Introduction
Breast cancer is one of the common malignant tumors
that occur among women, with a very high incidence rate (1). In addition, breast cancer has a high
metastasis rate, which is mainly instigated by lymphatic vessels
and reflux veins of the female breasts. Further, lung, liver and
brain metastases are often reported in breast cancer patients at an
early stage. Therefore, the clinical treatment effects of the
patients are quite poor, and the death rates are significantly high
(2). Currently, the clinical
treatment of breast cancer mainly includes surgical resection,
radiotherapy and chemotherapy. Although a certain effect has been
achieved, the relapse and metastasis rates of the breast cancer in
patients are still fairly high, leading to universally poor
prognosis of the patients (3–5).
Suppressor of cytokine signaling (SOCS) is a newly
discovered category of immunosuppressive molecules. Furthermore
SOCS-1 is a type of suppressor gene that has been widely studied.
It has been confirmed for its regulatory role in multiple cytokine
signal transduction pathways (6).
SOCS-1 gene is located on the chromosome 16p13.3 and could encode
proteins containing 211 amino acids (7). SOCS-1 could adjust the Janus
kinase/signal transducer and activator of transcription (JAK/STAT)
signaling pathway as it has the ability to interact with JAK
kinases, induce inactivation of JAK tyrosine kinases and further
inhibit the activation of STAT (8).
When the SOCS-1 expression is lowered, it could activate the
JAK/STAT pathway, causing proliferation, metastasis and invasion of
tumor tissues (9).
Current studies have found that SOCS-1 protein is
expressed slowly in cervical, prostate, liver, esophageal and
pancreatic cancer (10–13). However, there is no literature
reporting the expression of SOCS-1 in tissues of breast cancer
patients. As a result, the present study aimed to investigate the
expression of SOCS-1 in the tumor tissues of patients with breast
cancer. Also, its effects on the pathological parameters, molecular
subtypes and prognosis of breast cancer were evaluated. In this
experiment, the reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and immunohistochemical methods were utilized to
study the SOCS-1 expressions in tumor tissues and adjacent normal
tissues of the patients with breast cancer. Further, the effects of
SOCS-1 expressions on the pathological parameters, molecular
subtypes and prognosis of the patients with breast cancer were
analyzed in combination with the clinical data.
Materials and methods
Materials
In the present study, 60 women patients with breast
cancer who were admitted to Jining First People's Hospital (Jining,
China) from September, 2010 to September, 2011 were selected as
study subjects. Subjects aged 22–77 years old, with a median age of
53 years old. All the patients were clinically and pathologically
diagnosed with breast cancer and were given surgical treatments for
the first time without chemotherapy and radiotherapy. The tumor
tissues and cancer-adjacent normal tissues were located 10 cm away
from each other and were excised during the surgery. A part of the
tissues were stored in liquid nitrogen immediately, and the
remaining was fixed in 10% paraformaldehyde solution. This was
followed by formation of paraffin sections. All the patients had
complete follow-up records, including age, tumor size, lymph node
status, clinical staging and survival conditions. The present study
was reviewed and approved by the Medical Ethics Committee of Jining
First People's Hospital, and all the patients or their families had
signed the informed consent.
TRIzol RNA extraction kit, reverse transcription kit
and RT-qPCR kit (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA); primer synthesis (Takara Biotechnology Co.,
Ltd., Dalian, China); SOCS-1 antibodies, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) antibodies and horseradish peroxidase
(HRP)-labeled secondary antibodies (BD Pharmingen, San Diego, CA,
USA); immunohistochemical staining kit SP-9001 (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.; OriGene Technologies,
Beijing, China).
Detection of SOCS-1 messenger RNA
(mRNA) expressions in patients' tissue specimens via RT-qPCR
method
The TRIzol method was used to extract the total RNA
in the patients' frozen tissues. Further, the concentration and
purity of the extracted RNA were measured via ultraviolet
spectrophotometric assay. The samples with an A260/A280 ratio of
1.8–2.0 were selected for subsequent operations. One milligram
total RNA of every patient was measured and taken for reverse
transcription in accordance with the procedures as prescribed in
the kit instructions. This was followed by addition of SOCS-1
primers, and qPCR method was applied to detect the expression of
SOCS-1 mRNA. In this experiment, GAPDH was selected as the internal
control. The primer sequences are shown in Table I. Specific reaction conditions: 94°C
for 5 min, denaturation at 94°C for 30 sec, annealing at 60°C for
30 sec, extension at 72°C for 30 sec and amplification for 35
cycles. The experimental results were analyzed using
2−ΔΔCq method, of which ΔCq (target gene) = Cq (target
gene) - Cq (reference gene).
 | Table I.RT-qPCR primer sequences. |
Table I.
RT-qPCR primer sequences.
| Gene | Primer sequence |
|---|
| SOCS-1 | F
5′-CACGCACTTCCGCACATrCC-3′ |
|
| R
5′-TCCAGCAGCTCGAAGAGGCA-3′ |
| GAPDH | F
5′-GCACCGTCAAGGCTGAGAAC-3′ |
|
| R
5′-TGGTGAAGACGCCAGTGGA-3′ |
Detection of SOCS-1 protein
expressions in patients' tissues via immunohistochemical method.
The streptavidin-peroxidase (SP) two-step method was utilized to
perform immunohistochemical staining
Major steps: after deparaffinization via
conventional methods, 3% H2O2 was used to
block the endogenous peroxidase, citric acid solution was utilized
for hotfix, and goat serum for blocking. SOCS-1 primary antibodies
(diluted at 1:200; cat. no. 554002; BD Pharmingen, San Diego, CA,
USA) were added in drips and then kept at 4°C overnight. The
secondary polyclonal antibodies (diluted at 1: 1,000) were then
added and incubated at room temperature for 1 h. All the
above-mentioned steps underwent washing with phosphate-buffered
saline (PBS) for 3 min 3 times. Finally, the diaminobenzidine (DAB)
was added in drips for development and hematoxylin for
counterstaining. PBS as the negative control replaced the primary
antibodies.
The double-blind method was applied to analyze the
results observed by a microscope (Olympus, Tokyo, Japan). The
judgment of positive expression was based on the comprehensive
judgment of the percentage of positive cells and staining
intensity. Percentage of positive cells: <5%, 0 point; 6–25%, 1
point; 26–50%, 2 points; and >50%, 3 points; staining intensity:
not stained, 0 point; light yellow, 1 point; yellowish-brown, 2
points; and sepia, 3 points. Six high-power fields were selected
randomly. Further, the two kinds of scores were added together: ≤3
points, negative; and >3 points, positive.
Correlation of SOCS-1 expressions in
breast cancer patients' tumor tissues with patients' pathological
parameters and prognosis
A total of 60 patients with breast cancer were
divided into positive and negative SOCS-1 expression groups
according to the expression levels of SOCS-1 in breast cancer
tissues. The clinicopathological parameters of the patients were
recorded, and the correlation of SOCS-1 expressions with patients'
pathological parameters, molecular subtypes and prognosis were
analyzed. All the patients underwent modified radical mastectomy.
The follow-up time was counted from the first day after the surgery
and the survival time was calculated. The overall survival time
referred to the duration from the first day after the surgery to
the date of death or the last follow-up. The follow-up was
conducted once a month for 5 years.
Statistical processing
Statistical Product and Service Solutions (SPSS)
17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data
recording and processing. All the measurement data are expressed as
mean ± standard deviation. Further, t-test was utilized for
comparison between two groups; χ2 test was performed for
comparison of enumeration data between groups. The Kaplan-Meier
method was utilized to conduct univariate survival analysis. P≤0.05
suggested that the difference was statistically significant.
Results
Detection of SOCS-1 mRNA expressions
in patients' tissues via RT-qPCR method
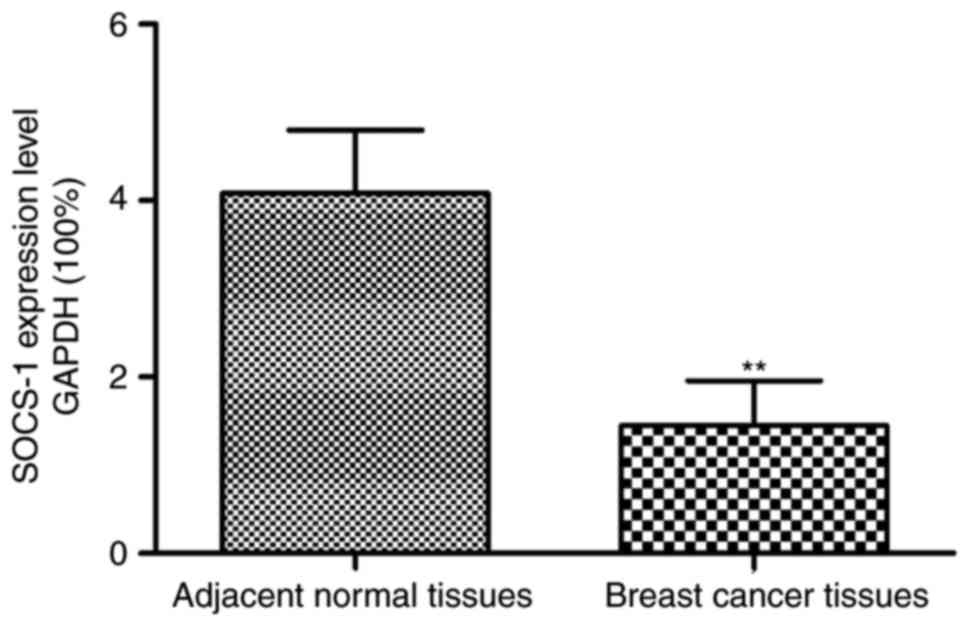
The results of SOCS-1 mRNA expressions in tumor
tissues and adjacent normal tissues of patients with breast cancer
are shown in Fig. 1. The relative
expression of SOCS-1 mRNA in tumor tissues of patients with breast
cancer was significantly decreased as compared to adjacent normal
tissues (p<0.01).
Detection of SOCS-1 protein
expressions in patients' tissue specimens via immunohistochemical
method
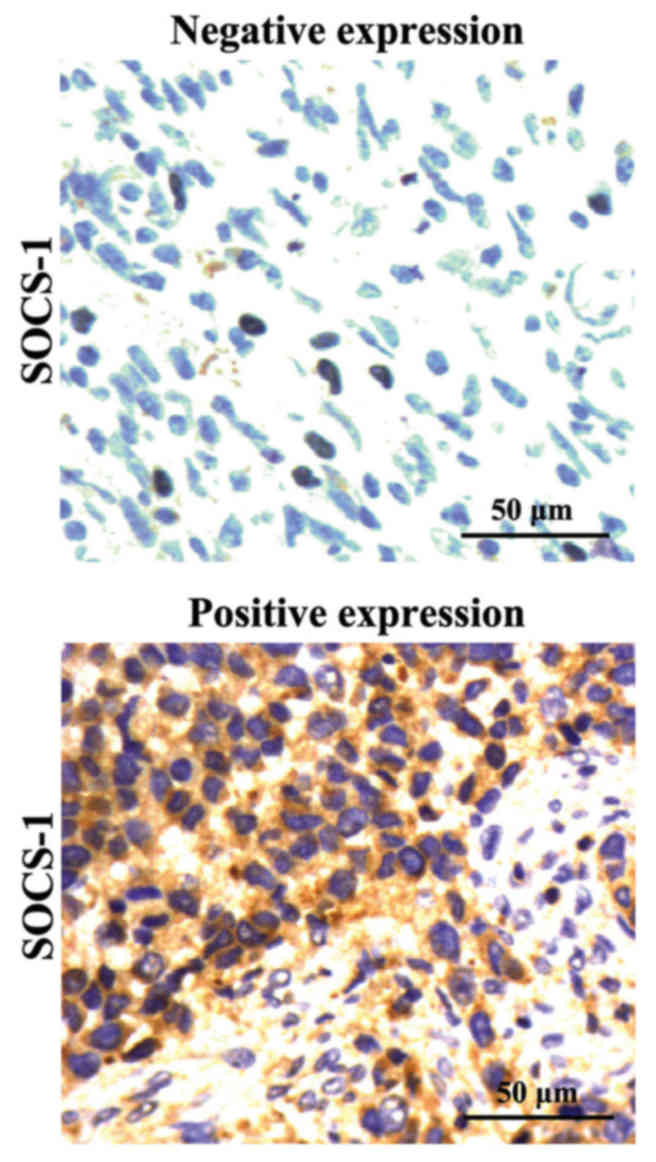
The immunohistochemical results are shown in
Fig. 2. The positive SOCS-1 protein
was manifested as sepia deposition of cytoplasm. According to the
statistical analysis on the staining results, the positive
expression rate of SOCS-1 proteins in tumor tissues of patients
with breast cancer was 23.33% (14/60), and in adjacent normal
tissues was 88.33% (53/60), respectively. The differences were
statistically significant (p<0.01).
Correlation of SOCS-1 expressions in
breast cancer patients' tumor tissues with clinicopathological
parameters
The patients with breast cancer were divided into
positive and negative SOCS-1 expression group in accordance with
the immunohistochemical results. χ2 test was performed
to analyze the relationship between the SOCS-1 expressions in the
tissues of patients with breast cancer and the patients'
clinicopathological parameters. The negative SOCS-1 expression in
patients' tumor tissues was associated with the lymph node
metastasis and clinical staging of the tumor (p<0.05) (Table II).
 | Table II.Correlation of abnormal SOCS-1
expressions with clinicopathological parameters of breast cancer
patients. |
Table II.
Correlation of abnormal SOCS-1
expressions with clinicopathological parameters of breast cancer
patients.
|
|
| SOCS-1 |
|---|
|
|
|
|
|---|
| Clinical data | Cases (n) | Negative (n, %) | Positive (n, %) | χ2
value | P-value |
|---|
| Age (years) |
| ≤40 | 32 | 26 (81.25) | 6
(18.75) | 0.81 | 0.12 |
|
>40 | 28 | 20 (71.43) | 8 (28.58) |
|
|
| Tumor size (cm) |
| ≤5 | 29 | 21 (72.41) | 8 (27.59) | 0.57 | 0.11 |
|
>5 | 31 | 25 (80.65) | 6 (19.35) |
|
|
| Lymphatic
metastasis |
| No | 24 | 15 (62.50) | 9
(37.50) | 4.49 | 0.03 |
| Yes | 36 | 31 (86.11) | 5 (13.89) |
|
|
| Clinical staging |
| I–II | 31 | 20 (64.52) | 11 (35.48) | 5.29 | 0.02 |
|
III–IV | 29 | 26 (89.66) | 3 (10.34) |
|
|
Correlation of SOCS-1 expressions with
molecular typing in breast cancer patients' tumor tissues
χ2 test was utilized to analyze the
relationship between the SOCS-1 expressions and molecular typing in
the tissues of patients with breast cancer; as shown in Table III. The positive expression rate of
luminal A SOCS-1 proteins was the highest (47.62%), and the SOCS-1
expressions in different molecular subtypes varied remarkably
(p<0.01).
 | Table III.Correlation of abnormal SOCS-1
expressions with molecular typing of breast cancer. |
Table III.
Correlation of abnormal SOCS-1
expressions with molecular typing of breast cancer.
|
|
| SOCS-1 |
|---|
|
|
|
|
|---|
| Molecular
subtype | Cases (n) | Negative (n, %) | Positive (n, %) | χ2
value | P-value |
|---|
| Luminal A | 21 | 11 (52.38) | 10 (47.62) | 11.62 | 0.067 |
| Luminal B | 18 | 17 (94.44) | 1 (5.56) |
|
|
| HER-2
overexpression | 12 | 11 (91.67) | 1 (8.33) |
|
|
| Basal-like | 9 | 7 (77.78) | 2 (22.22) |
|
|
Analyses on patients' survival
conditions and prognosis
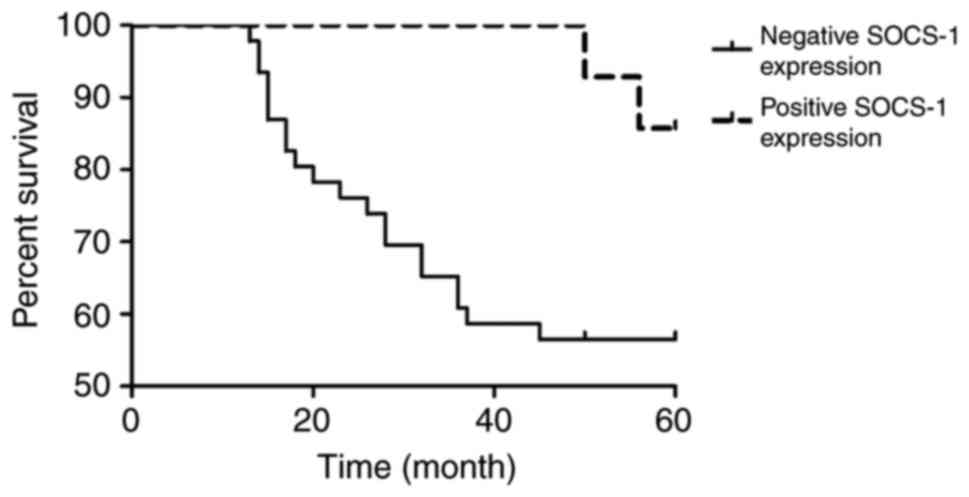
After 5 years of follow-up, among the 60 patients
with breast cancer, 38 survived and 22 were dead. The 5-year
overall survival rate of the patients was 63.33% (38/60). The
Kaplan-Meier survival analysis results are shown in Fig. 3. The prognosis of patients with
negative SOCS-1 expression was poor. The univariate survival
analysis results are shown in Table
IV. SOCS-1 could influence the overall survival rate of the
patients with breast cancer (p<0.05).
 | Table IV.Univariate analysis on correlation of
SOCS-1 expressions with overall survival rate of breast cancer
patients. |
Table IV.
Univariate analysis on correlation of
SOCS-1 expressions with overall survival rate of breast cancer
patients.
| SOCS-1 group | Cases (n) | Cases survived in 5
years (n) | 5-year survival rate
(%) | Wald (log-rank) | P-value |
|---|
| Positive
expression | 14 | 12 | 85.71 | 4.15 | <0.05 |
| Negative
expression | 46 | 26 | 56.52 |
|
|
Discussion
Recently, literature has shown significant rise in
the incidence of younger breast cancer patients. Moreover, its
death rate is also rising year by year. The onset of breast cancer
is related to variations in transcriptions and expressions of many
genes, and the clinical treatment effects fail to live up to
people's expectations. Therefore, breast cancer has posed a serious
threat to women's life and health (14–16). So
far, the individualized treatment of breast cancer is an emerging
treatment protocol that requires analysis of the gene expressions
of the breast cancer patients. This divides the patients into
different subtypes, which allow application of different
therapeutic methods and drugs according to the subtypes (17).
The N-terminal of SOCS-1 protein is the inhibition
section of kinase, in which a tyrosine residue is used as the
pseudosubstrate of JAK-2. Further, it could compete with STAT for
the binding sites in the JAK catalytic zone, thereby negatively
regulating the JAK/STAT signaling pathway (18). The JAK/STAT signaling pathway plays
key roles in the occurrence, proliferation, metastasis and invasion
processes of tumor tissues (19).
Some studies have found that SOCS-1 protein was slowly expressed in
the tumor tissues of patients with prostate cancer. Furtehr, the
low SOCS-1 expression could lead to upregulation of the expressions
of cyclins and cyclin-dependent kinases, thereby, enhancing the
proliferative capacity of prostate cancer cells (12). Moreover, the downregulated SOCS-1
expressions have also been discovered in multiple tumors, including
hepatocellular carcinoma, pancreatic cancer, multiple myeloma and
acute myeloid leukaemia. The proliferation activity of the tumor
tissues was suppressed when the SOCS-1 was highly expressed in
them. As a result, SOCS-1 is a suppressor gene (20–22).
The RT-qPCR method was used to detect the
expressions of SOCS-1 mRNA in the tumor tissues and adjacent normal
tissues of patients with breast cancer in order to investigate the
expression of SOCS-1 in the tumor tissues of patients. The results
showed that the SOCS-1 mRNA expression level in breast cancer
tissues was significantly lower than that of adjacent normal
tissues. The immunohistochemical results indicated that the
expression of the SOCS-1 proteins in breast cancer tumor tissues
was remarkably lower than that of adjacent normal tissues.
χ2 test was performed to analyze the relationship
between the SOCS-1 protein expressions and the patients'
pathological parameters along with molecular subtypes. The results
revealed that the negative expression of SOCS-1 protein in
patients' tumor tissues was associated with the lymph node
metastasis and clinical staging of the tumor. In addition, the
SOCS-1 expressions in different molecular subtypes varied
remarkably. Furthermore, univariate Kaplan-Meier survival analysis
was applied to study the impact of SOCS-1 expressions on the 5-year
overall survival rate of the patients. It was observed that the
SOCS-1 had a significant influence on the overall survival time of
breast cancer patients. The patients with lower expression of
SOCS-1 had a lower 5-year survival rate and poorer prognosis. The
5-year overall survival rate of the patients was 63.33%, and the
Kaplan-Meier survival analysis results showed that the prognosis of
patients with negative SOCS-1 expression was poor. So, SOCS-1 could
be regarded as an independent factor influencing the overall
survival rate of the breast cancer patients.
In conclusion, SOCS-1 has low expression in the
tumor tissues of patients with breast cancer, and plays a crucial
role in the pathogenesis of breast cancer. Therefore, the SOCS-1
expression in patients' tumor tissues could be regarded as a
reference for the prognostic estimation of breast cancer
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL extracted RNA. GS performed RT-qPCR. PL helped
with immunohistochemical method. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Medical Ethics Committee of Jining First People's Hospital (Jining,
China), and all the patients or their families had signed the
informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Murillas I, Schiavon G, Weigelt B,
Ng C, Hrebien S, Cutts RJ, Cheang M, Osin P, Nerurkar A, Kozarewa
I, et al: Mutation tracking in circulating tumor DNA predicts
relapse in early breast cancer. Sci Transl Med. 7:302ra1332015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Im NK, Jang WJ, Jeong CH and Jeong GS:
Delphinidin suppresses PMA-induced MMP-9 expression by blocking the
NF-κB activation through MAPK signaling pathways in MCF-7 human
breast carcinoma cells. J Med Food. 17:855–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shalaby MA, Nounou HA, Ms AOA, Azzam N and
Saeed HM: Associations between single nucleotide polymorphisms of
COX-2 and MMP-2 genes and colorectal cancer susceptibility in the
Saudi population. Asian Pac J Cancer Prev. 15:4989–4994. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Starr R, Willson TA, Viney EM, Murray LJ,
Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola
NA, et al: A family of cytokine-inducible inhibitors of signalling.
Nature. 387:917–921. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimura A, Naka T and Kubo M: SOCS
proteins, cytokine signalling and immune regulation. Nat Rev
Immunol. 7:454–465. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Endo TA, Masuhara M, Yokouchi M, Suzuki R,
Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H,
et al: A new protein containing an SH2 domain that inhibits JAK
kinases. Nature. 387:921–924. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senft C, Priester M, Polacin M, Schröder
K, Seifert V, Kögel D and Weissenberger J: Inhibition of the
JAK-2/STAT3 signaling pathway impedes the migratory and invasive
potential of human glioblastoma cells. J Neurooncol. 101:393–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyoshi H, Fujie H, Moriya K, Shintani Y,
Tsutsumi T, Makuuchi M, Kimura S and Koike K: Methylation status of
suppressor of cytokine signaling-1 gene in hepatocellular
carcinoma. J Gastroenterol. 39:563–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hussain S, Singh N, Salam I, Bandil K,
Yuvaraj M, Bhat Akbar M, Mir Muzaffar M, Siddiqi MA, Sobti RC,
Bharadwaj M, et al: Methylation-mediated gene silencing of
suppressor of cytokine signaling-1 (SOCS-1) gene in esophageal
squamous cell carcinoma patients of Kashmir valley. J Recept Signal
Transduct Res. 31:147–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neuwirt H, Puhr M, Santer FR, Susani M,
Doppler W, Marcias G, Rauch V, Brugger M, Hobisch A, Kenner L, et
al: Suppressor of cytokine signaling (SOCS)-1 is expressed in human
prostate cancer and exerts growth-inhibitory function through
down-regulation of cyclins and cyclin-dependent kinases. Am J
Pathol. 174:1921–1930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu PY, Yeh CM, Hsu NC, Chang YS, Chang JG
and Yeh KT: Epigenetic alteration of the SOCS1 gene in
hepatocellular carcinoma. Swiss Med Wkly. 140:w130652010.PubMed/NCBI
|
|
14
|
Huang S, Chen J and Jia Y: Research on the
correlation of MMP9 and p53 expression with the prognosis of triple
negative breast cancer. Xiandai Shengwu Yixue Jinzhan. 14:881–884.
2014.
|
|
15
|
Choi JS, Baek HM, Kim S, Kim MJ, Youk JH,
Moon HJ, Kim EK and Nam YK: Magnetic resonance metabolic profiling
of breast cancer tissue obtained with core needle biopsy for
predicting pathologic response to neoadjuvant chemotherapy. PLoS
One. 8:e838662013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yari K and Rahimi Z, Moradi MT and Rahimi
Z: The MMP-2 −735 C allele is a risk factor for susceptibility to
breast cancer. Asian Pac J Cancer Prev. 15:6199–6203. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamps LW and Folpe AL: The diagnostic
value of hepatocyte paraffin antibody 1 in differentiating
hepatocellular neoplasms from nonhepatic tumors: A review. Adv Anat
Pathol. 10:39–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kershaw NJ, Murphy JM, Liau NP, Varghese
LN, Laktyushin A, Whitlock EL, Lucet IS, Nicola NA and Babon JJ:
SOCS3 binds specific receptor-JAK complexes to control cytokine
signaling by direct kinase inhibition. Nat Struct Mol Biol.
20:469–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weissenberger J, Priester M, Bernreuther
C, Rakel S, Glatzel M, Seifert V and Kögel D: Dietary curcumin
attenuates glioma growth in a syngeneic mouse model by inhibition
of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res.
16:5781–5795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu TC, Lin SF, Chang JG, Yang MY, Hung SY
and Chang CS: Epigenetic alteration of the SOCS1 gene in chronic
myeloid leukaemia. Br J Haematol. 123:654–661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stanganelli C, Arbelbide J, Fantl DB,
Corrado C and Slavutsky I: DNA methylation analysis of tumor
suppressor genes in monoclonal gammopathy of undetermined
significance. Ann Hematol. 89:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe D, Ezoe S, Fujimoto M, Kimura A,
Saito Y, Nagai H, Tachibana I, Matsumura I, Tanaka T, Kanegane H,
et al: Suppressor of cytokine signalling-1 gene silencing in acute
myeloid leukaemia and human haematopoietic cell lines. Br J
Haematol. 126:726–735. 2004. View Article : Google Scholar : PubMed/NCBI
|