Introduction
Breast cancer is one of the most common malignant
tumours among women (1). There were
precise data from 2012 related to breast cancer incidence and
mortality. Across 5 years, from 2008 to 2012, the average incidence
rates for white women were the highest, followed by those of black
women (2,3). The current 5-year survival rate of
primary breast cancer is relatively high, ranging from 80 to 92% in
different populations (4). However,
it decreases to <25% when the disease becomes metastatic
(4,5).
The most important factor to improve the survival rate of patients
is to find the most effective treatment, which is guided by tumour
cell characteristics (6,7). Once a metastatic lesion is found,
accurate characterisation of the tumour cells must be obtained at
the start of treatment (8); a
possible way to do this is the use of biomarkers (9). Currently, a series of different
biomarkers, such as tissue markers, genetic markers, serum markers
and non-coding RNA (1,10,11), have
been found, but it is much more difficult to assess the
effectiveness of the targeted treatment or prognosis of the
disease. Therefore, we need to find more biomarkers and determine
their clinical utility in future research (9).
CXXC finger protein 5 (CXXC5) is a protein encoded
by the CXXC5 gene localised to the 5q31.3 chromosomal
region, which is often deleted in myeloid leukaemia (12). Kühnl et al (13) reported that CXXC5 could suppress
progression of acute myeloid leukaemia (AML) via inhibiting the Wnt
pathway and that downregulation of CXXC5 could predict a better
prognosis in AML. The study of Bruserud et al (14) showed that high CXXC5 expression was
related to the stem cell signature of AML that has a bad prognostic
impact. We know that 17β-oestradiol (E2) plays an important role in
the homeodynamic regulation of breast tissue functions, and the
oestrogen receptor (ERα) is the primary transcript expressed in
breast tissue. Yasar et al (15) reported that E2-ERα could regulate the
expression of CXXC5. Therefore, we knew that there was a certain
relationship between CXXC5 and breast cancer. Knappskog et
al (16) reported that the
overexpression of CXXC5 was significantly associated with a bad
prognosis in breast cancer. However, the prognostic implications of
CXXC5 expression in breast cancers of different molecular types
remain unclear. In our study, we used Breast Cancer Gene-Expression
Miner v4.0 (bc-GenExMiner v4.0,
bcgenex.centregauducheau.fr/BC-GEM/GEM-Accueil.php?js=1) (17), a database that includes a total of
5,861 patients, as the main tool to analyse the role of CXXC5
expression in different breast cancer subtypes. We aimed to show
that CXXC5 expression predicts the prognosis of different breast
cancer subtypes.
Materials and methods
GEO data analysis
We obtained the dataset of GDS5666 (18) from Gene Expression Omnibus (GEO;
https://www.ncbi.nlm.nih.gov/geo/)
(19) and analysed it using the Data
Analysis Tools of DATASET BROWSER in GEO. Probes of A_51_P234788
(ID_REF) and A_52_P633393 (ID_REF) represented the CXXC5
gene in the platform of GPL7202. We obtained 2 sets of CXXC5 mRNA
expression values from these probes. We used the average value of
each sample's CXXC5 mRNA expression as the expression value for
that sample.
Bioinformatics analysis by
bc-GenExMiner v4.0
Using bc-GenExMiner v4.0, we conducted CXXC5
expression analysis, prognostic analysis for CXXC5 through
univariate Cox analysis and Kaplan-Meier curve analysis, and gene
correlation analysis for CXXC5. Then, we obtained the gene ontology
(GO) term results through gene correlation exhaustive analysis. The
database of bc-GenExMiner v4.0 had 36 datasets, including a total
of 5,861 patients. There were 21 datasets including 3,524 patients
for CXXC5 expression analysis and gene correlation analysis among a
total of 36 datasets. A total of 3,472 patients from 21 datasets
were used for prognostic analysis for CXXC5 with any nodal status,
any ER status and any event (AE).
Statistical analysis
In the comparison of CXXC5 expression in primary and
metastatic tumours, we used SPSS version 19.0 (IBM SPSS, Armonk,
NY, USA) as the software for statistical analysis. Two-tailed
unpaired t-tests were used for statistical comparisons. Data are
represented as the means ± standard error of the mean. P<0.05
was considered significant. In other research, the statistical
analysis for comparison of CXXC5 expression according to ER and
Kaplan-Meier survival curves and univariate Cox analysis was
performed by bc-GenExMiner v4.0. Box and whiskers plots are
displayed, along with Dunett-Tukey-Kramer's test and Welch's t-test
for every possible clinical criteria for CXXC5 gene.
Results
CXXC5 expression is increased in
4T1-derived metastatic cancer compared to primary cancer
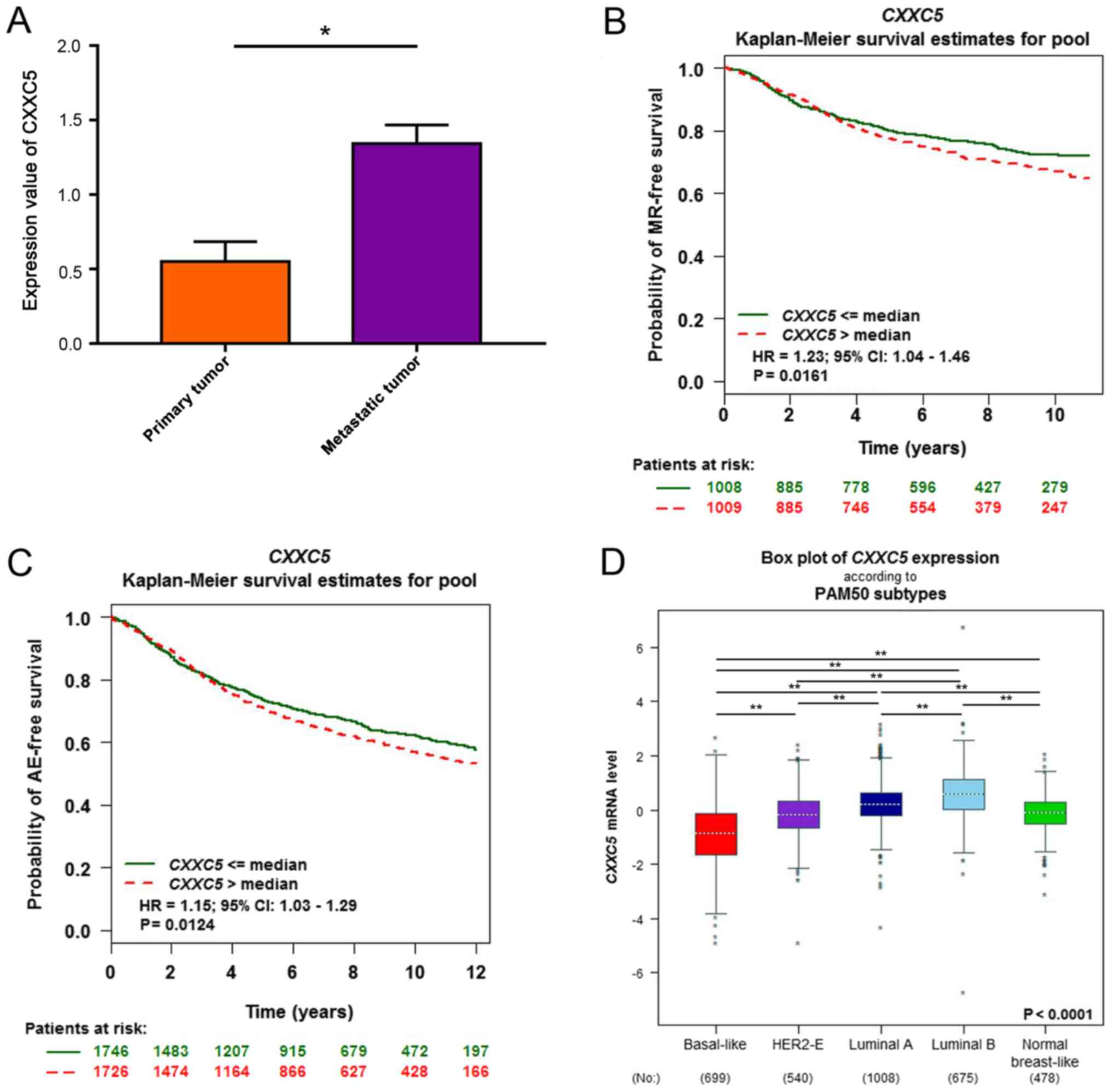
We observed that the expression values of CXXC5 were
higher in 4T1-derived metastatic populations than in primary
cancers (Fig. 1A). Then, we used
bc-GenExMiner v4.0 to determine that CXXC5 upregulation with
metastatic relapse (MR) or AE was associated with a poor prognosis
of breast cancer (Fig. 1B and C). In
the PAM50 breast cancer subtypes, the basal-like subtype had the
lowest CXXC5 expression, and CXXC5 expression of luminal tumours
was higher than in other types (Fig.
1D).
High level of CXXC5 is a poor
prognostic factor in oestrogen receptor positive (ER+) breast
cancer
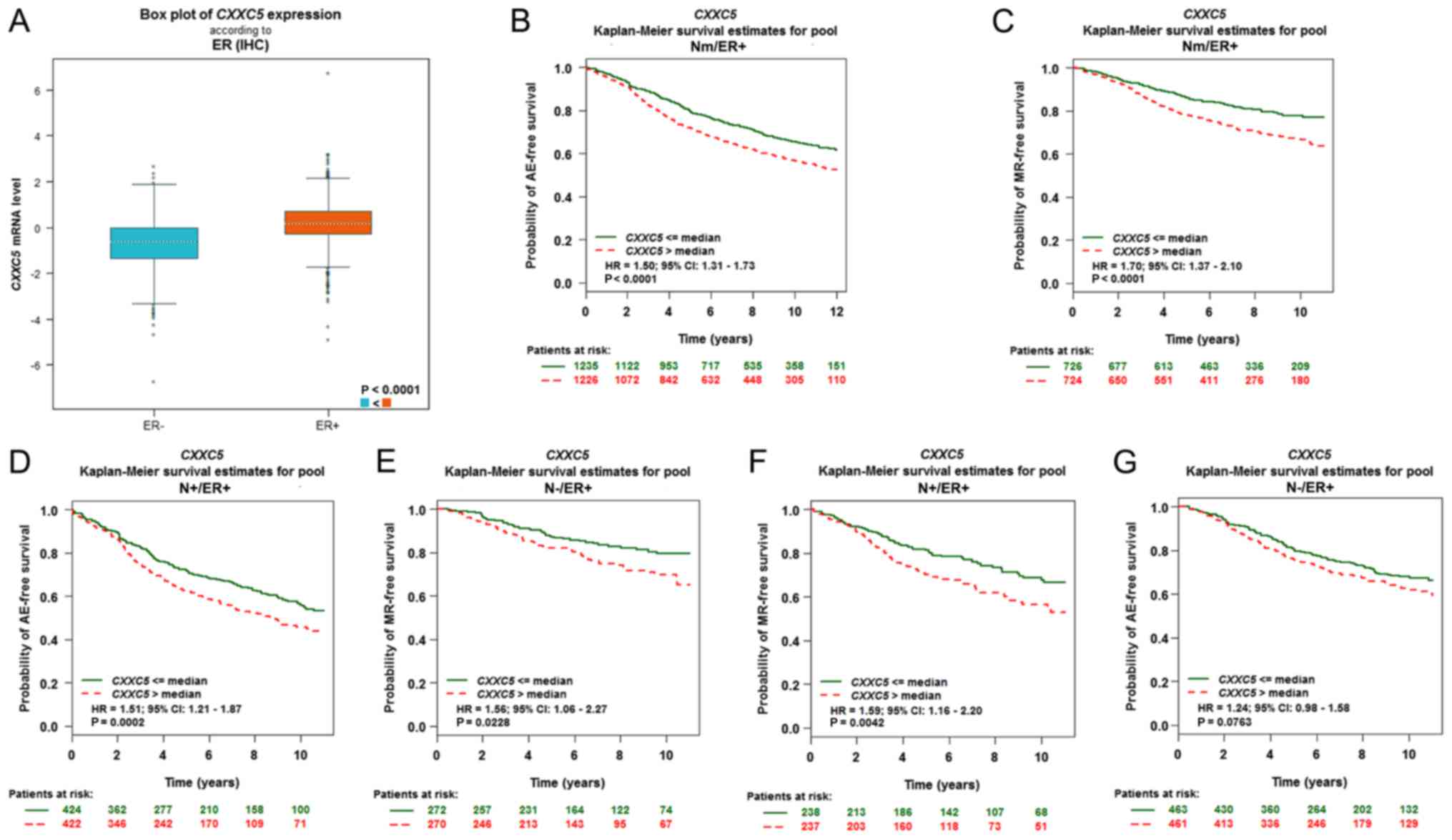
The impact of CXXC5 in breast cancer was considered
robust because there were 10 significant results (P<0.05) among
the 18 given results (Table I). We
determined that the high level of CXXC5 expression is associated
with poor prognosis of breast cancer with Nm/ER+/AE, Nm/ER+/MR,
N+/ER+/AE, N-/ER+/MR, N+/ER+/MR, Nm/ERm/MR, Nm/ERm/AE, N+/ERm/AE,
N+/ER-/AE and N-/ER+/AE through CXXC5 univariate Cox analysis
(Table I). In particular, all breast
cancer patients with ER+ status had a poor prognosis. CXXC5
expression was significantly higher in ER+ breast cancer than in
ER-breast cancer (Fig. 2A). Using the
Kaplan-Meier curve, we ascertained that high CXXC5 expression
predicted significantly poor AE-free survival in Nm/ER+ status
(HR=1.50; 95% CI, 1.31–1.73; P<0.0001) (Fig. 2B), MR-free survival in Nm/ER+ status
(HR=1.70; 95% CI, 1.37–2.10; P<0.0001) (Fig. 2C), AE-free survival in N+/ER+ status
(HR=1.51; 95% CI, 1.21–1.87; P=0.0002) (Fig. 2D), MR-free survival in N-/ER+ status
(HR=1.56; 95% CI, 1.06–2.27; P=0.0228) (Fig. 2E) and MR-free survival in N+/ER+
status (HR=1.59; 95% CI, 1.16–2.20; P=0.0042) (Fig. 2F). However, CXXC5 expression could not
predict AE-free survival in N-/ER+ status (P=0.0763) (Fig. 2G).
 | Table I.CXXC5 univariate Cox analysis. |
Table I.
CXXC5 univariate Cox analysis.
| No. | Nodal status | ER status | Event status | P-value | Hazard ratio | 95% CI | No. patients | No. events |
|---|
|
1 | Nm | ER+ | AE |
<0.0001 | 1.31 | 1.22–1.42 | 2,461 |
845 |
|
2 | Nm | ER+ | MR |
<0.0001 | 1.53 | 1.34–1.74 | 1,450 |
355 |
|
3 | N+ | ER+ | AE |
0.0001 | 1.30 | 1.14–1.48 |
846 |
348 |
|
4 | N- | ER+ | MR |
0.0002 | 1.61 | 1.25–2.08 |
542 |
113 |
|
5 | N+ | ER+ | MR |
0.0009 | 1.35 | 1.13–1.61 |
475 |
156 |
|
6 | Nm | ERm | MR |
0.0027 | 1.15 | 1.05–1.26 | 2,017 |
539 |
|
7 | Nm | ERm | AE |
0.0057 | 1.09 |
1.02–1.15 | 3,472 | 1,260 |
|
8 | N+ | ERm | AE |
0.0162 | 1.13 |
1.02–1.25 | 1,127 |
503 |
|
9 | N+ | ER- | AE |
0.0201 | 1.26 |
1.04–1.53 |
278 |
155 |
| 10 | N- | ER+ | AE |
0.0347 | 1.15 |
1.01–1.32 |
924 |
277 |
| 11 | N+ | ERm | MR |
0.0540 | 1.15 | 1.00–1.32 |
612 |
224 |
| 12 | N+ | ER- | MR |
0.0540 | 1.32 | 1.00–1.75 |
135 |
68 |
| 13 | Nm | ER- | AE |
0.0784 | 1.10 | 0.99–1.22 |
972 |
406 |
| 14 | Nm | ER- | MR |
0.1103 | 1.13 | 0.97–1.32 |
547 |
181 |
| 15 | N- | ERm | MR |
0.1563 | 1.12 | 0.96–1.32 |
762 |
167 |
| 16 | N- | ER- | MR |
0.5774 | 1.08 | 0.82–1.42 |
205 |
53 |
| 17 | N- | ER- | AE |
0.7933 | 0.98 | 0.81–1.17 |
361 |
118 |
| 18 | N- | ERm | AE |
0.8053 | 0.99 | 0.89–1.09 | 1,306 |
399 |
Basal-like breast cancer and/or TNBC
prognostic analysis for CXXC5
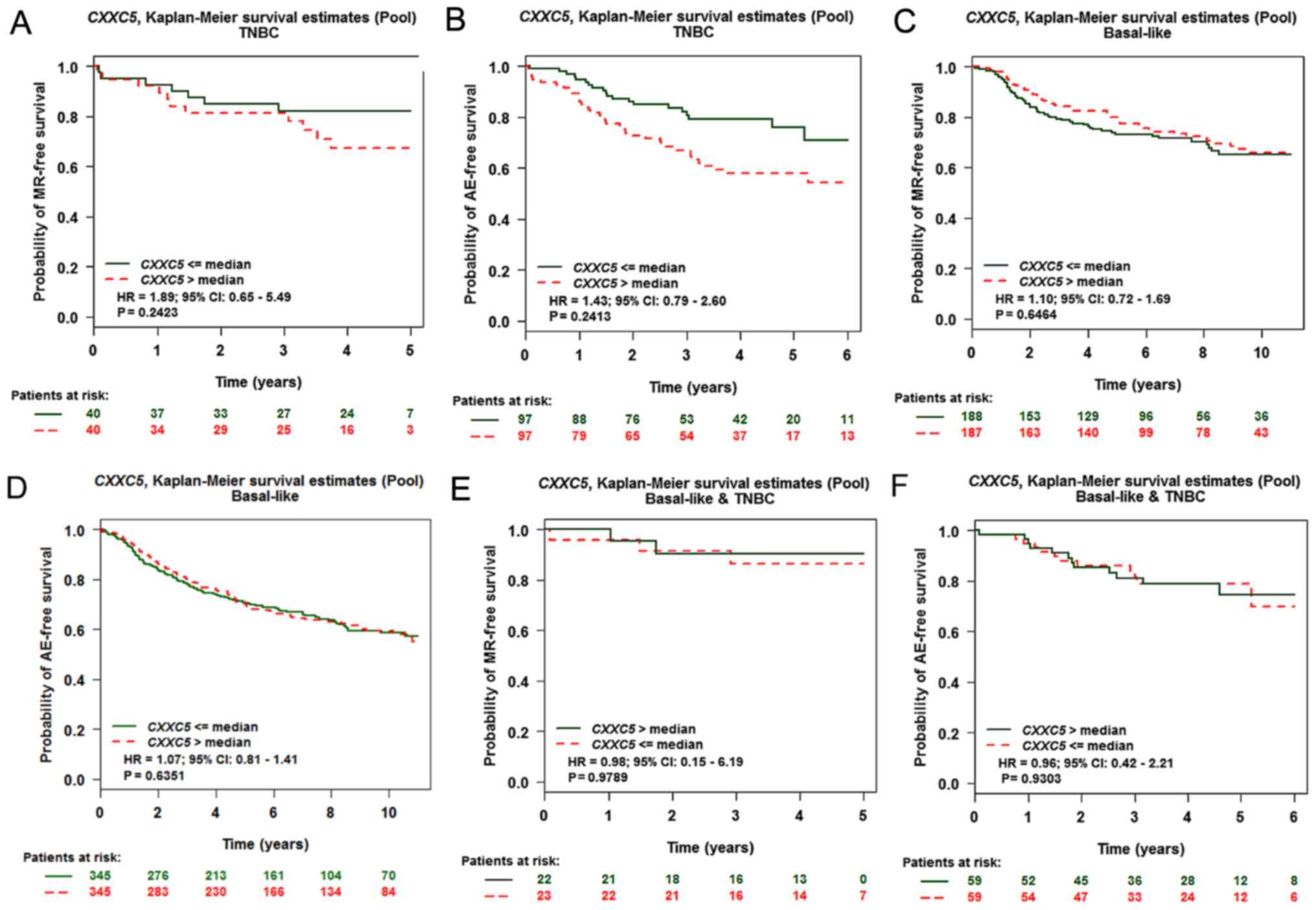
The basal-like breast cancer had lower CXXC5
expression than other subtypes (Fig.
1D). However, basal-like breast cancer could predict bad
prognosis, as 75–80% of the triple-negative breast cancers (TNBC)
belonged to the group of basal-like breast cancer (20). High levels of CXXC5 expression could
predict a bad prognosis in TNBC with MR via CXXC5 univariate Cox
analysis (basal-like and/or TNBC) but not in the other group
(Tables II and III). However, CXXC5 expression was not
associated with prognosis of TNBC with MR through the Kaplan-Meier
curve analysis (Fig. 3A). Consistent
with the results of CXXC5 univariate Cox analysis (basal-like
and/or TNBC), CXXC5 expression was not associated with the
prognosis of breast cancer in the other groups (Fig. 3B-F).
 | Table II.Univariate Cox analysis (basal-like
and/or TNBC) for CXXC5 with MR. |
Table II.
Univariate Cox analysis (basal-like
and/or TNBC) for CXXC5 with MR.
| Population | P-value | HR | 95% CI | No. patients | No. MR |
|---|
| Basal-like | 0.8288 | 1.02 | 0.84–1.24 | 375 | 109 |
| TNBC | 0.0369 | 1.66 |
1.03–2.67 |
80 |
18 |
| Basal-like +
TNBC | 0.7465 | 1.24 | 0.34–4.54 | 45 |
5 |
 | Table III.Univariate Cox analysis (basal-like
and/or TNBC) for CXXC5 with AE. |
Table III.
Univariate Cox analysis (basal-like
and/or TNBC) for CXXC5 with AE.
| Population | P-value | HR | 95% CI | No. patients | No. AE |
|---|
| Basal-like | 0.8185 | 0.99 | 0.87–1.12 | 690 | 251 |
| TNBC | 0.4321 | 1.12 | 0.84–1.50 | 194 | 58 |
| Basal-like +
TNBC | 0.7343 | 0.92 | 0.55–1.52 | 118 | 24 |
Correlated genes with CXXC5
We obtained the correlated genes with CXXC5 in
breast cancer through gene correlation exhaustive analysis.
Table IV shows the top 10 best
positive/negative correlations with CXXC5. Then, we obtained the GO
enrichments of the correlated genes with CXXC5 via GO analysis of
bc-GenExMiner v4.0 (Table V). Among
them, GO:0070062 (extracellular exosome) had the most associated
genes, and the associated genes of both GO:0000122 (negative
regulation of transcription from RNA polymerase II promoter) and
GO:0008134 (transcription factor binding) contained CXXC5.
 | Table IV.Top 10 best positive/negative
correlated genes with CXXC5. |
Table IV.
Top 10 best positive/negative
correlated genes with CXXC5.
| Gene symbol | Pearson's
correlation coefficient | P-value | No. of
patients |
|---|
| Positive
correlation |
|
|
|
|
FKBP9P1 |
0.5858 | <0.0001 |
214 |
|
LOC149401 |
0.5849 | <0.0001 |
155 |
|
LOC100288069 |
0.5806 | <0.0001 |
214 |
|
ACTG1P20 |
0.5784 | <0.0001 |
326 |
|
CA12 |
0.5474 | <0.0001 | 3,524 |
|
FOXA1 |
0.5377 | <0.0001 | 3,524 |
|
GATA3 |
0.5338 | <0.0001 | 3,524 |
|
AGR2 |
0.5287 | <0.0001 | 3,524 |
|
PRINS |
0.5245 | <0.0001 |
155 |
|
AGR3 |
0.5235 | <0.0001 | 3,023 |
| Negative
correlation |
|
|
|
|
FLJ44715 | −0.8160 | <0.0001 |
155 |
|
LOC100507412 | −0.8069 | <0.0001 |
155 |
|
LOC100133683 | −0.7873 | <0.0001 |
155 |
|
LOC729461 | −0.7741 | <0.0001 |
155 |
|
LOC728543 | −0.7698 | <0.0001 |
155 |
|
CEP170P1 | −0.6993 | <0.0001 |
155 |
|
LOC729324 | −0.6529 | <0.0001 |
155 |
|
CEP295NL | −0.6524 | <0.0001 |
155 |
|
LOC653739 | −0.5980 | <0.0001 |
155 |
|
LOC100507637 | −0.5718 | <0.0001 |
155 |
 | Table V.GO enrichments of correlated genes
with CXXC5. |
Table V.
GO enrichments of correlated genes
with CXXC5.
| Significant
terms | Description | P-value | Associated
genes |
|---|
| Biological
process |
|
|
|
|
GO:1902236 | Negative regulation
of endoplasmic reticulum stress-induced intrinsic apoptotic
signaling pathway |
4.53×10−5 | WFS1, TMBIM6,
XBP1 |
|
GO:0000122 | Negative regulation
of transcription from RNA polymerase II promoter |
7.14×10−4 | CXXC5, FOXA1,
GATA3, WFS1, CCND1, SPDEF, DACH1, XBP1, BCL11A, LPIN1 |
|
GO:0009653 | Anatomical
structure morphogenesis |
2.25×10−3 | FOXA1, GATA3,
KRT18, SOX10 |
|
GO:0043627 | Response to
estrogen |
2.40×10−3 | GATA3, ESR1,
CCND1 |
|
GO:0043433 | Negative regulation
of sequence-specific DNA binding transcription factor activity |
2.64×10−3 | WFS1, ESR1,
SIGIRR |
| Cellular
component |
|
9.57×10−4 |
|
|
GO:0005902 | Microvillus |
4.59×10−3 | FOXA1, STARD10,
SLC9A3R1 |
|
GO:0030176 | Integral component
of endoplasmic reticulum membrane |
4.53×10−5 | WFS1, PIGT,
XBP1 |
|
GO:0070062 | Extracellular
exosome |
6.21×10−3 | ANXA9, SLC9A3R1,
KRT18, TFF3, WWP1, GFRA1, FBP1, MLPH, NME3, CMBL, H2AFJ, HAGH,
PVRL2, HSPB1, SERPINA5, TSPAN1, GAMT, PSAT1, PM20D2, FAM171A1,
SFT2D2 |
|
GO:0071944 | Cell periphery |
7.21×10−3 | SLC9A3R1,
KRT18 |
| Molecular
function |
|
1.76×10−4 |
|
|
GO:0008134 | Transcription
factor binding |
4.10×10−3 | CXXC5, FOXA1,
GATA3, ESR1, CCND1, FOXC1, SOX10 |
|
GO:0000981 | Sequence-specific
DNA binding RNA polymerase II transcription factor activity |
7.43×10−3 | FOXA1, SPDEF, XBP1,
FOXC1 |
|
GO:0001078 | RNA polymerase II
core promoter proximal region sequence-specific DNA binding
transcription factor activity involved in negative regulation of
transcription |
8.49×10−3 | GATA3, DACH1,
BCL11A |
|
GO:0044212 | Transcription
regulatory region DNA binding | 8.49E-03 | FOXA1, GATA3, XBP1,
FOXC1 |
Discussion
CXXC5 is a newly identified CXXC-type zinc finger
family protein (21), which is
encoded by the CXXC5 gene localised to the 5q31.3
chromosomal region (12). Previous
studies showed that CXXC5 was related to AML, myelodysplastic
syndromes, human malignant peripheral nerve sheath tumours,
prostate cancer, breast cancer, thyroid cancers and metastatic
melanomas (16,22–25).
Knappskog et al (16) used
three independent public microarray datasets, including 599
patients from GEO, to find that CXXC5 was a bad prognostic factor
in breast cancer. However, they did not study the effects of CXXC5
on various subtypes in breast cancer.
In our study, using the dataset of GDS5666 from GEO,
we found that the expression of CXXC5 was higher in 4T1-derived
metastatic populations than in primary breast cancers. Therefore,
CXXC5 might be associated with metastasis. Then, we used expression
analysis of bc-GenExMiner v4.0 to find that the expression of CXXC5
was significantly different in PAM subtypes. We established that
the high level of CXXC5 expression is associated with poor
prognosis of ER+ breast cancer through CXXC5 univariate Cox
analysis and Kaplan-Meier curve analysis of bc-GenExMiner v4.0.
These results propose CXXC5 as a biomarker and potential
therapeutic target in ER+ breast cancer. Although basal-like breast
cancer and TNBC could predict bad prognosis, their CXXC5 expression
was low. In addition, CXXC5 could not predict their prognosis.
Finally, we obtained the CXXC5 correlated genes and enriched GO
terms of those genes through gene correlation exhaustive analysis
of bc-GenExMiner v4.0. Among those enriched GO terms, GO:0070062
(extracellular exosome) had the most associated genes, and the
associated genes of both GO:0000122 (negative regulation of
transcription from RNA polymerase II promoter) and GO:0008134
(transcription factor binding) contained CXXC5. These GO terms can
guide new investigations into understanding the mechanisms of CXXC5
in breast cancer and propose new treatments for ER+ breast
cancer.
There is a limitation to the present study. The
mechanism of CXXC5 in breast cancer requires further investigation
via in vitro and in vivo experiments.
In conclusion, we determined that overexpression of
CXXC5 was a strongly poor prognostic factor in ER+ breast cancer
through the tools of bc-GenExMiner V4.0 based on a database
including a total of 5,861 patients. This means that regardless of
the clinical stage of breast cancer, high expression of CXXC5 in
patients predicts that the disease is more significantly invasive.
As is known, gene expression can be measured in many ways. We hope
that measuring the expression of CXXC5 may become a routine
inspection to assess the prognosis of breast cancer in different
patients. In this way, early intervention and treatment could be
used, and the survival rate of patients could improve. However, the
pathways of CXXC5 in breast cancer require further investigation.
If in-depth research is conducted, we may find the pathways of
CXXC5 in breast cancer, and then CXXC5 can be utilized as a
potential therapeutic target.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from GEO database (GDS5666; www.ncbi.nlm.nih.gov/geo/) and Breast Cancer
Gene-Expression Miner
(bcgenex.centregauducheau.fr/BC-GEM/GEM-Accueil.php?js=1).
Authors' contributions
LF, YW and XC conceived and designed the study. LF
and YG analyzed and interpreted the data. LF and YW were the
contributors in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
2
|
DeSantis CE, Fedewa SA, Sauer Goding A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Siegel RL, Sauer AG, Miller
KD, Fedewa SA, Alcaraz KI and Jemal A: Cancer statistics for
African Americans, 2016: Progress and opportunities in reducing
racial disparities. CA Cancer J Clin. 66:290–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ufen MP, Köhne CH, Wischneswky M, Wolters
R, Novopashenny I, Fischer J, Constantinidou M, Possinger K and
Regierer AC: Metastatic breast cancer: Are we treating the same
patients as in the past? Ann Oncol. 25:95–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zervoudis S, Iatrakis G, Tomara E, Bothou
A, Papadopoulos G and Tsakiris G: Main controversies in breast
cancer. World J Clin Oncol. 5:359–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marino N, Woditschka S, Reed LT, Nakayama
J, Mayer M, Wetzel M and Steeg PS: Breast cancer metastasis: Issues
for the personalization of its prevention and treatment. Am J
Pathol. 183:1084–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nalejska E, Mączyńska E and Lewandowska
MA: Prognostic and predictive biomarkers: Tools in personalized
oncology. Mol Diagn Ther. 18:273–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gasparri ML, Casorelli A, Bardhi E,
Besharat AR, Savone D, Ruscito I, Farooqi AA, Papadia A, Mueller
MD, Ferretti E and Panici Benedetti P: Beyond circulating microRNA
biomarkers: Urinary microRNAs in ovarian and breast cancer. Tumor
Biol. 39:10104283176955252017. View Article : Google Scholar
|
|
11
|
Ye N, Wang B, Quan ZF, Cao SJ, Wen XT,
Huang Y, Huang XB, Wu R, Ma XP and Yan QG: Functional roles of long
non-coding RNA in human breast cancer. Asian Pac J Cancer Prev.
15:5993–5997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pendino F, Nguyen E, Jonassen I, Dysvik B,
Azouz A, Lanotte M, Ségal-Bendirdjian E and Lillehaug JR:
Functional involvement of RINF, retinoid-inducible nuclear factor
(CXXC5), in normal and tumoral human myelopoiesis. Blood.
113:3172–3181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kühnl A, Valk PJ, Sanders MA, Ivey A,
Hills RK, Mills KI, Gale RE, Kaiser MF, Dillon R, Joannides M, et
al: Downregulation of the Wnt inhibitor CXXC5 predicts a better
prognosis in acute myeloid leukemia. Blood. 125:2985–2994. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruserud Ø, Reikvam H, Fredly H, Skavland
J, Hagen KM, van Hoang TT, Brenner AK, Kadi A, Astori A, Gjertsen
BT and Pendino F: Expression of the potential therapeutic target
CXXC5 in primary acute myeloid leukemia cells-high expression is
associated with adverse prognosis as well as altered intracellular
signaling and transcriptional regulation. Oncotarget. 6:2794–2811.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasar P, Ayaz G and Muyan M:
Estradiol-estrogen receptor α mediates the expression of the CXXC5
gene through the estrogen response element-dependent signaling
pathway. Sci Rep. 6:378082016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knappskog S, Myklebust LM, Busch C,
Aloysius T, Varhaug JE, Lønning PE, Lillehaug JR and Pendino F:
RINF (CXXC5) is overexpressed in solid tumors and is an unfavorable
prognostic factor in breast cancer. Ann Oncol. 22:2208–2215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jézéquel P, Campone M, Gouraud W,
Guérin-Charbonnel C, Leux C, Ricolleau G and Campion L:
bc-GenExMiner: An easy-to-use online platform for gene prognostic
analyses in breast cancer. Breast Cancer Res Treat. 131:765–775.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tabariès S, Ouellet V, Hsu BE, Annis MG,
Rose AA, Meunier L, Carmona E, Tam CE, Mes-Masson AM and Siegel PM:
Granulocytic immune infiltrates are essential for the efficient
formation of breast cancer liver metastases. Breast Cancer Res.
17:452015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41:(Database Issue).
D991–D995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badowska-Kozakiewicz AM and Budzik MP:
Immunohistochemical characteristics of basal-like breast cancer.
Contemp Oncol (Pozn). 20:436–443. 2016.PubMed/NCBI
|
|
21
|
Zhang M, Wang R, Wang Y, Diao F, Lu F, Gao
D, Chen D, Zhai Z and Shu H: The CXXC finger 5 protein is required
for DNA damage-induced p53 activation. Sci China C Life Sci.
52:528–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Astori A, Fredly H, Aloysius TA, Bullinger
L, Mansat-De Mas V, de la Grange P, Delhommeau F, Hagen KM, Récher
C, Dusanter-Fourt I, et al: CXXC5 (retinoid-inducible nuclear
factor, RINF) is a potential therapeutic target in high-risk human
acute myeloid leukemia. Oncotarget. 4:1438–1448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stoddart A, Qian Z, Fernald AA, Bergerson
RJ, Wang J, Karrison T, Anastasi J, Bartom ET, Sarver AL, McNerney
ME, et al: Retroviral insertional mutagenesis identifies the
del(5q) genes, CXXC5, TIFAB and ETF1, as well as the Wnt pathway,
as potential targets in del(5q) myeloid neoplasms. Haematologica.
101:e232–e236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui Z, Shen Y, Chen KH, Mittal SK, Yang JY
and Zhang G: KANK1 inhibits cell growth by inducing apoptosis
though regulating CXXC5 in human malignant peripheral nerve sheath
tumors. Sci Rep. 7:403252017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bettin A, Reyes I and Reyes N: Gene
expression profiling of prostate cancer-associated genes identifies
fibromodulin as potential novel biomarker for prostate cancer. Int
J Biol Markers. 31:e153–e162. 2016. View Article : Google Scholar : PubMed/NCBI
|