Introduction
Hepatocellular carcinoma (HCC) is a common liver
cancer and its mortality rate ranks third among cancer-associated
mortalities worldwide in 2008 (1,2). A total
of >80% of HCC cases are from the Asian and African continents,
>50% of which are from mainland China (3). HCC is a complicated type of cancer,
involving numerous risk factors in its occurrence and development:
Hepatitis B virus (HBV) infection and genetic factors also serve
key roles in HCC carcinogenesis (4).
Despite intense study of the molecular carcinogenic mechanism of
HCC in previous years, it remains incompletely characterized
(5).
The Janus kinase-signal transducer and activator of
transcription (JAK-STAT) signal pathway plays a key role in HCC
(6). Up to 45.5% of cases of HCC
harbor genomic alterations in the JAK-STAT signal pathway (6) and a number of anti-HCC drugs also serve
their roles by blocking the JAK-STAT signal pathway. For example,
Murphy et al (7) suggested
that interferon-α (IFN-α) serves an anti-cancer role in HCC through
the JAK-STAT signal pathway, and Subramaniam et al (8) identified that that emodin suppressed
STAT3 activation by modulating the activation of the
upstream kinases, including Janus kinase 1 (JAK1), in HCC.
There are 4 JAKs in mammals, JAK1, JAK2, JAK3 and
Tyrosine kinase 2. JAK1, located on chromosome 1p31.3, was
first identified in 1991 (9). It is a
crucial component of diverse signal pathways, and polymorphisms in
JAK1 may be functional and serve a role in human cancer
development (10,11). Xie et al (12) demonstrated that mutations in
JAK1 may contribute to the development of HCC, and Yang
et al (13) revealed that
JAK1S703I was an activating mutation for the
JAK-STAT signaling pathway, which may represent a novel therapeutic
approach for HCC.
In view of the important roles served by JAK1
in HCC, the present study analyzed the association between the 4-bp
indel polymorphism rs112395617 in the 3′ untranslated region
(3′UTR) of JAK1 and HCC susceptibility in a Chinese
population, and the potential mechanisms through which the
insertion-deletion (indel) polymorphism affects JAK1
expression.
Materials and methods
Study populations
Genomic DNA was extracted from the peripheral blood
of patients with newly-diagnosed HCC, together with controls,
subsequent to gaining written informed consent. The cases and
controls recruited were non-consanguineous ethnic Han Chinese
individuals. All 290 HCC cases were diagnosed, hospitalized and
treated in the Suzhou Municipal Hospital (Suzhou, China) and the
First Affiliated Hospitals of Soochow University (Suzhou, China)
between May 2009 and January 2016. None of the patients with HCC
had been administered any medical treatment prior to providing
peripheral blood samples. The diagnosis of the HCC cases, the
exclusion and inclusion criteria for all the participants, and the
definitions of smokers and drinkers were the same as described
previously (14–16). Briefly, patients were excluded if they
had: (a) primary or secondary biliary cirrhosis or Budd-Chiari
syndrome, (b) autoimmune hepatitis or toxic hepatitis, (c)
recurrence of HCC, (d) tumors other than HCC or (e) liver disease
due to parasitosis, diabetes, fatty liver, metabolism disorders or
severe cardiovascular diseases. The diagnosis of these patients was
confirmed by a pathological examination combined with positive
imaging (Magnetic resonance imaging and/or computerized
tomography). The 320 cancer-free controls were selected during a
routine physical examination conducted in the same regions during
the same period as the recruitment of the cases. All controls had
no history of cancer, and were negative for antibodies to hepatitis
C virus, hepatitis D virus, and human immunodeficiency virus. The
70 newly-diagnosed and pathologically confirmed HCC tissues were
collected following surgical resection without preoperative
chemotherapy or radiotherapy. Tumor stages were determined using a
modified American Joint Committee on Cancer and International Union
against Cancer system (17). The
design of the study was approved by the Ethical Committee of Suzhou
Municipal Hospital and Soochow University (Suzhou, China).
DNA extraction and genotyping
Peripheral blood DNA was extracted using a DNeasy
Blood & Tissue kit (cat. no. 69504; Qiagen GmbH, Hilden,
Germany). DNA fragments containing the rs112395617 polymorphism
were amplified using the following primers:
5′-CAGGTTCTGGGAATGAGT-3′ (forward) and 5′-TGAGAAAGCTGGTTCTACAT-3′
(reverse). The polymerase chain reaction (PCR) was performed in a
total volume of 20 µl, containing 2.0 µl 10X PCR buffer, 1.5 mmol/l
MgCl2, 0.25 mmol/l for each dNTP, 0.5 mmol/l of each
primer, 50 ng genomic DNA, and 1.0 U Taq DNA polymerase (Tiangen
Biotech Co., Ltd., Beijing, China). The amplification protocol
consisted of an initial denaturation at 95°C for 5 min, followed by
35 cycles of denaturation at 95°C for 30 sec, annealing at 56°C for
40 sec and extension at 72°C for 48 sec, followed by a final
extension at 72°C for 5 min. A 7% percent non-denaturing PAGE and
silver staining method was used to analyze the PCR products
(18). The 4-base pair (bp)
deletion/insertion (del/ins) allele of rs112395617 yielded bands of
127 and 131 bp, respectively. Genotyping was performed without
knowledge of case or control status. The quality control was
performed as follows: In order to validate the genotyping method,
30 randomly selected DNA samples were sequenced by Genewiz Inc.
(South Plainfield, NJ, USA) following genotyping and the results
were all validated; to confirm 100% consistency with the PCR
results, ~10% of the total DNA samples were randomly selected for
genotyping in duplicate by 2 independent technicians.
Quantitative (q)PCR analysis
Total RNA was extracted from 70 HCC tissue samples
with different genotypes using a RNeasy Mini kit (cat. no. 74106;
Qiagen) and reverse transcribed used the Superscript II reverse
transcriptase (cat. no. 18064-014; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Next, to quantify the relative
mRNA expression level of JAK1 in these samples, FastStart
Universal SYBR-Green Master (Rox; cat. no. 04913914001; Roche
Diagnostics, Indianapolis, IN, USA) was used, and qPCR was
performed on a Roche Light Cycler 480 system. GAPDH was
selected as the internal control. The primer sequences used for
JAK1 and GAPDH were as follows: JAK1-Q forward (F),
5′-CCACTACCGGATGAGGTTCTA-3′; JAK1-Q reverse (R),
5′-GGGTCTCGAATAGGAGCCAG-3′; GAPDH-QF, 5′-CTCTCTGCTCCTCCTGTTCGAC-3′;
GAPDH-QR, 5′-TGAGCGATGTGGCTCGGCT-3′.
The 20 µl total volume final reaction mixture
consisted of: 1 µmol/l for each primer, 10 µl Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 2 µl cDNA. The
negative control experiments were performed using distilled
H2O as template. The 2−ΔΔCq algorithm was
used to calculate the mRNA expression levels in tissues with
different genotypes (19).
Plasmid construction
A DNA fragment of ~300 bp, including the ins/ins
genotype of rs112395617 in the 3′ UTR of JAK1 (sequence,
TCTAGAAAATGACTGTATTCTCTCACCAGTAGGACTTAAACTTTGTTTCTCCAGTGGCTTAGCTCCTGTTCCTTTGGGTGATCACTAGCACCCATTTTTGAGAAAGCTGGTTCTACATGGGGGGATAGCTGTGGAATAGATAATTTGCTGCATGTTAATTCTCAAGAACTAAGCCTGTGCCAGTGCTTTCCTAAGCAGTATACCTTTAATCAGAACTCATTCCCAGAACCTGGATGCTATTACACATGCTTTTAAGAAACGTCAATGTATATCCTTTTATAACTCTACCACTTTGGGGCAAGCTATTCCAGGCCGGCC),
was directly synthesized by Genewiz Inc. (South Plainfield, NJ,
USA) and cloned into Xba I and Fse III sites of a pGL3-control
expression vector (Promega Corporation, Madison, WI, USA), named
pGL3-JAK1-WT. A QuikChange Lightening Site-Directed Mutagenesis kit
(cat. no. 210518, Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA) was used to generate the mutant-type vector
(pGL3-JAK1-MT) including the del/del genotype of rs112395617
(sequence,
TCTAGAAAATGACTGTATTCTCTCACCAGTAGGACTTAAACTTTGTTTCTCCAGTGGCTTAGCTCCTGTTCCTTTGGGTGATCACTAGCACCCATTTTTGAGAAAGCTGGTTCTACATGGGGGGATAGCTGTGGAATAGATAATTTGCTGCATGTTCTCAAGAACTAAGCCTGTGCCAGTGCTTTCCTAAGCAGTATACCTTTAATCAGAACTCATTCCCAGAACCTGGATGCTATTACACATGCTTTTAAGAAACGTCAATGTATATCCTTTTATAACTCTACCACTTTGGGGCAAGCTATTCCAGGCCGGCC).
The sequence and direction of the resulting constructs
(pGL3-JAK1-WT and pGL3-JAK1-MT) were verified by direct sequencing
by Genewiz Inc.
In silico prediction of microRNA
(miRNA/miR) binding to rs112395617
Mature human miRNA sequences were obtained from
miRBase (http://microrna.sanger.ac.uk) in
2016. A 27-bp region comprising rs112395617 was analyzed for
hybridization of putative miRNAs using miRanda (version 3.3a)
software with default parameters as described previously (20).
Cell culture and luciferase reporter
assay
The Sk-Hep-1 cell line used in the present study was
obtained from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The Sk-Hep-1 cells were cultured in
Dulbecco's modified Eagle's medium (cat. no. 12491-015; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(cat. no. 10100147; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin at 37°C in a humidified chamber
supplemented with 5% CO2. Cells were seeded in 24-well
plates (cat. no. 3524; Corning Incorporated, Corning, NY, USA) at a
density of 1×105 cells/well and transfected following
culture at 37°C for 16 h using Lipofectamine® 2000 (cat.
no. 11668-019; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Subsequently, 500 ng pGL3-JAK1-WT or
pGL3-JAK1-MT reconstructed vector and 50 ng of pRL-SV40 (Promega
Corporation) were co-transfected using Lipofectamine®
2000 with 100 pmol miR-431-5p mimic or mimic control (Ambion;
Thermo Fisher Scientific, Inc.) with (~5×106
cells/well). Subsequent to 24 h, cells were harvested immediately
following addition of 100 µl passive lysis buffer (cat. no. E1910;
Promega Corporation). Dual-luciferase reporter assay (cat. no.
E1910; Promega Corporation) was used to measure the firefly
luciferase activity in cell lysates in a FilterMax F5, according to
the manufacturer's protocol, and Renilla luciferase activity was
used to normalize the data. All experiments were repeated at least
3 times with 6 replicates for each group.
Statistical analysis
The Hardy-Weinberg equilibrium was analyzed using
the χ2 test for the genotype distribution in the control
group. The association between rs112395617 and HCC risk was
analyzed by logistic regression, adjusted for age (mean ± standard
deviation), sex, smoking, drinking, tumor stage and HBV infection
status. A one-way analysis of variance followed by Dunnett's
post-hoc test was used to compare the relative JAK1 mRNA
expression levels in HCC tissues among different genotypic groups.
The difference in luciferase activity was examined using the
Student's t test. Statistical Analysis System software was used for
all the statistical analyses (version 8.0; SAS Institute, Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference, and all statistical tests were
two-sided.
Results
Association of rs112395617 with HCC
susceptibility
Table I summarizes the
demographic characteristics of the patients with HCC and the
controls. The distribution of age, sex, smoking and drinking status
was similar between the patients with HCC and controls. The
Hepatitis B surface antigen-positive rate was 70 and 10% in the HCC
patients and controls, respectively, supporting the assumption that
HBV infection is a risk factor for HCC (4). Fig. 1
presents the genotyping assays for rs112395617. The observed
rs112395617 genotypic frequencies in the controls were consistent
with the Hardy-Weinberg equilibrium (P>0.05). Table II indicates that the ins/del or
del/del genotype exhibited a significantly decreased risk of HCC
compared with the ins/ins genotype [P=0.00019; Odds ratio
(OR)=0.52; 95% confidence interval (CI): 0.36–0.74; and P=0.004;
OR=0.48; 95% CI, 0.28–0.82, respectively). In addition, the 4-bp
deletion allele was associated with a 36% decreased risk compared
with the insertion allele (P=0.00016; OR=0.64; 95% CI, 0.50–0.81).
Collectively, these results suggest an association between
rs112395617 and HCC susceptibility.
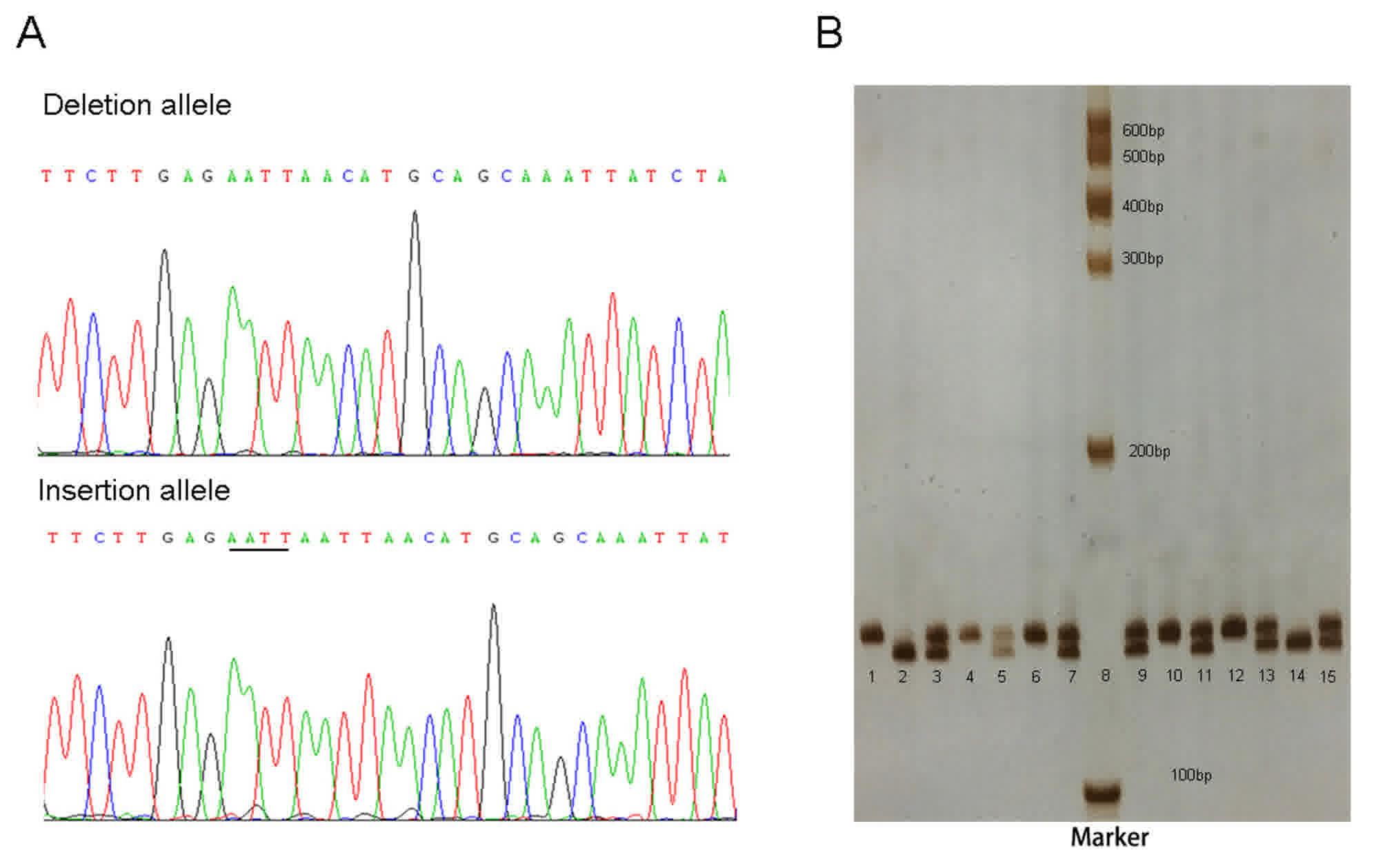 | Figure 1.Sequencing and genotyping example of
rs112395617. (A) Deletion and insertion allele sequences. The
inserted ‘AATT’ is underlined. (B) Genotyping results using 7%
non-denaturing PAGE and silver staining. Lanes 1, 4, 6, 10 and 12
ins/ins genotype; lanes 3, 5, 7, 9, 11, 13 and 15 ins/del genotype;
and lanes 2 and 14, del/del genotype; lanes 8 DNA marker. Ins,
insertion; del, deletion. |
 | Table I.Demographic characteristics of
hepatocellular carcinoma cases and controls. |
Table I.
Demographic characteristics of
hepatocellular carcinoma cases and controls.
|
Characteristics | Patients, %
(n=290) | Control, %
(n=320) |
|---|
| Age (mean ±
standard deviation) | 53.5±9.6 | 52.4±10.1 |
| Sex |
|
|
|
Male | 187 (0.64) | 208 (0.65) |
|
Female | 103 (0.36) | 112 (0.35) |
| Smoking status |
|
|
|
Non-smokers | 203 (0.70) | 218 (0.68) |
| Former
smokers | 44 (0.15) | 48 (0.15) |
| Current
smokers | 43 (0.15) | 54 (0.17) |
| Drinking
status |
|
|
|
Non-drinkers | 145 (0.50) | 166 (0.52) |
| Light
drinkers | 119 (0.41) | 128 (0.40) |
| Heavy
drinkers | 26 (0.09) | 26 (0.08) |
| Tumor stages |
|
|
| Ia +
Ib | 200 (0.69) |
|
| IIa +
IIb | 61 (0.21) |
|
| IIIa +
IIIb | 29 (0.10) |
|
| HBsAg |
|
|
|
Positive | 203 (0.70) | 31 (0.10) |
|
Negative | 87 (0.30) | 289 (0.90) |
 | Table II.Genotype distributions of the
rs112395617 polymorphism in patients with HCC and healthy
controls. |
Table II.
Genotype distributions of the
rs112395617 polymorphism in patients with HCC and healthy
controls.
| Genotype | HCC (n=290) n
(%) | Controls (n=320) n
(%) | OR (95%
CI)a | P-value |
|---|
| ins/ins | 146 (50.4) | 107 (33.4) | 1 (reference) |
|
| ins/del | 112 (38.6) | 162 (50.6) | 0.52
(0.36–0.74) | 0.0002 |
| del/del | 32 (11.0) | 51 (16.0) | 0.48
(0.28–0.82) | 0.004 |
| ins allele | 404 (69.7) | 376 (58.8) | 1 (reference) |
|
| del allele | 176 (30.3) | 264 (41.2) | 0.64
(0.50–0.81) | 0.0002 |
Association between rs112395617
genotype and JAK1 mRNA expression levels
As demonstrated in Fig.
2, the JAK1 mRNA level was highest in the AATT insertion
homozygous group (ins/ins), followed by the heterozygous (ins/del)
and AATT deletion homozygous groups (del/del). The average
JAK1 mRNA expression levels of the heterozygous and AATT
insertion homozygous group were increased by 1.75- and 3.36-fold,
respectively, compared with that of the AATT deletion homozygous
group. The data of the present study suggests an association
between different genotypes and JAK1 mRNA expression levels
in vivo.
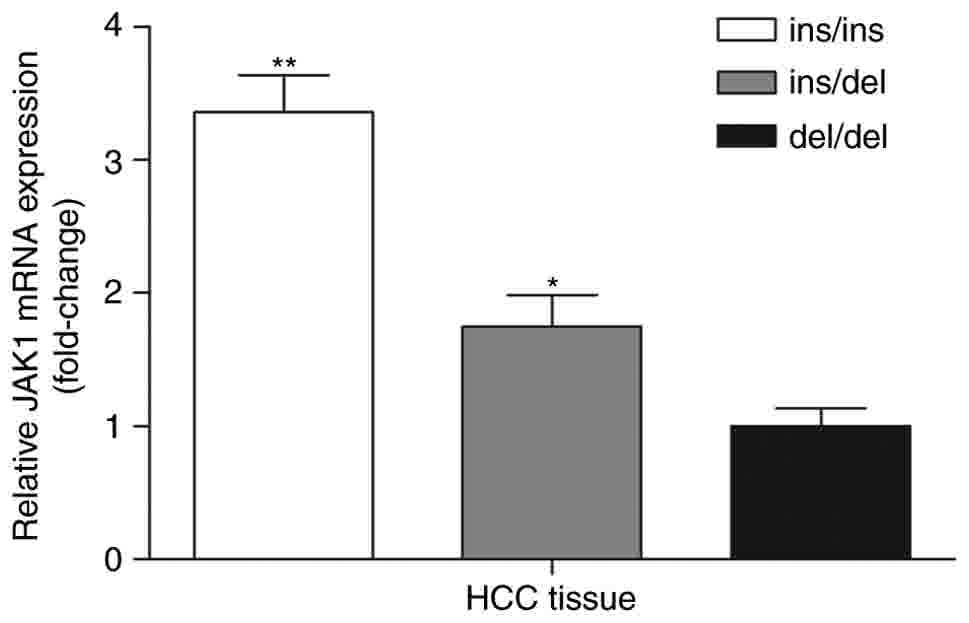 | Figure 2.JAK1 mRNA expression levels in HCC
tissues with different genotypes. The JAK1 mRNA expression level in
HCC tissues with ins/del and ins/ins geno›type was 1.75- and
3.36-fold higher compared with that of the del/del genotype,
respectively (*P<0.05 and **P<0.01 vs. del/del). ins/ins,
n=33, ins/del, n=29, del/del, n=8. JAK1, Janus kinase 1; HCC,
hepatocellular carcinoma; ins, insertion; del, deletion. |
rs112395617 polymorphism affects JAK1
transcription activity by regulating its binding with miR-431-5p in
vitro
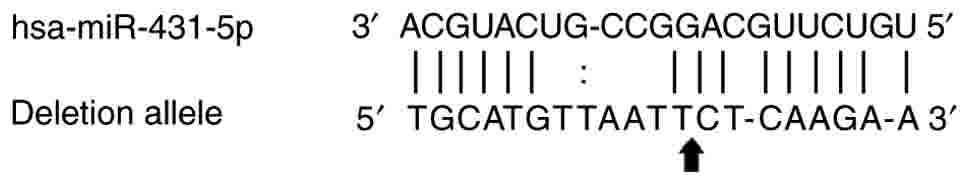
The bioinformatics analysis indicated that
rs112395617 lies within the predicted human miR-431-5p binding
site, and the insertion allele of rs112395617 may disrupt the
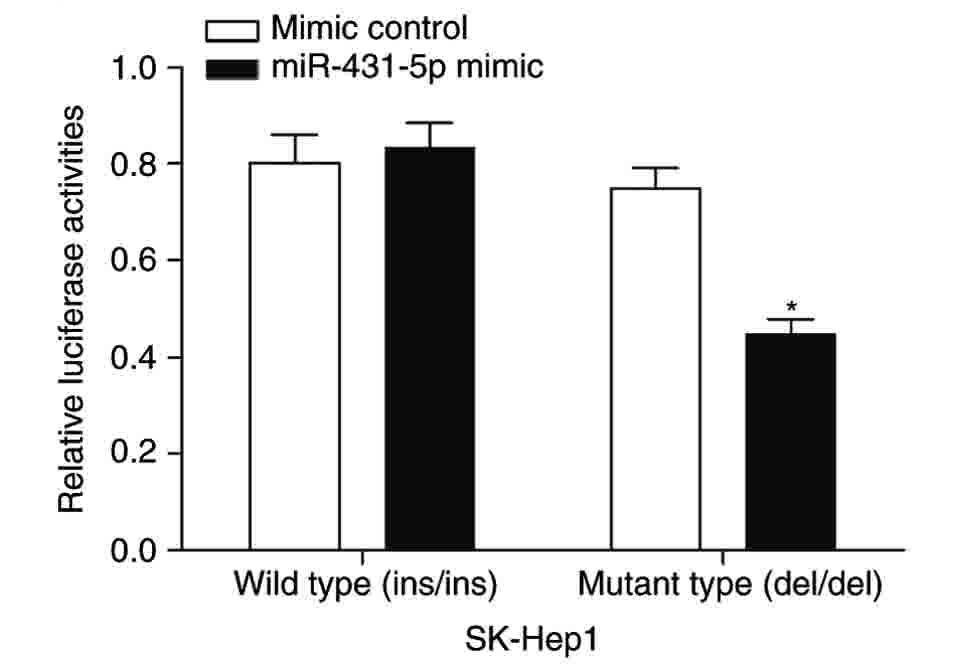
binding of miR-431-5p with the 3′ UTR of JAK1 (Fig. 3). To assess this prediction, 2
vectors, pGL3-JAK1-WT and pGL3-JAK1-MT, were constructed. In the
pGL3-JAK1-WT group, the expression of JAK1 mRNA, indicated
by firefly luciferase activity, in the miR-431-5p mimic-transfected
group was similar to that of the mimic control group. However, for
the pGL3-JAK1-MT group, the expression of JAK1 mRNA in the
miR-431-5p mimic transfected group was significantly lower compared
with that of the mimic control group (Fig. 4). These results suggest that
miR-431-5p may bind with the del/del mutant 3′ UTR of JAK1
and negatively regulate JAK1 transcription, and that the
AATT insertion allele may weaken its regulatory effect by
disrupting the binding of miR-431-5p to the 3′ UTR of JAK1.
Therefore, the in vitro study suggests that the polymorphism
rs112395617 affects JAK1 expression by altering the binding
of miR-431-5p to the 3′ UTR of JAK1.
Discussion
Although the association between polymorphisms in
the JAK1 exon and the risk of HCC or other types of cancer
has been investigated (10–13), to the best of our knowledge, no study
concerning the effect of polymorphisms in the 3′ UTR of JAK1
on HCC has been performed. The present study assessed the effect of
the rs112395617 polymorphism in the 3′ UTR of JAK1 on the
prevalence of HCC, and the potential mechanism of action. It was
identified that the del/del and del/ins genotypes of rs112395617
were significantly associated with a decreased risk of HCC. The
following phenotype-genotype association experiment indicated that
the level of JAK1 mRNA expression in HCC tissues with the
del/del or del/ins genotype was significantly lower compared with
that in HCC tissues with the ins/ins genotype. In addition, the
luciferase reporter assay suggested that the del/del or del/ins
genotype potentially worked in full, or at least partially, by
miR-431-5p mediated down-regulation of JAK1 mRNA expression.
To the best of our knowledge, this is the first study to
investigate the association between the indel polymorphism in the
JAK1 3′ UTR and the risk of HCC.
JAK1 is a member of the Janus kinase family,
which mediates the growth factor and cytokine-induced STAT signal
pathway and has effects on immunity, cell growth, and
differentiation (21). It has been
demonstrated that the JAK-STAT signal pathway serves a key role in
the development of HCC, and up to 45.5% of HCC cases harbor genomic
alterations in the JAK-STAT signal pathway (6). JAK1 expression in cancer cells
may enable individual cells to contract and metastasize to other
parts of the body (22). Activating
mutations in JAK1 have been identified in acute leukemia and
myeloproliferative neoplasms (23).
All of these data are consistent with the results demonstrated that
the protective deletion allele with a low level of JAK1 mRNA
expression may decrease the risk of HCC development.
As described above, abnormal activation of
JAK1 participates in the progression of HCC, and a number of
previous studies have indicated that polymorphisms in the 3′ UTR of
genes disturb their binding with miRNAs or reveal novel binding
sites for miRNAs (24–28). This affects the level of expression of
target genes and may be involved in numerous types of diseases,
including cancer (25–27). Gao et al (24) demonstrated that an indel polymorphism
at a miRNA-122-binding site in the 3′ UTR of interleukin (IL)-1a
conferred increased risk for HCC, and Wang et al (25) demonstrated that rs56288038 in the
IRF-1 3′ UTR regulated by miR-502-5p promoted the
development of gastric cancer. Certain previous studies have also
indicated that single nucleotide polymorphisms or indels in miRNA
binding sites of their target genes may participate in the
development or progression, or be associated with fluorouracil
resistance, of HCC by affecting binding with the target genes
(26–28). To explore the possible mechanism
through which rs112395617 is involved in HCC susceptibility, miRNAs
that would be able to bind with the JAK1 3′ UTR and be
affected by rs112395617 were predicted, and it was identified that
miR-431-5p was able to bind with the del/del genotype, but not the
ins/ins genotype, and consequently down regulated the expression of
JAK1, thus reducing the risk of HCC.
miR-431, formerly hypothesized to be a nervous
system-specific miRNA (29), has been
identified to have a role as a novel tumor-related miRNA (30–33).
Elevated expression of miR-431 was demonstrated to be able to act
as a biomarker in differentiating patients with colorectal cancer
(CRC) from healthy controls (30),
suggesting an oncogenic role in CRC. Studies on medulloblastoma and
glioblastoma have confirmed that miR-431 serves a role in the
inhibitory effect of human IFN-β on cell viability by modulating
the JAK-STAT signal pathway (31),
indicating its anti-tumor role in these types of cancer. A previous
study on HCC identified that the versican 3′ UTR may promote HCC
cell proliferation, migration, invasion and colony formation by
binding and arresting miR-431 functions (32). An additional study of HCC indicated
that miR-431 may inhibit HCC cell migration and invasion by
targeting ZEB1 (33). The
roles played by miR-431 in cancer are dependent on the type of
cancer, and the results of the present study are consistent with
the previous studies on medulloblastoma, glioblastoma and HCC. Due
to the bidirectional effects of miR-431 on different types of
cancer, and the complicated mechanism of HCC development,
additional functional analyses are required.
The present study contained multiple limitations:
The subjects in the study were all ethic Han, and the numbers were
limited, so additional large-scale studies in different populations
are required. An additional limitation is that the possible
mechanism of action of the indel was predicted by software;
additional experimental validation is required.
To conclude, the present study demonstrated that the
rs112395617 polymorphism in the JAK1 3′ UTR may contribute
to HCC susceptibility, possibly working in full, or at least
partially, through an effect on JAK1 transcriptional
activity, by disturbing its binding with miR-431-5p.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Suzhou
Science and Technology Development Project (grant no. SYS201652)
and the Science and Technology Development Project of Healthy and
Family Planning Commission of Changshu (grant no. csws201705).
Availability of data and materials
All the datasets generated in the present study are
included in the present manuscript and they are available upon
reasonable request from the corresponding author.
Authors' contributions
The experiments were designed by QY and WC; the
samples was collected by WQ; DNA extraction and genotyping was
performed by JW; qPCR was conducted by YW; plasmid construction,
in silico prediction of miRNA binding to rs112395617, cell
culture and luciferase reporter assay were conducted by JZ; data
analysis was performed by QY and was also the major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The design of the study was approved by the Ethical
Committee of Suzhou Municipal Hospital (Suzhou, China).
Consent for publication
Patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A, Minguez B, Forner A, Reig M
and Llovet JM: Hepatocellular carcinoma: Novel molecular approaches
for diagnosis, prognosis, and therapy. Annu Rev Med. 61:317–328.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McClune AC and Tong MJ: Chronic Hepatitis
B and hepatocellular carcinoma. Clin Liver Dis. 14:461–476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CJ and Chen DS: Interaction of
hepatitis B virus, chemical carcinogen, and genetic susceptibility:
Multistage hepatocarcinogenesis with multifactorial etiology.
Hepatology. 36:1046–1049. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aravalli RN, Steer CJ and Cressman EN:
Molecular mechanisms of hepatocellular carcinoma. Hepatology.
48:2047–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kan Z, Zheng H, Liu X, Li S, Barber TD,
Gong Z, Gao H, Hao K, Willard MD, Xu J, et al: Whole-genome
sequencing identifies recurrent mutations in hepatocellular
carcinoma. Genome Res. 23:1422–1433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy D, Detjen KM, Welzel M, Wiedenmann
B and Rosewicz S: Interferon-alpha delays S-phase progression in
human hepatocellular carcinoma cells via inhibition of specific
cyclin-dependent kinases. Hepatology. 33:346–356. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subramaniam A, Shanmugam MK, Ong TH, Li F,
Perumal E, Chen L, Vali S, Abbasi T, Kapoor S, Ahn KS, et al:
Emodin inhibits growth and induces apoptosis in an orthotopic
hepatocellular carcinoma model by blocking activation of STAT3. Br
J Pharmacol. 170:807–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilks AF, Harpur AG, Kurban RR, Ralph SJ,
Zürcher G and Ziemiecki A: Two novel protein-tyrosine kinases, each
with a second phosphotransferase-related catalytic domain, define a
new class of protein kinase. Mol Cell Biol. 11:2057–2065. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rane SG and Reddy EP: Janus kinases:
Components of multiple signaling pathways. Oncogene. 19:5662–5679.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staerk J, Kallin A, Demoulin JB,
Vainchenker W and Constantinescu SN: JAK1 and Tyk2 activation by
the homologous polycythemia vera JAK2 V617F mutation: Cross-talk
with IGF1 receptor. J Biol Chem. 280:41893–41899. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie HJ, Bae HJ, Noh JH, Eun JW, Kim JK,
Jung KH, Ryu JC, Ahn YM, Kim SY, Lee SH, et al: Mutational analysis
of JAK1 gene in human hepatocellular carcinoma. Neoplasma.
56:136–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Luo C, Gu Q, Xu Q, Wang G, Sun H,
Qian Z, Tan Y, Qin Y, Shen Y, et al: Activating JAK1 mutation may
predict the sensitivity of JAK-STAT inhibition in hepatocellular
carcinoma. Oncotarget. 7:5461–5469. 2016.PubMed/NCBI
|
|
14
|
Zhou C, Yu Q, Chen L, Wang J, Zheng S and
Zhang J: A miR-1231 binding site polymorphism in the 3′ UTR of
IFNAR1 is associated with hepatocellular carcinoma susceptibility.
Gene. 507:95–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Y, Yu Q, Zhou D, Chen L, Huang X, Xu
G, Huang J, Gao X, Gao Y and Shen L: The Mitochondrial DNA 9-bp
deletion polymorphism as a risk factor for hepatocellular carcinoma
in the Chinese population. Genet Test Mol Biomarkers. 16:330–334.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Q, Zhou CX, Chen NS, Zheng SD, Shen LM
and Zhang JK: A polymorphism within ErbB4 is associated with risk
for hepatocellular carcinoma in Chinese population. World J
Gastroenterol. 18:383–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allen RC, Graves G and Budowle B:
Polymerase chain reaction amplification products separated on
rehydratable polyacrylamide gels and stained with silver.
Biotechniques. 7:736–744. 1989.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(t)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh TC and Pellegrini S: The Janus kinase
family of protein tyrosine kinases and their role in signaling.
Cell Mol Life Sci. 55:1523–1534. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nordqvist C: Protein JAK makes cancer
cells contract, so they can squeeze out of a tumor. Medical News
Today. 2011.
|
|
23
|
Quintás-Cardama A, Kantarjian H, Cortes J
and Verstovsek S: Janus kinase inhibitors for the treatment of
myeloproliferative neoplasias and beyond. Nat Rev Drug Discov.
10:127–140. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, He Y, Ding J, Wu K, Hu B, Liu Y, Wu
Y, Guo B, Shen Y, Landi D, et al: An insertion/deletion
polymorphism at miRNA-122-binding site in the interleukin-1alpha 3′
untranslated region confers risk for hepatocellular carcinoma.
Carcinogenesis. 30:2064–2069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang B, Yang H, Shen L, Wang J, Pu W, Chen
Z, Shen X, Fu J and Zhuang Z: Rs56288038 (C/G) in 3′UTR of IRF-1
regulated by MiR-502-5p promotes gastric cancer development. Cell
Physiol Biochem. 40:391–399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan Y, Qian X and Zhang C: U/G SNP
rs111904020 in 3′UTR of STAT3 regulated by miR-214 promotes
hepatocellular carcinoma development in Chinese population. Tumour
Biol. 37:14629–14635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma J, Guo R, Wang T, Pan X and Lei X:
Let-7b binding site polymorphism in the B-cell lymphoma-extra large
3′UTR is associated with fluorouracil resistance of hepatocellular
carcinoma. Mol Med Rep. 11:677–681. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Zhao H, Zhao X, Wan J, Wang D, Bi
W, Jiang X and Gao Y: Association between an insertion/deletion
polymorphism within 3′UTR of SGSM3 and risk of hepatocellular
carcinoma. Tumour Biol. 35:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wheeler G, Ntounia-Fousara S, Granda B,
Rathjen T and Dalmay T: Identification of new central nervous
system specific mouse microRNAs. FEBS Lett. 580:2195–2200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanaan Z, Roberts H, Eichenberger MR,
Billeter A, Ocheretner G, Pan J, Rai SN, Jorden J, Williford A and
Galandiuk S: A plasma microRNA panel for detection of colorectal
adenomas: A step toward more precise screening for colorectal
cancer. Ann Surg. 258:400–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka T, Arai M, Jiang X, Sugaya S, Kanda
T, Fujii K, Kita K, Sugita K, Imazeki F, Miyashita T, et al:
Downregulation of microRNA-431 by human interferon-beta inhibits
viability of medulloblastoma and glioblastoma cells via
upregulation of SOCS6. Int J Oncol. 44:1685–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang L, Du WW, Yang X, Chen K, Ghanekar A,
Levy G, Yang W, Yee AJ, Lu WY, Xuan JW, et al: Versican
3′-untranslated region (3′-UTR) functions as a ceRNA in inducing
the development of hepatocellular carcinoma by regulating miRNA
activity. FASEB J. 27:907–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun K, Zeng T, Huang D, Liu Z, Huang S,
Liu J and Qu Z: MicroRNA-431 inhibits migration and invasion of
hepatocellular carcinoma cells by targeting the ZEB1-mediated
epithelial-mensenchymal transition. FEBS Open Bio. 5:900–907. 2015.
View Article : Google Scholar : PubMed/NCBI
|