Introduction
The development of molecular-targeted therapies has
remarkably changed the management of patients with advanced
non-small cell lung cancer (NSCLC) harboring specific genetic
alterations, including epidermal growth factor receptor (EGFR) gene
mutations and anaplastic lymphoma kinase rearrangements (1–7). Mutations
in the EGFR gene are identified in 40–50% of NSCLC in East Asian
(8) and in 10–15% in Caucasian
populations (9). Several phase III
clinical trials have indicated that EGFR-tyrosine kinase inhibitors
(TKIs) significantly improve median progression-free survival (PFS)
compared with chemotherapy in patients with NSCLC harboring EGFR
mutations (1–7). Tumors harboring these mutations,
however, generally acquire resistance to EGFR-TKIs after a median
time of 9–14 months, followed by disease progression. The ability
of several combination regimens containing EGFR-TKIs to improve
clinical outcomes in patients with NSCLC harboring EGFR mutations
has therefore been tested (10–12).
Bevacizumab is a recombinant humanized monoclonal
antibody that targets vascular endothelial growth factor, which has
been demonstrated to inhibit the growth and maintenance of tumor
blood vessels (13). The addition of
bevacizumab to platinum doublet chemotherapy has been a standard of
care for the first-line treatment of patients with advanced
non-squamous NSCLC (14). The
combination of EGFR-TKIs and bevacizumab is a promising treatment
in patients with EGFR mutation-positive NSCLC (11,12). A
multi-center randomized phase II trial reported that, compared with
erlotinib alone, erlotinib plus bevacizumab combination therapy
prolonged PFS in patients with NSCLC harboring EGFR mutations
(12). Common side effects of
bevacizumab include hypertension, proteinuria, thrombosis,
hemorrhages and wound-healing complications (15). Gastrointestinal perforation is a
relatively rare, but serious, adverse event associated with
bevacizumab, which is reported in 0.3–3.2% of patients (15). To date, however, to the best of our
knowledge this adverse event has not been reported during treatment
of NSCLC patients with erlotinib plus bevacizumab. The present
report describes the cases of two patients with advanced NSCLC, who
experienced gastrointestinal perforations following erlotinib plus
bevacizumab combination therapy.
Case report
Patient 1
A 67-year-old male observed to have an enlarged
right hilar lymph node during a medical check-up was admitted to
Fujita Health University Hospital (Toyoake, Aichi, Japan) in July
2014. The patient underwent a lymph node biopsy and was diagnosed
with lung adenocarcinoma. Computed tomography (CT) revealed a
primary tumor in the right lower lobe, which was diagnosed as stage
IIIB (cT1aN3M0) according to the 7th edition TNM classification
(16). A total of 80 mg/m2
cisplatin (Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan) on
day 1, plus 20 mg/m2 vinorelbine (Kyowa Hakko Kirin Co.,
Ltd., Tokyo, Japan) on day 1 and 8 of each 4 week cycle concurrent
with radiotherapy (60 Gy in 30 fractions) were immediately
administered, with a CT scan of the chest indicating a partial
response (PR) following two cycles of combination chemotherapy.
However, seven months later the disease relapsed in the lymph nodes
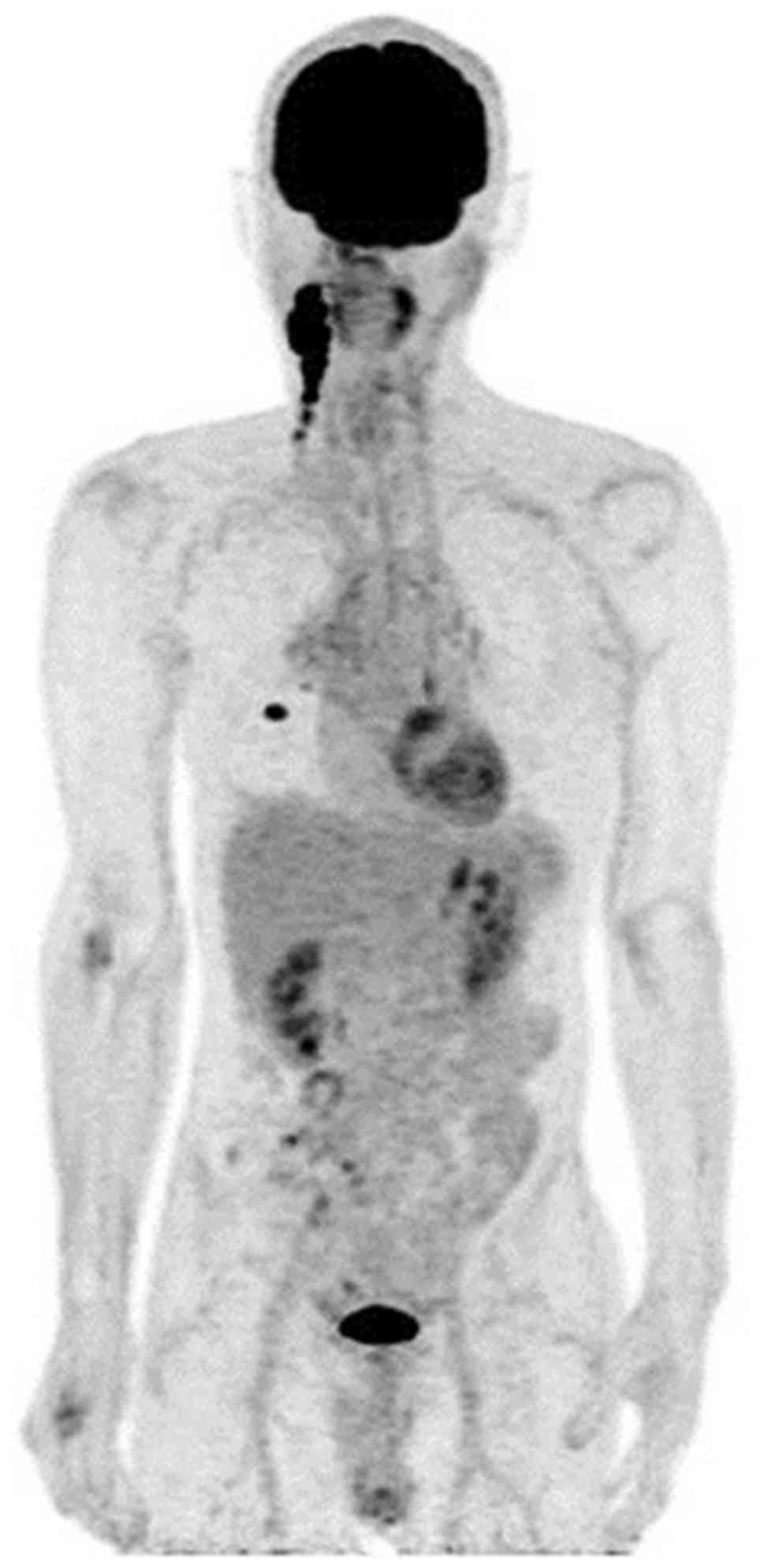
of the patient's right neck. Positron emission tomography revealed
fluorodeoxyglucose uptake only in the right lower lobe and neck
lymph nodes (Fig. 1). Mutation
analysis of the tumor biopsy revealed a L858R point mutation in
exon 21 of EGFR, and the patient was administered treatment with
150 mg/day erlotinib, plus 15 mg/kg bevacizumab (both Chugai
Pharmaceutical Co. Ltd., Tokyo, Japan) once every three weeks,
starting on day 1. A Grade 1 skin rash (Common Terminology Criteria
for Adverse Events Version 4.0; CTCAE v4.0) (17) was the only side effect during the
first two treatment cycles.
A total of two months later, the patient was
admitted to Fujita Health University Hospital due to acute upper
abdominal pain. Physical examination revealed generalized abdominal
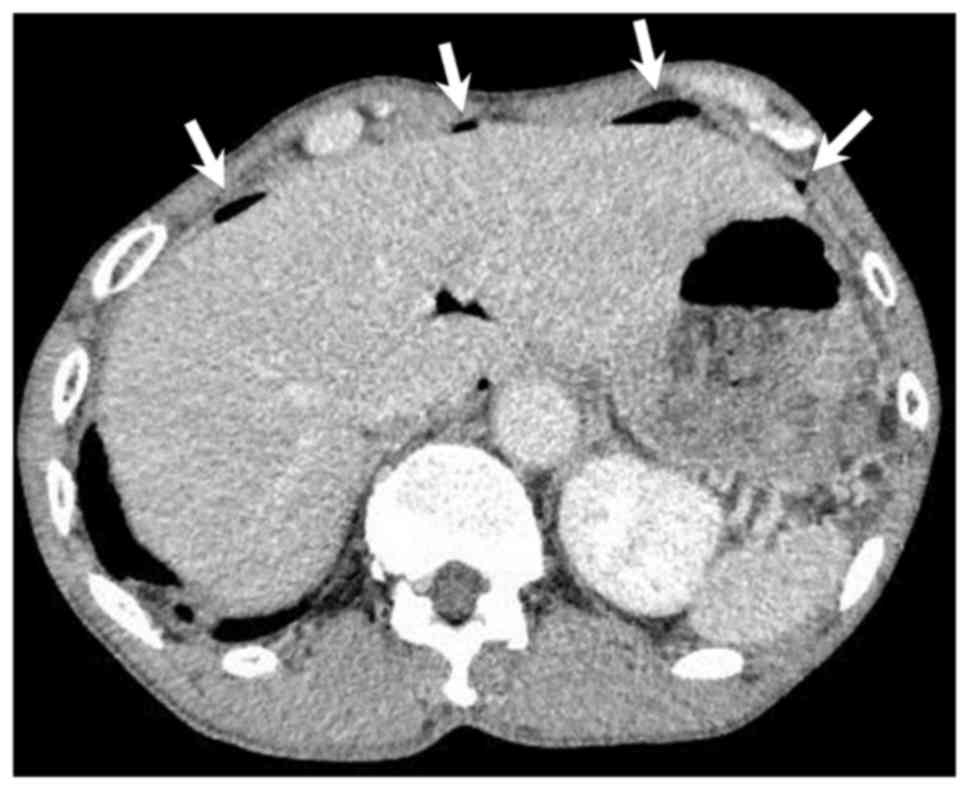
guarding and rebound tenderness. Abdominal CT showed spots of free
intraperitoneal air below the ventral abdominal wall (Fig. 2). These clinical findings suggested a
gastrointestinal perforation. Explorative laparoscopy revealed a
perforated duodenal ulcer, and laparoscopic repair was performed.
The patient resumed erlotinib treatment alone, without bevacizumab,
three weeks following emergency surgery. The treatment was
continued until the diagnosis of disease progression, and
progressive disease in neck lymph node metastasis was confirmed at
nine months.
The patient gave informed consent for the
publication of their data in the present report.
Patient 2
A 66-year-old male was referred to Fujita Health
University Hospital with increasing exertional dyspnea in March
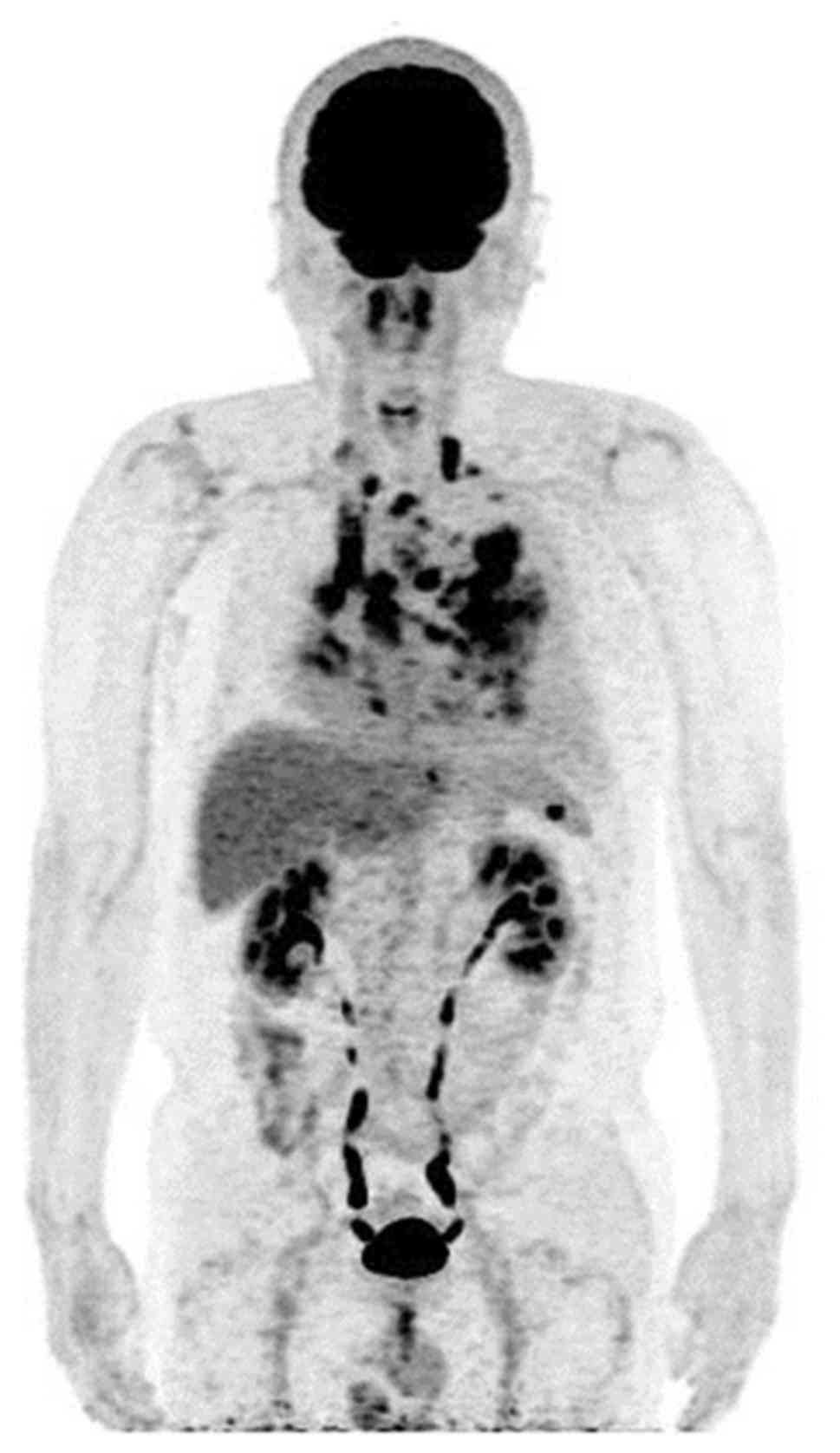
2015. A chest CT scan revealed a mass in the left lower lobe of the
lung, with multiple lung metastases, carcinomatous lymphangiosis
and malignant pleural effusion. The patient was diagnosed with lung
adenocarcinoma (cT4N3M1a, the 7th edition TNM classification;
Fig. 3) (16). Sequencing analysis of the patient's
pleural effusion revealed a deletion in EGFR exon 19 (E746-A750).
The patient's previous medical history included paroxysmal atrial
fibrillation, a risk factor for thromboembolism, and an inguinal
hernia repair three years earlier. The patient's pleural effusion
was uncontrollable following pleural drainage and pleurodesis.
Following an explanation of the risk and benefits of combination
bevacizumab treatment, the patient provided written informed
consent for treatment with erlotinib (150 mg/day) plus bevacizumab
(15 mg/kg every 3 weeks). Following the commencement of combination
treatment, the patient demonstrated marked improvement in multiple
pulmonary carcinomatous lymphangiosis and exertional dyspnea.
Following five months of treatment, the patient
experienced a grade 3 skin rash (CTCAE v4.0) (17), and the erlotinib dose was reduced to
100 mg/day. A total of seven months following initiation of
combination therapy, the patient experienced persistent lower
abdominal pain for several days and was admitted to the Fujita
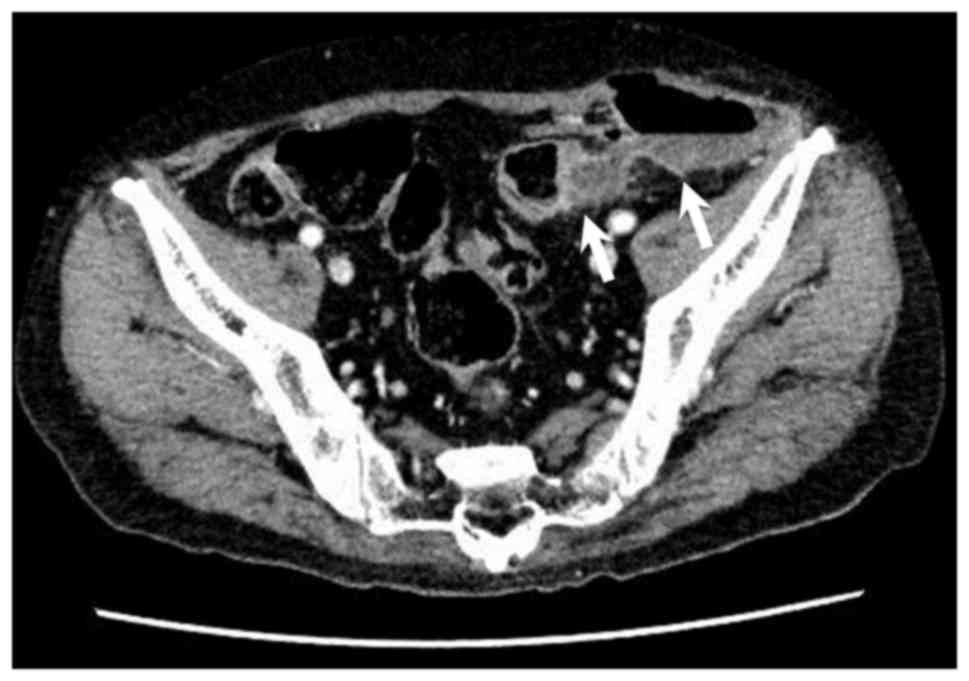
Health University Hospital. Abdominal CT scanning confirmed the
diagnosis of colon perforation and colocutaneous fistula
complicating sigmoid diverticulitis (Fig.
4). The patient underwent surgical drainage and ileostomy, and
was re-started on erlotinib treatment alone, without bevacizumab,
six weeks following the surgery. The treatment was continued for an
additional 18 weeks, and disease progression was observed in April
2016.
The patient gave informed consent for the
publication of their data in the present report.
Discussion
To the best of our knowledge, these are the first
reports of gastrointestinal perforation associated with erlotinib
plus bevacizumab therapy in patients with NSCLC. The previous
randomized phase II JO25567 trial demonstrated that treatment with
erlotinib plus bevacizumab increased median PFS by more than 6
months, compared with erlotinib alone, in patients with activating
EGFR mutation-positive NSCLC (12).
However, the addition of bevacizumab to erlotinib may increase the
numbers of potentially life-threatening adverse events, including
bleeding, arterial and venous thromboembolic events, wound-healing
complications, and gastrointestinal perforations (15). A meta-analysis of randomized
controlled trials that included bevacizumab revealed that
bevacizumab was associated with an increased risk of
gastrointestinal perforation (18).
The mechanism by which bevacizumab induces bowel perforation has
not been determined, but may be associated with delayed wound
healing (19).
In the present report, Patient 1 experienced a
sudden onset of duodenal perforation without use of aspirin or
other non-steroidal anti-inflammatory drugs. Had the patient not
been exposed to bevacizumab, the duodenal ulcer may not have
perforated. The patient was not taking any proton pump inhibitor
(PPI) or H2 antagonists. Although these agents increase
the pH of the upper gastrointestinal tract and heal ulcers, they
reduce the solubility of erlotinib, thereby reducing its
bioavailability (20,21). Thus, acid-reducing agents are
generally not administered to patients taking EGFR-TKIs.
The acidic beverage Coca-Cola (CC; The Coca-Cola
Company, Atlanta, GA, USA) was recently demonstrated to improve the
bioavailability of erlotinib in patients treated with a PPI
(22). When erlotinib is taken with
CC instead of water, the stomach is temporarily acidified, thereby
increasing the absorption rate of erlotinib in patients taking
esomeprazole. Taking erlotinib with CC instead of water is
therefore recommended for patients being treated with erlotinib and
a PPI (22).
Treatment with bevacizumab may lead to necrosis of
bowel tumors, which may result in bowel perforation (19). Colon perforation and colocutaneous
fistula in Patient 2 were complications of sigmoid diverticulitis.
As this patient underwent hernia repair 3 years previously, there
may have been some weakness in the sigmoid colon near the suture
site. However, the patient did not exhibit subjective symptoms
prior to commencing erlotinib plus bevacizumab therapy, suggesting
that this treatment had a significant effect on colon
perforation.
The incidence of gastrointestinal perforation has
been reported to vary by tumor type, being increased in patients
with colorectal carcinoma and renal cell cancer (18). These findings suggest that tumor
location in the abdominal cavity may be a risk factor for
gastrointestinal perforation. Bevacizumab-induced necrosis of these
tumors may lead to gastrointestinal perforation. However, there was
no evidence that intra-abdominal tumor in either of the present
patients triggered perforation.
Bevacizumab is frequently combined with cytotoxic
regimens, including taxanes, which may be associated with bowel
inflammation and necrosis (23).
Inflammation may be induced by tumor-promoting agents, mechanical
stimulation or chemotherapy. Delayed wound healing associated with
bevacizumab may lead to the perforation of bowels with acute
inflammation (19). However,
EGFR-TKIs have been associated with diarrhea. A phase II study of
erlotinib plus bevacizumab in 13 patients with recurrent ovarian,
primary peritoneal and fallopian tube cancer reported that two of
these patients experienced gastrointestinal perforation (24). These findings suggested that
erlotinib-induced diarrhea may increase the risk of bowel
perforation.
In conclusion, gastrointestinal perforation is a
potentially fatal adverse event associated with bevacizumab
combination therapy, and may occur even in the absence of tumor
spread in the abdomen. Although gastrointestinal perforation is
difficult to predict, careful patient monitoring may prevent a
fatal outcome.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TY designed the study and wrote the initial draft of
the manuscript with support from YG, HK and KI. HH contributed to
analysis computed tomography and assisted in the preparation of the
manuscript. All authors approved the final version of the
manuscript and agreed to be accountable for all aspects of the work
in ensuring that questions associated with the accuracy or
integrity of any part of the study are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
The patient provided written informed consent for
publication of this case report, and the privacy policy was fully
explained.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for european patients with
advanced EGFR mutations-positive non-small cell lung cancer
(EURTAC) a multicenter, open-label, randomized phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase III study of afatinib or cisplatin plus pemetrexed in
patients with metastatic lung adenocarcinoma with EGFR mutations. J
Clin Oncol. 31:3327–3334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugawara S, Oizumi S, Minato K, Harada T,
Inoue A, Fujita Y, Maemondo M, Yoshizawa H, Ito K, Gemma A, et al:
Randomized phase II study of concurrent versus sequential
alternating gefitinib and chemotherapy in previously untreated
non-small cell lung cancer with sensitive EGFR mutations:
NEJ005/TCOG0902. Ann Oncol. 26:888–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ichihara E, Hotta K, Nogami N, Kuyama S,
Kishino D, Fujii M, Kozuki T, Tabata M, Harada D, Chikamori K, et
al: Phase II trial of gefitinib in combination with bevacizumab as
first-line therapy for advanced non-small cell lung cancer with
activating EGFR gene mutations: The okayama lung cancer study group
trial 1001. J Thorac Oncol. 10:486–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seto T, Kato T, Nishio M, Goto K, Atagi S,
Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al:
Erlotinib alone or with bevacizumab as first-line therapy in
patients with advanced non-squamous non-small-cell lung cancer
harbouring EGFR mutations (JO25567): An open-label, randomised,
multicentre, phase 2 study. Lancet Oncol. 15:1236–1244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wedam SB, Low JA, Yang SX, Chow CK, Choyke
P, Danforth D, Hewitt SM, Berman A, Steinberg SM, Liewehr DJ, et
al: Antiangiogenic and antitumor effects of bevacizumab in patients
with inflammatory and locally advanced breast cancer. J Clin Oncol.
24:769–777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
US Food and Drug Administration, Center
for Drug Evaluation and Research: AVASTIN (bevacizumab) solution
for intravenous infusion [product label]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761028s000lbl.pdfApril
22–2018
|
|
16
|
Vallières E, Shepherd FA, Crowley J, Van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P: International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
IASLC lung cancer staging project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Common Terminology Criteria for Adverse
Events (CTCAE), Version 4.0 Published: May28, 2009 (v4.03: June 14,
2010). U.S. Department of Health and Human Services, . National
Institute of Health National Cancer Institute. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfApril
22–2018
|
|
18
|
Hapani S, Chu D and Wu S: Risk of
gastrointestinal perforation in patients with cancer treated with
bevacizumab: A meta-analysis. Lancet Oncol. 10:559–568. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gordon CR, Rojavin Y, Patel M, Zins JE,
Grana G, Kann B, Simons R and Atabek U: A review on bevacizumab and
surgical wound healing: An important warning to all surgeons. Ann
Plast Surg. 62:707–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
US Food and Drug Administration, Center
for Drug Evaluation and Research: Tarceva (erlotinib) tablets for
oral use [product label]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021743s019lbl.pdfApril
22–2018
|
|
21
|
Chu MP, Ghosh S, Chambers CR, Basappa N,
Butts CA, Chu Q, Fenton D, Joy AA, Sangha R, Smylie M and Sawyer
MB: Gastric acid suppression is associated with decreased erlotinib
efficacy in non-small-cell lung cancer. Clin Lung Cancer. 16:33–39.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Leeuwen RW, Peric R, Hussaarts KG,
Kienhuis E, IJzerman NS, de Bruijn P, van der Leest C, Codrington
H, Kloover JS, van der Holt B, et al: Influence of the acidic
beverage cola on the absorption of erlotinib in patients with
non-small-cell lung cancer. J Clin Oncol. 34:1309–1314. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seewaldt VL, Cain JM, Goff BA, Tamimi H,
Greer B and Figge D: A retrospective review of
paclitaxel-associated gastrointestinal necrosis in patients with
epithelial ovarian cancer. Gynecol Oncol. 67:137–140. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nimeiri HS, Oza AM, Morgan RJ, Friberg G,
Kasza K, Faoro L, Salgia R, Stadler WM, Vokes EE, Fleming GF, et
al: Efficacy and safety of bevacizumab plus erlotinib for patients
with recurrent ovarian, primary peritoneal, and fallopian tube
cancer: A trial of the Chicago, PMH, and California Phase II
Consortia. Gynecol Oncol. 110:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|