Introduction
Melanoma-associated antigen A (MAGEA) subfamily
proteins are members of cancer/testis antigens (CTAs), whose normal
expression is limited to germ cells, but ectopic expression can be
observed in tumor cells of different origins (1). The MAGEA genes were initially
identified as tumor antigens that can be recognized by cytotoxic
T-lymphocytes in melanoma patients (2). The MAGEA subgroup of CTA family
comprises eleven genes that show striking homology with each other
and are encoded as a cluster at the Xq28 region (3). Their normal expression is restricted to
the testis, trophoblast and placenta (3,4). MAGEA
expression in somatic cells is silenced by promoter DNA methylation
(5), but in tumor cells genome-wide
epigenetic reprogramming can result in promoter hypo-methylation,
leading to aberrant expression of one or more of these genes
(1,6).
MAGEA expression is observed mainly in cancers that
have acquired malignant phenotypes, invasiveness or metastasis, and
the expression of MAGEA family proteins has been linked to poor
prognosis in cancer patients. MAGEA family proteins have oncogenic
functions, including support of growth, survival and metastasis,
and are thought to contribute actively to malignancy (7). At the molecular level, MAGEA proteins
are involved, through direct and indirect mechanisms, in the
regulation of the tumor suppressor protein p53 pathway (8–12). MAGEA
proteins can also activate specific RING finger type E3 ubiquitin
ligases (13,14), thereby regulating the ubiquitin
signaling in cancer cells.
MAGEA proteins are known to be highly expressed in a
wide range on cancers including bladder, lung, skin and breast
malignancies (6,15–18).
Expression of these antigens may be highly heterogeneous in a
variety of tumors of different histological origin, with
percentages of positive cells ranging between 5 and 60% (18). MAGEA subfamily proteins are highly
conserved and it is very difficult to get antibodies that recognize
only one member of the family specifically. For example, MAGEA4 and
MAGEA10 proteins share more than 50% sequence identity on the amino
acid level, but have different sizes and cellular localizations
(19). Several antibodies used in
immunohistochemical studies cross-react with many MAGEA proteins
and have been seen in multiple cancer types to localize both in the
cytoplasm and in the nucleus (20–22). This
has complicated the immunohistochemical analysis of cancer tissues
and limited the analysis of specific subfamily members, which may
have different expression patterns, subcellular localizations and
impacts on the malignancy.
Melanoma is the most serious type of skin cancer and
its incidence has risen over the years. The etiology of melanoma is
multi-factorial, resulting from gene-environment interactions, with
the main environmental factor for melanoma development being
exposure to sunlight and UV radiation (23). The importance of the immune system in
the etiology of human skin cancer has been long recognized, based
primarily upon the increased incidence of skin cancers in organ
transplant recipients and mechanisms of ultraviolet (UV)
radiation-mediated immunomodulation (24). Although the rate of melanoma incidence
is rising, especially within young females, there is no direct
correlation with the increase of mortality. Histological regression
in primary cutaneous melanoma has been shown to occur in 10–35% of
cases (25). Thus, it can be
hypothesized that the immune system is involved in controlling the
melanoma progression, especially in younger individuals.
The aim of this study was to evaluate the presence
of naturally occurring antibodies against two MAGEA proteins in the
blood samples of melanoma patients with different stages of
disease. MAGEA proteins have oncogenic functions contributing to
malignancy, and they are known to be immunogenic proteins. The
MAGEA4 and MAGEA10 proteins were expressed in bacteria, purified
and used in the enzyme-linked immunosorbent assay (ELISA) for
detection of antibodies. We were curious to know i) whether the
melanoma patients have antibodies against these proteins, and ii)
whether these antibodies can be treated as a potential prognostic
marker.
Materials and methods
Patients and sera
Human sera were obtained from 185 patients with
melanoma attending the North Estonian Medical Centre (Tallinn,
Estonia) within two years (2013–2014). The melanoma stage was
assigned based on tumor thickness, ulceration and the involvement
of lymph nodes or organs. The characteristics of patients are shown
in Table I. As a control, we included
43 sera of healthy blood donors from the Estonian Blood Bank. We
have no data about the gender nor age of blood bank controls. All
samples were handled by standard procedures and stored at −80°C.
Approval for the use of blood samples for the study was obtained
from the Tallinn Medical Research Ethics Committee (Tallinn,
Estonia).
 | Table I.Characteristics of the melanoma
patients. |
Table I.
Characteristics of the melanoma
patients.
| Number | Stage 0 24 | Stage I 67 | Stage II 43 | Stage III 30 | Stage IV 21 | Total 185 |
|---|
| Sex |
|
|
|
|
|
|
| Male
(%) | 4
(16.7%) | 17 (25.4%) | 14 (32.6%) | 12 (40%) | 9
(42.9%) | 56
(30.3%) |
| Female
(%) | 20 (83.3%) | 50 (74.6%) | 29 (67.4%) | 18 (60%) | 12 (57.1%) | 129 (69.7%) |
| Disease
duration |
|
|
|
|
|
|
| <5
years | 21 (87.5%) | 47 (70.1%) | 29 (67.4%) | 20 (66.7%) | 18 (85.7%) | 135 (73%) |
| ≥5
years | 3
(12.5%) | 20 (29.9%) | 14 (32.6%) | 10 (33.3%) | 3
(14.3%) | 50
(27%) |
| Mean
(range) | 2.0 (0–13) | 4.5 (0–26) | 3.7 (0–18) | 4.5 (0–19) | 2.8 (0–25) | 3.8
(0–26) |
|
Median | 1 | 2 | 3 | 3 | 1 | 2 |
| Age |
|
|
|
|
|
|
| Mean
(range) | 51.9 (18–87) | 61.3 (28–87) | 64.3 (33–90) | 63.4 (43–82) | 73.1 (35–92) | 62.5 (18–92) |
|
Median | 51 | 65 | 66 | 65 | 78 | 65 |
Proteins
MAGEA4 and MAGEA10 coding sequences from
pQMCF-MAGEA4 and pQMCF-MAGEA10 vectors (19) were cloned in frame into pET28a vector
using NheI restriction enzyme to fuse the coding sequence with
His-tag. Recombinant His-tagged MAGEA4 and MAGEA10 proteins were
transformed into Escherichia coli (E coli) cells
BL-CodonPlus™RP (Invitrogen; USA); transformed bacteria
were grown at 37°C to the spectrophotometric density 0.6 (OD 600
nm; Ultraspec 7000; GE Healthcare Life Sciences, Little Chalfont,
UK) and induced with 1 mM IPTG for 2 h at room temperature. Then
the cells were collected by centrifugation (at 8,000 × g for 3 min
at 4°C; Centrifuge 5810R; Eppendorf, Hamburg, Germany) and
resuspended in buffer containing 50 mM Tris (pH 8.0) and 500 mM
NaCl. Proteins were purified with Ni-Sepharose™ 6 Fast
Flow beads (GE Healthcare Life Sciences) under standard native
conditions following manufacturer's recommendations; 20 mM
imidazole was added to the buffer for binding reactions, 25 mM for
wash buffers and 250 mM for elution of proteins from the beads.
Both proteins were purified using the same protocol. After
purification, the buffer was exchanged to PBS with
Amicon® Ultra centrifugal filters (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and the concentration of proteins was
determined by the Bradford assay using bovine serum albumin (BSA)
as a standard.
ELISA
Recombinant MAGEA4 or MAGEA10 protein (2 µg/ml) in
phosphate-buffered saline (PBS) containing 0.1% of Tween-20 was
adsorbed onto 96-well MaxiSorp NUNC-immunoplates (Sigma-Aldrich;
Merck KGaA) and incubated overnight at 4ºC. Plates were washed with
PBS/0.1% Tween-20 and blocked with 2% BSA in PBS/0.1% Tween-20.
Serial dilutions of human serum in 100 µl of 0.4% BSA/PBS/0.1%
Tween-20 were added to each well and incubated for one hour at room
temperature on the shaker (Titertek-Berthold; Berthold Detection
Systems GmbH, Pforzheim, Germany). Horseradish peroxidase
(HRP)-conjugated goat anti-human IgG (Zymax/Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used as a secondary
antibody for 45 min. After washing four times, the reaction was
developed with the TMB Peroxidase E1A substrate kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 10 min. and stopped with
H2SO4. The absorbance at 450 nm was measured
spectrophotometrically using the ELISA plate reader
Sunrise™ (Tecan, Männedorf, Switzerland). For quality
control, we included three reference sera which were analyzed on
every ELISA plate. The CVs of their ODs did not exceed 20%.
Statistical analysis
The data were analyzed in R (version 3.3.0).
Parameter estimates and corresponding CI (credible intervals) were
calculated using the BayesFirstAid package (26). Analysis of variance (ANOVA) with the
Tukey post-test was also done in R.
The patients with positive antibody response were
defined as follows: pooled MAGEA4 and MAGEA10 response values
obtained from the blood bank donors were log-transformed to ensure
normality, after which the mean and the standard deviation was
calculated from the control subjects only. Then the melanoma
patients, whose log-response value > mean + 2* SD, were
redefined as having a strong response. To classify subjects based
on their MAGEA protein levels a logistic regression model,
including both MAGEA proteins, sex, and age as additive predictors,
was trained on the subset of data containing stage 0, I, and II
patients. The pROC package was used to calculate the receiver
operating characteristic (ROC) curve (27).
Results
Antibody response against MAGEA4 and
MAGEA10 proteins in melanoma patients
We measured the anti-MAGEA4 and anti-MAGEA10
antibody levels by ELISA from 185 stage 0 (in situ) to stage
IV melanoma patients and from 43 healthy individuals, who had
donated their blood to the Estonian blood bank. The ELISA was
performed using MAGEA4 and MAGEA10 proteins, which were purified
from E. coli, immobilized on microtiter plates, and subsequently
probed with 1:200 to 1:800 human sera dilutions. The serums that
exhibited high OD values, indicating the presence of anti-MAGEA
antibodies, were tested at least three times on separate ELISA
plates and the mean OD value was used in further analysis. The OD
values obtained from 1:400 diluted serums were used in statistical
analysis.
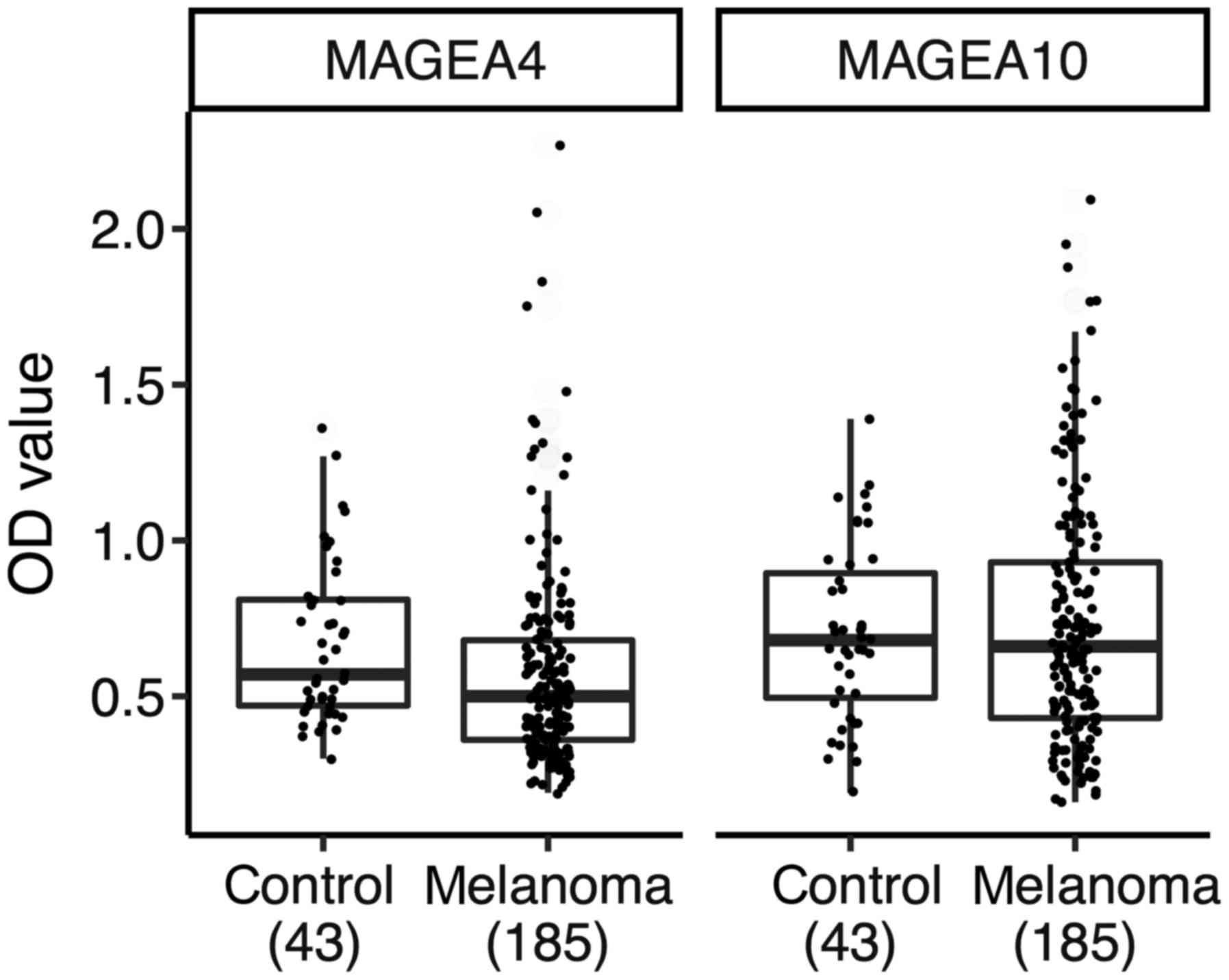
We first compared the OD values of the controls with
the melanoma patients separately for anti-MAGEA4 and anti-MAGEA10
response (Fig. 1). In Fig. 1, the Tukey box blots were used to show
the median and interquartile ranges, as well as dots corresponding
to individual patients and blood bank controls. The mean immune
responses of patients were not elevated as compared with the blood
bank controls. The mean OD value of the sera of melanoma patients
was 0.59 (SD 0.31) for MAGEA4 and 0.73 (0.38) for MAGEA10. For
blood bank controls, the mean OD value was 0.67 (0.26) for MAGEA4
and 0.70 (0.28) for MAGEA10. We could not find evidence for
elevated mean effects of melanoma patients over blood bank
controls, the probability that the patients mean value was higher
than the controls was 0.3% for MAGEA4 (two-sided P-value=0.004) and
62% for MAGEA10 (P=0.87). On the other hand, the patients had
higher variability at their anti-MAGEA4 and anti-MAGEA10 responses
than controls; the probability that the controls have higher
standard deviations was 2 and 6% for MAGEA4 and MAGEA10,
respectively. We suggest that the higher variability of the immune
response in patients could mean that in some patients the antibody
response is induced, while in others is not. The failure of the
patients to exhibit aggregate effects over the controls is likely
due to the voluntary blood donors, who make up the control group.
We have no data about their age and gender, but they are probably
younger than the melanoma patients. This limits the usefulness of
the blood donors as controls. Therefore, in the subsequent analysis
we omitted the blood bank controls and looked at melanoma stages 0
to IV as distinct groups.
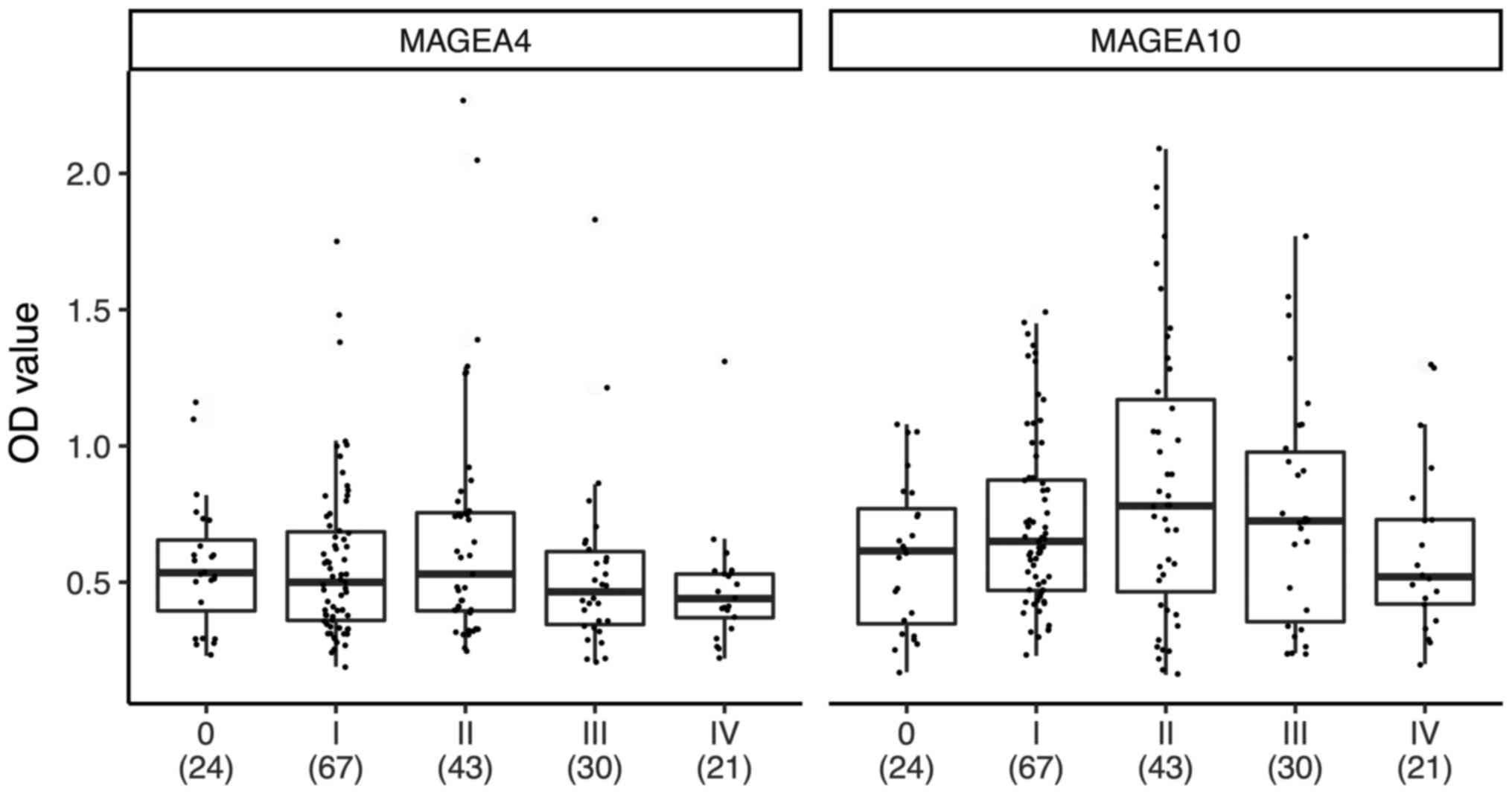
To follow the antibody response from limited to
advanced disease, we divided the patients into subgroups, depending
on the status of their disease. In Fig.
2, the Tukey box blots were used to show the median and
interquartile ranges, as well as dots corresponding to individual
patients; the number of patients is shown in parenthesis under the
stage number. As shown in Fig. 2,
some stage I, II and III patients exhibited elevated anti-MAGEA4
and/or anti-MAGEA10 immune responses. The one-way ANOVA P-value was
0.10 for MAGEA4 and 0.043 for MAGEA10, indicating that there are
statistically significant contrast(s) in the MAGEA10 data. We used
the Tukey HSD post-test to find groups that significantly differ
from each other. This showed that in the case of MAGEA10, there was
a single contrast, stage 0 vs. stage II patients, which had a
significant difference in mean OD values (P=0.047). The mean OD
values between stage II and stage IV patients were slightly
different (P=0.10), but no difference was observed between stage II
and III patients (P=0.78). In the case of MAGEA4, we did not
observe statistically significant differences in mean values
between patients with different stages of disease (P=0.74 for stage
0 vs. stage II; P=0.18 for stage II vs. stage IV and P=0.47 for
stage II vs. stage III). These data show that there is no strong
difference in mean OD values between stage II and III, but is a
slight difference between stage II and IV for MAGEA10. Although, we
found only a single statistically significant contrast, due to the
limited sample size this does not necessarily mean that there are
no real differences between stage II and III&IV. We sought to
clarify this point further by polynomial regression modelling. We
predicted anti-MAGEA4/A10 response levels (as measured by OD) from
the stage of melanoma modelled as a continuous ordinal variable.
These models indicate that for both proteins there is an initial
rise in optical density that peaks at stage II, and thereafter
falls again (data not shown).
As our samples were not balanced for age and sex
(Table I), we also looked for
associations between these variables and anti-MAGE response. By
applying linear regression towards our stage 0 to IV melanoma
samples, we could find no significant association between the age
of the subjects and anti-MAGEA4 or anti-MAGEA10 response (data not
shown). However, we found a weak association between anti-MAGEA10
levels and sex (r2=0.025; female melanoma patients have
on average 0.15 OD units higher anti-MAGEA10 response than male
patients, 95% CI: 0.04, 0.26). But the width of the CI indicates
that our sample size is not large enough to decide whether this
effect is scientifically relevant.
Patients with strong antibody
response
Next, we focused on patients with strong anti-MAGEA4
and/or anti-MAGEA10 immune responses. Here we included patients
whose OD values were higher than the mean OD of the healthy blood
bank donors plus 2 SD-s (28).
Table II shows the patients with
strong antibody response. The sera of 15 patients from 185 (8.2;
95% CI: 4.7, 13%) had a strong antibody response against the MAGEA4
and/or MAGEA10 protein (Table II).
Two patients (M38 and M111) had a strong response against both
proteins, that is why there are 17 patients listed in Table II. The mean age of strongly
responding patients was 67 years (median 67 years) and they were
first diagnosed from 0 to 18 years (mean 5 years, median 3 years)
before this analysis was performed. Most of them are women, there
are only 3 men (20; 95% CI: 5.4, 43%) among the patients with
strong antibody response, while the whole cohort consists of 30.3%
of men. Altogether, 5.6% of men and 9.3% of women had strong
antibody response against one or two of the MAGEA proteins.
 | Table II.Patients with strong antibody
response. |
Table II.
Patients with strong antibody
response.
| Patient | Gender | Age (years) | Disease duration
(years) | Stage | Protein | OD value |
|---|
| M35 | F | 61 | 2 | IIB | MAGEA4 | 2.05 |
| M38a | F | 73 | 18 | II | MAGEA4 | 2.27 |
| M111b | M | 67 | 1 | IIIC | MAGEA4 | 1.49 |
| M123 | F | 65 | 0 | IB | MAGEA4 | 1.48 |
| M162 | M | 64 | 3 | IA | MAGEA4 | 1.75 |
| M3 | F | 57 | 1 | IB | MAGEA10 | 1.49 |
| M38a | F | 73 | 18 | II | MAGEA10 | 1.67 |
| M47 | F | 71 | 4 | IIB | MAGEA10 | 2.09 |
| M63 | F | 80 | 3 | IIIA | MAGEA10 | 1.48 |
| M70 | F | 64 | 9 | IIB | MAGEA10 | 1.43 |
| M76 | F | 61 | 6 | IIB | MAGEA10 | 1.77 |
| M99 | F | 66 | 5 | IIB | MAGEA10 | 1.88 |
| M111b | M | 67 | 1 | IIIC | MAGEA10 | 1.55 |
| M115 | M | 72 | 1 | IIB | MAGEA10 | 1.95 |
| M119 | F | 76 | 11 | IB | MAGEA10 | 1.45 |
| M137 | F | 72 | 0 | IIB | MAGEA10 | 1.58 |
| M144 | F | 52 | 1 | IIIA | MAGEA10 | 1.77 |
MAGEA proteins are highly similar to each other with
half of the amino acids identical between MAGEA4 and MAGEA10
proteins. We have analyzed the sera separately for MAGEA4 and
MAGEA10 response, and the statistics was performed and cut-off
values calculated independently of each other. Interestingly, there
were 12 anti-MAGEA10 responses and 5 anti-MAGEA4 responses, out of
the total of 17 strong responses (estimated relative frequency of
MAGEA4 is 0.31; 95% CI: 0.13, 0.52; 5% probability of relative
frequency >0.5) (Table II).
Consequently, only two patients out of 15 (13.3, 95% CI: 2.7, 35%)
had strong and statistically significant responses against both,
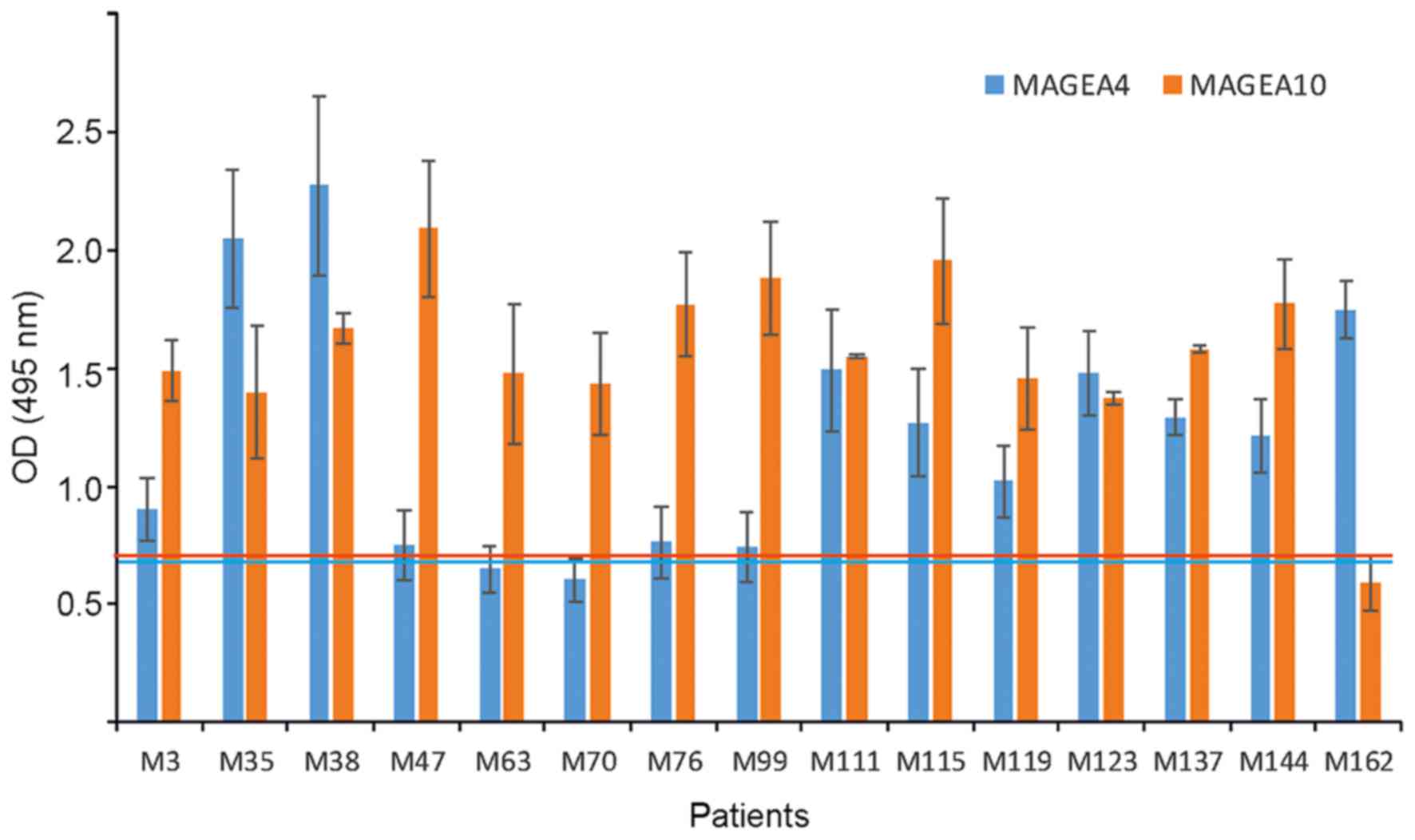
MAGEA4 and MAGEA10 proteins. In Fig.
3, we compare the antibody response against two different
antigens among the same patients. Some patients (M38, M111, but
also M35, M123 and M137) had antibodies against both MAGE-A
proteins, the others (M47, M63, M70, M76, M99 and M162) against
only one of the two proteins, either MAGEA4 or MAGEA10. Five
patients of 15 (33%) had a statistically significant higher OD
value (P<0.01) against MAGEA10 than MAGEA4, while only one
patient, M162, had a better immune response against MAGEA4
(Fig. 3). These data show that among
strongly responding patients, there are more anti-MAGEA10 than
anti-MAGE4 responses.
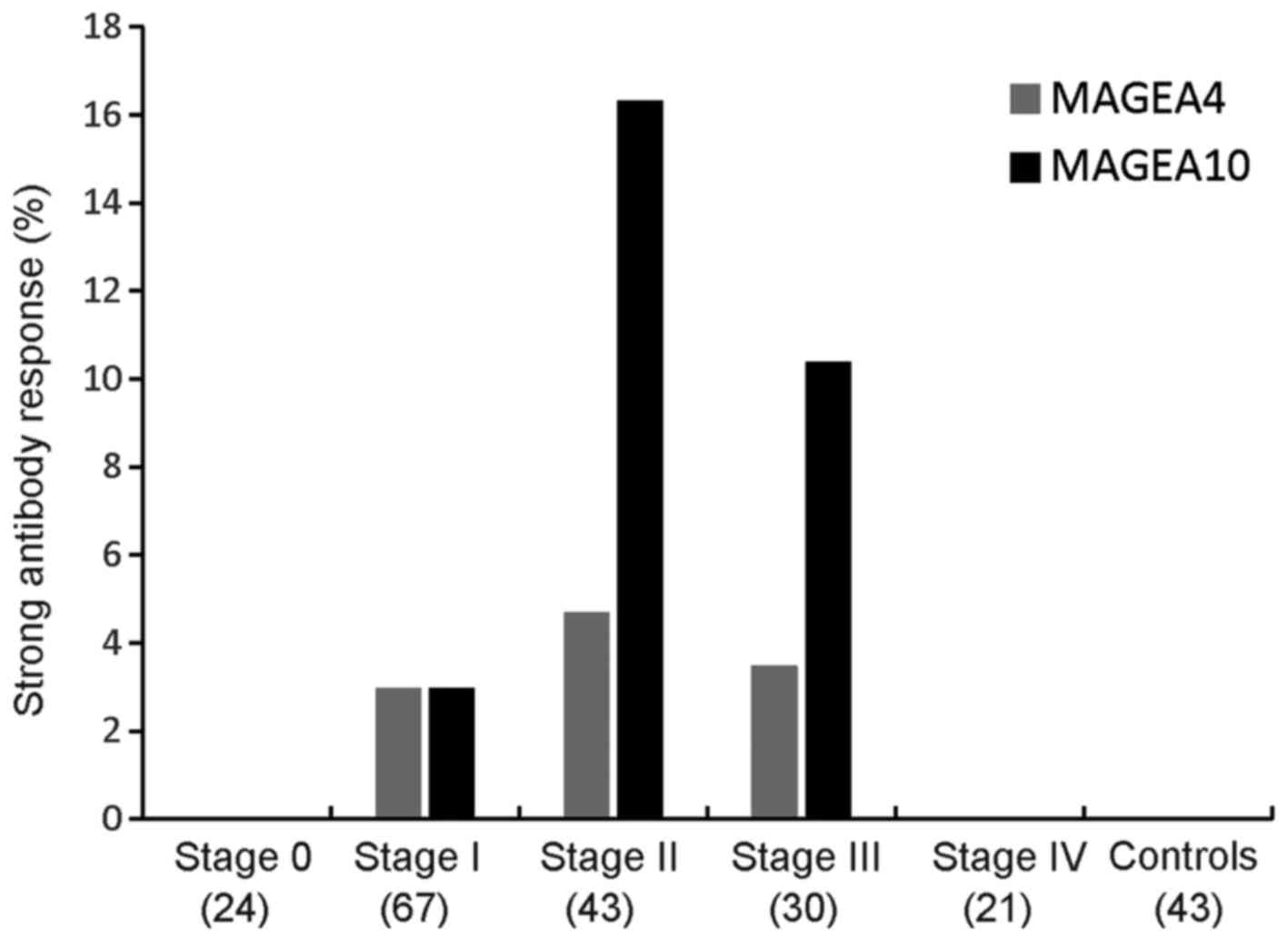
Comparing the number of strongly responding patients
between different stages of disease revealed that the highest
number of strong responses was detected among stage II melanoma
patients (Fig. 4). In the case of
MAGEA10, 7 of 43 (16.3, 95% CI: 7.3, 29%) sera were positive among
stage II patients, and 3 of 29 (10.3, 95% CI: 2.4, 24%) in stage
III patients. In the case of MAGEA4, there was no clear preference
to any stage, strongly responding sera belonged to patients of
stages I, II and III. We could not detect any strong response from
the blood samples of melanoma patients with stage 0 and IV.
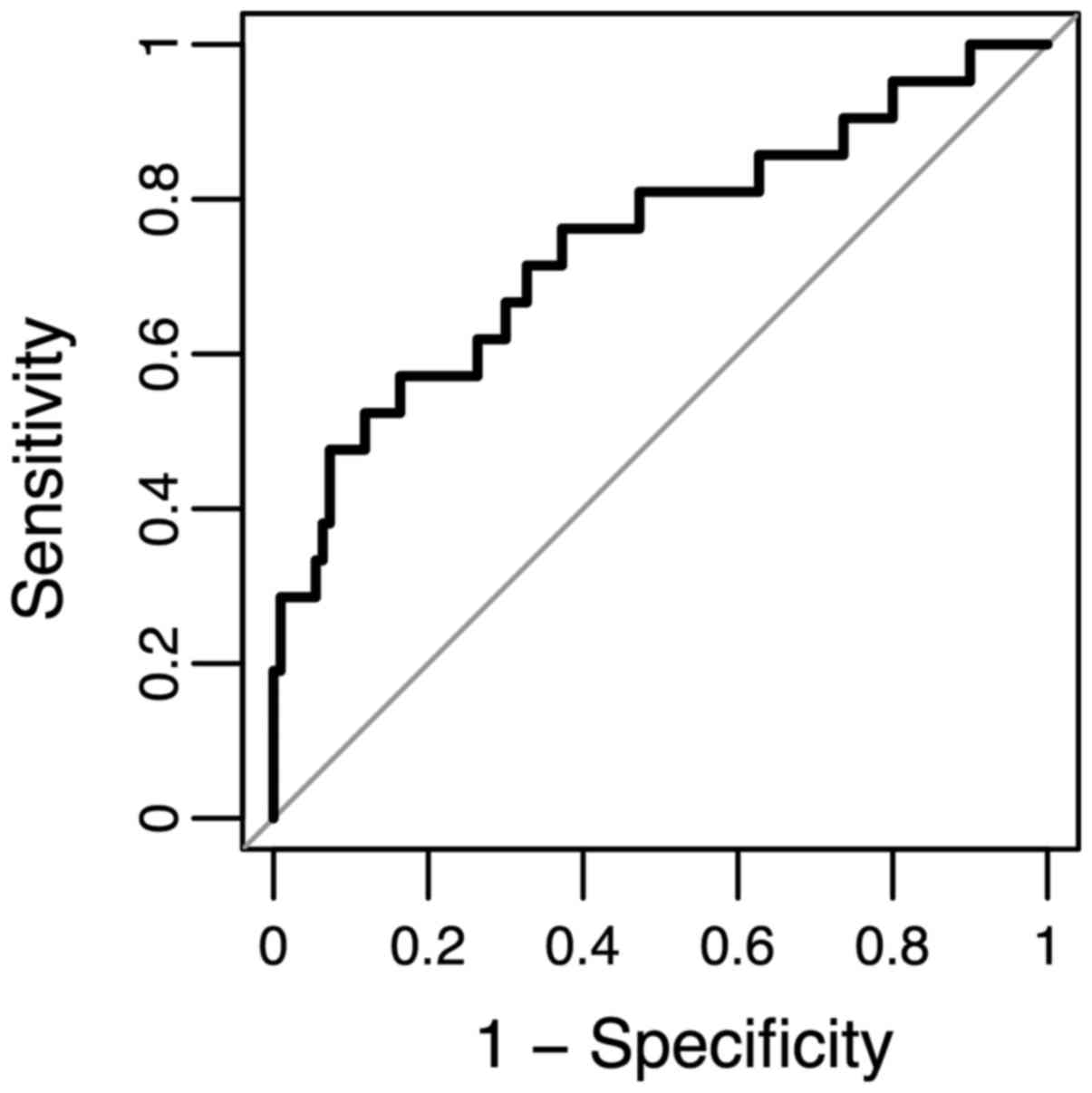
Predictive modelling of anti-MAGE-A
responses
To explore the potential diagnostic value of
anti-MAGEA antibodies, we classified all stage 0 vs. pooled stage I
and II patients using an additive logistic regression model that
includes both MAGEA proteins, age, and sex. We summarized the model
performance in a ROC curve where we plotted the sensitivity (true
positive rate) values against 1-specificity (false positive rate)
values for each possible cut-point (Fig.
5). The area under the curve (AUC) is 0.74, suggesting that
anti-MAGEA antibodies can be treated as potential diagnostic
biomarkers.
Discussion
MAGEA proteins are cancer-testis antigens (CTAs),
which elicit both cellular and humoral responses. In this study, we
have analyzed the presence of naturally occurring antibodies
against two MAGEA family proteins, MAGEA4 and MAGEA10, in melanoma
patients with different stages of disease. Our data showed that
sera of 15 patients out of 185 (8%) had a strong antibody response
against the MAGEA4 and/or MAGEA10 protein. The highest antibody
response was detected in stage II melanoma patients.
CTAs are named after their typical pattern of
expression in a variety of malignant tumors. Their expression in
normal tissues is restricted to germ cells of the testis. Male germ
cells are devoid of HLA-class I molecules and cannot present
antigens to T cells. Therefore, MAGEA antigens can be considered
neo-antigens when expressed in cancer cells (29). Recent studies have shown that the
induction of MAGEA4-specific immune responses correlated well with
the prognosis of patients vaccinated with MAGEA4 protein and that
antibody response could be a marker for a good prognosis (28).
Our study revealed that 8% of patients had strong
antibody responses against the MAGEA4 or/and MAGEA10 protein. When
we grouped patients according to the level of the disease, then
stage II patients had more antibodies than others, reaching to 16%
in case of MAGEA10. Scultz-Thater et al have studied the
prevalence of MAGEA10 in different cancers and shown that it is
expressed in 38% of malignant melanomas (18). Several studies have shown that MAGEA
proteins are associated with or contribute to solid malignancies,
MAGE-A expression is considered to be an important predictor of
malignant transformation (21). For
instance, MAGEA4 is expressed in 9% of primary tumors, but reaching
to 44% in distant metastasis (30)
and MAGEA1 expression has been found in 16% of primary melanomas
and 48% of metastatic melanomas (15). However, in another study no
correlation was observed, and MAGEA3/4 protein was present in 25%
of primary invasive and metastatic tumors, but not in in
situ melanomas (31). In our
study, the prevalence of MAGEA antibodies was highest in stage II
and lowest in stage 0 and stage IV patients. We have not determined
the expression of MAGEA proteins in the tissue samples of our
patients, but it is very unlikely that MAGEA expression declines in
advanced stages. Previous studies have shown that MAGEA4 is rarely
lost when once acquired (30). We
favor the explanation that stage II melanoma patients have a better
immune response than patients with more advanced stages of disease.
This is consistent with the immune evasion seen in metastatic
cancers (32). Interestingly, there
were also very few responses amongst in situ and stage I
melanoma patients. This can be explained by the localization of the
primary tumor. Stage 0 or in situ and stage I melanoma are
found mostly on the outer layer of the skin, in epidermis. Stage II
melanoma has spread to the lower part of the inner layer of skin
(dermis), but not yet into the tissue below the dermis or into
nearby lymph nodes. The dermis contains many antigen presenting
cells, which may help to boost the immune response.
In our study, some patients had strong antibody
response against both MAGE-A proteins, the others exhibited
antibodies against either MAGEA4 or MAGEA10 protein. One of the
limitations of this study is that we do not have biopsies of
patients and we were not able to perform neither qPCR nor
immunochemical analysis to confirm that the antibodies are specific
to MAGEA4 or MAGEA10. However, tumor cells very often express more
than one MAGEA protein. Simultaneous expression of five or more
MAGEA proteins occurs in more than half of oral squamous cell
carcinomas (33) and simultaneous
expression of MAGEA1 and MAGEA4 expression occurs in 60–70% of
melanomas (30). We favor the
explanation that the two MAGEA proteins used in our study have
different immunogenic properties, so that the MAGEA10 protein is a
better antigen than MAGEA4. Thus, our work is consistent with the
studies, which have suggested that MAGEA10 is the most immunogenic
antigen of the MAGEA family (34–36). It is
well known that obtaining antibodies against one specific MAGEA
protein may be challenging; we cannot rule out the possibility that
antibodies detected in our assay are formed against some other
member of the family. MAGEA proteins are highly similar to each
other, with half of the amino acids identical between MAGEA4 and
MAGEA10. The MAGEA subfamily consists of 11 MAGE-A proteins and in
addition, there are MAGE-B, MAGE-C, MAGE-D etc. families which all
share the MHG (MAGE homology) domain (37). All these proteins are to some extent
similar to each other (MHG domains has similarities from 25 to 80%)
and may give some cross-reactivity. This may also explain the
immune response against MAGEA proteins in some healthy donors seen
in our study. The other limitation of our study is that we have
used only ELISA assay for screening of the sera. We choose ELISA,
because it is suitable for high-throughput analysis, but have not
analyzed the sera by western blotting. However, by doing so we
might have missed some antibody responses generated against linear
and/or denatured epitopes of MAGEA proteins.
The existence of strongly responding patients
suggests that their immune system has been activated and has
started to generate antibodies against the primary tumor. So, our
data support the hypothesis that the immune system is involved in
the control of melanoma, at least in the early stages. Several
studies have shown spontaneous regression of primary melanomas, but
regression of metastatic tumors is very rare. A good antibody
response at early stages can stop the growth of primary tumor and
further spreading to the lymph nodes and other organs. Among the 15
patients of our study with the positive status for MAGEA
antibodies, only one has died and one has disease progression
during the 2-year post-study follow-up period (data not shown). So,
the disease of the majority of patients with strong antibody
response is under control. However, this cohort is too small to
make long-term conclusions about the prognosis. Longitudinal
time-course studies on larger cohorts are needed to establish the
prognostic significance of the presence of MAGEA antibodies in
patients. We plan to follow the patients and their antibody
response for at least five more years and perform then the survival
analysis.
The sensitivity and specificity calculations suggest
that the anti-MAGEA antibody response can be treated as a potential
diagnostic biomarker. One of the limitations for use in clinics is
that MAGE-A proteins are expressed only in a portion of cancer
cells; different works have shown that the amount of expressing
cells is between 25 and 50% (30–31). When
we assume that only half of these people have a strong immune
response, then the expected % of strongly responding patients will
be 12 to 25%. Is this enough for clinical diagnostics? On the other
hand, these antibodies are so-called early markers and there is a
great need for early cancer markers. So, when the presence of
strong antibody response correlates with good prognosis, then they
are/will be useful for clinics.
In addition, from the clinical aspect, the
longitudinal detection of MAGEA antibody levels could be utilized
for profiling of disease status or of effectiveness of novel
immunotherapies, as there exists a great need for biomarkers which
could assist in discrimination of patients suitable for
immunotherapy or for monitoring the therapy effectiveness of these
expensive drugs. For example, it has been shown that during
immunotherapy with ipilimumab the MAGEA protein levels declined and
elevation correlated with either treatment response or failure,
respectively (38). However, the
anti-MAGEA antibody status of patients prior to and after
checkpoint therapy has never been evaluated.
In summary, our study supports the role of the host
immune response in the progression of melanoma. To the best of our
knowledge, the present study is the first report on following the
antibody response against MAGEA-s and comparing it with the disease
progression. A healthy immune system enables to create antibodies
against cancer antigens that are expressed specifically by tumor
cells. The link between MAGEA antigens and cancer is widely known
and accepted; several works have shown a good cellular and humoral
response against MAGEAs (1,6,37). Due to
their relatively high tumor specificity, they represent attractive
targets for active specific and adoptive cancer immunotherapies
(39). In the current study, we are
not interested in antigens, but we focus on the antibody response
against the antigens. There are some studies who have analyzed the
antibodies against melanoma antigens (tyrosinase, and TRPs) in
melanoma patients, but not against MAGE-A proteins (40,41). In
the current study, we focused on naturally occurring antibody
response against MAGEA proteins. We compared the number of strongly
responding patients between different stages of disease and found
that the highest number of strong responses was detected among
stage II melanoma patients. The strong antibody response could be a
marker for a good prognosis (28) as
well as an early marker which can be used for cancer diagnostics
from liquid biopsy.
Acknowledgements
Not applicable.
Funding
This study was supported by institutional research
funding (IUT20-27) of the Estonian Ministry of Education and
Research and by the European Regional Development Fund through the
Center of Excellence in Molecular Cell Engineering, and by Estonian
Health Program TerVe project IMGEMEL.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KÕ, KK and RK designed the experiments; KÕ, KK and
LV conducted the experiments; MT and AP collected the clinical data
and were responsible for ethics approvals and consent of patients
to participate in the study; ÜM performed the statistical analysis;
MU was responsible for overall the design and funding of the
project and KÕ, ÜM, AP and RK prepared the figures and wrote the
manuscript. All authors read and have approved the final
manuscript.
Ethics approval and consent to
participate
Approval no. 2781 (from June 21, 2012) for the use
of blood samples of melanoma patients, and no. 254 (from December
13, 2012) for controls were obtained from the Tallinn Medical
Research Ethics Committee of Estonian National Institute for Health
Development. All the patients, whose blood samples have been used,
had signed the consent to participate in the study.
Consent for publication
The patients have provided written informed consent
that the results of the study are published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meek DW and Marcar L: MAGE-A antigens as
targets in tumour therapy. Cancer Lett. 324:126–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde B, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chomez P, De Backer O, Bertrand M, De
Plaen E, Boon T and Lucas S: An overview of the MAGE gene family
with the identification of all human members of the family. Cancer
Res. 61:5544–5551. 2001.PubMed/NCBI
|
|
4
|
Kalejs M and Erenpreisa J: Cancer/testis
antigens and gametogenesis: A review and ‘brain-storming’ session.
Cancer Cell Int. 5:42005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Smet C, Lurquin C, Lethé B, Martelange
V and Boon T: DNA methylation is the primary silencing mechanism
for a set of germ line- and tumor-specific genes with a CpG-rich
promoter. Mol Cell Biol. 19:7327–7335. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sang M, Lian Y, Zhou X and Shan B: MAGE-A
family: Attractive targets for cancer immunotherapy. Vaccine.
29:8496–8500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Cheng S, Asa SL and Ezzat S: The
melanoma-associated antigen A3 mediates fibronectin-controlled
cancer progression and metastasis. Cancer Res. 68:8104–8112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marcar L, Ihrig B, Hourihan J, Bray SE,
Quinlan PR, Jordan LB, Thompson AM, Hupp TR and Meek DW: MAGE-A
cancer/testis antigens inhibit MDM2 ubiquitylation function and
promote increased levels of MDM4. PLoS One. 10:e01277132015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcar L, Maclaine NJ, Hupp TR and Meek
DW: Mage-A cancer/testis antigens inhibit p53 function by blocking
its interaction with chromatin. Cancer Res. 70:10362–10370. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang B, O'Herrin SM, Wu J, Reagan-Shaw S,
Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, et
al: MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1
and suppress p53-dependent apoptosis in MAGE-positive cell lines.
Cancer Res. 67:9954–9962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monte M, Simonatto M, Peche LY, Bublik DR,
Gobessi S, Pierotti MA, Rodolfo M and Schneider C: MAGE-A tumor
antigens target p53 transactivation function through histone
deacetylase recruitment and confer resistance to chemotherapeutic
agents. Proc Natl Acad Sci USA. 103:11160–11165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ladelfa M, Peche LY, Toledo MF, Laiseca
JE, Schneider C and Monte M: Tumor-specific MAGE proteins as
regulators of p53 function. Cancer Lett. 325:11–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doyle JM, Gao J, Wang J, Yang M and Potts
PR: MAGE-RING protein complexes comprise a family of E3 ubiquitin
ligases. Mol Cell. 39:963–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Mutter-Rottmayer E, Greenwalt AM,
Goldfarb D, Yan F, Yang Y, Martinez-Chacin RC, Pearce KH, Tateishi
S, Major MB and Vaziri C: A neomorphic cancer cell-specific role of
MAGE-A4 in trans-lesion synthesis. Nat Commun. 7:121052016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brasseur F, Rimoldi D, Liénard D, Lethé B,
Carrel S, Arienti F, Suter L, Vanwijck R, Bourlond A, Humblet Y, et
al: Expression of MAGE genes in primary and metastatic cutaneous
melanoma. Int J Cancer. 63:375–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergeron A, Picard V, LaRue H, Harel F,
Hovington H, Lacombe L and Fradet Y: High frequency of MAGE-A4 and
MAGE-A9 expression in high-risk bladder cancer. Int J Cancer.
125:1365–1371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Črnjević Badovinac T, Spagnoli G, Juretić
A, Jakić-Razumović J, Podolski P and Šarić N: High expression of
MAGE-A10 cancer-testis antigen in triple-negative breast cancer.
Med Oncol. 29:1586–1591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schultz-Thater E, Piscuoglio S, Iezzi G,
Le Magnen C, Zajac P, Carafa V, Terracciano L, Tornillo L and
Spagnoli GC: MAGE-A10 is a nuclear protein frequently expressed in
high percentages of tumor cells in lung, skin and urothelial
malignancies. Int J Cancer. 129:1137–1148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurg R, Reinsalu O, Jagur S, Õunap K, Võsa
L, Kasvandik S, Padari K, Gildemann K and Ustav M: Biochemical and
proteomic characterization of retrovirus Gag based microparticles
carrying melanoma antigens. Sci Rep. 6:294252016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rimoldi D, Salvi S, Schultz-Thater E,
Spagnoli G and Cerottini J: Anti-MAGE-3 antibody 57B and
anti-MAGE-1 antibody 6C1 can be used to study different proteins of
the MAGE-A family. Int J Cancer. 86:749–751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laban S, Atanackovic D, Luetkens T, Knecht
R, Busch CJ, Freytag M, Spagnoli G, Ritter G, Hoffmann TK, Knuth A,
et al: Simultaneous cytoplasmic and nuclear protein expression of
melanoma antigen-A family and NY-ESO-1 cancer-testis antigens
represents an independent marker for poor survival in head and neck
cancer. Int J Cancer. 135:1142–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piotti KC, Scognamiglio T, Chiu R and Chen
YT: Expression of cancer/testis (CT) antigens in squamous cell
carcinoma of the head and neck: Evaluation as markers of squamous
dysplasia. Pathol Res Pract. 209:721–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Russo AE, Torrisi E, Bevelacqua Y,
Perrotta R, Libra M, McCubrey JA, Spandidos DA, Stivala F and
Malaponte G: Melanoma: Molecular pathogenesis and emerging target
therapies (Review). Int J Oncol. 34:1481–1489. 2009.PubMed/NCBI
|
|
24
|
Rangwala S and Tsai KY: Roles of the
immune system in skin cancer. Br J Dermatol. 165:953–965. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ribero S, Moscarella E, Ferrara G, Piana
S, Argenziano G and Longo C: Regression in cutaneous melanoma: A
comprehensive review from diagnosis to prognosis. J Eur Acad
Dermatol Venereol. 30:2030–2037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bååth R: Bayesian First Aid: A package
that implements Bayesian alternatives to the classical*. Test
functions in R. Proc Use R. 2014.
|
|
27
|
Robin X, Turck N, Hainard A, Tiberti N,
Lisacek F, Sanchez JC and Müller M: pROC: An open-source package
for R and S+ to analyze and compare ROC curves. BMC Bioinformatics.
12:772011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito T, Wada H, Yamasaki M, Miyata H,
Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M and Doki Y: High
expression of MAGE-A4 and MHC class I antigens in tumor cells and
induction of MAGE-A4 immune responses are prognostic markers of
CHP-MAGE-A4 cancer vaccine. Vaccine. 32:5901–5907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gjerstorff MF, Andersen MH and Ditzel HJ:
Oncogenic cancer/testis antigens: Prime candidates for
immunotherapy. Oncotarget. 6:15772–15787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barrow C, Browning J, MacGregor D, Davis
ID, Sturrock S, Jungbluth AA and Cebon J: Tumor antigen expression
in melanoma varies according to antigen and stage. Clin Cancer Res.
12:764–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Busam K, Iversen K, Berwick M, Spagnoli
GC, Old LL and Jungbluth AA: Immunoreactivity with the anti-MAGE
antibody 57B in malignant melanoma: Frequency of expression and
correlation with prognostic parameters. Mod Pathol. 13:459–465.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vinay DS, Ryan EP, Pawelec G, Talib WH,
Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et
al: Immune evasion in cancer: Mechanistic basis and therapeutic
strategies. Semin Cancer Biol. 35 Suppl:S185–S198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brisam M, Rauthe S, Hartmann S, Linz C,
Brands RC, Kübler AC, Rosenwald A and Müller-Richter UD: Expression
of MAGE-A1-A12 subgroups in the invasive tumor front and tumor
center in oral squamous cell carcinoma. Oncol Rep. 35:1979–1986.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Groeper C, Gambazzi F, Zajac P, Bubendorf
L, Adamina M, Rosenthal R, Zerkowski HR, Heberer M and Spagnoli GC:
Cancer/testis antigen expression and specific cytotoxic T
lymphocyte responses in non small cell lung cancer. Int J Cancer.
120:337–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bricard G, Bouzourene H, Martinet O,
Rimoldi D, Halkic N, Gillet M, Chaubert P, Macdonald HR, Romero P,
Cerottini JC and Speiser DE: Naturally acquired MAGE-A10- and
SSX-2-specific CD8+ T cell responses in patients with
hepatocellular carcinoma. J Immunol. 174:1709–1716. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valmori D, Dutoit V, Rubio-Godoy V,
Chambaz C, Liénard D, Guillaume P, Romero P, Cerottini JC and
Rimoldi D: Frequent cytolytic T-cell responses to peptide
MAGE-A10(254–262) in melanoma. Cancer Res. 61:509–512.
2001.PubMed/NCBI
|
|
37
|
Lee AK and Potts PR: A comprehensive guide
to the MAGE family of ubiquitin ligases. J Mol Biol. 429:1114–1142.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arenberger P, Fialova A, Gkalpakiotis S,
Pavlikova A, Puzanov I and Arenbergerova M: Melanoma antigens are
biomarkers for ipilimumab response. J Eur Acad Dermatol Venereol.
31:252–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zajac P, Schultz-Thater E, Tornillo L,
Sadowski C, Trella E, Mengus C, Iezzi G and Spagnoli GC: MAGE-A
antigens and cancer immunotherapy. Front Med (Lausanne).
4:182017.PubMed/NCBI
|
|
40
|
Huang S, Okamoto T, Morton DL and Hoon DS:
Antibody responses to melanoma/melanocyte autoantigens in melanoma
patients. J Invest Dermatol. 111:662–667. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fishman P, Merimski O, Baharav E and
Shoenfeld Y: Autoantibodies to tyrosinase: The bridge between
melanoma and vitiligo. Cancer. 79:1461–1464. 1997. View Article : Google Scholar : PubMed/NCBI
|