Introduction
Breast cancer (BC) is one of the most common types
of cancer worldwide, accounting for ~20–23% of total cancer cases,
and the incidence is increasing each year (1,2). Although
the diagnostic techniques and therapeutic strategies have improved
over the past decades, BC remains the leading cause of cancer death
in females worldwide (3).
Triple-negative breast cancers (TNBCs), accounting
for ~25% of BC cases, are characterized by tumors that lack
expression of the estrogen receptor (ER), progesterone receptor
(PR) and the human epidermal growth factor receptor 2 (HER2)
(4,5).
Because the majority of basal-like cancers are also ER-, PR-, and
HER2-negative (~80%), it has been suggested that the
triple-negative and basal-like phenotypes are effectively
synonymous, but data from immunohistochemical analysis have
revealed that this is not the case (6–8).
Triple-negative is a phenotype that encompasses more than one
molecular subtype: its major components are the basal-like, normal,
and claudin-low molecular subtypes, including BRCA1-deficient
breast tumors (9). Hormone
receptor-positive breast tumors are treated with endocrine
manipulations, such as tamoxifen and aromatase inhibitors (10). However, patients with TNBC cannot
benefit from endocrine therapy because of the lack of ER, PR, and
HER2 expression. The current treatment options for TNBC are
limited, and include surgery, radiation therapy, and systemic
treatment. Chemotherapy is currently the only approved systemic
treatment for TNBC, which is frequently accompanied by considerable
side effects (1,11). Therefore, there is an urgent need to
investigate the molecular mechanisms and to explore novel
diagnostic and therapeutic strategies for TNBC.
The B7 family of proteins consists of co-stimulatory
or co-inhibitory molecules that are expressed mainly on the cell
surface of antigen presenting cells and serve a key role in the
regulation of the immune system (12,13). Among
the B7 family, natural killer cell cytotoxicity receptor 3 ligand 1
(NCR3LG1, also known as B7H6) was identified as a tumor-specific
ligand for the natural cytotoxicity triggering receptor 3 (NCR3)
(14). It has been reported that B7H6
is expressed in various types of tumor cells, including lung,
ovarian and renal cell cancer, but not expressed in normal tissues
(14,15). Thus, B7H6 holds therapeutic potential
for cancer, but its exact role in cancer is not fully elucidated
yet. In specific, the mechanisms by which B7H6 may affect tumor
progression have not been identified fully in TNBC cells.
In the present study, it was hypothesized that
knockdown of B7H6 might be involved in inhibiting tumor progression
of TNBC cells. Suppression of B7H6 could be used as a potential
target for treatment of TNBC. Therefore, the role of B7H6 in cell
viability, apoptosis and migration of TNBC cells was
investigated.
Materials and methods
Cell culture
Human normal mammary epithelial cell line (MCF-10A),
BC cell lines (MCF-7, AU565), and TNBC cell lines (MDA-MB-231,
MDA-MB-468) were all obtained from the American Tissue Cell Culture
collection (ATCC; Manassas, VA, USA). MCF-10A cells were maintained
in Dulbecco's modified Eagle's medium (DMEM)/F12 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10 µg/ml insulin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 100 ng/ml cholera enterotoxin, 0.5 µg/ml
hydrocortisol, and 20 ng/ml epidermal growth factor (EGF). MCF-7
and AU565 cells were cultured in RPMI 1640 (Invitrogen; Thermo
Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA). MDA-MB-231 and MDA-MB-468 cells were
propagated in DMEM/H-21 (Invitrogen; Thermo Fisher Scientific,
Inc.) with 10% FBS. All cells were cultured in a humidified 37°C
incubator with 5% CO2.
Small interfering (si) RNA
transfection
siRNAs were commercially designed and purchased from
Thermo Fisher Scientific, Inc. The sequence of the siRNA for B7H6
was 5′-CGGCACAGTCTTTCTGAAACT-3′. Cells were transfected with 50 nM
scramble siRNA (negative control, or NC sense:
5′-UUCUCCGAACGUGUCACGUTT-3′, antisense:
5′-ACGUGACACGUUCGGAGAATT-3′) or B7H6-siRNA with Lipofectamine 3000
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. After 6 h, the transfection medium (Opti-MEM; Gibco;
Thermo Fisher Scientific, Inc.) was removed and the cells were
maintained in normal medium for an additional 24 h. Western blot
assay was performed to assess the transfection efficiency.
Western blot analysis
Cells were washed with cold PBS and treated with
cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA,
USA) at 4°C or on ice for 2 h. Total proteins were prepared using
the Extraction kit (Beyotime Institute of Biotechnology, Shanghai,
China), and the concentration was determined by a BCA Protein assay
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Then proteins
(40 µg) were separated by SDS-PAGE and transferred to
polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc.). To
block the nonspecific signals, membranes were incubated with 5% low
fat milk for at least 2 h at room temperature. Then membranes were
incubated with primary antibody overnight at 4°C. The membranes
were then washed with 5% bovine serum albumin in TBS/0.1% Tween-20
(TBS/T), and incubated with a horseradish peroxidase-conjugated
secondary antibody (1:1,000; ab205718; Abcam, Cambridge, MA, USA)
for 2 h at 37°C. The results were visualized using the enhanced
chemiluminescence substrate kit (Amersham; GE Healthcare, Chicago,
IL, USA). The protein quantity was analyzed by Image Lab software
(version 5.0; Bio-Rad, Hercules, CA, USA). The antibodies used in
the present study were: anti-GAPDH (1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-SMAD family member 4
(SMAD4; 1:300; ab40759; Abcam, Cambridge, MA, USA), anti-β-Catenin
(1:1,000; ab32572; Abcam), anti-BCL2 associated X (Bax; 1:1,000;
ab32503; Abcam), anti-BCL2 apoptosis regulator (Bcl-2; ab32124;
1:1,000; Abcam), and anti-Caspase-3 (1:1,000; ab13585; Abcam).
Cell viability assay (MTT assay)
MTT assay was performed in order to evaluate the
effects of B7H6 siRNA on TNBC cell lines, as previously described
(16). Briefly, cells were harvested
with trypsin and seeded at a concentration of 3×103
cells/well into a 96-well plate. Then, 20 µl of MTT reagent was
added to each well, and incubated at 37°C for 4 h, followed by the
addition of 150 µl dimethyl sulfoxide. Finally, absorbance was
determined by using a microplate reader at 490 nm (Sunrise; Tecan
Group Ltd., Männedorf, Switzerland) at 48 h.
Flow cytometry analysis
Cell apoptosis was investigated by flow cytometry
using Annexin V/propidium iodide (PI) double staining (BD
Biosciences, Franklin Lakes, NJ, USA). The cells were plated onto
6-well plates at a concentration of 1×106 cells/well.
Following incubation for 24 h, cells were washed twice with cold
PBS, and resuspended in 500 µl binding buffer containing 5 µl
fluorescein isothiocyanate (FITC)-labeled Annexin V and 5 µl PI for
at least 10 min at room temperature in the dark. Finally, cells
were analyzed by flow cytometry and data were analyzed using Cell
Quest Pro software (BD Biosciences).
Transwell assay
The cell migration assay was performed in 6-well
Transwell chambers with 8-µm pore size polycarbonate membranes
(Corning Inc., Corning, NY, USA). Cells were suspended in
serum-free medium at a density of 1×105 cells/well, then
100 µl of this cell suspension was seeded in the upper chamber. The
lower chamber was filled with 500 µl medium supplemented with 20%
fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.). Following
24 h of incubation at 37°C in 5% CO2, cells on the upper
surface of the membrane were removed, and the lower surface of the
inserts was fixed in methanol for 10 min and stained with crystal
violet. The cells that had migrated to the lower surface were
photographed and measured using an inverted phase contrast
microscope (magnification, ×40; Olympus Corporation, Tokyo,
Japan).
Wound-healing assay
Cells were seeded in 6-well plates at the density of
1.5×105 cells/well, and cultured overnight until
confluent. Then the cells were treated with siRNAs (50 nM negative
control siRNA or 50 nM B7H6 siRNA) for 48 h. A straight scratch was
made through the confluent cells with a pipette tip. Cells were
washed twice with PBS, and images (magnification, ×100) were
captured immediately as baseline. Fresh medium was then added and
images of the same location were captured at 48 h. The ratio of the
remaining wound area relative to the initial wound area was
calculated using Image-Pro Plus software (version 6.0; Media
Cybernetics, Bethesda, MD, USA).
Statistical analysis
Data are presented as mean ± standard deviation.
SPSS 21.0 software (IBM Corp., Armonk, NY, USA) and GraphPad
(GraphPad Software, Inc., La Jolla, CA, USA) were used to conduct
the analysis. Statistical differences between two groups were
examined with unpaired Student's t-test and multiple groups were
compared using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
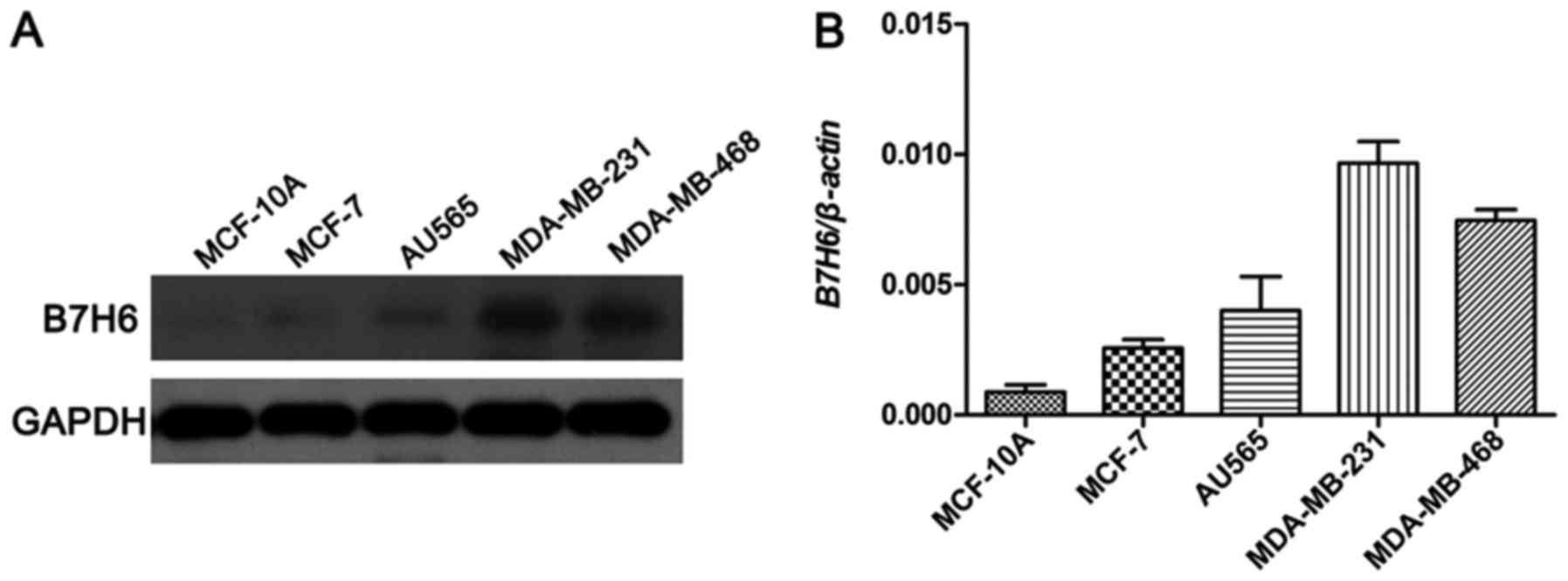
B7H6 is highly expressed in TNBC cell
lines
To investigate the potential biological roles of
B7H6 in TNBC, the expression levels of B7H6 were examined using
western blot analysis in a normal mammary epithelial cell line
(MCF-10A), a ER+/PR+ BC cell line (MCF-7), a HER2+ BC cell line
(AU565), and two TNBC cell lines (MDA-MB-231 and MDA-MB-468). Among
these, the results demonstrated that B7H6 levels were markedly
higher in the MDA-MB-231 and MDA-MB-468 cell lines compared to
MCF-10A, MCF-7 and AU565 cell lines (Fig.
1).
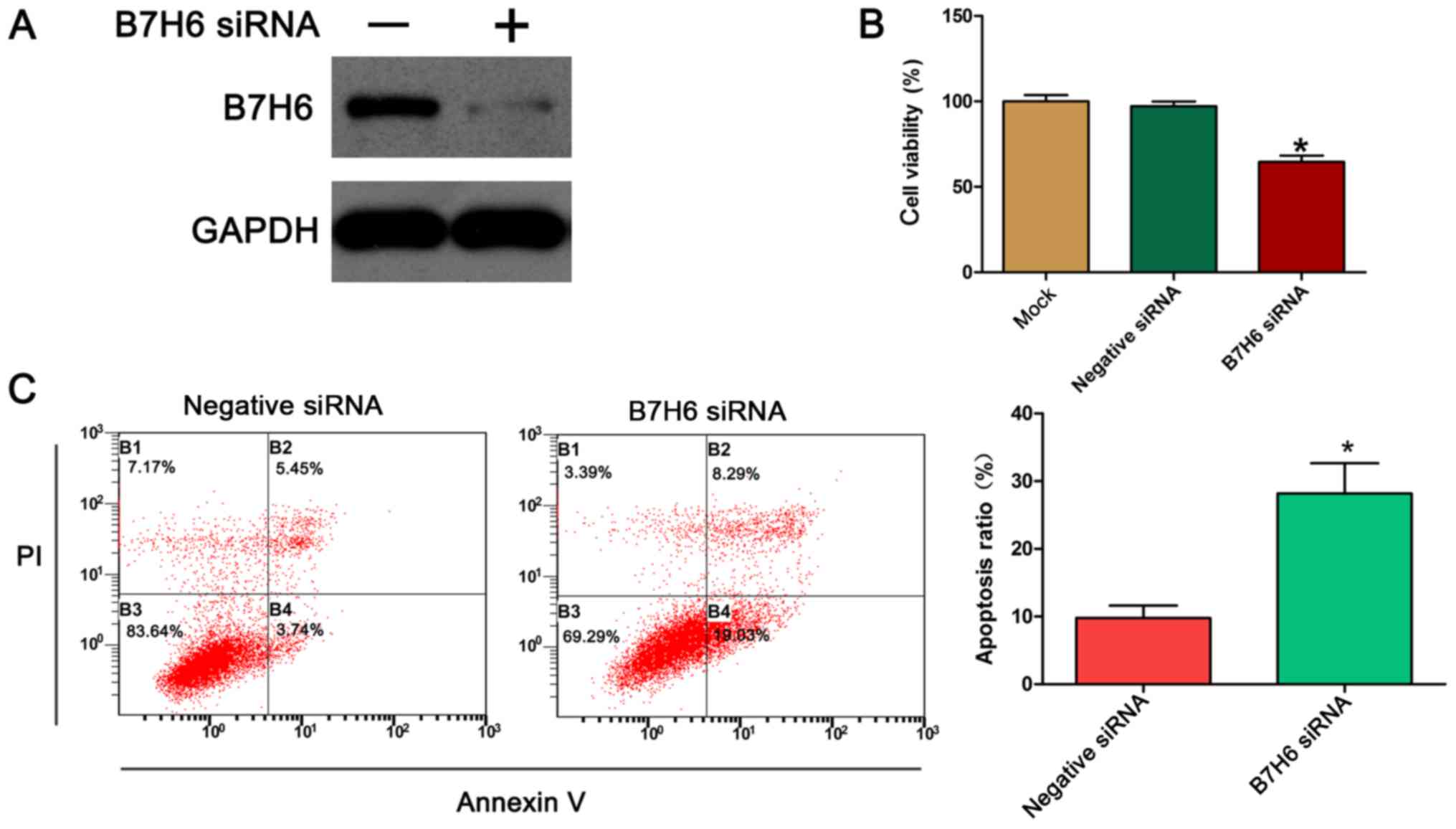
B7H6 knockdown inhibits cell
proliferation and promotes apoptosis in TNBC cells
To further assess the biological function of B7H6 in
TNBC cells, the expression of B7H6 was silenced in MDA-MB-231
cells, and the effects of B7H6 silencing on cell proliferation were
examined using the MTT assay. First, the efficiency of the
B7H6-specific siRNA was confirmed by western blotting. The results
demonstrated that transfection of the MDA-MB-231 cells with the
B7H6 siRNA resulted in a marked downregulation of B7H6 expression
compared with control (Fig. 2A). The
results from the MTT assay indicated that knockdown of B7H6
significantly inhibited the proliferation of MDA-MB-231 cells
compared with cells transfected with negative control siRNA
(Fig. 2B). Apoptosis is a significant
aspect of cancer cell progression, so to determine whether
apoptosis could be induced by B7H6 siRNA, annexin V/PI double
staining was performed followed by flow cytometry analysis. The
results demonstrated that the % of apoptotic cells significantly
increased following transfection with B7H6 siRNA compared with
control cells (Fig. 2C).
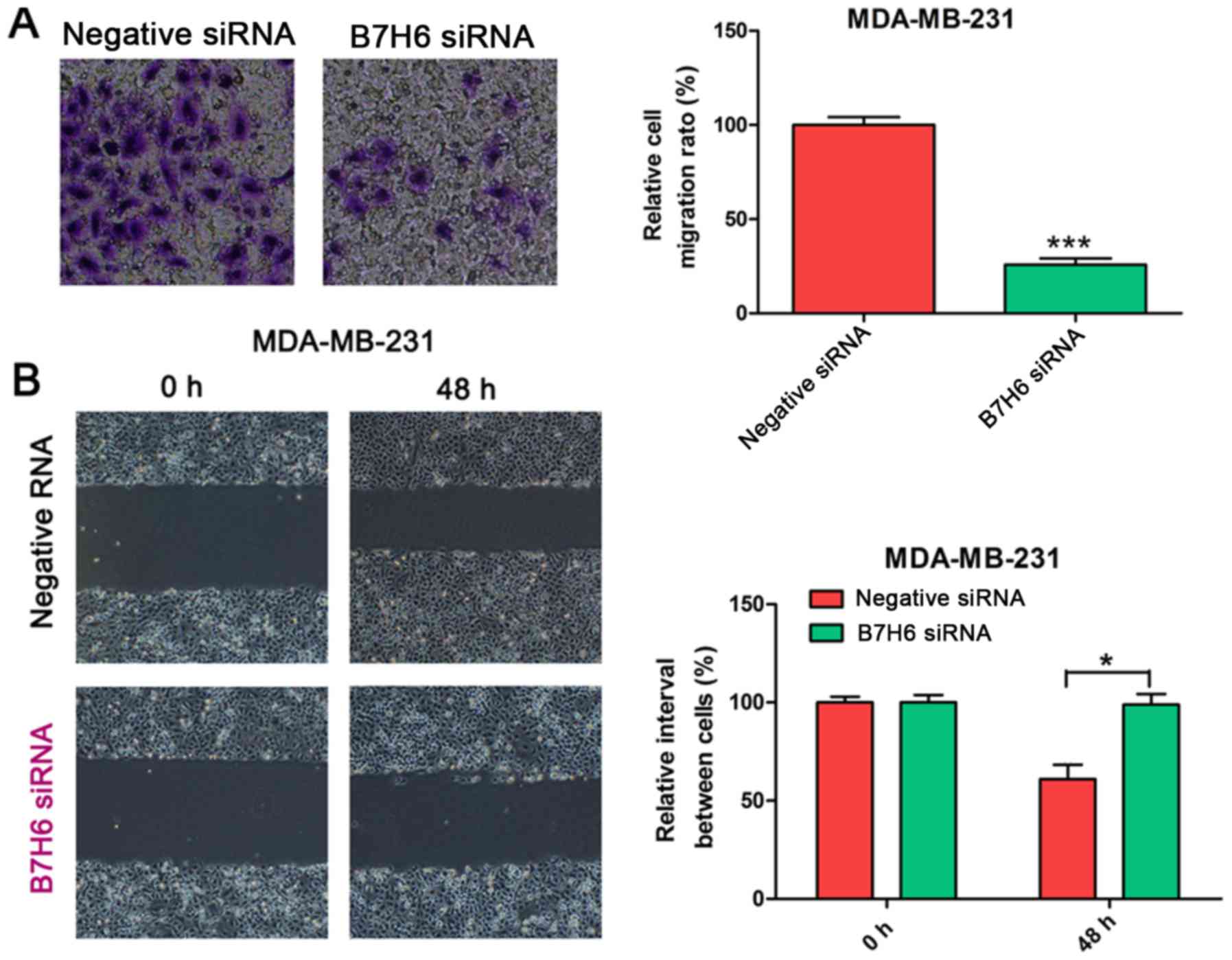
B7H6 knockdown suppresses cell
migration in MDA-MB-231 cells
To validate the effects of B7H6 on cell migration,
Transwell and wound-healing assays were conducted in MDA-MB-231
cells transfected with B7H6 or negative control siRNA. Results from
the Transwell assay demonstrated that cell migration was
significantly decreased in the B7H6 knockdown group compared with
the control group (Fig. 3A). In the
wound-healing assay, the scratch healed slower in B7H6-knockdown
MDA-MB-231 cells compared with the control group (Fig. 3B), suggesting that B7H6 inhibits cell
migration in TNBC cells.
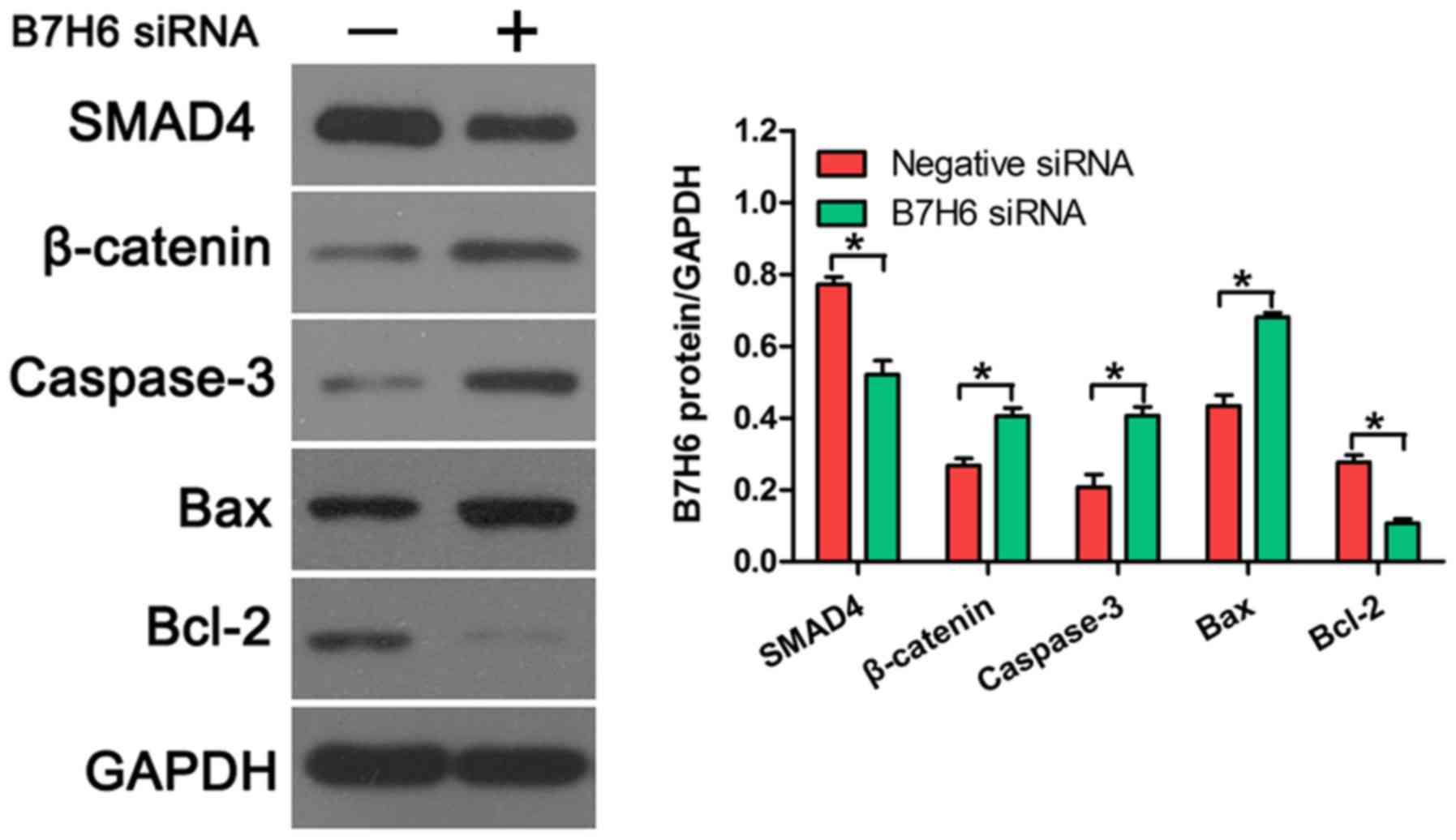
B7H6 knockdown upregulates the
expression of β-catenin, caspase-3, and Bax, and downregulates the
expression of SMAD4 and Bcl-2 in TNBC cells
To further confirm the effects of B7H6 on cell
proliferation and apoptosis, the expression of several
proliferation and apoptosis-related proteins was examined in TNBC
cells transfected with B7H6 siRNA. Western blot analysis revealed
that SMAD4 expression was downregulated and β-catenin expression
was upregulated following B7H6 knockdown, compared with cells
transfected with negative control siRNA (Fig. 4), indicating that B7H6 promoted
proliferation in TNBC cells. In addition, the results demonstrated
that knockdown of B7H6 increased the production of caspase-3 and
Bax, and decreased the production of Bcl-2 in TNBC cells (Fig. 4), suggesting that B7H6 inhibited TNBC
cell apoptosis.
Discussion
Despite an overall decreased mortality of TNBC, the
incidence appears to be on the rise and the prognosis remains
poorer than the other types of BC (17). Given the lack of molecular targets,
traditional treatments have limited effect, and the possibility of
metastasis of TNBC to visceral sites, including the brain, is
higher than the other subtypes (18).
Recent studies have demonstrated that EGF receptor (EGFR) and
vascular endothelial growth factor (VEGF) are involved in the
progression of TNBC cells in vitro (19). It was reported that bevacizumab, an
anti-angiogenic agent that targets VEGF, could induce a significant
improvement in progression-free survival of TNBC (20). Similar reports have suggested that
EGFR is expressed on approximately half of the basal-like BC cells,
and is indispensable for the proliferation of basal-like cell lines
(21,22). Recently, an antibody against EGFR
(cetuximab) was designed to treat breast cancer (23). Currently, the therapeutic strategies
for TNBC primarily include surgery (breast conservation therapy and
mastectomy) and chemotherapy (24,25). But
outcome still remains poor for patients with TNBC and therefore
targeted agents need to be investigated to explore novel treatments
for TNBC.
The immune system has been demonstrated to crosstalk
with tumor cells, and the aberrant expression of co-stimulatory or
co-inhibitory molecules that belong to the B7 family has been
considered to be involved in the suppression of antitumor immunity
(26,27). Therefore, the relationship between the
B7 family and antitumor immunity is currently being studied to
explore potentially novel effective therapeutic targets for various
human cancers. Despite the undetermined function of B7H3,
experiments with tumor models tend to support a co-stimulatory role
in antitumor immunity. For example, mouse P815 tumor cells
transfected with B7H3 display enhanced immunogenicity (28). It was reported that B7H4 is
overexpressed in human BCs, and knockdown of B7H4 by specific siRNA
induced tumor cell apoptosis, suggesting a co-inhibitory role of
B7H4 in BC cells (29). B7H6, a
recently discovered member of the B7 family, has been reported to
be expressed on tumors despite its absence in normal tissues,
suggesting that it may be a potential target for cancer treatment
(14). B7H6 was identified as a
tumor-specific ligand for NCR3 (14).
It has been reported that B7H6 mRNA expression is upregulated in
leukemia and gastrointestinal stromal tumors and associated with
clinical observations of NCR3-mediated immunosurveillance in these
tumors (30,31). However, the mechanism by which B7H6
affects tumor progression has not been identified completely in
TNBC cells. Previously, it has been reported that several types of
primary human tumors, including lymphoma, leukemia, ovarian cancer,
breast cancer, renal cell carcinoma, various sarcomas and brain
tumors, express high levels of B7H6 mRNA (32).
To investigate the exact role of B7H6 in BC first
the expression levels of B7H6 were examined in several BC cell
lines. The results demonstrated that B7H6 was highly expressed in
the TNBC cell lines compared with non-TNBC and normal human mammary
epithelial cell lines. To determine the function of B7H6 in TNBC
cells, cell viability, apoptosis, and migration were measured
following B7H6 knockdown in TNBC cells. The results demonstrated
that B7H6 promoted TNBC cell proliferation and migration, and
inhibited apoptosis.
Previous studies have demonstrated that SMAD4 is a
tumor suppressor involved in the proliferation of many human
cancers, including gastric, colorectal, and breast cancers
(33–35). SMAD4 suppresses invasiveness and
mediates reversion of SW480 cells from a mesenchymal-like to a
polarized epithelial phenotype, with features of enterocyte-like
differentiation (36). Therefore, the
present study investigated whether B7H6 could regulate the
production of SMAD4, and western blot analysis revealed that B7H6
knockdown decreased the expression of SMAD4. Because SMAD4 was
reported to prevent tumor progression by diminishing the β-catenin
signal, production of β-catenin was also measured and revealed to
be upregulated following B7H6 knockdown in TNBC cells. Furthermore,
the production of several apoptosis-related proteins was examined
in B7H6-knockdown TNBC cells, and the results demonstrated that
capase-3 and Bax were upregulated, while Bcl-2 was downregulated.
These data further confirmed the inhibitory effects of B7H6 in TNBC
cells. The present results suggest that B7H6 might have important
roles in the regulation of cell proliferation, apoptosis and
migration in TNBC cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BZ and SS designed the study. JS, XY and JL
performed the experiments. YY and FY analyzed the data. BZ wrote
the manuscript. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar P and Aggarwal R: An overview of
triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyle P: The globalisation of cancer.
Lancet. 368:629–630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Autier P, Boniol M, LaVecchia C, Vatten L,
Gavin A, Héry C and Heanue M: Disparities in breast cancer
mortality trends between 30 European countries: Retrospective trend
analysis of WHO mortality database. Br Med J. 341:c36202010.
View Article : Google Scholar
|
|
4
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sotiriou C and Pusztai L: Gene-expression
signatures in breast cancer. N Engl J Med. 360:790–800. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kreike B, van Kouwenhove M, Horlings H,
Weigelt B, Peterse H, Bartelink H and van de Vijver MJ: Gene
expression profiling and histopathological characterization of
triple-negative/basal-like breast carcinomas. Breast Cancer Res.
9:R652007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krishnamurthy S, Poornima R, Challa VR and
Goud YG: Triple negative breast cancer-our experience and review.
Indian J Surg Oncol. 3:12–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sethi S, Sarkar FH, Ahmed Q, Bandyopadhyay
S, Nahleh ZA, Semaan A, Sakr W, Munkarah A and Ali-Fehmi R:
Molecular markers of epithelial-to-mesenchymal transition are
associated with tumor aggressiveness in breast carcinoma. Transl
Oncol. 4:222–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Podo F, Buydens LM, Degani H, Hilhorst R,
Klipp E, Gribbestad IS, Van Huffel S, van Laarhoven HW, Luts J,
Monleon D, et al: Triple-negative breast cancer: Present challenges
and new perspectives. Mol Oncol. 4:209–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collins M, Ling V and Carreno BM: The B7
family of immune-regulatory ligands. Genome Biol. 6:2232005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flajnik MF, Tlapakova T, Criscitiello MF,
Krylov V and Ohta Y: Evolution of the B7 family: Co-evolution of
B7H6 and NKp30, identification of a new B7 family member, B7H7, and
of B7's historical relationship with the MHC. Immunogenetics.
64:571–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brandt CS, Baratin M, Yi EC, Kennedy J,
Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, et
al: The B7 family member B7-H6 is a tumor cell ligand for the
activating natural killer cell receptor NKp30 in humans. J Exp Med.
206:1495–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu MR, Zhang T, Gacerez AT, Coupet TA,
DeMars LR and Sentman CL: B7H6-specific bispecific T cell engagers
lead to tumor elimination and host antitumor immunity. J Immunol.
194:5305–5311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Ye Y, Yang X, Liao H, Zhao C and
Liang S: Expression-based in silico screening of candidate
therapeutic compounds for lung adenocarcinoma. PLoS One.
6:e145732011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elias AD: Triple-negative breast cancer: A
short review. Am J Clin Oncol. 33:637–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herold CI and Anders CK: New targets for
triple-negative breast cancer. Oncology (Williston Park).
27:846–854. 2013.PubMed/NCBI
|
|
19
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siziopikou KP and Cobteigh M: The basal
subtype of breast carcinomas may represent the group of breast
tumors that could benefit from EGFR-targeted therapies. Breast.
16:104–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salazar N, Muñoz D, Kallifatidis G, Singh
RK, Jordà M and Lokeshwar BL: The chemokine receptor CXCR7
interacts with EGFR to promote breast cancer cell proliferation.
Mol Cancer. 13:1982014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui W, Zhang S, Shan C, Zhou L and Zhou Z:
microRNA-133a regulates the cell cycle and proliferation of breast
cancer cells by targeting epidermal growth factor receptor through
the EGFR/Akt signaling pathway. FEBS J. 280:3962–3974. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carey LA, Rugo HS, Marcom PK, Mayer EL,
Esteva FJ, Ma CX, Liu MC, Storniolo AM, Rimawi MF, Forero-Torres A,
et al: TBCRC 001: Randomized phase II study of cetuximab in
combination with carboplatin in stage IV triple-negative breast
cancer. J Clin Oncol. 30:2615–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Freedman GM, Anderson PR, Li T and
Nicolaou N: Locoregional recurrence of triple-negative breast
cancer after breast-conserving surgery and radiation. Cancer.
115:946–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung J and Suh WK: The CD28-B7 family in
anti-tumor immunity: Emerging concepts in cancer immunotherapy.
Immune Netw. 14:265–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo L, Chapoval AI, Flies DB, Zhu G,
Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, et al: B7-H3
enhances tumor immunity in vivo by costimulating rapid clonal
expansion of antigen-specific CD8+ cytolytic T cells. J Immunol.
173:5445–5450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delahaye NF, Rusakiewicz S, Martins I,
Ménard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro
M, et al: Alternatively spliced NKp30 isoforms affect the prognosis
of gastrointestinal stromal tumors. Nat Med. 17:700–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fauriat C, Just-Landi S, Mallet F,
Arnoulet C, Sainty D, Olive D and Costello RT: Deficient expression
of NCR in NK cells from acute myeloid leukemia: Evolution during
leukemia treatment and impact of leukemia cells in NCRdull
phenotype induction. Blood. 109:323–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu MR, Zhang T, DeMars LR and Sentman CL:
B7H6-specific chimeric antigen receptors lead to tumor elimination
and host antitumor immunity. Gene Ther. 22:675–684. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyaki M, Iijima T, Konishi M, Sakai K,
Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, et al:
Higher frequency of Smad4 gene mutation in human colorectal cancer
with distant metastasis. Oncogene. 18:3098–3103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kundu J, Wahab SM, Kundu JK, Choi YL,
Erkin OC, Lee HS, Park SG and Shin YK: Tob1 induces apoptosis and
inhibits proliferation, migration and invasion of gastric cancer
cells by activating Smad4 and inhibiting betacatenin signaling. Int
J Oncol. 41:839–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deckers M, van Dinther M, Buijs J, Que I,
Löwik C, van der Pluijm G and ten Dijke P: The tumor suppressor
Smad4 is required for transforming growth factor beta-induced
epithelial to mesenchymal transition and bone metastasis of breast
cancer cells. Cancer Res. 66:2202–2209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pohl M, Radacz Y, Pawlik N, Schoeneck A,
Baldus SE, Munding J, Schmiegel W, Schwarte-Waldhoff I and
Reinacher-Schick A: SMAD4 mediates mesenchymal-epithelial reversion
in SW480 colon carcinoma cells. Anticancer Res. 30:2603–2613.
2010.PubMed/NCBI
|