Introduction
Hepatocellular carcinoma (HCC) is the second leading
cause of cancer-associated mortality worldwide (1). Of patients diagnosed with liver cancer,
>70% are diagnosed too late for successful surgical treatment,
and, of patients with HCC who undergo surgery, between 60 and 70%
relapse within 5 years (2,3). Therefore, there is an urgent requirement
to improve antitumor drugs for advanced and recurrent HCC.
Sorafenib, a multi-kinase inhibitor that prevents tumor cell
proliferation by targeting the RAF proto-oncogene/mitogen-activated
protein kinase signaling pathway, also inhibits the vascular
endothelial growth factor receptor and platelet-derived growth
factor receptor-β, thereby inhibiting angiogenesis (4). Sorafenib is the first line of treatment
against unresectable HCC (5,6). However, widespread sorafenib-resistance
exists in patients with advanced HCC, which significantly decreases
the therapeutic efficacy of the drug (7,8). The
third-generation platinum drug oxaliplatin has been widely used for
the treatment of various types of cancer, including colon, gastric
and metastatic liver cancer (9).
Clinical trials of oxaliplatin in advanced HCC revealed moderate
activity, but limited efficacy (10).
The reason for this is partly intrinsic multidrug resistance and
acquired drug resistance following chemotherapy (11). Therefore, elucidation of the mechanism
of drug resistance is required to improve the prognosis of patients
with HCC.
The glutathione transferases (GSTs) are a gene
superfamily of phase II metabolic enzymes that detoxify free
radicals derived from tobacco smoke, oxidative stress and
carcinogens, including benzopyrene and other polycyclic aromatic
hydrocarbons (12). A previous study
by our group demonstrated that the GST Mu 1 (GSTM1)-null genotype
may increase the risk of HCC (13).
It is speculated that the expression of GSTM1 is associated with
tumor suppression in HCC tumorigenesis. However, the GSTs are
considered to be directly involved in the metabolic pathways of
drug resistance (14). It was also
demonstrated that GSTs were able to function in vivo and
in vitro as endogenous repressors of the opening of a
permeability transition pore complex, which contributed to
chemotherapy-induced apoptosis (15).
Thus, GSTs may protect tumor cells from chemotherapy drugs via
metabolic or non-metabolic pathways. In the present study, the
underlying molecular mechanism of GSTM1-mediated chemoresistance in
HCC was investigated.
A previous study by our group demonstrated that
autophagy defects during early stages of oncogenesis may contribute
to the malignant differentiation and invasive phenotype of HCC
(16). Furthermore, autophagy is able
to protect HCC tumor cells against anti-neoplastic agent-induced
apoptosis (17). In the present
study, oxaliplatin and sorafenib were selected as chemotherapeutic
agents, and the levels of GSTM1 were analyzed in HCC cell lines
with various metastatic potentials. It was hypothesized that there
would be an association between GSTM1 expression and autophagy
following oxaliplatin treatment.
Materials and methods
Reagents
Oxaliplatin was purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt Germany). Sorafenib was synthesized at Bayer
(Newbury, UK). Oxaliplatin and sorafenib were dissolved in
Dulbecco's modified Eagle's medium (DMEM) containing 0.1%
dimethylsulfoxide (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Cell lines
The HCC cell lines HCCLM3 and MHCC97-H (established
in Liver Cancer Institute of Zhongshan Hospital, Fudan University,
Shanghai China) have different lung metastatic potentials and the
same genetic background. The HCC cell line Huh-7 has low metastatic
potential, and was purchased from the Institute of Biochemistry and
Cell Biology (The Chinese Academy of Sciences, Shanghai, China).
All cells were maintained in high-glucose DMEM supplemented with
10% heat-inactivated fetal bovine serum, 100 units/ml penicillin
and 100 mg/ml streptomycin. All cells were cultured at 37°C in a
humidified incubator containing 5% CO2.
MTT assay
The cell viability was assessed using an MTT cell
proliferation assay kit (Trevigen Inc., Gaithersburg, MD, USA),
according to the manufacturer's protocol. In total,
5×103 cells were plated in 96-well plates, incubated for
24 h at 37°C. After 24 h incubation and attachment, the cells were
treated with 10 µmol/l oxaliplatin or 20 µmol/l sorafenib for an
additional 12, 24, 36, and 48 h, respectively. Then, 20 µl of MTT
solution (5 mg/ml) was added to each well for and incubated for 4 h
at 37°C. The supernatant in each well was then gently aspirated,
and 150 µl dimethyl sulfoxide was added to each well to dissolve
the crystals, and the plate was shaken on a horizontal shaker for
10 min. The optical density (OD) at 570 nm were measured using a
microplate reader, and the inhibition ratio was calculated using
the following equation: Inhibition ratio (%)=(1-OD value of the
experimental group/OD value of the control group) ×100.
Annexin V/propidium iodide (PI)
assay
The number of apoptotic cells was determined using
annexin V/PI staining (Annexin V, Alexa Fluor 555 conjugate; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
(18). Cells were analyzed using a
flow cytometer, and data were analyzed using CellQuest software
version 3.3 (BD Bioscience, Franklin Lakes, NJ, USA).
Western blot analysis
Protein extraction from the HCCLM3, MHCC97-H and
Huh-7 cells was performed using a radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing 1% protease inhibitor. Total protein was measured using
a bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. A total of, 200
µg/well protein was loaded in 5% acrylamide and separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were washed in TBS 3 times and blocked in TBS with
0.05% Tween-20 (ST825, Beyotime Institute of Biotechnology)
containing 5% non-fat dried milk for 1 h at room temperature, and
the membrane was then incubated with primary antibodies against the
following: GSTM1 (dilution, 1:1,000; cat. no. ab113432; Abcam,
Cambridge, UK), autophagy related 5 (ATG5) (dilution, 1:1,000; cat.
no. 12994; CST Biological Reagents Co., Ltd., Shanghai, China) and
GAPDH (dilution, 1:10,000; cat. no. AP0063; Bioworld Technology,
Inc., St. Louis Park, MN, USA) at 4°C overnight. The membranes were
then incubated with a horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (dilution, 1:10,000; cat. no.
A16110; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2 h
at room temperature. The protein bands were visualized using
enhanced chemiluminescence western blotting substrate (Pierce;
Thermo Fisher Scientific, Inc.,), and captured by ChemiDoc™ XRS+
system (Bio-Rad Laboratories, Hercules, CA, USA) and the
densitometry of the protein bands were determined by ImageLab
version 3.0 (Bio-Rad Laboratories).
RNA interference
High-performance purity grade small interfering RNA
(siRNA; >90% pure) against GSTM1 (GSTM1-siRNA) was obtained from
OriGene Technologies, Inc. (cat. no. SR301988; Rockville, MD, USA).
A non-silencing oligonucleotide sequence was used as a negative
control (negative control siRNA, cat. no. SR30004; OriGene
Technologies, Inc.). MHCC97-H and Huh-7 cells were seeded at a
density of 5×104 cells/well in 6-well plates and
cultured in DMEM containing 10% FBS. At 1 day after seeding, cells
were transfected with 100 pmol GSTM1-siRNA or negative siRNA using
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Cells were lysed 72 h after
transfection, and protein was analyzed by western blotting.
Autophagy analysis
For the quantitative analysis of autophagy,
2×105 of MHCC97-H or Huh-7 cells were seeded in 6-well
plates at 37°C and transfected with 4 µg GFP-LC3 plasmid
(concentration, 1 µg/µl; Beyotime Institute of Biotechnology,
Haimen, China) at 25°C using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
for 24 h and then the cells were exposed to 10 µmol/l oxaliplatin
for 12 h at 37°C. Autophagy was assessed by green fluorescent
protein (GFP)-light chain 3 (LC3) redistribution analysis.
Redistribution of GFP-LC3 was assessed by determining the number of
GFP-LC3-positive dots per transfected cell in three independent
experiments using an inverted fluorescence microscope
(magnification, ×200; Nikon Corporation, Tokyo, Japan). In total,
eight randomly selected fields, each containing ~200 cells, were
analyzed per well.
Statistical analysis
All data are presented as the mean ± standard
deviation. An unpaired two-tailed Student's t-test was used to
compare the difference between two groups, and one-way analysis of
variance was used to compare ≥3 groups, followed by the Dunnett's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
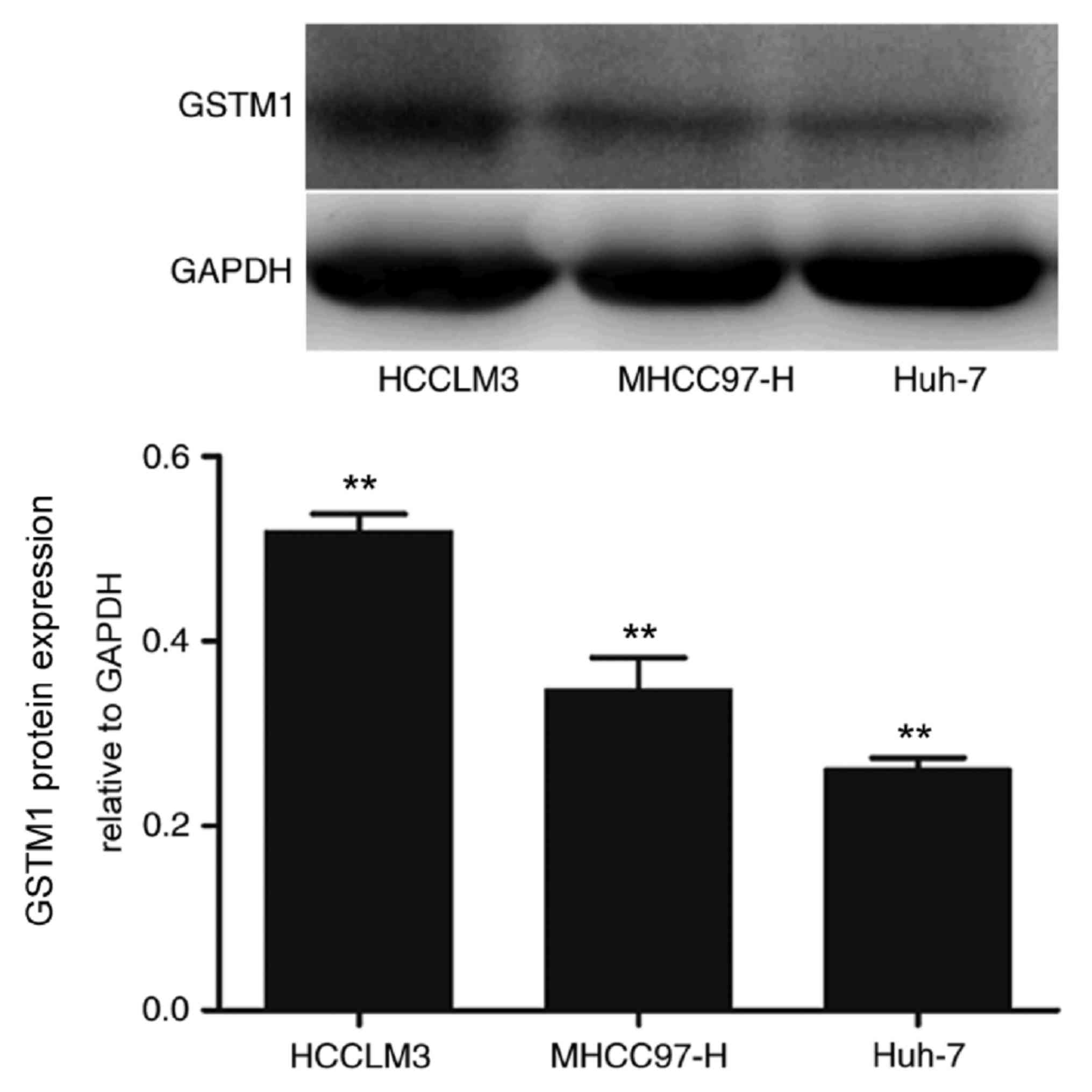
GSTM1 expression in HCC cell
lines
Western blotting revealed a 26-kDa band,
corresponding to membrane-bound GSTM1 protein in human HCC cell
lines. Semi-quantitative analysis demonstrated that the GSTM1
protein expression level increased with the increasing metastatic
potential of HCCLM3, MHCC97-H and Huh-7 cells (P<0.01) (Fig. 1).
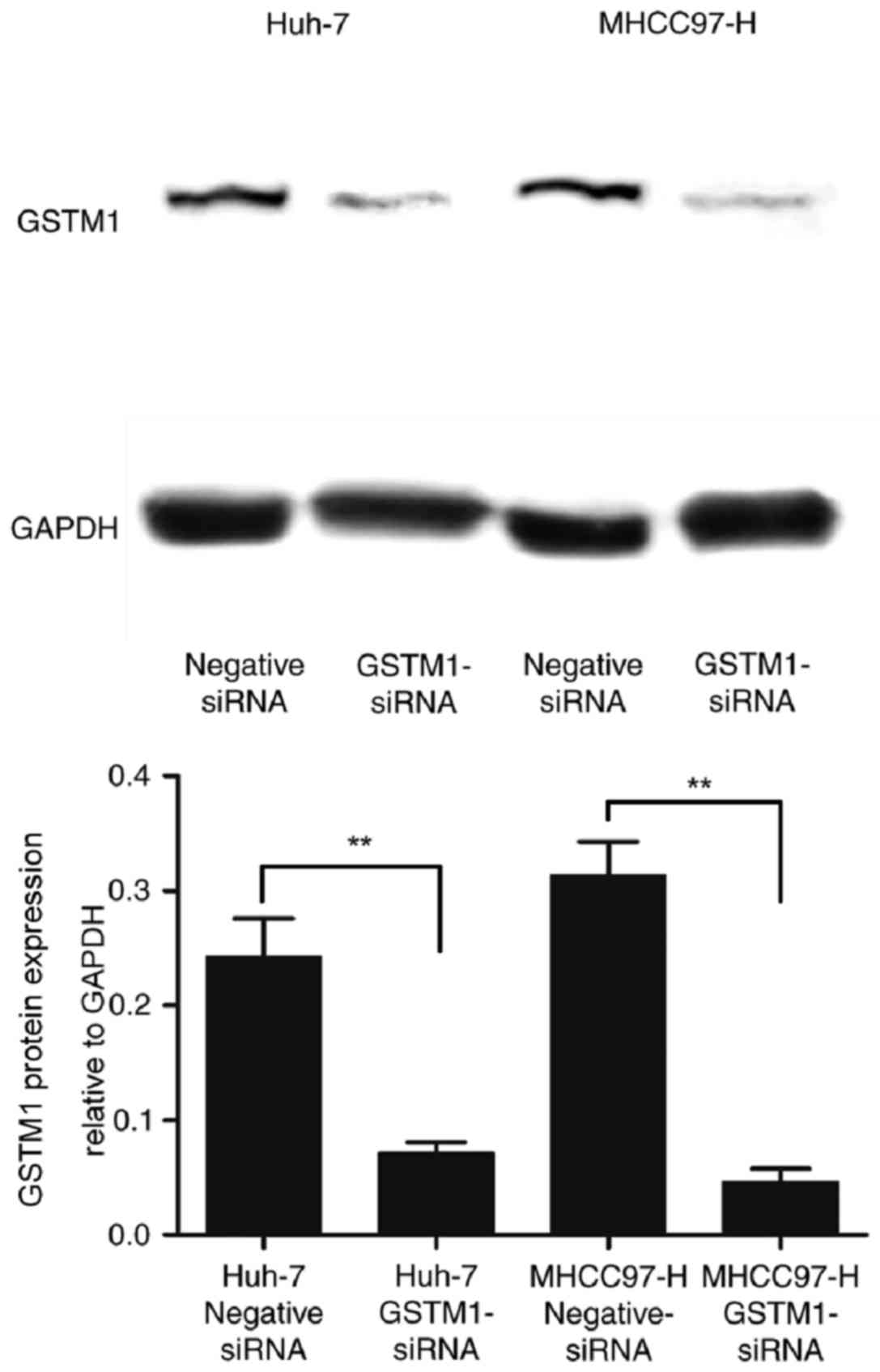
Inhibition of GSTM1 promotes apoptosis
following treatment with oxaliplatin
To investigate further the function of GSTM1 in
chemotherapy-induced cell death, the expression of GSTM1 was
inhibited using GSTM1-siRNA transfection of highly invasive
MHCC97-H cells and low-invasive Huh-7 cells. Western blotting
revealed that the protein expression level of GSTM1 was markedly
inhibited following transfection, indicating that GSTM1 expression
was successfully silenced by RNA interference (Fig. 2).
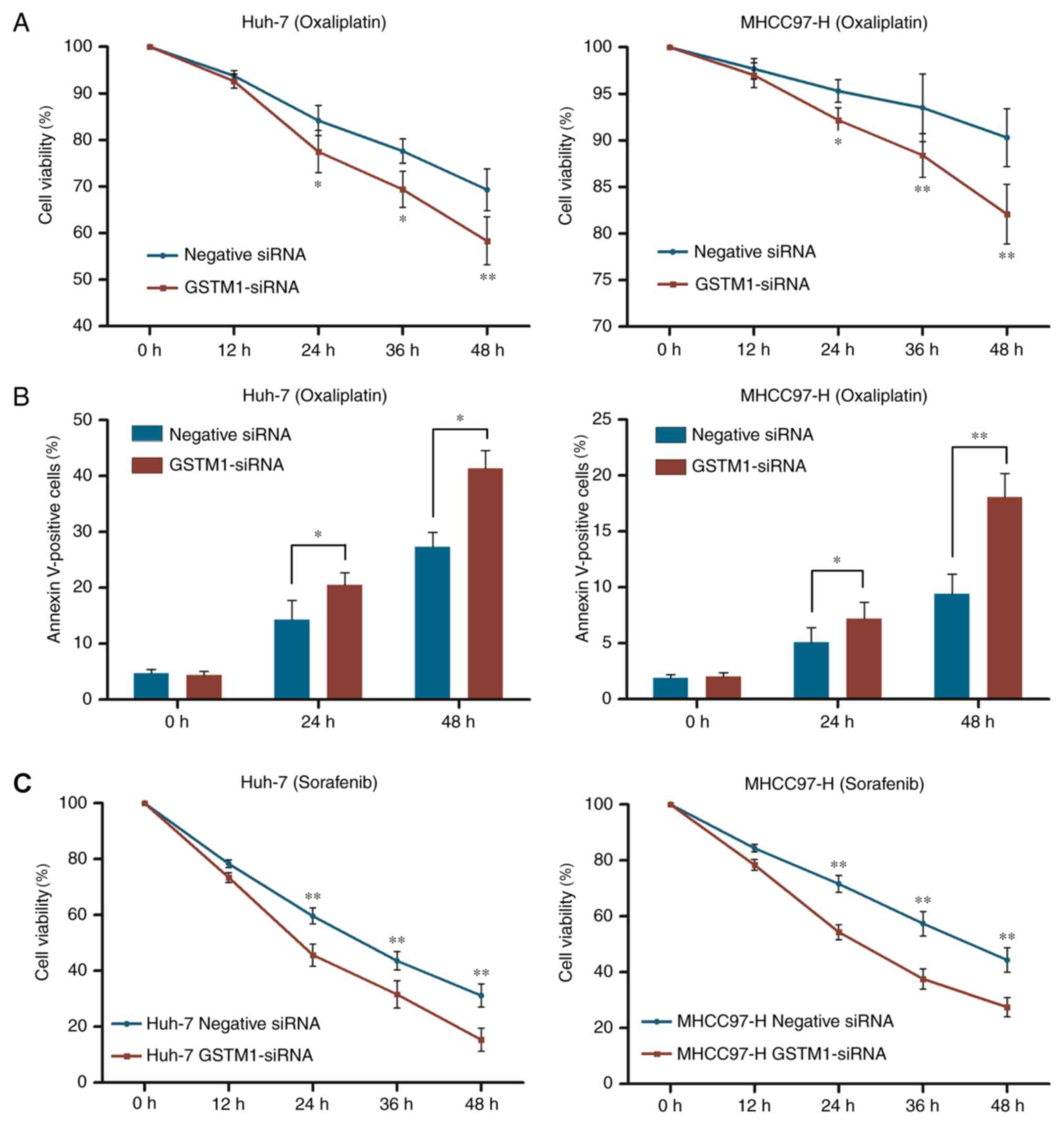
There was no difference in viability prior to
oxaliplatin or sorafenib treatment. However, inhibition of GSTM1
expression in Huh-7 cells abolished the protective effect of GSTM1
and decreased cell viability by 6.7 and 11%, respectively,
following treatment with oxaliplatin for 24 and 48 h, compared with
control cells. Similarly, silencing GSTM1 expression in MHCC97-H
cells markedly decreased cell viability following treatment with
oxaliplatin for 24 and 48 h by 3.3 and 8.2%, respectively, compared
with control cells (Fig. 3A).
Induction of apoptosis by oxaliplatin was further evaluated by
annexin V staining. As presented in Fig.
3B, silencing of GSTM1 significantly increased the proportion
of annexin V-positive MHCC97-H and Huh-7 cells following exposure
to oxaliplatin. In addition, Huh-7 and MHCC97-H cells transfected
with GSTM1-siRNA also demonstrated increased sensitivity to
sorafenib (Fig. 3C).
Inhibition of GSTM1 protects against
autophagy following oxaliplatin treatment
GFP-LC3 expression shifted from a diffuse
cytoplasmic pattern to a punctate membranous pattern following
exposure of MHCC97-H and Huh-7 cells to oxaliplatin for 12 h,
indicating the formation of autophagic vacuoles.
Oxaliplatin-treated cells exhibited a significantly increased
number of GFP-LC3-positive fluorescent autophagy vesicles per cell
compared with untreated cells (Huh-7, P<0.05; MHCC97-H,
P<0.01). GSTM1-siRNA transfection significantly decreased the
number of fluorescent autophagy vesicles in oxaliplatin-treated
cells, compared with negative control cells. No significant
difference was identified between GSTM1-siRNA-transfected cells and
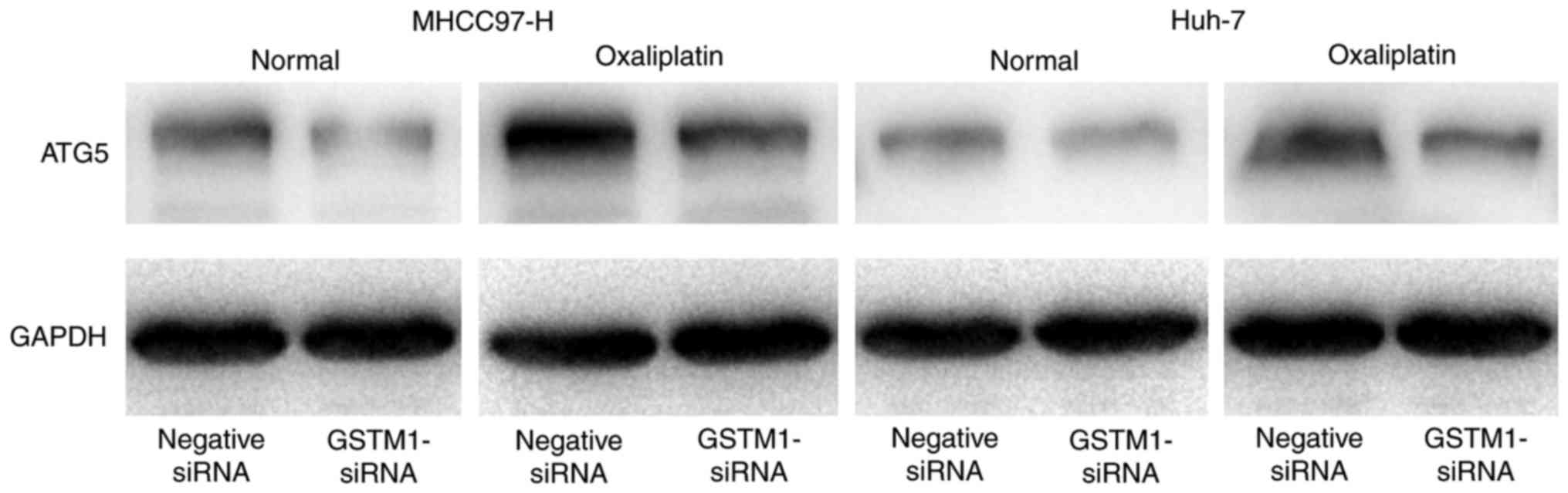
negative control cells when not exposed to oxaliplatin (Fig. 4A and B). Western blotting revealed
that the expression of ATG5 was markedly decreased in
GSTM1-siRNA-transfected cells compared with negative control cells
following oxaliplatin treatment (Fig.
5).
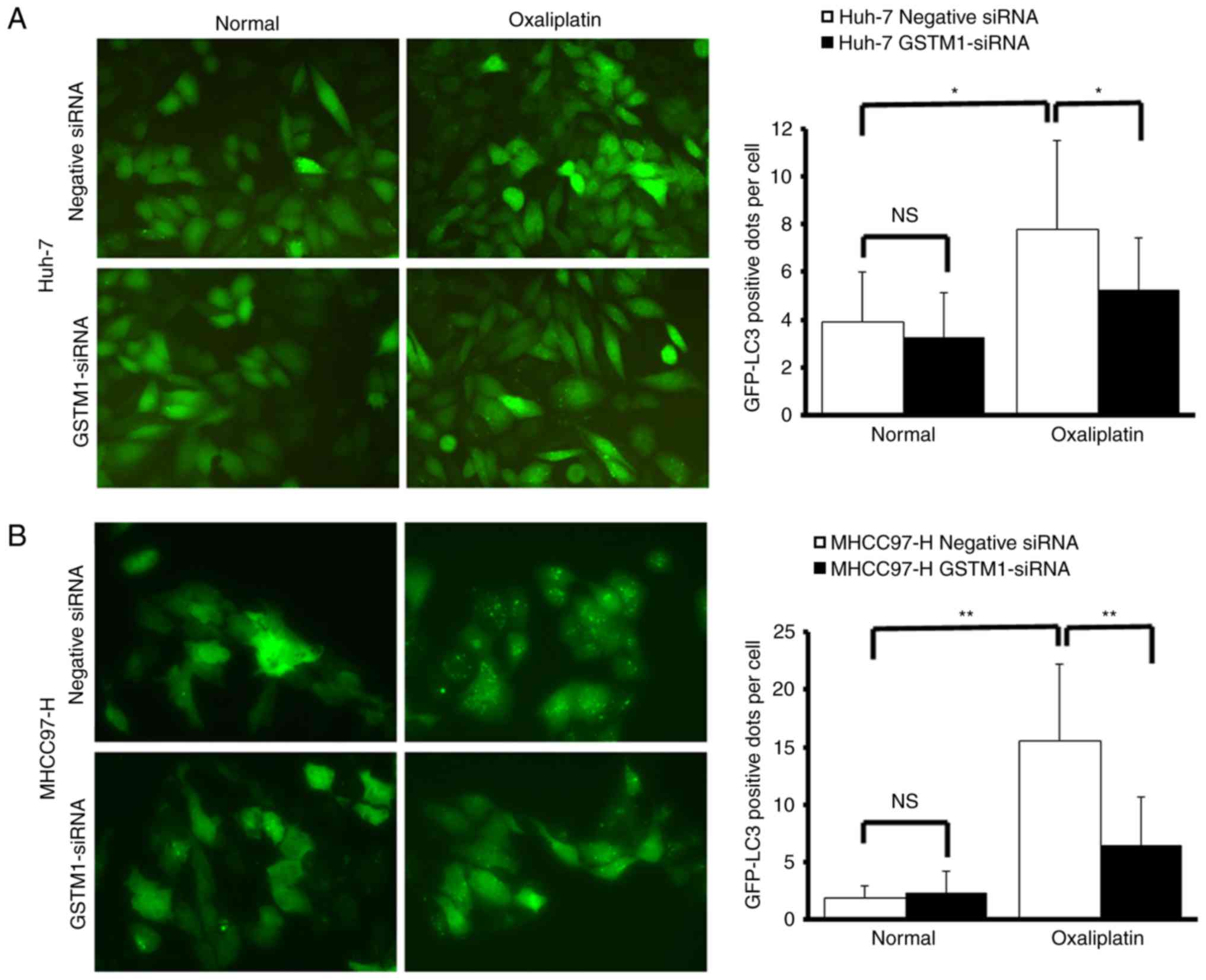 | Figure 4.Silencing of GSTM1 expression protects
against autophagy following oxaliplatin treatment. (A) Huh-7 and
(B) MHCC97-H stably expressing the GFP-LC3 fusion protein were
established. Following transfection with negative siRNA or
GSTM1-siRNA, cells were exposed to 10 µmol/l oxaliplatin for 12 h
and imaged using fluorescent microscopy. Magnification, ×200. The
number of GFP-LC3-positive dots per transfected cell were
determined in three independent experiments. In total, eight random
fields representing 200 cells were examined. The data are presented
as the mean ± standard deviation of three independent experiments.
GSTM1-siRNA transfection significantly decreased the number of
fluorescent autophagy vesicles in oxaliplatin-treated cells,
compared with negative control cells. No significant difference was
identified between GSTM1-siRNA-transfected cells and negative
control cells when not exposed to oxaliplatin. *P<0.05,
**P<0.01. GSTM, glutathione transferase Mu 1; GFP, green
fluorescent protein; LC3, light chain 3; negative siRNA, negative
control small interfering RNA; GSTM1-siRNA, GSTM1-targeted small
interfering RNA; NS, no significant difference. |
Discussion
Resistance to anticancer agents is a major obstacle
in the improvement of cancer therapy. It is widely accepted that
GSTs participate in drug resistance processes by catalyzing the
conjugation of glutathione (GSH) to drugs, including cisplatin,
oxaliplatin, cyclophosphamide and doxorubicin (19,20).
GS-drug conjugates are hydrophilic and easily fluxed out of the
cell through transporter proteins, which often contribute to the
mechanism of drug resistance (21).
The results of the present study indicate that the expression of
GSTM1 was low in HCC cells with low metastatic potential, compared
with in HCC cells with high metastatic potential. It has been
demonstrated previously that HCC cell lines with high metastatic
potential are less sensitive to chemotherapy (22). Therefore, it is speculated that GSTM1
expression is associated with cancer drug resistance in HCC
cells.
MHCC97-H cells exhibit high metastatic potential
(23,24), and it has been demonstrated that
MHCC97-H cells exhibit increased resistance to chemotherapeutics
compared with HCC cell lines with low metastatic potential
(23–25). Similarly, previous studies have
demonstrated that Huh-7 cells exhibit low metastatic potential and
relatively weak chemoresistance (26,27). In
the present study, it was revealed that knockdown of GSTM1 in
MHCC97-H and Huh-7 cells increased resistance to oxaliplatin and
sorafenib. Furthermore, our previous studies revealed that
oxaliplatin and sorafenib could induce autophagy of HCC cells which
contributed to chemo-resistance (28,29), and
it is reasonable to presume that this effect was associated with
GSTM1-derived anti-apoptotic activity; however, it may not be
associated with the general property of glutathione conjugation by
GST.
Autophagy serves as a dynamic recycling system,
which provides resources for cell homeostasis and repair (30). It has been demonstrated that autophagy
is able to protect cancer cells against hypoxia, metabolic stress,
detachment-induced anoikis and apoptosis or necrosis induced by
antitumor therapy or other cell death stimuli (17,31–34). ATG5
forms a conjugate with autophagy-related 12 to function in
autophagosome formation, and is considered to be a marker of
autophagy (29,35). To directly determine the level of
autophagy in MHCC97-H and Huh-7 cells following treatment with
oxaliplatin, GFP-LC3 redistribution and the expression of ATG5 were
analyzed. The increased number of fluorescent autophagy vesicles
per cell in oxaliplatin-treated cells compared with in untreated
cells indicated that oxaliplatin was able to induce autophagy in
HCC cells. Silencing of GSTM1 expression using siRNA allowed the
investigation of the association between GSTM1 and autophagy in HCC
cells. The results revealed that oxaliplatin-induced autophagy
could be downregulated by silencing GSTM1. It can be hypothesized
that GSTM1 may affect autophagic activity in HCC cells. To the best
of our knowledge, the present study is the first to investigate the
GSTM1-mediated chemoresistance via autophagy in HCC. However, the
molecular mechanism underlying the effect of GSTM1 on autophagy
regulation remains unknown, and future research should focus on its
elucidation.
A previous study revealed that activation of
autophagy in HCC could contribute to the tolerance of oxaliplatin
via regulation of the level of reactive oxygen species (ROS)
(28). It has been demonstrated that
GSTs serve an important function in cellular protection against
oxidative stress in the respiratory tract via inhibition of the
endogenous production of ROS, which is increased by exposure to
sulfur mustard (36). Thus, it is
speculated that regulation of ROS levels may involve GSTM1 and
autophagic activity.
There are limitations to the present study.
Overexpression of the gene of interest, GSTM1, may be more
effective than gene knockdown. Thus, future studies will
investigate the effect of overexpression of GSTM1 in HCC cell
lines, and aim to identify a potential chemoresistance target in
vivo. Furthermore, the present study would be improved by
including tissues collected from patients with HCC exhibiting drug
resistance.
In summary, GSTM1 may protect HCC cells against
oxaliplatin treatment through activating autophagy. This provides a
novel perspective to the investigation of drug resistance of
HCC.
Acknowledgements
The authors would like to thank Dr Yan Zhao (Liver
Cancer Institute of Zhongshan Hospital, Fudan University, Shanghai
China) for her assistance with cell culture.
Funding
The present study was supported by the National
Natural Science Fund of China (grant nos. 81472219 and
81602037).
Availability of data and materials
he datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XF, ZD and JF designed the study and performed the
literature review, supervised statistical analysis and
interpretation of data and drafted the manuscript; KS, JZ, YS GS
and QG contributed to the study design and drafting of the
manuscript. WL, MT and LJ performed the molecular biological
experiment. All authors critically revised the manuscript for
intellectual and significant contents and approved the final
manuscript for submission.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142(1264–1273): e12612012.
|
|
2
|
Hatzaras I, Bischof DA, Fahy B, Cosgrove D
and Pawlik TM: Treatment options and surveillance strategies after
therapy for hepatocellular carcinoma. Ann Surg Oncol. 21:758–766.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen MF, Hwang TL, Jeng LB, Wang CS, Jan
YY and Chen SC: Postoperative recurrence of hepatocellular
carcinoma. Two hundred five consecutive patients who underwent
hepatic resection in 15 years. Arch Surg. 129:738–742. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chao Y, Chung YH, Han G, Yoon JH, Yang J,
Wang J, Shao GL, Kim BI and Lee TY: The combination of
transcatheter arterial chemoembolization and sorafenib is well
tolerated and effective in Asian patients with hepatocellular
carcinoma: Final results of the START trial. Int J Cancer.
136:1458–1467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY,
Kang YK, Shin YM, Kim KM, Lim YS and Lee HC: Sorafenib alone versus
sorafenib combined with transarterial chemoembolization for
advanced-stage hepatocellular carcinoma: Results of propensity
score analyses. Radiology. 269:603–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hua L, Hu B, Yan D, Liu J, Shen Y, Zhao F,
Shen C, Chen B and Cui X: Upregulated expression of
Nucleostemin/GNL3 is associated with poor prognosis and Sorafenib
Resistance in Hepatocellular Carcinoma. Pathol Res Pract.
213:688–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu YJ, Zheng B, Wang HY and Chen L: New
knowledge of the mechanisms of sorafenib resistance in liver
cancer. Acta Pharmacol Sin. 38:614–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin S, Bai Y, Lim HY, Thongprasert S, Chao
Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, et al:
Randomized, multicenter, open-label study of oxaliplatin plus
fluorouracil/leucovorin versus doxorubicin as palliative
chemotherapy in patients with advanced hepatocellular carcinoma
from Asia. J Clin Oncol. 31:3501–3508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaanan A, Williet N, Hebbar M, Dabakuyo
TS, Fartoux L, Mansourbakht T, Dubreuil O, Rosmorduc O, Cattan S,
Bonnetain F, et al: Gemcitabine plus oxaliplatin in advanced
hepatocellular carcinoma: A large multicenter AGEO study. J
Hepatol. 58:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gobel T, Blondin D, Kolligs F, Bolke E and
Erhardt A: Current therapy of hepatocellular carcinoma with special
consideration of new and multimodal treatment concepts. Dtsch Med
Wochenschr. 138:1425–1430. 2013.(In German). PubMed/NCBI
|
|
12
|
Yang JX, Luo Y, Qiu HM and Tang WX:
Characterization and resistance mechanisms of cisplatin-resistant
human hepatocellular carcinoma cell line. Saudi Med J. 30:35–40.
2009.PubMed/NCBI
|
|
13
|
Song K, Yi J, Shen X and Cai Y: Genetic
polymorphisms of glutathione S-transferase genes GSTM1, GSTT1 and
risk of hepatocellular carcinoma. PLoS One. 7:e489242012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Pietro G, Magno LA and Rios-Santos F:
Glutathione S-transferases: An overview in cancer research. Expert
Opin Drug Metab Toxicol. 6:153–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verrier F, Deniaud A, Lebras M, Métivier
D, Kroemer G, Mignotte B, Jan G and Brenner C: Dynamic evolution of
the adenine nucleotide translocase interactome during
chemotherapy-induced apoptosis. Oncogene. 23:8049–8064. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai
Z, Shi GM, Wang XY, Ke AW, Wu B and Fan J: Association of autophagy
defect with a malignant phenotype and poor prognosis of
hepatocellular carcinoma. Cancer Res. 68:9167–9175. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin S and White E: Role of autophagy in
cancer: Management of metabolic stress. Autophagy. 3:28–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
King FW, Fong S, Griffin C, Shoemaker M,
Staub R, Zhang YL, Cohen I and Shtivelman E: Timosaponin AIII is
preferentially cytotoxic to tumor cells through inhibition of mTOR
and induction of ER stress. PLoS One. 4:e72832009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tew KD: Glutathione-associated enzymes in
anticancer drug resistance. Cancer Res. 54:4313–4320.
1994.PubMed/NCBI
|
|
20
|
Ang WH, Khalaila I, Allardyce CS,
Juillerat-Jeanneret L and Dyson PJ: Rational design of platinum(IV)
compounds to overcome glutathione-S-transferase mediated drug
resistance. J Am Chem Soc. 127:1382–1383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Townsend DM and Tew KD: The role of
glutathione-S-transferase in anti-cancer drug resistance. Oncogene.
22:7369–7375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang T, Zheng ZM, Li XN, Li ZF, Wang Y,
Geng YF, Bai L and Zhang XB: MiR-223 modulates multidrug resistance
via downregulation of ABCB1 in hepatocellular carcinoma cells. Exp
Biol Med (Maywood). 238:1024–1032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao N, Wang R, Zhou L, Zhu Y, Gong J and
Zhuang SM: MicroRNA-26b suppresses the NF-κB signaling and enhances
the chemosensitivity of hepatocellular carcinoma cells by targeting
TAK1 and TAB3. Mol Cancer. 13:352014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Z, Deng H, Yan W, Luo M, Tu W, Xia Y,
He J, Han P, Fu Y and Tian D: AEG-1 promotes anoikis resistance and
orientation chemotaxis in hepatocellular carcinoma cells. PLoS One.
9:e1003722014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu Z, Wu J, Wu J, Luo D, Jiang C and Ding
Y: Exosomes derived from HCC cells induce sorafenib resistance in
hepatocellular carcinoma both in vivo and in vitro. J Exp Clin
Cancer Res. 35:1592016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu X, Liu RF, Zhang X, Huang LY, Chen F,
Fei QL and Han ZG: DLK1 as a potential target against cancer
stem/progenitor cells of hepatocellular carcinoma. Mol Cancer Ther.
11:629–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Lingala S, Khoobyari S, Nolta J,
Zern MA and Wu J: Epithelial mesenchymal transition and hedgehog
signaling activation are associated with chemoresistance and
invasion of hepatoma subpopulations. J Hepatol. 55:838–845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding ZB, Hui B, Shi YH, Zhou J, Peng YF,
Gu CY, Yang H, Shi GM, Ke AW, Wang XY, et al: Autophagy activation
in hepatocellular carcinoma contributes to the tolerance of
oxaliplatin via reactive oxygen species modulation. Clin Cancer
Res. 17:6229–6238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke
AW, Wang XY, Dai Z, Peng YF, Gu CY, et al: Targeting autophagy
enhances sorafenib lethality for hepatocellular carcinoma via ER
stress-related apoptosis. Autophagy. 7:1159–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moreau K, Luo S and Rubinsztein DC:
Cytoprotective roles for autophagy. Curr Opin Cell Biol.
22:206–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Hou N, Faried A, Tsutsumi S,
Takeuchi T and Kuwano H: Inhibition of autophagy by 3-MA enhances
the effect of 5-FU-induced apoptosis in colon cancer cells. Ann
Surg Oncol. 16:761–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian F, Deguchi K, Yamashita T, Ohta Y,
Morimoto N, Shang J, Zhang X, Liu N, Ikeda Y, Matsuura T and Abe K:
In vivo imaging of autophagy in a mouse stroke model. Autophagy.
6:1107–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nourani MR, Azimzadeh S, Ghanei M and
Fooladi Imani AA: Expression of glutathione S-transferase variants
in human airway wall after long-term response to sulfur mustard. J
Recept Signal Transduct Res. 34:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|