Introduction
Cervical cancer is the fourth most common cancer in
women, and the seventh overall, with an estimated 528,000 new cases
and 266,000 deaths worldwide in 2012 (1). Currently, for locally advanced cervical
cancer standard therapy is cisplatin-based concurrent
chemoradiotherapy, with an overall survival (OS) of approximately
66% at 5 years (2–5).
Chemotherapy and radiotherapy are both considered as
DNA damage agents, more precisely capable to introduce DNA
double-strand breaks (DSBs) in order to induce cell death (6–8). When
cellular DNA damage is not repaired one alternative response is
apoptosis, which is the objective of the current therapeutic
approach with cytotoxic agents and radiotherapy, but genetic
alterations at key proteins in the pathway may result in the
development of resistance to therapy (9,10).
Therefore, studies involving variations in genes involved in
cellular response to damage are important to understand how the
development of resistant phenotypes occurs. One example may be the
TP53 gene, the ‘guardian of genome’ due to its role on cell
cycle arrest, DNA repair activation and regulation of apoptosis
(11–13). This suppressor gene is located on
chromosome 17 (17p13.1) and encodes a phosphoprotein of 393 long
amino acids (14,15).
Polymorphic variants are the substitution of a
single base which results in alteration of the codon may have
different conformation and function, i.e., no changes cannot occur
or can be gain or loss of protein function (16,17).
Several TP53 mutant proteins associated with tumors, have
gained oncogenic function besides losing the suppressive function
(18,19). The most studied polymorphism of the
TP53 gene is the TP53 Arg72Pro (rs1042522), which
influences the protein expression of TP53 protein expression
(20). This variant results from a
change of guanine (G) to cytosine (C) in codon 72 in exon 4, that
leads to the replacement of arginine (Arg) by proline (Pro)
(21–23). It should be noted that due to the
location in the proline-rich region of the TP53 gene, this
single nucleotide polymorphism (SNP) may interfere with protein
stability (24,25). The two allelic variants confer
different susceptibilities to cancer progression, because they are
structurally and functionally different (26). In studies in vivo and in
vitro, the Arg allele has a higher capacity to induce
apoptosis than the Pro allele. The functions associated with Pro
allele include higher induction of cell cycle arrest in G1 and
better activation of TP53 dependent DNA repair (27). It has also been mentioned that this
polymorphism can influence the individual response (28).
Concerning cervical cancer, few studies have
evaluated the predictive role of TP53 Arg72Pro polymorphism
in clinical outcome and the results are contradictory (29,30).
Therefore, we have conducted this study to assess the possible
influence of the TP53 Arg72Pro polymorphism (rs1042522) in
OS and disease-free survival (DFS) in patients with advanced
cervical cancer.
Materials and methods
Patients
We conducted a retrospective hospital-based study
analyzing a total of 260 Caucasians patients with histologically
confirmed locally advanced cervical carcinoma (FIGO stage IB2-IVA).
These patients were recruited between February 2002 and October
2009, from the north region of Portugal and treated with
cisplatin-based chemotherapy (40 mg/m2 per week) and
concomitant external radiotherapy and/or brachytherapy in
Portuguese Institute of Oncology Francisco Gentil (Porto,
Portugal). All women were selected consecutively according to the
following inclusion criteria: Women with histological and cytology
diagnosis of cervical cancer, age greater than or equal to 18
years, stage IB2-IVA and QTRT concomitant. Regarding exclusion
criteria, these were surgery before treatment; absence of informed
consent; failure to comply with any of the inclusion criteria.
Patients' clinical characteristics obtained from
medical records are described in Table
I. The median age at diagnosis was 48.00 years, the more
frequent histological type was squamous cancer cell, the stage more
common was IIB and the median follow up time was 63.5 months. The
tumor stage was evaluated according to the International Federation
of Gynecology and Obstetrics (FIGO) classification system, and the
assessment of histology type was based the on system of Bethesda
classification. Genomic DNA was extracted from peripheral blood
samples by using FavorPrep™ Genomic DNA Mini kit (FABGK®
300; Favorgen Biotech Corp., Ping-Tung, Taiwan), according to the
manufacturers protocol. All samples were obtained with the informed
consent of the participants prior to their inclusion in the study,
according to Helsinki Declaration principles and after approval of
the Portuguese Institute of Oncology ethics committee
(CES.287/014).
 | Table I.Distribution of patients'
clinicopathologic characteristics. |
Table I.
Distribution of patients'
clinicopathologic characteristics.
|
Characteristics | No. of patients
(%) |
|---|
| Age (years) | 260 (100) |
| Median,
48.00 |
|
| Mean ±
SD, 49.00±11.50 |
|
| Follow-up time
(months) |
|
| Median,
63.5 (range 3–115) |
|
| Number of
chemotherapy cycles |
|
| Median,
6 (range 1–6) |
|
| Total dose of
radiotherapy (Gy) |
|
| Median,
80 (range 45–88) |
|
| Tumor stage |
|
|
IB2 | 22
(8.5) |
|
IIA2 | 10
(3.8) |
|
IIB | 163
(62.7) |
|
IIIA | 5
(1.9) |
|
IIIB | 53
(20.4) |
|
IVA | 7
(2.7) |
| Histologic
type |
|
|
Squamous cell carcinoma | 216
(83.1) |
|
Adenocarcinoma | 32
(12.3) |
|
Adenosquamous carcinoma | 7
(2.7) |
| Small
cell carcinoma | 5
(1.9) |
| Nodal
involvement |
|
|
Present | 14
(5.4) |
| Not
present | 246
(94.6) |
| Smoking habits |
|
|
Smoker/former smoker | 35
(13.5) |
|
Non-smoker | 152
(58.5) |
|
Unknown | 73
(28.0) |
| Response to
therapy |
|
|
Complete | 197
(75.8) |
|
Parcial | 45
(17.3) |
|
Persistent/stable | 12
(4.6) |
|
Progression | 6
(2.3) |
| Recurrence |
|
|
Yes | 50
(19.2) |
| No | 210
(80.8) |
Evaluation of chemoradiotherapy
response
The therapy response was evaluated according to
RECIST criteria (31). Complete
response (CR) indicates disappearance of the disease, partial
response (PR) indicates at least 50% reduction in tumor load,
stable disease (SD) indicates that the lesion showed ≤25%
progression or <50% shrinkage, and progression of disease (PD)
indicates >25% enlargement of the lesion, or appearance of a new
lesion. CR and PR were considered to be a good response; SD and PD,
a poor response.
Genotyping of TP53 Arg72Pro
(rs1042522) polymorphism
The selected SNP was chosen from the best evidence
from published studies (29,30,32,33),
through public databases who provide information on the phenotypic
risks, had a minor allele frequency of an at least 10 to 20% and
the SNP biological effect. The genotyping was performed using
Taqman™ Allelic Discrimination methodology by quantitative
polymerase chain reaction (qPCR). This method uses probes labeled
with fluorochromes specific for each allele, thus VIC probe is
allele C and the FAM probe is allele G (AGGAGCTGCTGGvTGCAGGGGCCACG
[C/G] GGGGAGCAGCCTCTGGCATTCTGGG). The allelic discrimination PCR
reactions were carried out in 6 µl volumes using 2.5 µl of
TaqMan® Universal PCR Master Mix (2X), 0.125 µl of 40×
assay mix 2.375 µl of sterile H2O and 1 µl of genomic
DNA. Amplification of DNA was carried out using the following
amplification conditions: 95°C for 10 min, followed by 45 cycles of
95°C for 15 sec and 60°C for 1 min. Data capture and analysis was
carried thought the ABI 7300 Real Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
the Sequence Detection Systems software (version 1.2.3; Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Quality control included the use of negative
controls in all runs, double sampling in at least 10% of the
samples, genotyping performed blindly regarding to clinical and
pathologic characteristics of patients and the results
independently evaluated by two researchers. We observed complete
concordance among duplicates.
Statistical analysis
Difference in frequencies of the TP53
Arg72Pro genotypes between the different chemoradiotherapy
responses groups were evaluated by χ2 test. The OS and
the overall survival at 5 years was defined from the date of
diagnosis to the date of death and the percentage of patients alive
after 5 years of diagnosis, respectively. The DFS times were
defined from the data from the date of diagnosis to the date of
disease recurrence. Patients without progression, lost to follow-up
or died from other causes were censored at their last date of
record. In the evaluation of OS and DFS was used Kaplan-Meier
survival estimate and log-rank test. We applied a multivariate
analysis using COX regression method to calculate hazard ratio (HR)
and 95% confidence intervals (CI) for the association between the
genotypes and the risk of death in advanced cervical cancer
patients. This analysis was used to adjust for potential
confounders, such as age (<48 years vs. ≥48 years), stage
(<IIB vs. ≥IIB), smoking habits (non-smokers vs. smokers and
former smokers) and histological type (adenosquamous cell
carcinomas and small cell carcinoma vs. adenocarcinoma and squamous
cell carcinoma), with TP53 Arg72Pro genotypes fitted as
indicator variables. A level of P<0.05 was considered
statistically significant. All analysis of data was performed using
the computer software Statistical Package for Social Sciences
(SPSS) for Windows (version 22.0; IBM Corp., Armonk, NY, USA).
As our study was performed based on DNA
availability, we did not carry out any power analysis before the
study. Therefore, we cannot report on any original study power.
However, two-way analysis of variance followed by post hoc analysis
was performed as follows: The power to detect a hazard ratio of
2.001 obtained by multivariate analysis, depended on the
distribution of the polymorphism genotypes, patients' median
survival time, recruitment period (93 months) and additional
follow-up time (63.5 months). Consequently, assuming a type I error
probability of 0.05, we estimate a post hoc power higher than 80%.
This analysis was performed using the Power and Sample Size program
(version 3.1.2).
Results
As mentioned before, the TP53 Arg72Pro
polymorphism studied in this work results from a change of guanine
(G) to cytosine (C) in codon 72 in exon 4 that leads to the
replacement of arginine (Arg) by proline (Pro). Besides that, all
results are presented for an analysis comparing heterozygote
genotypes (Arg/Pro) with homozygous genotypes (Arg/Arg and
Pro/Pro).
Of the 260 patients included in this study (Table I), only 249 patients have results for
genotyping. The frequencies of Arg/Arg, Arg/Pro and Pro/Pro
genotypes were 0.10, 0.33 and 0.56, respectively. The allele
frequency for Arg allele and Pro allele was 27.11 and 72.89%,
respectively. The good treatment response rate was 10.7, 33.9 and
55.4% for Arg/Arg, Arg/Pro and Pro/Pro genotypes, respectively.
Poor treatment response rate for Arg/Arg, Arg/Pro and Pro/Pro
genotypes was 6.3, 25.0 and 68.8%, respectively. This polymorphism
were found to be not associated with response to therapy (P=0.571)
(Table II).
 | Table II.Response to treatment of the advanced
cervical cancer patients treated with chemoradiotherapy according
to genotypes of the TP53 Arg72Pro polymorphism. |
Table II.
Response to treatment of the advanced
cervical cancer patients treated with chemoradiotherapy according
to genotypes of the TP53 Arg72Pro polymorphism.
| TP53
Arg72Pro polymorphism | Good response (CR +
PR), no (%) | Poor response (SD +
PD), no. (%) | P-value |
|---|
| Genotype |
|
| 0.571 |
|
Arg/Arg | 25
(10.7) | 1 (6.3) |
|
|
Arg/Pro | 79
(33.9) | 4
(25.0) |
|
|
Pro/Pro | 129 (55.4) | 11 (68.8) |
|
| Allele |
|
| 0.271 |
|
Arg | 129 (27.7) | 6
(18.8) |
|
|
Pro | 337 (72.3) | 26 (81.2) |
|
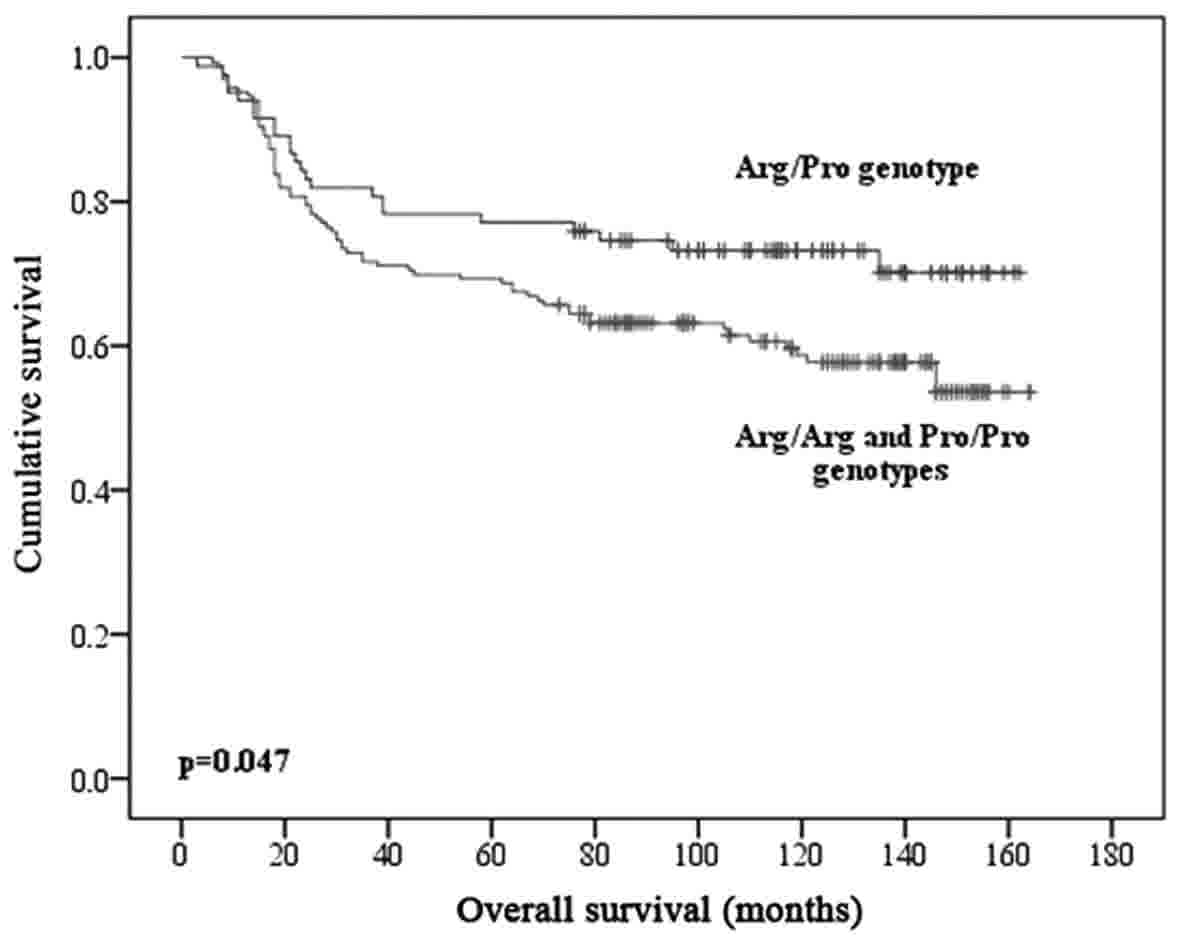
Regarding OS rates found using Kaplan-Meier method
and log-rank test, we observed that the mean survival rates were
not statistically different according to the patients TP53
Arg72Pro genotypes (P=0.058), age (P=0.630) and histology
(P=0.758). Stage (P=0.008) and recurrence (P<0.001) were
independent prognostic factors that influenced significantly OS of
women with advanced cervical cancer treated with chemoradiotherapy
(QTRT). Moreover, there are significant differences in mean
survival between heterozygote genotypes (Arg/Pro) and homozygous
genotypes (Arg/Arg and Pro/Pro). The group of patients carrying
heterozygous genotype present a higher mean survival rate than the
other patients (126 vs. 111 months, P=0.047) (Fig. 1).
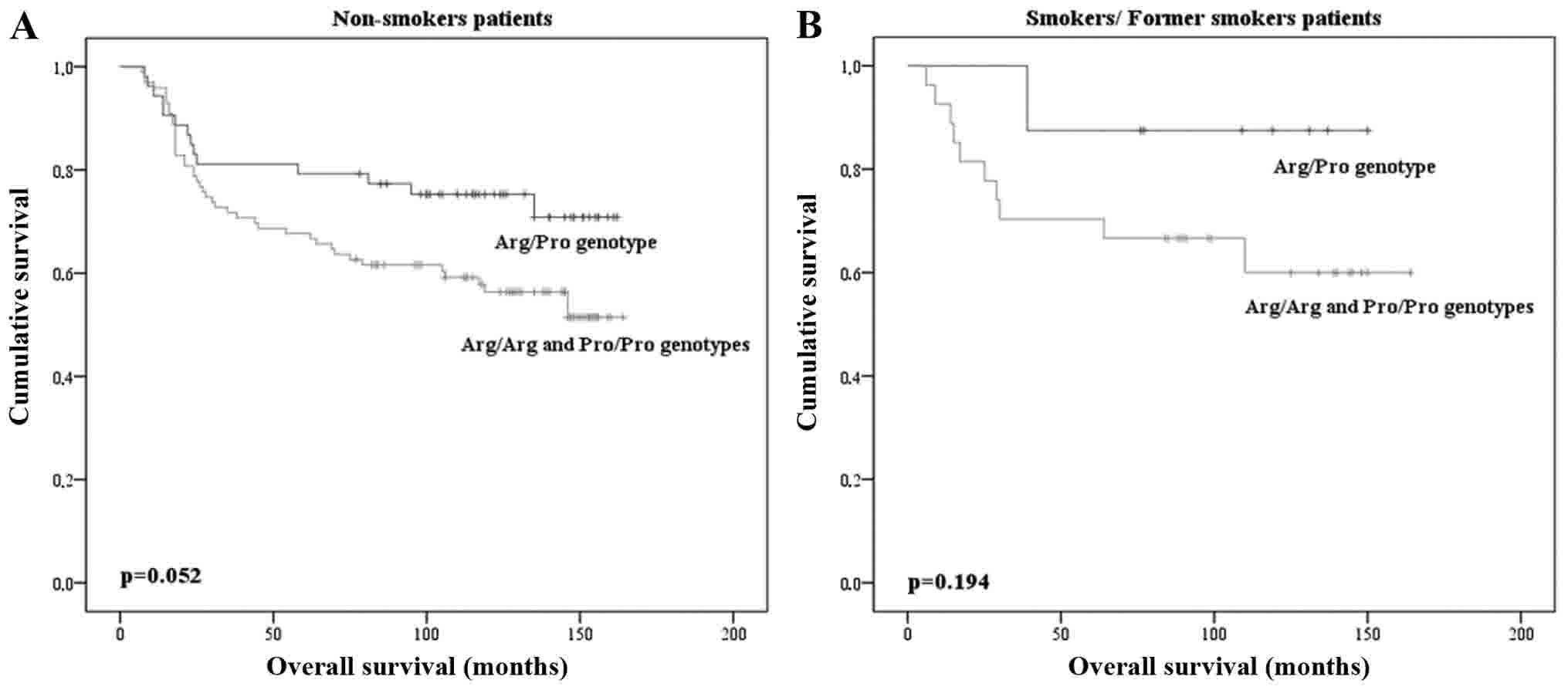
Concerning smoking history, our results demonstrate
that OS time differed according to the Arg/Arg and Pro/Pro
homozygous genotype vs Arg/Pro heterozygous genotypes carriers in
non-smoker individuals, but these results are in the threshold for
statistical significance (P=0.052; Fig.
2A). No statistically significant differences were found in the
genotype frequencies and OS rate among smokers and former smokers
(P=0.194; Fig. 2B). Using the Cox
regression analysis, we found that carriers of TP53 Arg72Pro
homozygous genotypes (Arg/Arg and Pro/Pro) present a 2-fold
increase of risk of death which is not statistically significant,
when compared with TP53 Arg72Pro heterozygous genotypes,
with tumor stage, median age, histology and smoking history as
covariates [hazard ratio (HR), 2.001; 95% CI, 0.917–4.368; P=0.082]
(Table III).
 | Table III.Multivariate analysis of death risk
at 5 years by Cox regression for the TP53 genotypes,
adjusted to different clinical and pathological variables. |
Table III.
Multivariate analysis of death risk
at 5 years by Cox regression for the TP53 genotypes,
adjusted to different clinical and pathological variables.
| Clinicopathological
characteristics | HR | 95% CI | P-value |
|---|
| Median age (<48
years/≥48 years) | 1.319 | 0.693–2.511 | 0.400 |
| Tumor stage
(<IIB/≥IIB) | 3.212 | 0.767–13.445 | 0.110 |
| Tobacco
(non-smokers/smokers and former smokers) | 0.938 | 0.817–1.077 | 0.364 |
| Histology
(adenosquamous cell carcinomas and small cell
carcinoma/adenocarcinoma and squamous cell carcinoma) | 0.680 | 0.092–5.001 | 0.705 |
| TP53
Arg72Pro genotypes (Homozygous/heterozygous) | 2.001 | 0.917–4.368 | 0.082 |
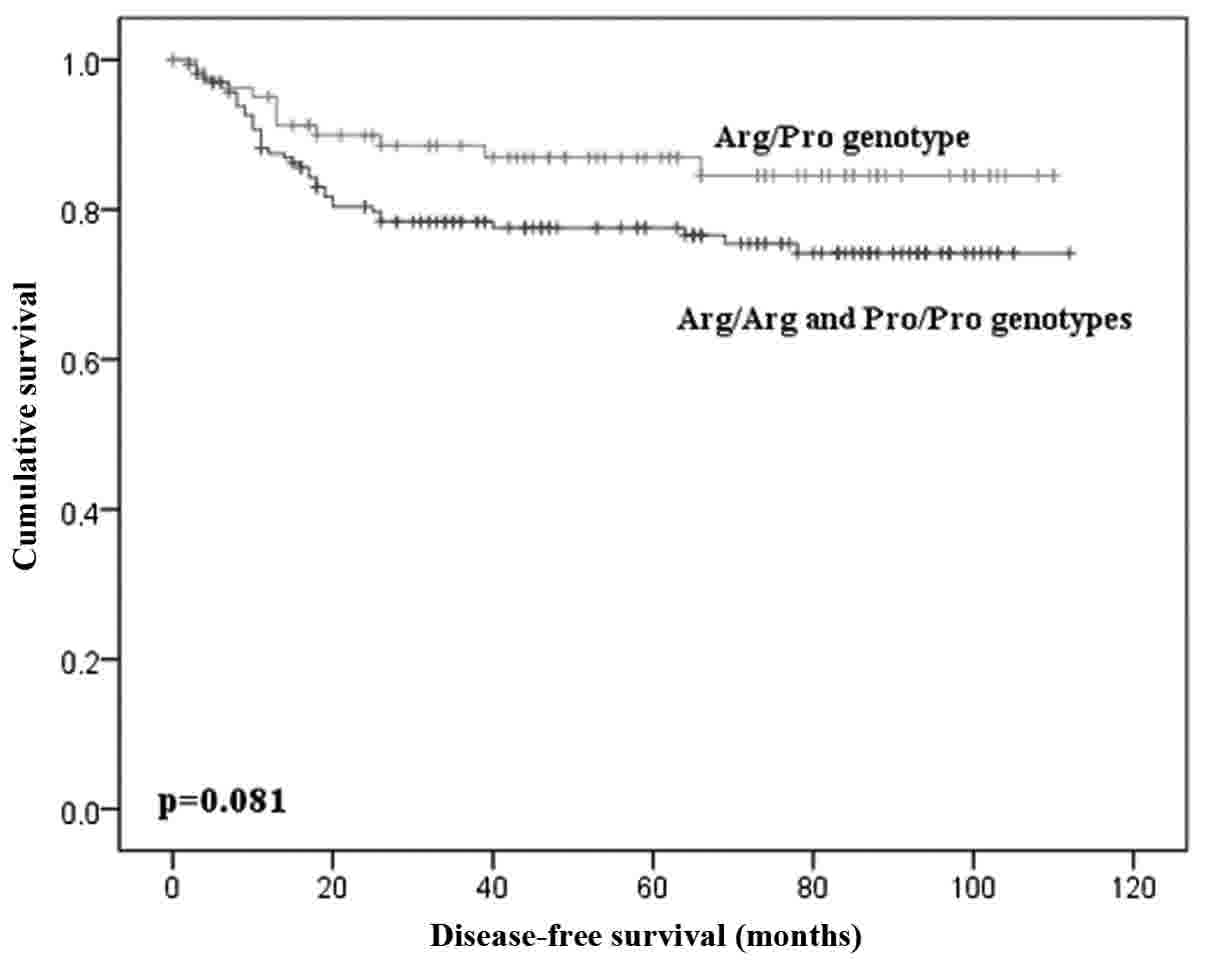
No difference was found for DFS according to the
distribution of genotypes of TP53 Arg72Pro polymorphism
(P=0.205), same when comparing patients with heterozygous and
homozygous genotypes (P=0.081; Fig.
3).
Discussion
The activation of the response to DNA damage aims to
cell cycle arrest and DNA repair, and the lesions correction
failure can result in the senescence or apoptosis (34). Assuming that cells respond differently
to DNA damage taking into account whether or not they are tumor
cells, understanding this mechanisms will allow selection of
therapeutic strategies to individualize response to DNA damage in
altered in cancer cells (35).
Apoptosis is the main mechanism by which anti-cancer agents
originate toxicity (36).
One major mechanism of resistance to therapy and
cell survival is the inactivation of the function of TP53
gene, so observed resistance in tumor cells harboring wild type
TP53 for a large variety of agents, such as ionizing
radiation and several classes of cytotoxic drugs, can occur
directly by factors that regulate or negate the functional activity
of this protein, or indirectly, by deregulation of pathways
downstream of this gene (37).
The role of the TP53 Arg72Pro polymorphism
remains controversial (17). The
segregation of this polymorphism shows pronounced ethnic
differences, so results will be dependent of the study population
(32,38). It is also important to refer that in
this study we did not consider HPV infections, although it might be
a relevant issue for additional studies. However, Medeiros and
colleagues (39) studied HPV
genotyping profile in squamous cervical lesions in Portugal, and
then they find high prevalence of HPV-16 and −18, approximately 80
and 15%, respectively, in cases with invasive cervical cancer.
Thus, future studies may include HPV genotyping to evaluate its
role in disease progression and clinical outcome under the
influence of the genetic background.
In our study, carriers of the heterozygous genotype
(Arg/Pro) had a higher mean survival overall than patients with
both homozygous genotypes (Arg/Arg and Pro/Pro). Several studies
have evaluated the influence of TP53 codon 72 polymorphism in the
clinical outcome of cancer patients with controversial results
(29,30,33,40).
Investigation of Piña-Sánchez et al (30) in Mexican women with cervical cancer
found a higher survival in heterozygous women (Arg/Pro) than in
homozygous women (Arg/Arg and Pro/Pro), however without significant
statistical differences. In study of Liu et al (29), they did not find association of
TP53 Arg72Pro polymorphism and clinical outcome in Chinese
women with cervical carcinoma.
In patients with pancreatic, testicular and prostate
cancer no significant effect of this polymorphism was found
(40,41). Pro allele homozygosis has been linked
with lower sensitivity to chemotherapy in breast and head and neck
cancer and lower survival in breast, lung and colorectal cancer
(42–46). Moreover other studies show that
carriers of Arg genotype have a higher treatment response rate and
survival after chemoradiotherapy in advanced head and neck cancer
and lung and breast cancer (43,44,47). The
presence of one mutated Arg allele may be associated with a reduced
sensitivity to cancer therapy in head and neck cancer as well as
retention of the Arg allele in heterozygous women with breast
cancer are associated with a reduced OS and progression-free
disease (48). In this sense,
Sullivan and colleagues (44) found
that drugs exert their cytotoxic effect in different ways,
according to the two codon 72 mutant variant of TP53 gene,
thus verifying a differentiated cell resistance.
There are four possible reasons that may explain the
fact that homozygous patients had lower survival than heterozygous
patients: i) As seen in other types of cancer, patients with
homozygous Pro allele have lower survival than heterozygous
carriers, since this allele has a major role in cell cycle arrest
and DNA repair than Arg allele (27).
Wild type TP53 Pro variant activates several genes involved
in DNA repair more effectively than TP53 Arg variant. At the
same time, cells expressing the TP53 Pro allele were able to
repair the DNA damage much more effectively than cells expressing
TP53 Arg allele (25); ii)
Patients with homozygous Arg allele showed lower survival rates
compared to heterozygous. It is important refer that the Arg
variant has been correlated with a higher affinity of binding and
degradation of TP53 protein by E6 oncoprotein of HPV-16/HPV-18
(38,49–51). One
of the better well-known functions of the HPV E6 is the ability to
increase the tolerance of normal response to DNA damage or then the
independent regulation of cellular growth (52,53). These
actions are promoted by TP53 degradation as well as the
inhibition of the gene and the multiple repair pathways.
Furthermore, the expression of E6 decreases the ability to repair
DSBs (53). In this sense, it is
believed that carriers of the Arg/Arg homozygous genotype have
lower apoptotic capacity, which results in poor survival; iii) the
low survival for Arg/Arg homozygous patients have a greater
affinity for the mutant TP53 creating mutants with
gain-of-function. Furthermore this variant seems to inhibit the
pro-apoptotic activity of the TP73 gene, which determines
the cellular response to different anticancer drugs. In head and
neck tumors where TP53 gene is frequently mutated it is
noted that Arg allele carriers had higher resistance to
chemotherapy leads to shorter survival. It should be noted that the
Pro allele is more frequent with TP53 wild type and tumors
are less sensitive to apoptosis (25); d) it is known that tumors with Arg
allele were associated with insufficient or absence of apoptosis,
because it was observed the absence of coexpression of Fas and
FasL, as well as high expression of Bcl-2 protein. In heterozygous
carriers of Arg72Pro polymorphism the absence of expression of
Bcl-2 and co-expression of Fas/FasL is not found. The Bcl-2, Fas
and FasL are three apoptosis-related proteins and the down
regulation of Fas expression is common in vary type of cancers,
including gynecological cancers (54).
This study also indicates that the influence of
Arg72Pro polymorphism in treatment response of cervical cancer
patients seems to be modulated by smoking history. Our results
demonstrate that non-smoker carriers of homozygous genotype present
a lower mean OS time comparing with patients with heterozygous
genotype (P=0.052), but these results are in the threshold for
statistical significance. However, this potential association was
not observed in smoker or former-smokers (P=0.194). In a similar
study in lung cancer no association was found between genotype of
the polymorphism and OS of patients, taking into account their
smoking history (P=0.850) (55).
Moreover, the biological mechanism that may explain these
differences in results is not yet known.
One of the possible limitations of the present study
was the exclusion of participants with cervical intraepithelial
neoplasia (CIN) or uninfected controls. Therefore, future studies
including these types of participants will be relevant to make this
study more complete, so to compare the impact of the TP53
Arg72Pro polymorphism in clinical outcome between pre-invasive
cancer patients and advanced cervical cancer patients.
In conclusion, our results demonstrate survival
advantage in heterozygous carriers of Arg72Pro polymorphism and a
trend to greater risk of death in the homozygous carriers of this
polymorphism. Furthermore, TP53 genotypes could be a useful
molecular tools for predicting the clinical outcome of cervical
cancer patients and may allow to evaluate optional therapeutic
regimens in patients with lower survival. Therefore, in the attempt
of optimizing responses and minimizing toxicities associated with
chemoradiotherapy, the analysis of a wide range of genetic
polymorphisms in DNA damages response genes may indicate the more
suitable therapeutic procedure for each cancer patient.
Acknowledgements
This authors thank the Research
Department-Portuguese League against Cancer (NRNorte) for their
general support provided.
Funding
The present study was funded by a research grant for
Doctoral project of Augusto Nogueira from the Ministério da
Ciência, Tecnologia e Ensino Superior-FCT (Fundação para a Ciência
e a Tecnologia: SFRH/BD/124155/2016) and by Project no.
CI-IPOP-22-2015 from the Research Center the Portuguese Institute
of Oncology of Porto, (Porto, Portugal).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AC and AN conceived and designed the experiments. AC
and AN performed the experiments. AC, AN and RM analyzed the data.
SS, JA, IB and RC contributed to the interpretation of results
obtained and manuscript construction. DP analyzed and interpreted
the patients' data regarding the clinical characteristics. AC and
AN wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All samples were obtained with the informed consent
of the participants prior to their inclusion in the study,
according to Helsinki Declaration principles and after approval of
the ethics committee of Portuguese Oncology Institute of Porto
(CES.287/014).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noordhuis MG, Eijsink JJH, Roossink F, de
Graeff P, Pras E, Schuuring E, Wisman GB, de Bock GH and van der
Zee AG: Prognostic cell biological markers in cervical cancer
patients primarily treated with (chemo)radiation: A systematic
review. Int J Radiat Oncol Biol Phys. 79:325–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Downs L: Advances in cervical cancer
treatment. Gynecol Oncol. 121:431–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujiwara M, Isohashi F, Mabuchi S,
Yoshioka Y, Seo Y, Suzuki O, Sumida I, Hayashi K, Kimura T and
Ogawa K: Efficacy and safety of nedaplatin-based concurrent
chemoradiotherapy for FIGO Stage IB2-IVA cervical cancer and its
clinical prognostic factors. J Radiat Res. 56:305–314. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tangjitgamol S, Katanyoo K, Laopaiboon M,
Lumbiganon P, Manusirivithaya S and Supawattanabodee B: Adjuvant
chemotherapy after concurrent chemoradiation for locally advanced
cervical cancer. Cochrane Database Syst Rev. 3:CD0104012014.
|
|
6
|
Hosoya N and Miyagawa K: Targeting DNA
damage response in cancer therapy. Cancer Sci. 105:370–388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bajinskis A, Natarajan AT, Erixon K and
Harms-Ringdahl M: DNA double strand breaks induced by the indirect
effect of radiation are more efficiently repaired by non-homologous
end joining compared to homologous recombination repair. Mutat Res.
756:21–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldstein M and Kastan MB: The DNA damage
response: Implications for tumor responses to radiation and
chemotherapy. Annu Rev Med. 66:129–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan KH, Blanco-Codesido M and Molife LR:
Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev
Oncol Hematol. 90:200–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jekimovs C, Bolderson E, Suraweera A,
Adams M, O'Byrne KJ and Richard DJ: Chemotherapeutic compounds
targeting the DNA double-strand break repair pathways: The good,
the bad and the promising. Front Oncol. 4:862014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basu A and Krishnamurthy S: Cellular
responses to Cisplatin-induced DNA damage. J nucleic acids.
2010:2013672010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Gu Y and Zhang SL: Association
between p53 codon 72 polymorphism and cervical cancer risk among
Asians: A huge review and meta-analysis. Asian Pac J Cancer Prev.
13:4909–4914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sionov RV and Haupt Y: The cellular
response to p53: The decision between life and death. Oncogene.
18:6145–6157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naccarati A, Polakova V, Pardini B,
Vodickova L, Hemminki K, Kumar R and Vodicka P: Mutations and
polymorphisms in TP53 gene-an overview on the role in colorectal
cancer. Mutagenesis. 27:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golubovskaya VM and Cance WG: Targeting
the p53 pathway. Surg Oncol Clin N Am. 22:747–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dos Santos HG, Nunez-Castilla J and
Siltberg-Liberles J: Functional diversification after gene
duplication: Paralog specific regions of structural disorder and
phosphorylation in p53, p63 and p73. PLoS One. 11:e01519612016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donehower LA: p53: Guardian and suppressor
of longevity? Exp Gerontol. 40:7–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haupt S, Raghu D and Haupt Y: Mutant p53
drives cancer by subverting multiple tumor suppression pathways.
Front Oncol. 6:122016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zhang C and Feng Z: Tumor
suppressor p53 and its gain-of-function mutants in cancer. Acta
biochim biophys Sin (Shanghai). 46:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dastjerdi MN: TP53 codon 72 polymorphism
and P53 protein expression in colorectal cancer specimens in
Isfahan. Acta Med Iran. 49:71–77. 2011.PubMed/NCBI
|
|
21
|
Hu X, Zhang Z, Ma D, Huettner PC, Massad
LS, Nguyen L, Borecki I and Rader JS: TP53, MDM2, NQO1 and
susceptibility to cervical cancer. Cancer Epidemiol Biomarkers
Prev. 19:755–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santos AM, Sousa H, Catarino R, Pinto D,
Pereira D, Vasconcelos A, Matos A, Lopes C and Medeiros R: TP53
codon 72 polymorphism and risk for cervical cancer in Portugal.
Cancer Genet Cytogenet. 159:143–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dokianakis DN and Spandidos DA: P53 codon
72 polymorphism as a risk factor in the development of
HPV-associated cervical cancer. Mol Cell Biol Res Commun.
3:111–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sousa H, Santos AM, Pinto D and Medeiros
R: Is there a biological plausability for p53 codon 72 polymorphism
influence on cervical cancer development? Acta Med Port.
24:127–134. 2011.PubMed/NCBI
|
|
25
|
Hrstka R, Coates PJ and Vojtesek B:
Polymorphisms in p53 and the p53 pathway: Roles in cancer
susceptibility and response to treatment. J Cell Mol Med.
13:440–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dahabreh IJ, Schmid CH, Lau J, Varvarigou
V, Murray S and Trikalinos TA: Genotype misclassification in
genetic association studies of the rs1042522 TP53 (Arg72Pro)
polymorphism: A systematic review of studies of breast, lung,
colorectal, ovarian and endometrial cancer. Am J Epidemiol.
177:1317–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bojesen SE and Nordestgaard BG: The common
germline Arg72Pro polymorphism of p53 and increased longevity in
humans. Cell Cycle. 7:158–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bergamaschi D, Gasco M, Hiller L, Sullivan
A, Syed N, Trigiante G, Yulug I, Merlano M, Numico G, Comino A, et
al: p53 polymorphism influences response in cancer chemotherapy via
modulation of p73-dependent apoptosis. Cancer Cell. 3:387–402.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu JH, Xi P, Chai YL, Wang J, Wang T, Liu
Z and Dai PG: Association of DNA repair gene polymorphisms with
response to cisplatin-based concurrent chemoradiotherapy in
patients with cervical carcinoma. DNA Repair (Amst). 41:69–72.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piña-Sánchez P, Hernández-Hernández DM,
Taja-Chayeb L, Cerda-Flores RM, González-Herrera AL, Rodea-Avila C,
Apresa-García T, Ostrosky-Wegman P, Vázquez-Ortíz G,
Mendoza-Lorenzo P, et al: Polymorphism in exon 4 of TP53 gene
associated to HPV 16 and 18 in Mexican women with cervical cancer.
Med Oncol. 28:1507–1513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sousa H, Santos AM, Pinto D and Medeiros
R: Is the p53 codon 72 polymorphism a key biomarker for cervical
cancer development? A meta-analysis review within European
populations. Int J Mol Med. 20:731–741. 2007.PubMed/NCBI
|
|
33
|
Papadakis ED, Soulitzis N and Spandidos
DA: Association of p53 codon 72 polymorphism with advanced lung
cancer: The Arg allele is preferentially retained in tumours
arising in Arg/Pro germline heterozygotes. Br J Cancer.
87:1013–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hollingworth R and Grand RJ: Modulation of
DNA damage and repair pathways by human tumour viruses. Viruses.
7:2542–2591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rassool FV and Tomkinson AE: Targeting
abnormal DNA double strand break repair in cancer. Cell Mol Life
Sci. 67:3699–3710. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinez-Rivera M and Siddik ZH:
Resistance and gain-of-resistance phenotypes in cancers harboring
wild-type p53. Biochem Pharmacol. 83:1049–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petitjean A, Achatz MI, Borresen-Dale AL,
Hainaut P and Olivier M: TP53 mutations in human cancers:
Functional selection and impact on cancer prognosis and outcomes.
Oncogene. 26:2157–2165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Medeiros R, Prazeres H, Pinto D,
Macedo-Pinto I, Lacerda M, Lopes C and Cruz E: Characterization of
HPV genotype profile in squamous cervical lesions in Portugal, a
southern European population at high risk of cervical cancer. Eur J
Cancer Prev. 14:467–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong M, Nio Y, Yamasawa K, Toga T, Yue L
and Harada T: p53 alteration is not an independent prognostic
indicator, but affects the efficacy of adjuvant chemotherapy in
human pancreatic cancer. J Surg Oncol. 82:111–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu WJ, Kakehi Y, Habuchi T, Kinoshita H,
Ogawa O, Terachi T, Huang CH, Chiang CP and Yoshida O: Allelic
frequency of p53 gene codon 72 polymorphism in urologic cancers.
Jpn J Cancer Res. 86:730–736. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang YC, Chen CY, Chen SK, Chang YY and
Lin P: p53 codon 72 polymorphism in Taiwanese lung cancer patients:
Association with lung cancer susceptibility and prognosis. Clin
Cancer Res. 5:129–134. 1999.PubMed/NCBI
|
|
43
|
Xu Y, Yao L, Ouyang T, Li J, Wang T, Fan
Z, Lin B, Lu Y and Xie Y: p53 Codon 72 polymorphism predicts the
pathologic response to neoadjuvant chemotherapy in patients with
breast cancer. Clin Cancer Res. 11:7328–7333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sullivan A, Syed N, Gasco M, Bergamaschi
D, Trigiante G, Attard M, Hiller L, Farrell PJ, Smith P, Lu X and
Crook T: Polymorphism in wild-type p53 modulates response to
chemotherapy in vitro and in vivo. Oncogene. 23:3328–3337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tommiska J, Eerola H, Heinonen M, Salonen
L, Kaare M, Tallila J, Ristimäki A, von Smitten K, Aittomäki K,
Heikkilä P, et al: Breast cancer patients with p53 Pro72 homozygous
genotype have a poorer survival. Clin Cancer Res. 11:5098–5103.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Starinsky S, Figer A, Ben-Asher E, Geva R,
Flex D, Fidder HH, Zidan J, Lancet D and Friedman E: Genotype
phenotype correlations in Israeli colorectal cancer patients. Int J
Cancer. 114:58–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pietsch EC, Humbey O and Murphy ME:
Polymorphisms in the p53 pathway. Oncogene. 25:1602–1611. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bonafé M, Ceccarelli C, Farabegoli F,
Santini D, Taffurelli M, Barbi C, Marzi E, Trapassi C, Storci G,
Olivieri F and Franceschi C: Retention of the p53 codon 72 arginine
allele is associated with a reduction of disease-free and overall
survival in arginine/proline heterozygous breast cancer patients.
Clin Cancer Res. 9:4860–4864. 2003.PubMed/NCBI
|
|
49
|
Storey A, Thomas M, Kalita A, Harwood C,
Gardiol D, Mantovani F, Breuer J, Leigh IM, Matlashewski G and
Banks L: Role of a p53 polymorphism in the development of human
papillomavirus-associated cancer. Nature. 393:229–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chansaenroj J, Theamboonlers A,
Junyangdikul P, Swangvaree S, Karalak A, Chinchai T and Poovorawan
Y: Polymorphisms in TP53 (rs1042522), p16 (rs11515 and rs3088440)
and NQO1 (rs1800566) genes in thai cervical cancer patients with
HPV 16 infection. Asian Pac J Cancer Prev. 14:341–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Santos AM, Sousa H, Pinto D, Portela C,
Pereira D, Catarino R, Duarte I, Lopes C and Medeiros R: Linking
TP53 codon 72 and P21 nt590 genotypes to the development of
cervical and ovarian cancer. Eur J Cancer. 42:958–963. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Peralta-Zaragoza O, Bermúdez-Morales VH,
Pérez-Plasencia C, Salazar-León J, Gómez-Cerón C and Madrid-Marina
V: Targeted treatments for cervical cancer: A review. Onco Targets
Ther. 5:315–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wallace NA and Galloway DA: Manipulation
of cellular DNA damage repair machinery facilitates propagation of
human papillomaviruses. Semin Cancer Biol. 26:30–42. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schneider-Stock R, Mawrin C, Motsch C,
Boltze C, Peters B, Hartig R, Buhtz P, Giers A, Rohrbeck A,
Freigang B and Roessner A: Retention of the arginine allele in
codon 72 of the p53 gene correlates with poor apoptosis in head and
neck cancer. Am J Pathol. 164:1233–1241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matakidou A, El Galta R, Webb EL, Rudd MF,
Bridle H, Eisen T and Houlston RS: GELCAPS Consortium: Lack of
evidence that p53 Arg72Pro influences lung cancer prognosis: An
analysis of survival in 619 female patients. Lung Cancer.
57:207–212. 2007. View Article : Google Scholar : PubMed/NCBI
|