Introduction
Breast cancer is the most common cause for
cancer-associated mortality among women (1), and the treatment of breast cancer
continues to present a difficult clinical challenge. Although
radiotherapy with surgery may significantly prolong the survival
period of patients with breast cancer, the 10-year loco-regional
recurrence rate remains 38% for women <40 years; the reasons for
this have yet to be established. The existence of loco-regional
recurrence was associated with a worse distant disease-free
survival and with a worse overall survival compared with those who
did not (2). At present, the majority
of patients with breast cancer in early stages are treated with
breast conserving surgery followed by radiation therapy, with
appropriate adjuvant systemic therapy. However, it remains a
challenge for clinicians to predict the response to radiotherapy,
and there is a demand to develop novel strategies to reduce tumor
radioresistance. Therefore, an improved understanding of the
molecular characteristics associated with intrinsic radioresistance
is required. The tumor microenvironment has been increasingly
recognized to serve an important role in the pathogenesis of breast
cancer, in which stromal cells may produce extracellular matrix
(ECM) components and growth factors to promote cancer cell
activation, reprogramming and survival (3).
The ECM protein periostin (POSTN) is a bone adhesion
molecule that regulates osteoblast adhesion and differentiation
that may be upregulated in a wide range of cancer types, including
colon, pancreatic, breast, gastric and non-small cell lung cancer,
and neuroblastoma (4,5). POSTN is required for cancer stem cell
maintenance and may act as a critical regulator of tumorigenesis
and progression (6,7). It has been reported that the
overexpression of POSTN is associated with metastasis and
chemotherapy resistance by interacting with integrin receptors, as
well as with other signals, particularly via the phosphoinositide
3-kinase/AKT pathway (8,9).
A previous study demonstrated that POSTN protein
expression was increased in CD44+/CD24−
breast cancer stem cells compared with control cells, which was
associated with cancer stem cell chemotherapy resistance (10). In the serum of patients with
early-stage breast cancer, POSTN expression could be detected prior
to surgery, and increased baseline serum POSTN levels were
predictive of worse long-term survival outcomes in specific
subgroups of patients (11). However,
the relevance of POSTN expression status to clinical implications,
particularly the response to radiotherapy for the management of
breast cancer, is poorly understood.
In the present study, a tissue microarray (TMA) with
tissue from 259 tumors from patients with breast cancer was used to
evaluate the prognostic value of POSTN, including its association
with clinical outcomes, and therefore, radiotherapy response. These
data may provide clinical evidence of a method for predicting the
tumor response to radiotherapy in patients with breast cancer and
optimizing therapeutic strategies against radioresistance.
Materials and methods
Patient characteristics and
histological review
Subsequent to obtaining approval from the
institutional review board of Qingdao University School of Medicine
Ethics Committee, 259 patients with early stage breast cancer were
recruited to the study between January 2010 to January 2011 at the
Department of Breast Surgery, Qingdao Central Hospital, the Second
Affiliated Hospital of Qingdao University (Qingdao, China). All
patients provided written informed consent prior to their inclusion
in the study. Information about the patients' clinical history was
obtained from the patient records database of Qingdao Central
Hospital.
The age of each patient was defined at diagnosis.
The size of the primary tumor was considered to be the largest
tumor diameter reported by a pathologist subsequent to surgical
excision. Patients with early stage breast cancer were staged as I
and II based on the tumor-node-metastases (TNM) classification of
the International Union against Cancer, revised in 2010 (12). The histological grade of the tumors
was classified according to the World Health Organization criteria
(13). The lymph node status was
determined by the presence of histological evidence for lymph node
metastasis. All patients in the present study were treated with
breast conserving surgery with or without axillary lymph node
dissection. Following surgery, the patients received standard whole
breast irradiation at the radiation oncology facilities of the
Department of Therapeutic Radiology, Qingdao Central Hospital, the
Second Affiliated Hospital of Qingdao University. The median total
dose was 50 Gy to the whole breast. Adjuvant systemic chemotherapy
and/or adjuvant hormone therapy were administered as clinically
indicated in accordance with standard practice during the time of
the study. Local recurrence-free survival (RFS), distant
metastasis-free survival (DFS) and overall survival (OS) were the
endpoints evaluated.
TMA construction
Formalin-fixed, paraffin-embedded tissues from the
Department of Pathology, Qingdao Central Hospital were used in the
construction of the breast cancer sample TMA. Briefly,
representative areas of breast tumor samples were selected from
whole tissue sections by pathologists. A cylindrical specimen core
of 2-mm diameter was extracted from these regions and precisely
arrayed into a new recipient paraffin block using a custom-built
precision instrument (Beecher Instruments; Estigen OÜ, Tartu,
Estonia) as previously described (14). The resulting TMA blocks were cut to
4-µm thickness with a microtome and placed on slides with an
adhesive tape-transfer method (InstruMedics; Stryker Corporation,
Kalamazoo, MI, USA). One section from each TMA was stained with
hematoxylin and eosin (in hematoxylin for up to 2 min and eosin for
30 sec at room temperature) to verify the presence of tumor cells.
The TMA was assessed for the staining intensity, and the percentage
of stained cells in the nucleus, cytoplasm or membrane.
Immunohistochemical study
Immunohistochemical analysis was performed on the
4-µm thick paraffin-embedded tissue sections of the TMA using a
standard streptavidin-peroxidase method. The subsequent levels of
tissue sections were stained with Ventana Benchmark XT Autostainer
according to the manufacturer's instructions (Ventana Medical
Systems, Inc., Tucson, AZ, USA). The slides were incubated with a
rabbit anti-human primary antibody against POSTN (dilution 1:500,
cat. no. sc-67233; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), ER (cat. no. G07268), PR (cat. no. Y12992), HER2 (cat. no.
Y08422) and Ki-67 (cat. no. Y13569) (all from Roche Diagnostics,
Indianapolis, IN, USA) for 28–32 min at 37°C. The primary
antibodies for ER, PR, HER2, Ki-67 and the goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (Ultraview
Universal DAB detection kit; cat. no. 05269806001; Roche
Diagnostics) were ready-to-use for 8 min at 37°C. A known positive
case was stained as a positive control. The primary antibody was
replaced with nonimmune mouse serum (Santa Cruz Biotechnology,
Inc.) as a negative control. Immunohistochemical staining was
developed in a Dako Autostainer Plus using an LSAB detection kit
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). The
individuals performing immunohistochemical evaluation were blinded
to the clinical information. The staining results for all markers
were first determined independently, then reviewed together at a
multi-head microscope. According to the criteria for
immunohistochemical evaluation reported previously (10), the presence of 1% of positively
stained neoplastic cells was defined as positive POSTN
expression.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
(IBM Corp., Armonk, NY, USA). The association of POSTN expression
with categorical clinicopathological parameters was assessed by
χ2 tests. Estimates of RFS, DFS and OS were calculated
by the Kaplan-Meier product-limit method, and the differences were
assessed by the log-rank test. Multivariate analysis was performed
using the Cox proportional hazards regression model to determine
the prognostic effect on the independent contribution of each
variable to survival; hazard ratios (HRs) and their corresponding
95% confidence intervals (CIs) were calculated. All Р-values are
two-sided; Р<0.05 was considered to indicate a statistically
significant difference.
Results
Immunostaining results of POSTN in
breast cancer tissues
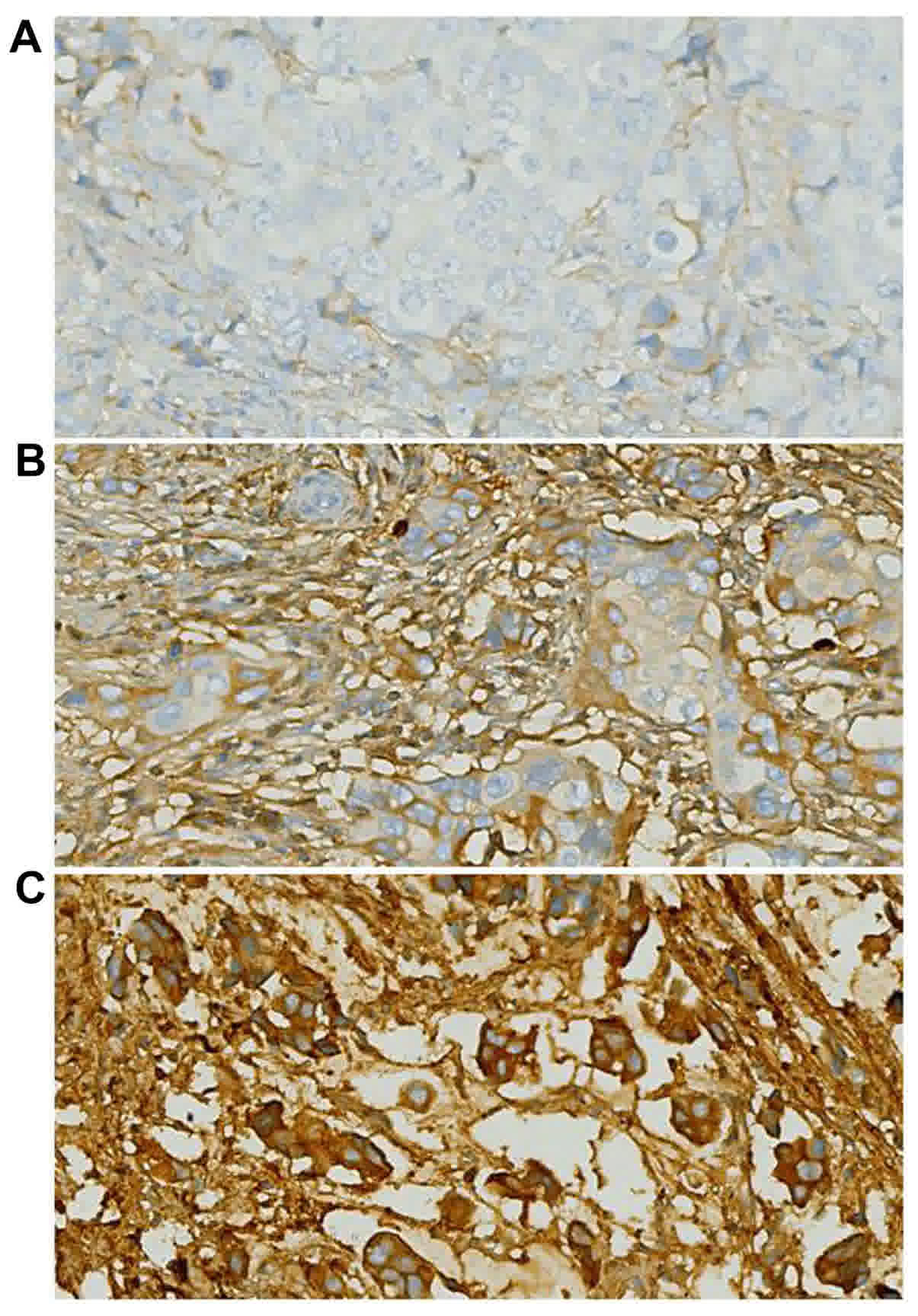
The intracellular localization of POSTN protein
staining was examined, as its location may affect the mechanism of
action and potentially, the response to treatment. In the breast
cancer tissue samples, there was a heterogeneous distribution of
POSTN in the cytoplasm. Nuclear POSTN expression was relatively
infrequent. Representative POSTN immunostaining results are
included in Fig. 1.
Descriptive statistics
The clinical and pathological variables of the
patients are included in Table I. The
median age at diagnosis for all patients was 53 years (range,
27–93), with 44.4% of the patients (n=115) <50 years at the time
of diagnosis. A total of 45% (n=117) of the patients presented with
lymph node metastases (N1 or 2) at the time of surgery. Of the 259
patients, 27% had the Luminal A molecular subtype, 33% Luminal B,
13% HER2-overexpressed and 25% triple-negative; the remaining
patients were uncategorized.
 | Table I.Clinicopathological features of 259
patients with breast cancer, and the association with the
expression of POSTN. |
Table I.
Clinicopathological features of 259
patients with breast cancer, and the association with the
expression of POSTN.
|
|
| POSTN expression,
n |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | n (%) | Negative | Positive | P-value |
|---|
| Total | 259 | 168 | 91 |
|
| Age (years) |
|
|
| 0.248 |
| ≤50 | 115 (44.4) | 79 | 36 |
|
|
>50 | 144 (55.6) | 89 | 55 |
|
| Tumor size |
|
|
| 0.123 |
| T1 | 142 (54.8) | 98 | 44 |
|
| T2 | 117 (45.2) | 70 | 47 |
|
| Histological
grade |
|
|
| 0.001a |
| I | 29 (11.2) | 26 | 3 |
|
| II | 160 (61.8) | 106 | 54 |
|
| III | 70 (27.0) | 36 | 34 |
|
| Nodal status |
|
|
| 0.023a |
|
Negative | 146 (56.4) | 99 | 47 |
|
|
Positive | 113 (43.6) | 61 | 52 |
|
| Molecular
subtype |
|
|
|
<0.001a |
| Luminal
A | 71 (27.4) | 59 | 12 |
|
| Luminal
B | 84 (32.4) | 55 | 29 |
|
|
HER2-overexpressed | 34 (13.1) | 22 | 12 |
|
|
Triple-negative | 65 (25.1) | 28 | 37 |
|
|
Unclassified | 5 (1.9) | 4 | 1 |
|
| Estrogen receptor
status |
|
|
|
<0.001a |
|
Negative | 106 (40.9) | 54 | 52 |
|
|
Positive | 153 (59.1) | 114 | 39 |
|
| Progesterone
receptor status |
|
|
|
<0.001a |
|
Negative | 124 (47.9) | 67 | 57 |
|
|
Positive | 135 (52.1) | 101 | 34 |
|
| HER2 status |
|
|
| 0.897 |
|
Negative | 178 (68.7) | 115 | 63 |
|
|
Positive | 81 (31.3) | 53 | 28 |
|
| Ki-67
expression |
|
|
| 0.011a |
|
Low | 104 (40.2) | 77 | 27 |
|
|
High | 155 (59.8) | 91 | 64 |
|
| Local
recurrence |
|
|
| 0.034a |
| No | 242 (93.4) | 161 | 81 |
|
|
Yes | 17 (6.6) | 7 | 10 |
|
| Postoperative
distant metastasis |
|
|
| 0.002a |
|
Negative | 227 (87.6) | 155 | 72 |
|
|
Positive | 32 (12.4) | 13 | 19 |
|
| Survival
status |
|
|
| 0.001a |
|
Alive | 230 (88.8) | 157 | 73 |
|
|
Deceased | 29 (11.2) | 11 | 18 |
|
As of February 2015, the median follow-up time was
51 months. Of all the patients in the study, 7% (n=17) of patients
experienced local relapse, 12% (n=32) experienced distant
metastasis and 11% (n=29) succumbed to the disease.
POSTN expression in breast cancer and
its association with clinicopathological characteristics
Clinicopathological characteristics and molecular
features were compared with the POSTN expression status for the
patients in the cohort of the present study. The results are
summarized in Table I. The POSTN
status was significantly associated with the histological grade
(P<0.01), nodal status (P<0.05), molecular subtype
(P<0.01), estrogen receptor (ER) status (P<0.01),
progesterone receptor (PR) status (P<0.01) and Ki-67 expression
(P<0.05). No significant association between POSTN expression
and other parameters could be established.
Association between POSTN and
postoperative local relapse and distant metastasis
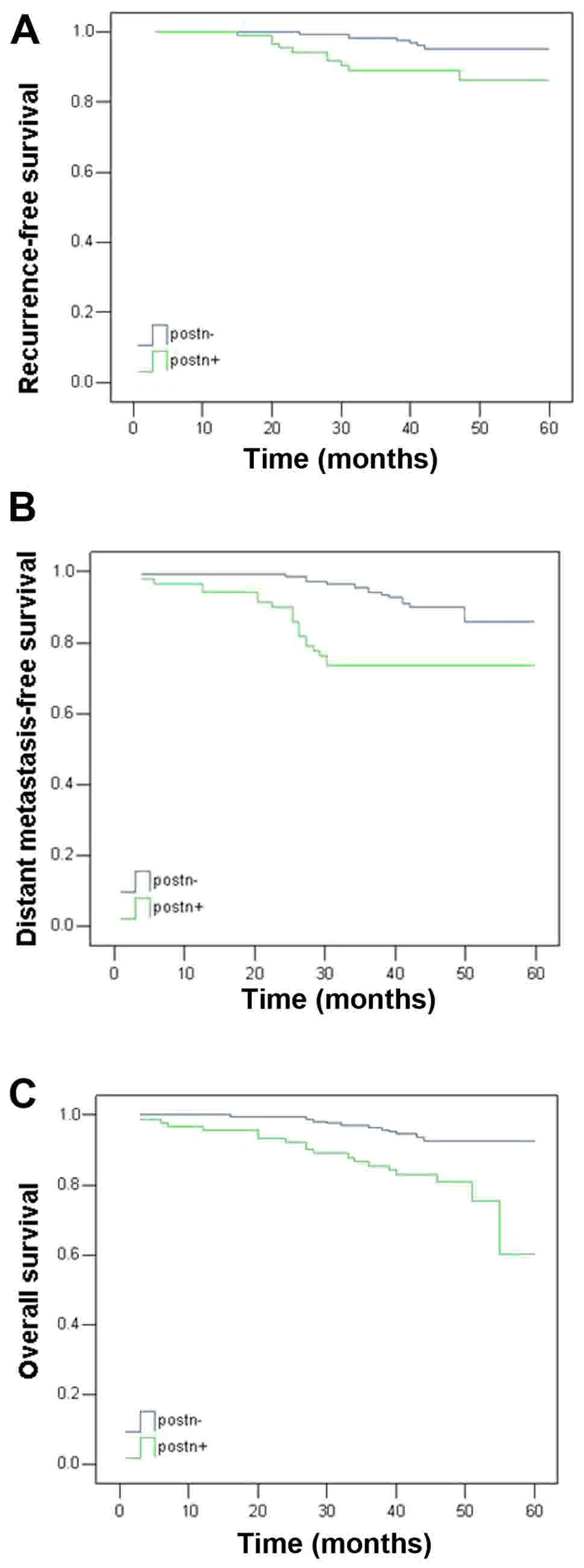
Five-year survival analysis was performed for the
259 patients, considering the RFS, DFS and OS rates. As summarized
in Table II and illustrated in
Fig. 2, tumors with positive POSTN
expression were associated with poorer outcomes for all endpoints,
including RFS (P<0.05), DFS (P<0.01) and OS (P<0.01).
 | Table II.Five-year outcomes as a function of
POSTN expression. |
Table II.
Five-year outcomes as a function of
POSTN expression.
|
| Patients with POSTN
status (%) |
|
|---|
|
|
|
|
|---|
| Clinical
outcome | Negative | Positive | P-value |
|---|
| Local
recurrence-free survival | 95.8 | 89.0 | 0.017 |
| Distant
metastasis-free survival | 92.3 | 79.1 | 0.001 |
| Overall
survival | 93.5 | 80.2 | 0.001 |
Prognostic analysis of POSTN
expression in breast cancer
Multivariate analysis with the Cox proportional
hazards model was performed using the following variables: POSTN
expression, age, tumor size, histological grade, nodal status,
disease subtype, ER, PR and HER2 status, and Ki-67 expression
(Table III). Positive POSTN
expression was associated with a relatively poor outcome for all
endpoints. A larger tumor size, the tumor subtype and high Ki-67
expression were associated with reduced RFS time (P=0.007, P=0.005
and P=0.012, respectively). Positive nodal status was also
associated with a reduced DFS and OS time (P=0.005 and P=0.003,
respectively). A higher histological grade and high Ki-67
expression were further associated with a reduced OS time (P=0.043
and P=0.016, respectively).
 | Table III.Multivariate analysis of the
association of prognostic factors with five-year outcomes. |
Table III.
Multivariate analysis of the
association of prognostic factors with five-year outcomes.
|
| Recurrence-free
survival | Distant
metastasis-free survival | Overall
survival |
|---|
|
|
|
|
|
|---|
|
|
| 95% CI |
|
| 95% CI |
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value |
|---|
| Periostin
expression | 3.450 | 1.240 | 9.603 | 0.018a | 2.305 | 1.111 | 4.781 | 0.025a | 2.298 | 1.013 | 5.213 | 0.047a |
| Age | 0.992 | 0.952 | 1.035 | 0.721 | 1.014 | 0.986 | 1.042 | 0.339 | 1.027 | 0.999 | 1.056 | 0.061 |
| Tumor size | 5.569 | 1.616 | 19.194 | 0.007a | 1.244 | 0.588 | 2.630 | 0.569 | 0.935 | 0.414 | 2.112 | 0.872 |
| Histological
grade | 1.128 | 0.378 | 3.366 | 0.829 | 1.535 | 0.723 | 3.259 | 0.265 | 2.435 | 1.030 | 5.759 | 0.043a |
| Nodal status | 1.609 | 0.566 | 4.575 | 0.373 | 3.169 | 1.421 | 7.067 | 0.005a | 3.986 | 1.618 | 9.823 | 0.003a |
| Phenotype | 0.226 | 0.079 | 0.644 | 0.005a | 1.187 | 0.636 | 2.215 | 0.590 | 0.998 | 0.533 | 1.866 | 0.994 |
| ER status | 0.190 | 0.029 | 1.232 | 0.082 | 1.362 | 0.291 | 6.376 | 0.695 | 0.259 | 0.042 | 1.611 | 0.147 |
| PR status | 1.044 | 0.191 | 5.723 | 0.960 | 1.002 | 0.335 | 2.999 | 0.997 | 2.959 | 0.795 | 11.015 | 0.106 |
| HER2 status | 0.738 | 0.248 | 2.196 | 0.585 | 0.801 | 0.342 | 1.877 | 0.610 | 0.918 | 0.374 | 2.253 | 0.851 |
| Ki-67
expression | 8.800 | 1.623 | 47.731 | 0.012a | 1.078 | 0.452 | 2.573 | 0.865 | 0.336 | 0.138 | 0.816 | 0.016a |
Therefore, it was demonstrated by the multivariate
analysis that POSTN expression retained significance independent of
other prognostic factors, including as an independent prognostic
marker for local relapse, distant metastasis and OS in the patients
with early stage breast cancer of the present study.
Discussion
Radiation therapy subsequent to conserving surgery
has made notable contributions to oncotherapy; it remains the
standard therapeutic modality for breast cancer patients in early
stages, and has been demonstrated to improve the overall survival
(15). Nevertheless, tumor relapse
and therapy failure continue to occur in a high proportion of
patients. One emerging explanation posits that cancer stem cells
may be responsible for the resistance to chemotherapeutic agents
and radiation therapy (16). The
exposure to radiation may reprogram differentiated breast cancer
cells into induced breast cancer stem cells, which express the same
stemness-related genes and exhibit enhanced tumorigenicity compared
with non-irradiated samples (17).
Phillips et al (18)
previously reported that CD44+CD24−/low
breast cancer stem cells are less radiosensitive, supporting the
hypothesis that cancer stem cells are more radioresistant than
non-stem cancer cells. Specifically, breast cancer stem cells with
the HER2+/CD44+/CD24−/low
phenotype exhibited increased aggressiveness, tumorigenesis and
radioresistance compared with
HER2−/CD44+/CD24−/low breast
cancer cells, thus providing a potential therapeutic target for the
radiosensitization of breast cancer cells (19). One mechanism for radioresistance in
cancer stem cells appears to be associated with their enhanced DNA
repair capacity, reactive oxygen species defenses and self-renewal
potential (20).
The extracellular matrix protein POSTN is highly
expressed in a subset of basal-like breast cancer (BLBC) cell
lines, as well as in cancer stem cell-enriched populations within
tumors. When produced by cancer stem cells, POSTN acts to maintain
the stem-like state through the activation of integrin receptors
and the production of the key cytokines, interleukin-6 and −8
(21). POSTN is required for cancer
stem cell maintenance and increases Wnt signalling in cancer stem
cells, and therefore, is a critical limiting factor during
metastatic colonization (6).
Recent studies have demonstrated that POSTN may be
critical for the interactions between cancer stem cells and their
metastatic niche (4). It is necessary
for cancer stem cells to maintain vitality, and blocking its
function prevents metastasis (7). Xu
et al (10) reported that
POSTN was highly expressed in cancer stem cells and could be a
potential biomarker for the bone metastasis and chemotherapy
resistance of breast cancer tumors. To the best of our knowledge,
no study has examined the relationship between POSTN,
radioresistance and the prognosis of patients with breast cancer
receiving radiotherapy.
In the present study, in a cohort of breast cancer
patients treated with conserving surgery and radiation therapy,
positive POSTN expression was associated with higher rates of local
recurrence and distant metastasis. It was considered to be an
independent predictor for all endpoints based on multivariate
analysis, and therefore, may be suitable as a biomarker of
radioresistance. An association was also identified between POSTN
expression and certain clinicopathological characteristics of the
patients with breast cancer, although previous studies have
reported that serum POSTN levels are not associated with
clinicopathological parameters in early-stage colorectal cancer,
lung cancer and hepatocellular carcinoma (22–24).
However, Sasaki et al (25)
reported on the presence of elevated levels of serum POSTN in
patients with breast cancer affected by bone metastases, suggesting
that serum POSTN may represent a marker of bone metastasis in
patients with breast cancer. The present study identified that
POSTN expression in the tumor was associated with histological
grade, nodal status, molecular subtype, ER status, PR status and
Ki-67 levels in these patients. In addition, through survival
analysis, positive POSTN expression was associated with a higher
propensity for local and distant relapse and poorer overall
survival. Cox's proportional hazard regression model analysis
identified POSTN as an independent prognostic factor for breast
cancer outcomes following conserving surgery and radiotherapy. This
suggests that POSTN may be critical for breast cancer
tumorigenesis, and may be a potential biomarker for the metastasis
and radiotherapy resistance of breast cancer.
The limitations of the present study include a
relatively small sample size and a short follow-up time. Further
studies with a larger number of subjects will be required. In
addition, a more detailed analysis with a focus on identifying
associations between POSTN expression and cohort subgroups,
particularly patients with triple-negative breast cancer treated
with radiotherapy, will be required. The association of POSTN
expression with the breast cancer stem cell ratio in tissues, and
the molecular mechanisms by which POSTN regulates the effect of
cancer stem cells in the radiotherapeutic resistance of breast
tumors, also require more extensive investigation.
Acknowledgements
The present study was supported by Qingdao Health
Science and Technology Project (grant no. 2016-WJZD039).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gaffan J, Dacre J and Jones A: Educating
undergraduate medical students about oncology: A literature review.
J Clin Oncol. 24:1932–1939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bollet MA, Sigal-Zafrani B, Mazeau V,
Savignoni A, de la Rochefordière A, Vincent-Salomon A, Salmon R,
Campana F, Kirova YM, Dendale R and Fourquet A: Age remains the
first prognostic factor for loco-regional breast cancer recurrence
in young (<40 years) women treated with breast conserving
surgery first. Radiother Oncol. 82:272–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z and Ouyang G: Periostin: A bridge
between cancer stem cells and their metastatic niche. Cell Stem
Cell. 10:111–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Zhang G, Li J, Tao Q and Tang W:
The expression analysis of periostin in human breast cancer. J Surg
Res. 160:102–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malanchi I, Santamaria-Martínez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyutoku M, Taniyama Y, Katsuragi N,
Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y
and Morishita R: Role of periostin in cancer progression and
metastasis: Inhibition of breast cancer progression and metastasis
by anti-periostin antibody in a murine model. Int J Mol Med.
28:181–186. 2011.PubMed/NCBI
|
|
8
|
Xiao ZM, Wang XY and Wang AM: Periostin
induces chemoresistance in colon cancer cells through activation of
the pi3k/akt/survivin pathway. Biotechnol Appl Biochem. 62:401–406.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung PL, Jan YH, Lin SC, Huang CC, Lin H,
Wen KC, Chao KC, Lai CR, Wang PH, Chuang CM, et al: Periostin in
tumor microenvironment is associated with poor prognosis and
platinum resistance in epithelial ovarian carcinoma. Oncotarget.
7:4036–4047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H
and Lu P: Cancer stem cell-related gene periostin: A novel
prognostic marker for breast cancer. PLoS One. 7:e466702012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nuzzo PV, Rubagotti A, Argellati F, Di
Meglio A, Zanardi E, Zinoli L, Comite P, Mussap M and Boccardo F:
Prognostic value of preoperative serum levels of periostin (PN) in
early breast cancer (BCa). Int J Mol Sci. 16:17181–17192. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
American Joint Committee on Cancer: Cancer
Staging Handbook: From the AJCC Cancer Staging Manual. Edge SB,
Byrd DR, Compton CC, Fritz AG, Greene F and Trotti A: 7th edition.
Springer-Verlag; New York: pp. 3472010
|
|
13
|
Lakhani SR, Ellis IO, Schnitee SJ, Tan PH
and van de Vijver MJ: WHO Classification of Tumours of the Breast.
4. 4th. IARC Press; Lyon: 2012
|
|
14
|
Diallo-Danebrock R, Ting E, Gluz O, Herr
A, Mohrmann S, Geddert H, Rody A, Schaefer KL, Baldus SE, Hartmann
A, et al: Protein expression profiling in high-risk breast cancer
patients treated with high-dose or conventional dose-dense
chemotherapy. Clin Cancer Res. 13:488–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gebski V, Lagleva M, Keech A, Simes J and
Langlands AO: Survival effects of postmastectomy adjuvant radiation
therapy using biologically equivalent doses: A clinical
perspective. J Natl Cancer Inst. 98:26–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosen JM and Jordan CT: The increasing
complexity of the cancer stem cell paradigm. Science.
324:1670–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lagadec C, Vlashi E, Della Donna L,
Dekmezian C and Pajonk F: Radiation-induced reprogramming of breast
cancer cells. Stem Cells. 30:833–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips TM, McBride WH and Pajonk F: The
response of cd24−/low/cd44+ breast
cancer-initiating cells to radiation. J Natl Cancer Inst.
98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duru N, Fan M, Candas D, Menaa C, Liu HC,
Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al:
HER2-associated radioresistance of breast cancer stem cells
isolated from HER2-negative breast cancer cells. Clin Cancer Res.
18:6634–6647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lambert AW, Wong CK, Ozturk S, Papageorgis
P, Raghunathan R, Alekseyev Y, Gower AC, Reinhard BM, Abdolmaleky
HM and Thiagalingam S: Tumor cell-derived periostin regulates
cytokines that maintain breast cancer stem cells. Mol Cancer Res.
14:103–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F and
Yuan YZ: Circulating levels of periostin may help identify patients
with more aggressive colorectal cancer. Int J Oncol. 34:821–828.
2009.PubMed/NCBI
|
|
23
|
Hong L, Sun H, Lv X, Yang D, Zhang J and
Shi Y: Expression of periostin in the serum of nsclc and its
function on proliferation and migration of human lung
adenocarcinoma cell line (a549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv Y, Wang W, Jia WD, Sun QK, Huang M,
Zhou HC, Xia HH, Liu WB, Chen H, Sun SN and Xu GL: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sasaki H, Yu CY, Dai M, Tam C, Loda M,
Auclair D, Chen LB and Elias A: Elevated serum periostin levels in
patients with bone metastases from breast but not lung cancer.
Breast Cancer Res Treat. 77:245–252. 2003. View Article : Google Scholar : PubMed/NCBI
|