suggestions. Of 24 patients (except
for the NA case), 3 (12.5%) reported recurrence and 1 (our case)
developed SPM (4.2%); no patient with regional recurrence or
distant metastasis was identified. However, case reports with
long-term follow-up are limited. Of the 20 patients whose follow-up
duration was reported, only 6 (30%) and 8 (40%) patients were
followed up for >5 and 2 years, respectively. Meanwhile, cases
of recurrence after 23 years of follow-up (16) and six recurrences over 26 years of
follow-up (22) have been reported.
Our case exhibited no recurrence or metastasis during 5 years of
follow-up; however, SPM (pleomorphic LS of the chest wall)
developed at 5 years after the H&N DDLS resection. We could not
determine why the current patient developed SPM because there have
been no reports of SPM in H&N DDLS cases to date. Lupo et
al (43), reported on the
statistical analysis of 8,785 sarcoma (at all regions of the body,
including H&N) survivors diagnosed between 1992 and 2012 from
the Surveillance, Epidemiology, and End Results database, using
standardized incidence ratios. Among these, LS survivors (257
patients) had a relatively high SPM risk; however, there were no
details regarding the DDLS survivors (30 patients) (43). To date, reports of SPMs in DDLS (at
all regions of the body) cases are sparse (44). Therefore, our case indicates the
possibility of SPM developing not only in the H&N region but
also at all DDLS sites. According to the size of oral region LSs,
lesions of >5.0 or >3.6 cm were reported as prognostic
factors for recurrence, metastasis, or death (22,45). We
researched the relationship between the size of H&N DDLS
lesions and recurrence; however, no definitive data were found
because of the sparsity of clinical information.
 | Table I.DDLSs in the head and neck
region. |
Table I.
DDLSs in the head and neck
region.
| First author | Year | Age | Gender | Site | Size (cm) | Type of on
biopsy | Histological
diagnosis based on biopsy findings | Grade and
histological type of DDLS | Postoperative
RT | Follow-up data | (Refs.) |
|---|
| Tobey | 1979 | 61 | M | Larynx | NA | (+) | LS | (−) | (−) | Approximately 6
months; . recurrence and mortality | (15) |
| McCormick | 1994 | 62 | M | Larynx | NA | NA | NA | NA | NA | 23 years;
recurrence | (16) |
| Henricks | 1997 | NA | NA | H&N | NA | NA | NA | NA | NA | NA | (17) |
| Henricks | 1997 | NA | NA | Larynx | NA | NA | NA | NA | NA | NA | (17) |
| Henricks | 1997 | NA | NA | Buccal | NA | NA | NA | NA | NA | NA | (17) |
| Cai | 2001 | 54 | F | Orbit | >2 | NA | NA | (−) | NA | NA | (18) |
| Nascimento | 2002 | 83 | F | Tongue | 2.5 | NA | NA | NA | NA | NA | (19) |
| Diamond | 2002 | 57 | M | Cheek | NA | (+) | Suggestive of
neurofibroma | (−) | (+): 66 Gy | 12 months; NED | (20) |
| Gonzalez-Lois | 2002 | 69 | M | Pyriform sinus | >3 | (+) | Lipoma | (−) | (−) | 6 months; NED | (21) |
| Fanburg-Smith | 2002 | 39 | M | Tongue | 6 | NA | NA | Low-grade | NA | 6 years; NED | (22) |
| Fanburg-Smith | 2002 | 56 | M | Buccal
(mucosa) | 5 | NA | NA | High-grade, focal
myxoid features | NA | 26 years; 6
recurrences, but alive | (22) |
| Fanburg-Smith | 2002 | 67 | F | Parotid grand | 5.5 | NA | NA | High-grade | NA | 17 years; NED | (22) |
| Roza | 2004 | 61 | M | Cheek | 7 | (−) | (−) | (−) | (+) | Lost to
follow-up | (23) |
| Cunha | 2005 | 42 | F | Cheek | 6 | (−) | (−) | (−) | (+) | 1 year; NED | (24) |
| Angiero | 2006 | 62 | M | Cheek | 3 | Incisional | LS | NA | (−) | 7 years; NED | (25) |
| Giordano | 2006 | 50 | M | Pyriform sinus | 5 | (−) | (−) | Low-grade | (−) | 6 months;, NED | (26) |
| Powitzky | 2007 | 63 | M | Larynx | 4.5 | (+) | Myxoid LS | High-grade, with
myxomatous degeneration and clement rhabdomyosarcoma | (+): 70.2 Gy | 16 months; NED | (11) |
| Saeed | 2007 | 56 | F | Orbit | NA | (+) | DDLS grade 2 | Grade 2 | (+): 60 Gy | NED | (27) |
| Rogers | 2010 | 83 | M | Pharyngeal
space | 8.6 | FNA | No evidence of
malignancy | NA | (+): 64 Gy | 19 months; NED | (28) |
| Gritli | 2010 | NA | NA | Neck | NA | NA | NA | NA | (+) | NED | (29) |
| Endo | 2010 | 48 | M | Neck | 5 | (−) | (−) | Low-grade | (−) | 1 year; NED | (30) |
| Makeieff | 2010 | 62 | F | Larynx | 8 | (+) | A possible
gastrointestinal stromal tumor (malignant) | NA | (+) | NED | (31) |
| Stomeo | 2012 | 76 | M | Cheek | 12+10 | Incisional | Lipomatous
lesion | NA | (Refused by the
patient) | 2 years; death with
NED | (32) |
| Zhang | 2011 | 23 | F | Orbit | NA | (−) | (−) | NA | (+) | 16 months; NED | (33) |
| Blumberg | 2012 | 65 | M | Paratracheal | 4.7 | FNA | Failure | Low-grade, with
meningothelial-like whorling | (−) | NED | (34) |
| Wang | 2012 | 20 | F | Neck | 5 | (−) | (−) | With an
osteosarcomatous component | (−) | 5 months; NED | (35) |
| Zreik | 2015 | 86 | M | Posterior neck | 9.3 | US guided FNA | Suggestive of
DDLS | NA | (+) | 4 months; NED | (36) |
| Gerry | 2014 | NA | NA | H&N (number of
cases, 16) | NA | NA | NA | NA | NA | NA | (7) |
| Petersson | 2014 | 61 | F | Paralaryngeal | 6 | CT guided | Deceptively mild
histopathological features (benign) | Suggestive of a
partially benign dedifferentiated component | (+) | The case was
reported during postoperative RT | (37) |
| Jour | 2015 | NA | NA | Larynx | NA | NA | NA | NA | NA | NA | (38) |
| Tirumani | 2015 | NA | NA | H&N (number of
cases, NA) | NA | NA | NA | NA | NA | NA | (12) |
| Saâda-Bouzid | 2015 | 63 | M | Nose | NA | NA | NA | NA | NA | NA | (39) |
| Ishii | 2016 | NA | NA | H&N (number of
cases, 2) | NA | NA | NA | NA | NA | NA | (40) |
| Riva | 2016 | 81 | M | Pyriform sinus | 21 | (−) | (−) | Grade 2 according
to FNCLCC | (−) | 1 year; NED | (41) |
| Enomoto | 2017 | 28 | F | Maxillary
gingiva | NA | (+) | DDLS | Grade 3 according
to FNCLCC | (−) | 30 months; NED | (42) |
| Current case | / | 70 | M | Oral floor | 6 | (−) | (−) | Grade 2 according
to FNCLCC | (+): 60 Gy | 5 years; second
primary cancer of the chest wall (pleomorphic LS) |
|
So far, no accurate protocol for DDLS (in all
regions of the body, including H&N) management has been
established (5,9). For both LS of the whole body and
H&N, surgical resection is the standard treatment (7). However, the effects of pre- and
postoperative therapy have been inaccurately reported so far
(38). DDLS is a rare condition, and
experimental DDLS models are lacking, leading to a delay in the
development of suitable therapeutic strategies (46). Furthermore, DDLS may have
site-specific characteristics. Henricks et al (17), studied 155 DDLS cases and concluded
that retroperitoneal DDLS has a significantly worse prognosis than
does DDLS at other sites. However, reports of H&N DDLS cases
remain sparse because this is a relatively rare site for this tumor
(7,37). Therefore, the accumulation of H&N
DDLS cases with detailed clinical information and long-term
follow-up is needed to establish a novel therapeutic protocol. We
speculate that hidden H&N DDLS cases of recurrence, metastasis,
or SPM exist.
Another important issue highlighted in this study is
that biopsy (either incisional biopsy or fine needle aspiration) is
not reliable for the diagnosis of DDLS. Table I shows that biopsy results have
reported in 13 cases; however, DDLS was diagnosed in only 3 cases
(23.1%). Even worse, 6 cases (46.2%) were misdiagnosed as benign
lesions (5 cases) or ‘failures’ (1 case). DDLS generally involves
heterogeneous lesions and occasionally presents as kinds of lesions
(11,34,35,37).
Petersson and Murugasu (37),
reported a case of a unique DDLS lesion with a partly deceptively
benign-appearing dedifferentiated component, leading to the
misdiagnosis of DDLS on biopsy. Some studies have confirmed that
WDLS and DDLS belong to the same group (14,47,48)
because DDLS is well defined as a disease caused by progression
from WDLS to a high- or low-grade lesion (34,38).
Importantly, DDLS has a poorer 5-year disease-specific and overall
survival rates compared with WDLS (7). Therefore, accurate pathological
diagnosis with total resection is preferred to clearly distinguish
DDLS from other LSs.
In conclusion, the current patient was the first
documented case of oral floor DDLS. Furthermore, our case was the
first reported case of SPM development after the treatment of
H&N DDLS. After the first DDLS description in 1979 (49), the present study detected 50 cases of
H&N DDLS. Our literature review indicated that preoperative
biopsy is not reliable for the diagnosis of H&N DDLS, and
accurate pathological diagnosis with total resection is preferred.
Statistical analyses could not be performed, due to the small
number of patients and sparse clinical information. Therefore,
additional cases with long-term follow-up and well-described
clinical information are needed to develop new protocols for
H&N DDLS patients.
Acknowledgements
The authors would like to thank Professor Masaki
Fujita, Dr Akira Nakao, Dr Hisako Kushima (Department of
Respiratory Medicine, Fukuoka University Hospital, Fukuoka, Japan)
and Dr Asahi Nagata (Departments of General Thoracic Breast and
Pediatric Surgery, Fukuoka University Hospital, Fukuoka, Japan) who
contributed to clinical patient diagnosis and treatment of
pleomorphic LS of the chest wall.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FN and TM acquired the data, performed the
literature review and edited the manuscript. AA made substantial
contributions to the concept and design of the study. TN, AM, NM
and KN acquired the data and gave clinical advice. HM, NM and AA
revised the manuscript. HM and NY evaluated the specimens and gave
histopathological advice. TM was a major contributor in writing the
manuscript.
Ethics approval and consent to
participate
The report was submitted for ethical review to the
Ethics Committee of the University of the Ryukyus (Okinawa, Japan),
which waived the requirement for a review, since the study does not
contain any protocols requiring ethical approval. The Ethics
Committee approved the submission and publication of the
manuscript.
Consent for publication
Written informed consent was obtained from the
patient for the publication of this case report, including their
clinical data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DDLS
|
dedifferentiated liposarcoma
|
|
LS
|
liposarcoma
|
|
ALT
|
atypical lipomatous tumor
|
|
WDLS
|
well-differentiated liposarcoma
|
|
H&N
|
head and neck
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
RT
|
radiotherapy
|
|
SPM
|
second primary malignancy
|
|
NA
|
not applicable
|
|
PET
|
positron emission tomography
|
References
|
1
|
Crago AM, Socci ND, DeCarolis P, O'Connor
R, Taylor BS, Qin LX, Antonescu CR and Singer S: Copy number losses
define subgroups of dedifferentiated liposarcoma with poor
prognosis and genomic instability. Clin Cancer Res. 18:1334–1340.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Virchow R: A case of malignant occurring
in part in the form of fat neuroma tumors. Virchows Arch Pathol
Anat. 11:281–288. 1857.(In Deutsch). View Article : Google Scholar
|
|
3
|
Zagars GK, Goswitz MS and Pollack A:
Liposarcoma: Outcome and prognostic factors following conservation
surgery and radiation therapy. Int J Radiat Oncol Biol Phys.
36:311–319. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brennan MF, Antonescu CR, Alektiar KM and
Maki RG: Liposarcoma. Management of soft tissue sarcoma. Springer
Int Publ. PP105–124. 2016.
|
|
5
|
Dei Tos AP, Marino-Enriquez A, Pedeutour F
and Rossi S: Dedifferentiated Liposarcoma. WHO Classification of
Tumours of Soft Tissue and Bone. Fletcher C, Bridge J, Hogendoorn P
and Mertens F: 4th. Lyon, IARC Press; pp. PP37–38. 2013
|
|
6
|
Darouichi M, Garibotto V, Christen B, Roud
AF, Pazera A, Renggli JC, Willi JP, Ratib O and Qanadli S: FDG
PET-CT in detection of diaphragmatic metastasis of dedifferentiated
liposarcoma: A case report. Eur J Radiol Extra. 77:e35–e38. 2011.
View Article : Google Scholar
|
|
7
|
Gerry D, Fox NF, Spruill LS and Lentsch
EJ: Liposarcoma of the head and neck: Analysis of 318 cases with
comparison to non-head and neck sites. Head Neck. 36:393–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mariño-Enríquez A, Hornick JL, Dal Cin P,
Cibas ES and Qian X: Dedifferentiated liposarcoma and pleomorphic
liposarcoma: A comparative study of cytomorphology and MDM2/CDK4
expression on fine-needle aspiration. Cancer Cytopathol.
122:128–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldblum J, Weiss S and Folpe AL:
Liposarcoma. In: Enzinger and Weiss's soft tissue tumors. 6th.
Elsevier Saunders; Philadelphia, PA: pp. 484–523. 2013
|
|
10
|
Ariji Y, Gotoh M, Naitoh M, Izumi M,
Shimozato K, Kurita K, Maeda H and Ariji E: Magnetic resonance
imaging assessment of tumorous lesions in the floor of the mouth:
Case reports and review of the literature. Oral Radiol. 22:182006.
View Article : Google Scholar
|
|
11
|
Powitzky R, Powitzky ES and Garcia R:
Liposarcoma of the larynx. Ann Otol Rhinol Laryngol. 116:418–424.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tirumani SH, Tirumani H, Jagannathan JP,
Shinagare AB, Hornick JL, Ramaiya NH and Wagner AJ: Metastasis in
dedifferentiated liposarcoma: Predictors and outcome in 148
patients. Eur J Surg Oncol. 41:899–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McElderry J, McKenney JK and Stack BC:
High-grade liposarcoma metastatic to the gingival mucosa: Case
report and literature review. Am J Otolaryngol. 29:130–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sioletic S, Dal Cin P, Fletcher CD and
Hornick JL: Well-differentiated and dedifferentiated liposarcomas
with prominent myxoid stroma: Analysis of 56 cases. Histopathology.
62:287–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tobey DN, Wheelis RF and Yarington CT Jr:
Electron microscopy in the diagnosis of liposarcoma and
fibrosarcoma of the larynx. Ann Otol Rhinol Laryngol. 88:867–871.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCormick D, Mentzel T, Beham A and
Fletcher CD: Dedifferentiated liposarcoma. Clinicopathologic
analysis of 32 cases suggesting a better prognostic subgroup among
pleomorphic sarcomas. Am J Surg Pathol. 18:1213–1223. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henricks WH, Chu YC, Goldblum JR and Weiss
SW: Dedifferentiated liposarcoma: A clinicopathological analysis of
155 cases with a proposal for an expanded definition of
dedifferentiation. Am J Surg Pathol. 21:271–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai YC, McMenamin ME, Rose G, Sandy CJ,
Cree IA and Fletcher CD: Primary liposarcoma of the orbit: A
clinicopathologic study of seven cases. Ann Diagn Pathol.
5:255–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nascimento AF, McMenamin ME and Fletcher
CD: Liposarcomas/atypical lipomatous tumors of the oral cavity: A
clinicopathologic study of 23 cases. Ann Diagn Pathol. 6:83–93.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diamond C, Prince ME, Covert AA and Morris
SF: Dedifferentiated liposarcoma of the cheek: Case report and
literature review. J Otolaryngol. 31:125–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
González-Lois C, Ibarrola C, Ballestín C
and Martánez-Tello FJ: Dedifferentiated liposarcoma of the pyriform
sinus: Report of a case and review of the literature. Int J Surg
Pathol. 10:75–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fanburg-Smith JC, Furlong MA and Childers
EL: Liposarcoma of the oral and salivary gland region: A
clinicopathologic study of 18 cases with emphasis on specific
sites, morphologic subtypes, and clinical outcome. Mod Pathol.
15:1020–1031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de la Roza G, Baredes S and Aisner SC:
Dedifferentiated liposarcoma of the cheek. Ann Diagn Pathol.
8:352–357. 2004. View Article : Google Scholar
|
|
24
|
da Cunha IW, Kowalski LP and Soares FA:
Dedifferentiated liposarcoma of the oral cavity with
angiosarcomatous dedifferentiation. Virchows Arch. 446:456–459.
2005. View Article : Google Scholar
|
|
25
|
Angiero F, Sidoni A and Stefani M:
Liposarcoma of the oral cavity-case reports of the pleomorphic and
the dedifferentiated variants and a review of the literature.
Anticancer Res. 26:4857–4867. 2006.PubMed/NCBI
|
|
26
|
Giordano G, Corcione L, Letizia G,
Mercante G and Ferri T: Dedifferentiated liposarcoma of the
pyriform sinus. Oral Oncol Extra. 42:176–180. 2006. View Article : Google Scholar
|
|
27
|
Saeed MU, Chang BY, Atherley C, Khandwala
M, Merchant DW and Liddington M: A rare diagnosis of
dedifferentiated liposarcoma of the orbit. Orbit. 26:43–45. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rogers J, Patil Y, Strickland-Marmol L and
Padhya T: Lipomatous tumors of the parapharyngeal space: Case
series and literature review. Arch Otolaryngol Head Neck Surg.
136:621–624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gritli S, Khamassi K, Lachkhem A, Touati
S, Chorfa A, Ben Makhlouf T, El May A and Gammoudi A: Head and neck
liposarcomas: A 32 years experience. Auris Nasus Larynx.
37:347–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Endo M, Oda Y, Harimaya K, Tamiya S,
Yamamoto H, Kohashi K, Kurihara S, Setsu N, Matsuura S, Matono H,
et al: Low-grade dedifferentiated liposarcoma of the neck: Magnetic
resonance imaging and pathological correlation. J Orthop Sci.
15:148–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Makeieff M, Pelliccia P, Poizat F, Arnaud
S, Rat F, Cupissol D, Guerrier B and Costes V: Laryngeal
dedifferentiated liposarcoma. Eur Arch Otorhinolaryngol.
267:991–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stomeo F, Bianchini C, Ciorba A, Padovani
D, Pedriali M, Pelucchi S and Pastore A: Giant dedifferentiated
liposarcoma of the right hemifacial area involving the oral cavity.
Gerodontology. 29:e1152–e1156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang JX, Ma JM and Wang NL:
Dedifferentiated Orbital liposarcoma: A case report. Int J
Ophthalmol. 4:452–453. 2011.PubMed/NCBI
|
|
34
|
Blumberg JM, Jedrych J, Costa J and Judson
B: Cervical dedifferentiated liposarcoma with meningothelial-like
whorling. Head Neck Pathol. 6:476–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y and Shi H: Dedifferentiated
liposarcoma of the neck: CT findings. AJNR Am J Neuroradiol.
33:E4–E6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zreik R, Soyalp K, Ruiz S, Ward R, Dobin
S, Chen X, Liu L and Rao A: Ultrasound-guided fine-needle
aspiration of a posterior neck dedifferentiated liposarcoma with
MDM2 fluorescence in situ hybridization performed on a Pap-stained
smear. Diagn Cytopathol. 43:320–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petersson F and Murugasu E:
Dedifferentiated liposarcoma of the deep (paralaryngeal) soft
tissue: Lessons learnt from a case with a partly deceptively benign
appearing dedifferentiated component. Head Neck Pathol. 8:171–177.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jour G, Gullet A, Liu M and Hoch BL:
Prognostic relevance of Fédération Nationale des centres de lutte
contre le cancer grade and MDM2 amplification levels in
dedifferentiated liposarcoma: A study of 50 cases. Mod Pathol.
28:37–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saâda-Bouzid E, Burel-Vandenbos F,
Ranchère-Vince D, Birtwisle-Peyrottes I, Chetaille B, Bouvier C,
Château MC, Peoc'h M, Battistella M, Bazin A, et al: Prognostic
value of HMGA2, CDK4, and JUN amplification in well-differentiated
and dedifferentiated liposarcomas. Mod Pathol. 28:1404–1414. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishii T, Kohashi K, Iura K, Maekawa A,
Bekki H, Yamada Y, Yamamoto H, Nabeshima K, Kawashima H, Iwamoto Y
and Oda Y: Activation of the Akt-mTOR and MAPK pathways in
dedifferentiated liposarcomas. Tumour Biol. 37:4767–4776. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Riva G, Sensini M, Corvino A, Vittone F,
Garzaro M and Pecorari G: Rare giant pedunculated liposarcoma of
the hypopharynx: Case report and review of literature. J
Gastrointest Cancer. 47:449–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Enomoto A, Matsunaga K, Shimoide T, Mukai
T, Uchihashi T and Hamada S: Dedifferentiated liposarcoma in the
maxillary gingiva: A clinical report and review of the literature.
J Oral Maxillofac Surg Med Pathol. 29:542–545. 2017. View Article : Google Scholar
|
|
43
|
Lupo PJ, Brown AL and Hettmer S: Second
malignancy risk among pediatric, adolescent, and young adult
survivors of fusion-positive and fusion-negative sarcomas: Results
from the SEER database, 1992 through 2012. Cancer. Aug 2–2016.(Epub
ahead of print). View Article : Google Scholar
|
|
44
|
Özcan B, Çevener M, Yildiz A, Özdoğan M
and Erdoğan O: Case report on the coincidence of retroperitoneal
dedifferentiated giant liposarcoma and renal papillary cell
carcinoma. Marmara Med J. 30:50–53. 2017. View Article : Google Scholar
|
|
45
|
Cheng J, Yu H, Wang L, Wang X and Shen G:
Primary oral and maxillofacial liposarcoma: A clinicopathological
and immunohistochemical study of eleven cases. Arch Med Sci.
8:316–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li H, Wozniak A, Sciot R, Cornillie J,
Wellens J, Van Looy T, Vanleeuw U, Stas M, Hompes D, Debiec-Rychter
M and Schöffski P: Pazopanib, a receptor tyrosine kinase inhibitor,
suppresses tumor growth through angiogenesis in dedifferentiated
liposarcoma xenograft models. Transl Oncol. 7:665–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ray-Coquard I, Blay JY, Italiano A, Le
Cesne A, Penel N, Zhi J, Heil F, Rueger R, Graves B, Ding M, et al:
Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients
with MDM2-amplified, well-differentiated or dedifferentiated
liposarcoma: An exploratory proof-of-mechanism study. Lancet Oncol.
13:1133–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Louis-Brennetot C, Coindre JM, Ferreira C,
Pérot G, Terrier P and Aurias A: The CDKN2A/CDKN2B/CDK4/CCND1
pathway is pivotal in well-differentiated and dedifferentiated
liposarcoma oncogenesis: An analysis of 104 tumors. Genes
Chromosomes Cancer. 50:896–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Evans HL, Soule EH and Winkelmann RK:
Atypical lipoma, atypical intramuscular lipoma, and well
differentiated retroperitoneal liposarcoma: A reappraisal of 30
cases formerly classified as well differentiated liposarcoma.
Cancer. 43:574–584. 1979. View Article : Google Scholar : PubMed/NCBI
|