Introduction
Prostate cancer (PCa) remains a leading cause of
cancer death in males (1). The
mainstay treatment for this disease is androgen deprivation therapy
(ADT), which has been practiced for many decades and showed
promising therapeutic effect on PCa patients (2). Androgen receptor (AR) has been widely
recognized as a key factor in determining PCa progression and
continues to exert biological functions even in the castrated
condition, in which androgen level is very low (3–6).
Therefore, further suppression of AR activity remains a therapeutic
goal. For instance, abiraterone and enzalutamide have been approved
to treat metastatic castration-resistant prostate cancer (mCRPC)
owing to their potent capacities to inhibit AR signaling (7–10).
PCa is a heterogeneous mass consisting of
fibroblasts, endothelial cells, stem progenitor cells and immune
cells, which together play profound roles in the pathogenesis of
prostate cancer (11,12). It is widely accepted that immune cells
are highly associated with PCa progression and clinical outcomes
(13). Natural killer (NK) cells
belong to innate immune system and exhibit antitumor function
either by directly killing tumor cells or by influencing other
immune cells via secreted cytokines such as TNFα and IFNr (14). Studies have been demonstrated that
cytotoxic activity of NK cells is highly correlated with castration
resistance and overall survival: patients with more NK cells
infiltration would have better prognosis and take longer time to
develop castration resistance (13).
According to these clinical phenomenon, it is urgent to find new
agents that can accelerate the proliferating rate of NK cells or
enhance their activity to cure PCa.
Calf spleen-derived lienal polypeptide (LP)
solution, consisting of small peptides, nucleic acids and
polysaccharides, has been clinically used in China and proved to
benefit patients who have leukemia, leukopenia, lymphoma and
advanced tumors (15). Stimulation of
innate immune response including NK cells is one of LP's
pharmacologic effects. Nevertheless, the detailed mechanism(s) by
which LP boosts the lysis activity of NK cells and how it affects
PCa progression is still largely unknown.
In this study, we found that LP can enhance the
cytotoxic activity of NK cells to kill PCa cells. LP-mediated NK
activity bore the ability to downregulate AR expression levels,
which in turn caused a released expression of MICA/MICB. Our
original findings suggest that LP may be developed alone or in
combination with other drugs to better suppress PCa growth.
Materials and methods
Cell culture
C4-2 and CRW22Rv1 cells (ATCC, Manassas, VA, USA)
were cultured in 10% FBS RPMI-1640 with 100 U/ml penicillin, 100
µg/ml streptomycin and 1% L-glutamine. NK-92MI cells (ATCC), were
maintained in α-MEM (Invitrogen Life Technologies, Carlsbad, CA,
USA) with 0.2 mmol/l inositol, 0.1 mmol/l 2-mercaptoethanol, 0.02
mmol/l folic acid, horse serum to a final concentration of 12.5%
and FBS to a final concentration of 12.5%. Cells were maintained in
humidified 5% CO2 environment at 37°C. For transient
transfection experiments, Lipofectamine 3000 (Invitrogen, Grand
Island, NY, USA) was used.
Plasmids construction and lentivirus
generation
Twenty-onenucleotides against the target gene will
be synthesized and cloned into the plKO vector. AR cDNA was cloned
into PWPI vector. Lentiviral particles will be generated by
transfection of lentiviral expressing plasmid, packaging plasmid
psPAX2, and pMD2.G envelope plasmid into HEK293 cells. Lentiviral
particles will be collected to transduce target cells according to
Addgene's lentiviral protocol.
Site-directed gene mutagenesis
The promoter of MICA/MICB was cloned into basic pGL3
using and site-directed gene mutagenesis was performed as
previously described (16). Briefly,
PCR was conducted in 20 µl volume system: DNA template (50 ng)
(pGL3-promoter-MICA or pGL3-promoter-MICB), 2 µl 10X phusion
buffer, 0.25 µl phusion enzyme, 1 µl 10 µM forward or reverse
primers. The reaction was done as follows: 98°C for 5 min, 30
cycles of: 98°C, 30 sec; 60°C, 30 sec; 72°C, 2 min. PCR products
was digested with DpnI and subjected to PNK treatment, then
was ligated with T4 ligase before transformation. Mutated clone was
confirmed by HindIII restriction enzyme. The primers used in
mutagenesis are:
pGL3-promoter-MICA forward,
5′-GGGGTACCGGGATTATAGTCATGAACCACTG and reverse,
5′-CCCTCGAGCTCAGAATGCGGTGACAGC; pGL3-promoter-MICB forward,
5′-GGGGTACCCCAGTCTCTGAAGTCACTGTCA and reverse,
5′-CCCTCGAGCCTCGGCGCCCGAAAGCTTT; pGL3-promoter-MICA-mut forward,
5′-AGATCTTCCCAATAAGATGATTTA and reverse, 5′-TTGCATGCAATAAACTGCATCT;
pGL3-promoter-MICB-mut forward, 5′-AGATCTTCCCAATATGATGATTTA and
reverse, 5′-TTGCATGCAATAAACTGCATCT.
MTT cell viability assay
C4-2 or CRW22Rv1 cells (1X104)were loaded
into 24-well plates and treated with LP with or without NK-92MI
cells. After 24 h later, NK-92MI cells were washed away and 0.5 ml
MTT (0.5 mg/ml) was added to each well and incubated for another 2
h. The absorbance at 570/630 nm was detected.
RNA extraction and qRT-PCR assay
Total RNAs (1 µg), which were extracted using TRIzol
reagent (Invitrogen Life Technologies), were subjected to reverse
transcription using SuperScript III Transcriptase (Invitrogen Life
Technologies). qRT-PCR was conducted using a Bio-Rad CFX96 system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with SYBR-Green to
determine the mRNA expression level of genes of interest. GAPDH was
used as control.
Western blotting
Cells were lysed in ice-cold RIPA lysis buffer at
4°C for 30 min. After centrifugation, equal amounts of the protein
were loaded for electrophoresis on 8–12% denaturing SDS-PAGE gels
and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). After being blocked, membranes were
incubated with appropriate dilutions of specific primary antibodies
(1:1,000) overnight before their one-hour incubation with
HRP-conjugated secondary antibodies. Signal was visualized using
ECL system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). AR
(N20), and GAPDH (8C2) (both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) were used in this study.
Chromatin immunoprecipitation
(ChIP)
Briefly, protein/DNA complexes were cross-linked by
1% formaldehyde for 10 min, then quenched using 125 mM glycine for
10 min. Cells were lysed and subjected to sonication to get
chromatin DNA fragments (~500 bp in length). After centrifugation,
the supernatant was incubated with AR antibody overnight at 4°C.
Next, pre-blocked A/G beads were added to AR/DNA complex for
another 1 h, and chromatin DNA was purified using gel extraction
kit (Invitrogen Life Technologies) and subjected to
semi-quantitative PCR analysis.
Luciferase assay
Cells were plated in 24-well plates and transfected
with MICA-pGL3/MICB-pGL3 or its corresponding mutant (100 ng/well)
using Lipofectamine 3000 (Invitrogen Life Technologies) according
to the manufacturer's instructions, pRL-TK (1 ng/well) was
co-transfected as control. Two days later, cells were lysed for
luciferase activity detection by the dual luciferase assay. Each
Luciferase reading was performed in triplicate.
Statistical analysis
All values are reported as the mean ± SD and all
comparisons were analyzed with a t-test or a one way ANOVA followed
by t-test.
Results
LP enhances the cytotoxicity of NK
cells to kill PCa cells
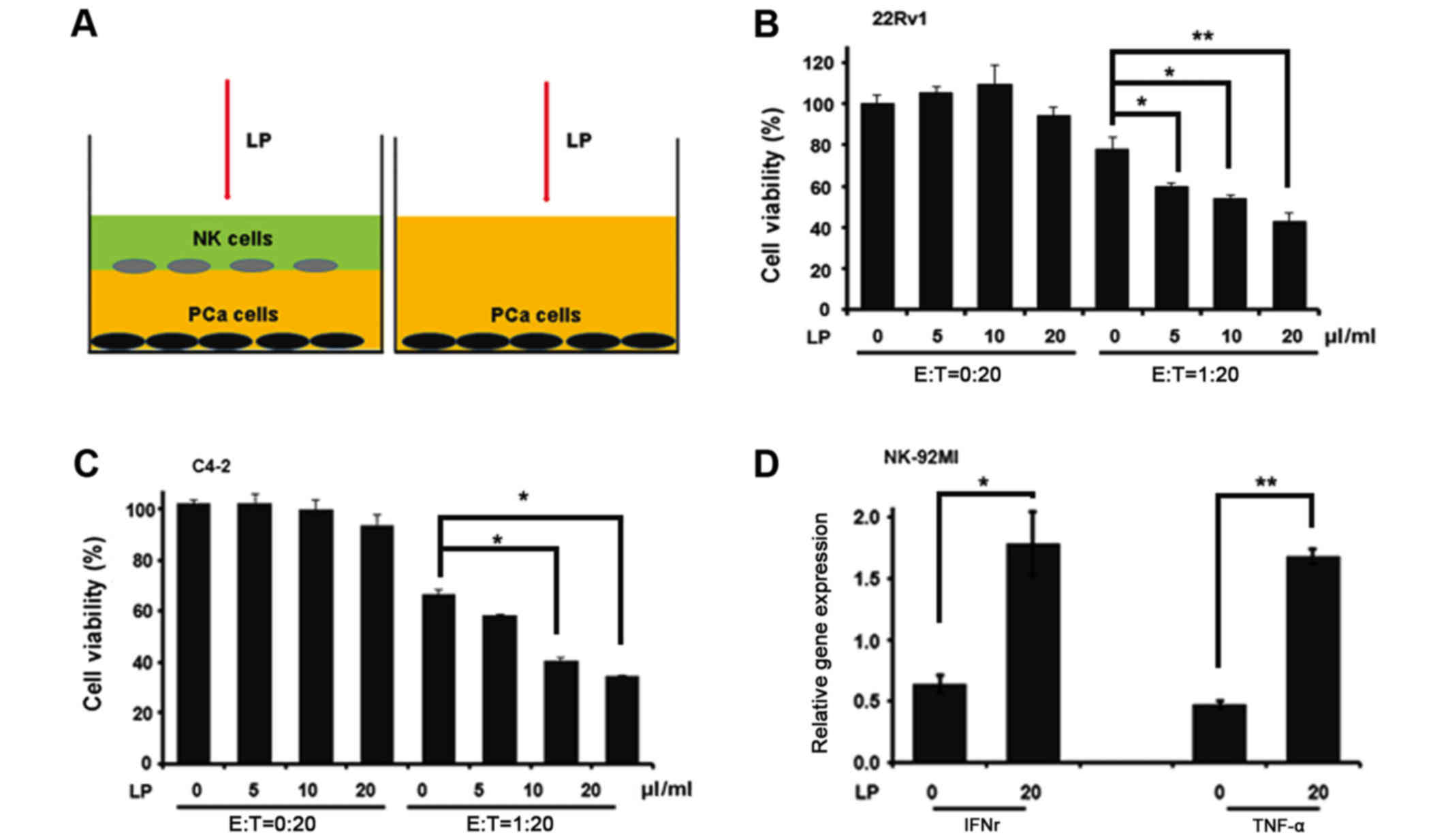
In China, LP solution has been applied to treat
advanced cancer alone or in combination with chemotherapy drugs. To
explore whether LP can alter the capacity of NK cells to lyse PCa
cells, we applied co-culture system as indicated in Fig. 1A to end this. Interestingly, LP alone
failed to influence CWR22Rv1 cell viability while combined with
NK-92MI cells dramatically reduced the cell number of CWR22Rv1
after 24-hour co-culture (Fig. 1B),
indicating that LP indeed could enhance the killing ability of
NK-92MI cells. We also obtained similar result when CWR22Rv1 cell
line was replaced by C4-2 cell line (Fig.
1C). One hallmark of NK activation is cytokine production. To
investigate this, we collected NK-92MI cells after their co-culture
with CWR22Rv1 cells and extracted RNAs for the measurement of TNFα
and IFNr, two important cytokines determining the cytotoxic
activity of NK cells. Consistently, the expression levels of TNFα
and IFNr were much more abundant in cells which were treated by LP
compared to these in non-treated ones (Fig. 1D). Taken together, all these data
validate the notion that LP could activate NK cells to kill PCa
cells.
AR renders LP-mediated NK cytotoxicity
to PCa cells
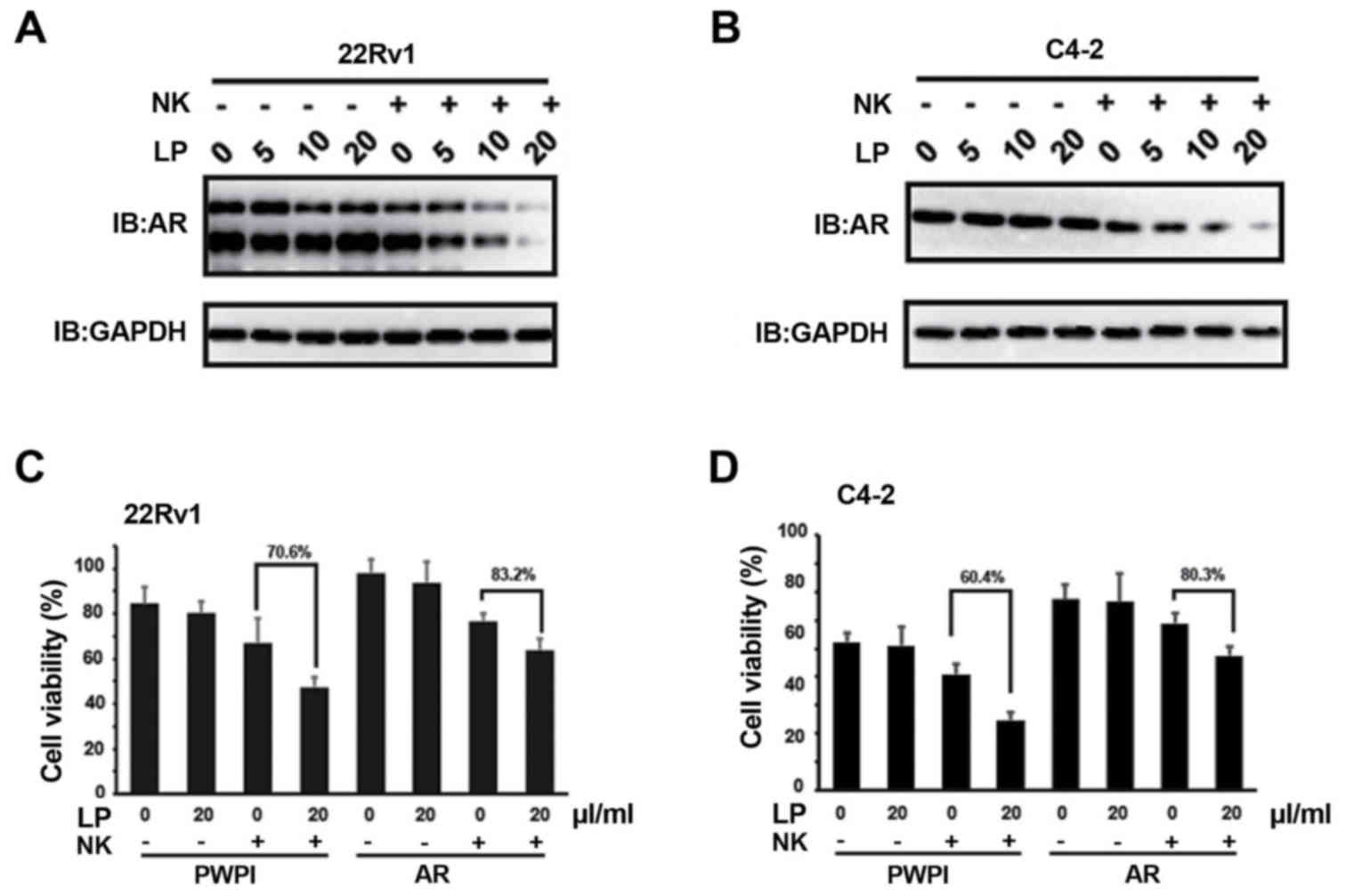
Previous study has documented that NK cells could
suppress AR expression (17).
Consistently, we also found that the protein levels of AR in both
CWR22Rv1 (Fig. 2A) and C4-2 (Fig. 2B) were markedly suppressed when LP was
present in the co-culture system. However, AR mRNA levels are
indiscriminate in this system (data not shown), suggesting NK
cells/LP mediated AR decrease is independent of transcriptional
regulation. To validate the involvement of AR signaling in
conferring the cytotoxicity of NK cells to PCa cells, we
exogenously introduced AR cDNA into CWR22Rv1 cells and found that
the cytotoxicity of NK cells to PCa cells was attenuated even when
LP was present in the co-culture system (Fig. 2C). Similar phenomenon was observed in
C4-2 cells (Fig. 2D). Collectively,
these data indicate that AR signaling in PCa cells is one of the
effectors rendering NK cytotoxicity to PCa cells, which is mediated
by LP solution in this case.
MICA and MICB are directly regulated
by AR
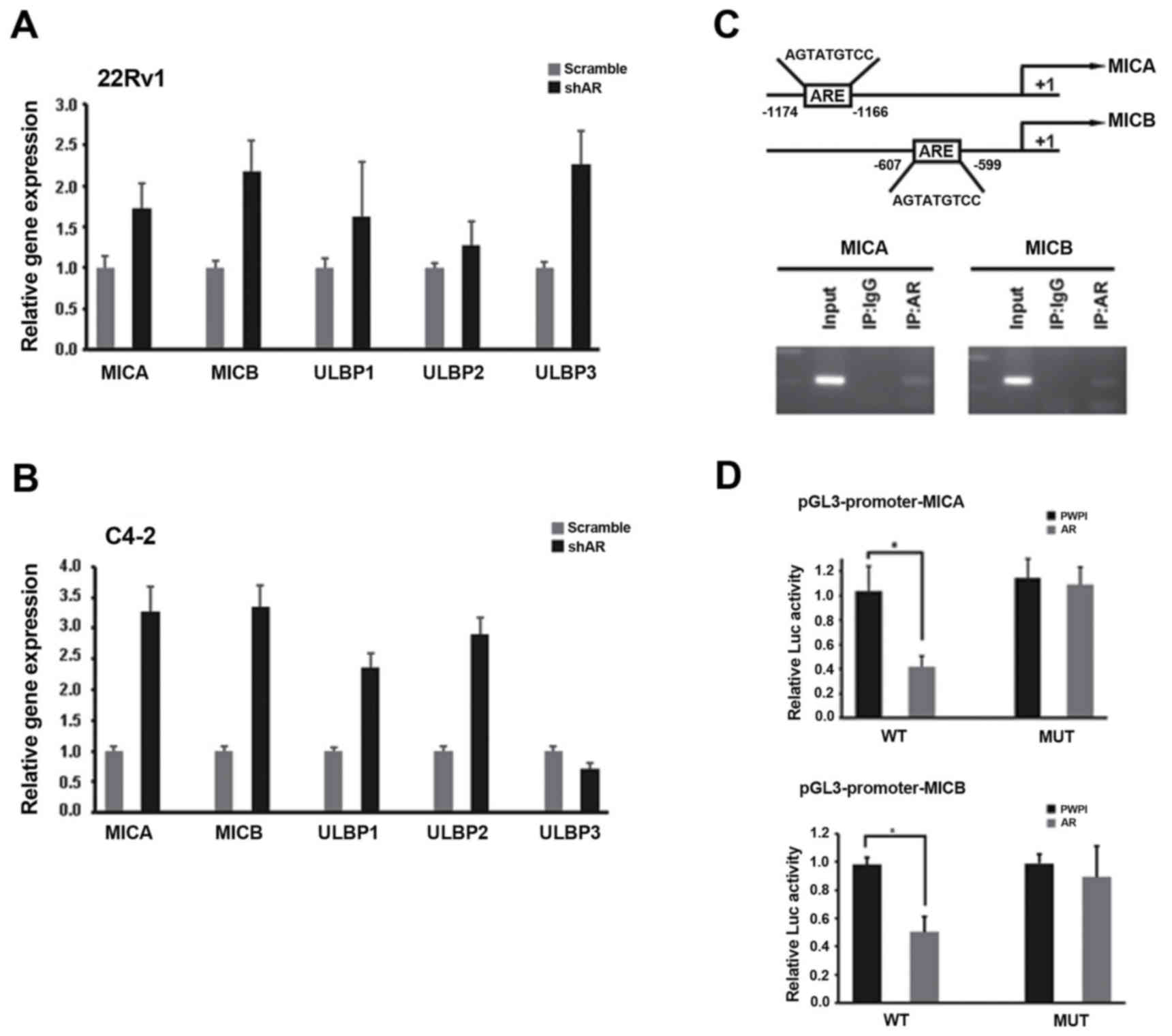
Given that fact that AR is implicated into
LP-mediated NK cytotoxicity, we continue to explore the downstream
genes those are responsible for LP-mediated NK activation. To end
this, we first focused several candidates (MICA, MICB, ULBP1, ULBP2
and ULBP3) because they are NKG2D ligands and can stimulate NK
cells activation (18). As shown in
Fig. 3A and B, MICA and MICB were
consistently elevated at mRNA levels when AR was suppressed in
CWR22Rv1 and C4-2 cells. According to online software analysis
indicating that there is one AR response element (ARE) in the
proximal promoters of MICA and MICB (Fig.
3C, top), we postulate that AR transcriptionally regulates
their expression levels. Indeed, ChIP assay revealed that AR
directly bound the proximal promoters of MICA and MICB (Fig. 3C, bottom). To test whether AR could
regulate the promoters' activities of MICA and MICB, we performed
luciferase-based promoter's activity assay, which showed that AR
could suppress the promoters' activities of MICA and MICB while the
suppression on their corresponding mutants could not be observed
(Fig. 3D). All these data suggest
that AR transcriptionally suppresses MICA and MICB expression,
which can be reversed by LP-mediated NK activation.
MICA and MICB are involved in
LP-mediated NK activation
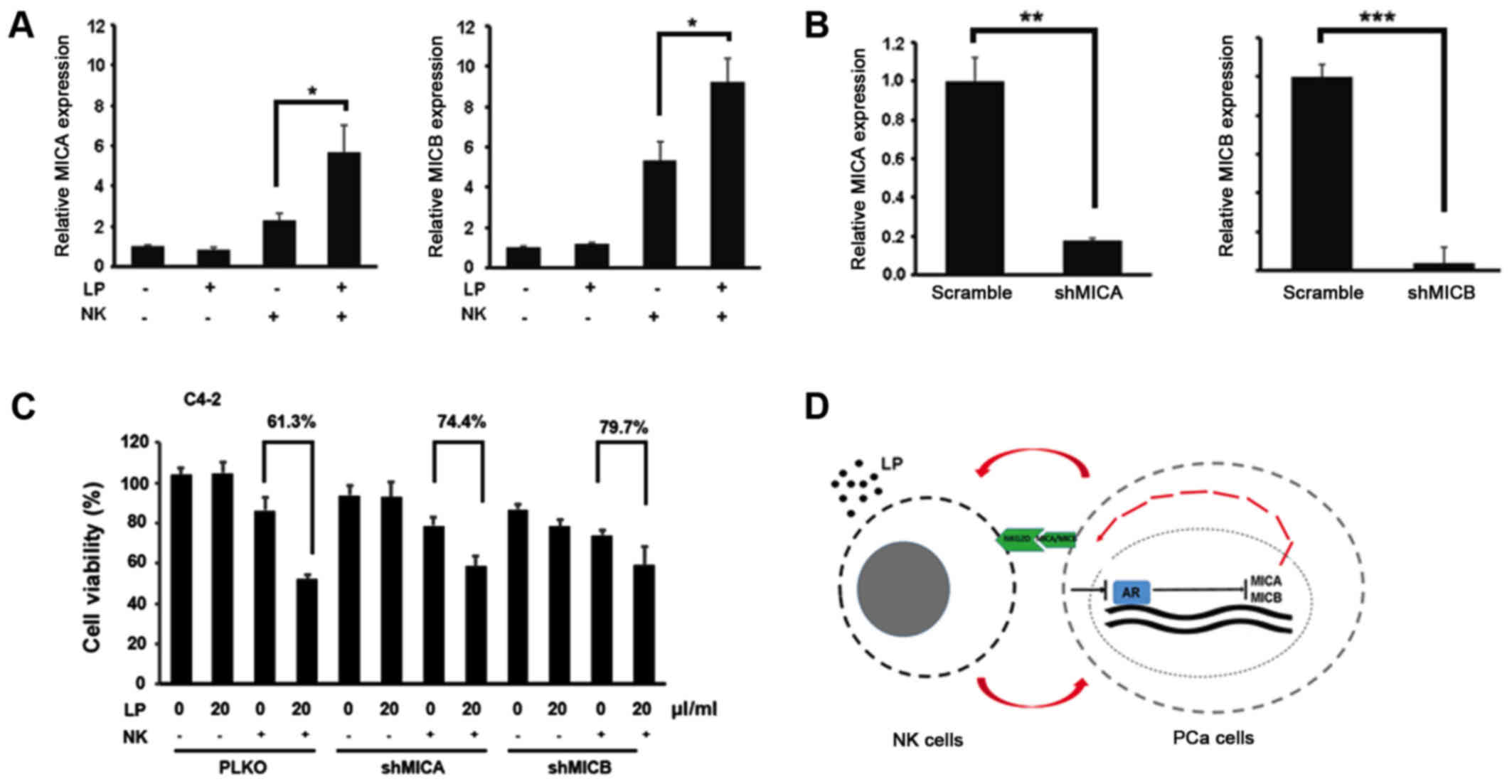
To further confirm the role of MICA and MICB in
LP-mediated NK cytotoxicity, we first measured the expression
levels of MICA and MICB in PCa cells upon NK treatment in the
presence or absence of LP. Fig. 4A
demonstrated that combination of NK cells and LP could elevate MICA
and MICB expression in C4-2 cells. More importantly, LP-mediated NK
cytotoxic activity against PCa cells was partially abolished by
knockdown of MICA and MICB in C4-2 cells (Fig. 4B and C). All these data prove that
MICA/MICB is indeed involved in LP-mediated NK activation, probably
by binding to NKG2D receptor on the surface of NK cells.
In conclusion, LP could activate NK cells to kill
PCa cells. In return, PCa cells undergo an AR downregulation
process to boost MICA/MICB expression, which further triggers NK
cells activation to form a positive loop (Fig. 4D).
Discussion
PCa continues to threaten male's health for many
decades. Although drugs targeting AR signaling have shown promising
results in the treatment of PCa patients, the complex
micro-environment surrounding tumor is one of the obstacles that
affects their therapeutic efficacy. As a key player in innate
immune response, NK cells play fundamental roles in the progression
of PCa and their infiltration is highly associated with clinical
outcomes. Here, we found that LP solution can boost the cytotoxic
activity of NK cells to lyse PCa cells. Interestingly, we also
observed that LP-mediated NK activation can downregulate AR
expression, leading to the released expression of its downstream
genes: MICA and MICB. Overexpression of AR or knockdown of
MICA/MICB could attenuate LP-mediated cytotoxicity of NK cells to
both C4-2 and CWR22Rv1 cells, suggesting that AR-MICA/MICB pathway
is the downstream effector of NK cells and LP solution.
The population of NK cells in PCa microenvironment
is highly related to disease progression. Study has demonstrated
that patients who harbor higher amount of NK cells would have much
delayed castration resistance. Since LP solution can activate the
cytotoxicity of NK cells, we believe that LP solution, if applied
clinically, would synergize ADT treatment. More importantly,
amplification of AR signaling due to gene amplification, gene
mutation and occurrence of AR-v7 in castration resistant stage are
the major concerns for current treatments (19–22), which
would be alleviated by LP solution because it enhances the capacity
of NK cells to downregulate both AR-FL and AR-v7. All these data
suggest that LP is the potential agent that can alter castration
resistance via stimulating the activity of NK cells.
We also found that LP can upregulate MICA/MICB
expression via degrading AR. MICA and MICB, which interact with
their common receptor NKG2D on NK cells to trigger cytotoxic event
or cytokine production, are frequently observed in PCa patients,
indicating its potential role in linking PCa tumor to NK cells
(23). The upregulation of MICA and
MICB mediated by LP would provide a positive feedback loop between
PCa and NK cells. In this case, PCa cells undergo a self-destroyed
mechanism to recruit more NK cells to kill themselves. Also, in
castration resistant stage, the amplification of AR signaling
probably suppresses MICA/MICB expression so that the interaction
between NK cells and PCa cells is marginal, resulting in
insufficient cytotoxicity of NK cells to PCa cells. In summary, our
data provide strong rational to develop LP solution as new agent to
battle PCa.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huggins C: Effect of orchiectomy and
irradiation on cancer of the prostate. Ann Surg. 115:1192–1200.
1942. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coutinho I, Day TK, Tilley WD and Selth
LA: Androgen receptor signaling in castration-resistant prostate
cancer: A lesson in persistence. Endocr Relat Cancer. 23:T179–T197.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bishop JL, Davies A, Ketola K and Zoubeidi
A: Regulation of tumor cell plasticity by the androgen receptor in
prostate cancer. Endocr Relat Cancer. 22:R165–R182. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaarbø M, Klokk TI and Saatcioglu F:
Androgen signaling and its interactions with other signaling
pathways in prostate cancer. Bioessays. 29:1227–1238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Efstathiou E, Titus M, Tsavachidou D,
Tzelepi V, Wen S, Hoang A, Molina A, Chieffo N, Smith LA, Karlou M,
et al: Effects of abiraterone acetate on androgen signaling in
castrate-resistant prostate cancer in bone. J Clin Oncol.
30:637–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasaitis TS, Bruno RD and Njar VC: CYP17
inhibitors for prostate cancer therapy. J Steroid Biochem Mol Biol.
125:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tran C, Ouk S, Clegg NJ, Chen Y, Watson
PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al:
Development of a second-generation antiandrogen for treatment of
advanced prostate cancer. Science. 324:787–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corn PG: The tumor microenvironment in
prostate cancer: Elucidating molecular pathways for therapy
development. Cancer Manag Res. 4:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karlou M, Tzelepi V and Efstathiou E:
Therapeutic targeting of the prostate cancer microenvironment. Nat
Rev Urol. 7:494–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasero C, Gravis G, Granjeaud S, Guerin M,
Thomassin-Piana J, Rocchi P, Salem N, Walz J, Moretta A and Olive
D: Highly effective NK cells are associated with good prognosis in
patients with metastatic prostate cancer. Oncotarget.
6:14360–14373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang R, Jaw JJ, Stutzman NC, Zou Z and Sun
PD: Natural killer cell-produced IFN-γ and TNF-α induce target cell
cytolysis through up-regulation of ICAM-1. J Leukoc Biol.
91:299–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Y, Hu Q, Sun C, Gu B, Xu K and Xia G:
Postoperative renormalization of C-reactive protein with adjuvant
lienal polypeptide and its association with tumour recurrence in T1
clear cell renal cell carcinoma. J Int Med Res. 44:620–626. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brett A, Pandey S and Fraizer G: The
Wilms' tumor gene (WT1) regulates E-cadherin expression and
migration of prostate cancer cells. Mol Cancer. 12:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin SJ, Chou FJ, Li L, Lin CY, Yeh S and
Chang C: Natural killer cells suppress enzalutamide resistance and
cell invasion in the castration resistant prostate cancer via
targeting the androgen receptor splicing variant 7 (ARv7). Cancer
Lett. 398:62–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nausch N and Cerwenka A: NKG2D ligands in
tumor immunity. Oncogene. 27:5944–5958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koivisto P, Kononen J, Palmberg C, Tammela
T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A,
Visakorpi T and Kallioniemi OP: Androgen receptor gene
amplification: A possible molecular mechanism for androgen
deprivation therapy failure in prostate cancer. Cancer Res.
57:314–319. 1997.PubMed/NCBI
|
|
20
|
Joseph JD, Lu N, Qian J, Sensintaffar J,
Shao G, Brigham D, Moon M, Maneval EC, Chen I, Darimont B and Hager
JH: A clinically relevant androgen receptor mutation confers
resistance to second-generation antiandrogens enzalutamide and
ARN-509. Cancer Discov. 3:1020–1029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dehm SM, Schmidt LJ, Heemers HV, Vessella
RL and Tindall DJ: Splicing of a novel androgen receptor exon
generates a constitutively active androgen receptor that mediates
prostate cancer therapy resistance. Cancer Res. 68:5469–5477. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang R, Sun Y, Li L, Niu Y, Lin W, Lin C,
Antonarakis ES, Luo J, Yeh S and Chang C: Preclinical study using
malat1 small interfering RNA or androgen receptor splicing variant
7 degradation enhancer ASC-J9®to suppress
enzalutamide-resistant prostate cancer progression. Eur Urol.
72:835–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu JD, Higgins LM, Steinle A, Cosman D,
Haugk K and Plymate SR: Prevalent expression of the
immunostimulatory MHC class I chain-related molecule is
counteracted by shedding in prostate cancer. J Clin Invest.
114:560–568. 2004. View Article : Google Scholar : PubMed/NCBI
|