Introduction
Esophageal cancer is a common fatal cancer
worldwide, often diagnosed at an advanced stage. Long-term outcomes
remain poor despite advances in chemotherapy, indicating the need
for new therapeutic targets to improve prognosis (1–3).
Immunotherapy is an emerging treatment against cancer. Inhibition
of immune-checkpoints using programmed death-1, programmed death
ligand-1, and cytotoxic T-lymphocyte (CTL)-associated protein 4
antibodies appear to be promising immunotherapy approaches.
However, the efficacy of immune-checkpoint inhibition is
approximately 20% in solid cancer (4). Therefore, the development of other
immunotherapy approaches such as vaccination remains a priority.
Vaccination strategies involving dendritic cells (DCs)-important
antigen-presenting cells for T-cell activation-have been developed.
Antigen-pulsed autologous DCs have been applied for therapeutic
cancer vaccination (5). An autologous
DC vaccine stimulated with prostatic acid phosphatase and
granulocyte-macrophage colony-stimulating factor (GM-CSF), recently
approved by the United States Food and Drug Administration, has
been shown to prolong overall survival of prostate cancer patients
(6).
The Wilms tumor gene 1 (WT1) antigen is a well-known
cancer antigen expressed in many types of solid tumors and
hematological malignancies (7). The
WT1 protein has been reported to be overexpressed in 95% of
esophageal cancer patients (8). A WT1
peptide vaccine has been tested in a variety of solid tumors
(9,10). In particular, the
HLA-A*2402-restricted modified 9-mer WT1 peptide (CYTWNQMNL)
stimulates WT1-specific CTLs more effectively than the natural
9-mer WT1 peptide (11). We
previously conducted a pilot study of CYTWNQMNL-pulsed DC
vaccination combined with gemcitabine (DCGEM) as the first-line
therapy in chemotherapy-naive pancreatic cancer patients with local
advancement or metastasis (12). We
found the therapy to be feasible, tolerable, and effective as a
first-line therapy for inducing antitumor T-cell responses in
patients with advanced pancreatic cancer without liver metastases.
Next, we planned a pilot study of WT1 peptide-pulsed DC vaccination
therapy in esophageal cancer. A combination of 5-fluorouracil
(5-FU) and cisplatin is presently used in Japan as first-line
therapy, followed by docetaxel as second-line therapy (13). Single-agent docetaxel in patients with
metastatic esophageal cancer revealed effectiveness in a phase II
study; however, the response rate was low (20.7%) (14). At the same time, the immune
enhancement effect of docetaxel has been reported (15–17).
Therefore, we planned a pilot study of WT1 peptide-pulsed DC
vaccination combined with docetaxel (DCDOC) in advanced esophageal
cancer patients who had already received first-line
chemotherapy.
Patients and methods
Study design
Ten esophageal cancer patients were enrolled in the
pilot study, which was performed at Tokyo Midtown Clinic (Tokyo,
Japan) and Keio University (Tokyo, Japan). The primary endpoints
were adverse events. Common Terminology Criteria for Adverse Events
v4.0 was used to grade the adverse events. The secondary endpoints
were immune response to the WT1 peptide, overall survival, and
response rate. Response Evaluation Criteria in Solid Tumors
(RECIST) (v1.1) was used to evaluate the clinical response
(18). Overall survival was defined
as the time between the date when informed consent was obtained and
the date of death.
At the baseline, complete history examination,
physical examination, computed tomography (CT), and laboratory
tests were performed. The clinical stage of the tumors was
determined according to the TNM classification of the International
Union against Cancer. A repeat CT study was carried out before each
cycle and at one month after the third cycle of treatment. Therapy
was stopped when patients were diagnosed with disease
progression.
The institutional review board of Keio University
approved the study, and all patients provided written informed
consent. This clinical trial was registered in the University
Hospital Medical Information Network (UMIN) Clinical Trials
Registry, no. UMIN-000007925.
Inclusion and exclusion criteria
Inclusion criteria were i) histological or
cytological diagnosis of esophageal cancer (squamous cell
carcinoma, adenocarcinoma, adenosquamous carcinoma); ii)
unresectable esophageal cancer or recurrence after resection of
esophageal cancer; iii) HLA-A*2402; iv) an Eastern Cooperative
Oncology Group performance status of 0-2; v) no immediate allergy
to the WT1 peptide; vi) a measurable target lesion that could be
evaluated according to RECIST; vii) a lesion refractory to
treatment with first-line chemotherapy or chemoradiotherapy; and
viii) adequate cardiac, hepatic, hematologic, and renal function.
Exclusion criteria were i) tracheo-esophageal fistula; ii) symptom
of brain metastasis; iii) other active primary malignancies; iv)
history of allergic disease; v) severe comorbidity (cardiovascular
disease, fibroid lung, infections, interstitial pneumonia, liver
disease, renal disease, uncontrolled diabetes); vi) pericardial
fluid or pleural effusion requiring treatment; vii) pregnant or
nursing women; viii) men planning conception; ix) severe
psychiatric disease; x) active autoimmune disease; xi) current
treatment with immunosuppressive agents; and xii) other reasons for
unsuitability.
DC vaccine preparation and DCDOC
A detailed protocol for the preparation of the DC
vaccine has been reported previously (12,19).
Isolated peripheral blood mononuclear cells (PBMCs) were incubated
in plastic tissue culture plates in AIM-V medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). After a 30-min
incubation at 37°C, non-adherent cells were washed, and adherent
cells were placed in AIM-V containing interleukin 4 (IL-4; 50
ng/ml; Primmune Inc., Kobe, Japan) and GM-CSF (50 ng/ml; Primmune
Inc.) to generate immature DCs. Five days after culture, OK-432 (10
µg/ml; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) and
prostaglandin E2 (50 ng/ml; Daiichi Fine Chemical Co., Ltd.,
Toyama, Japan) were added to stimulate immature DCs for 24 h. The
generated DCs were pulsed with 100 µg of WT1 peptide (NeoMPS, San
Diego, CA, USA) for 1 h. HLA-DR+, HLA-ABC+
CD11c+, CD14−, CD40+,
CD80+, CD83+, CD86+, and
CCR7+ phenotypes were considered as indicating mature
DCs (20). A fixed dose of
107 WT1 peptide-pulsed DCs was injected into the dermis
close to the inguinal or axillary lymph nodes on days 15 and 22.
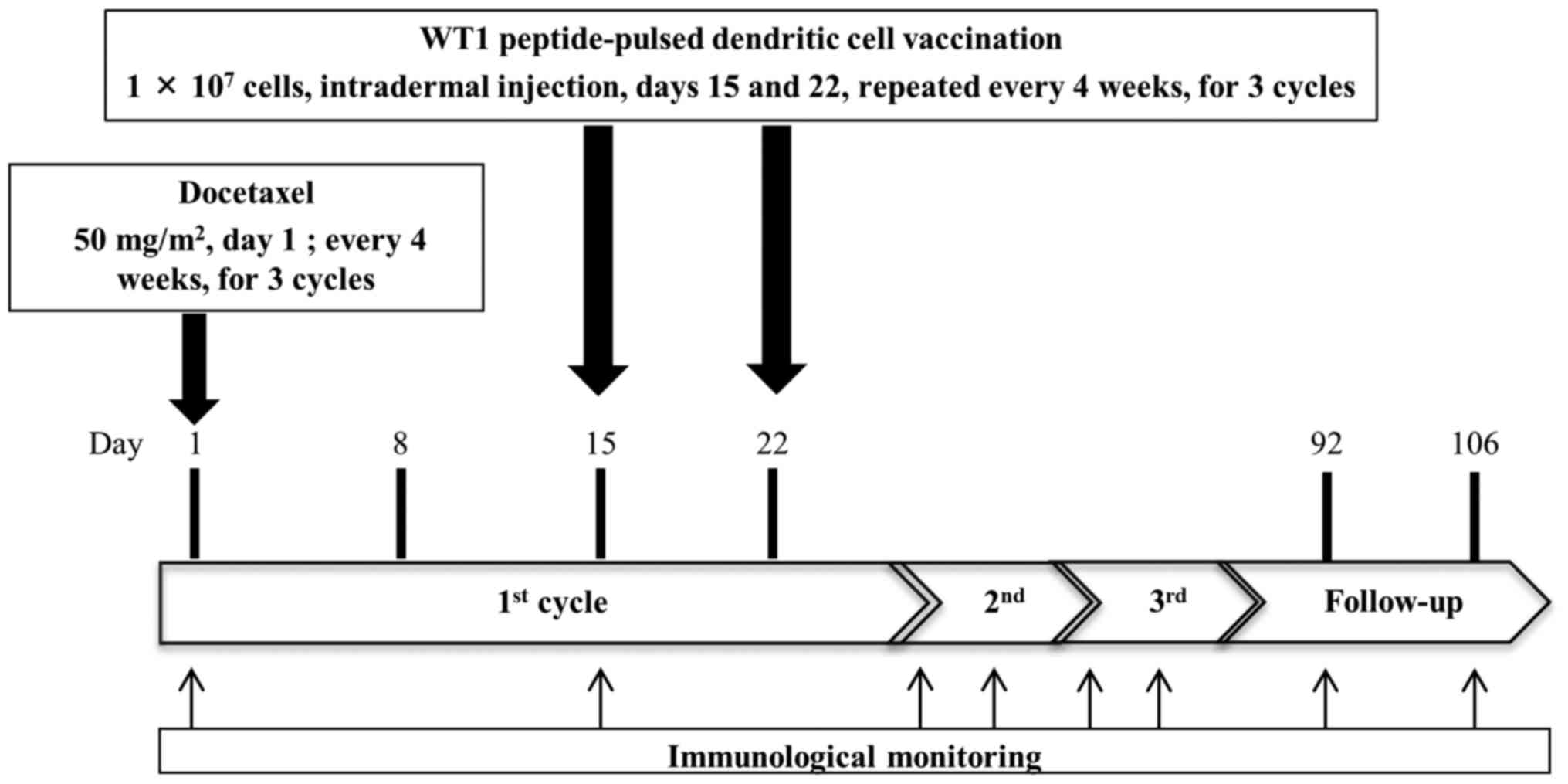
Docetaxel (50 mg/m2) was administered every 4 weeks by
intravenous drip infusion on day 1 (Fig.
1). Docetaxel was administered before vaccination, because we
expected immune enhancement effect of docetaxel (15–17). DC
vaccination was repeated over 3 cycles in patients who were not
diagnosed progressive disease. After termination or completion of
the protocol therapy, post-protocol DC vaccination could continue
with patients' consent. Dendritic cells were generated in the cell
processing facility in Tokyo Midtown Clinic, headed by Dr. Junichi
Taguchi.
Immunological monitoring
Peripheral blood was collected from patients on days
1 and 15 of each cycle and twice for 4 weeks after the third cycle
(Fig. 1). We analyzed the PBMCs on
day 1 of first cycle as pre-vaccination samples and PBMCs after day
1 of second cycle as post-vaccination samples. Immune response to
the WT1 peptide was analyzed using the interferon (IFN)-γ
enzyme-linked immunospot (ELISPOT) assay, HLA tetramer staining
assay, and delayed-type hypersensitivity (DTH) skin test (12).
DTH test
Induration diameters and erythema were measured 48 h
after peptide injection on day 1 of each cycle and at 4 weeks after
the third cycle. Erythema diameter >5 mm was defined as a
positive reaction.
In vitro generation of peptide
cocktail-cultured PBMCs
After thawing cryopreserved PBMCs from patients,
these were stimulated with 10 µg/ml modified-type WT1 peptide
(CYTWNQMNL; Merck Bioscience AG, Läufelfingen, Switzerland) and 16
µg/ml CE control peptide pool HLA-A 24 (8 peptides; Bio-Synthesis
Inc., Lewisville, TX, USA) in AIM-V CTS medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10 ng/ml IL-7
(PeproTech, Inc., Rocky Hill, NJ, USA), 20 U/ml IL-2 (Shionogi,
Osaka, Japan), and 10% human AB serum (MP Biomedicals, Solon, OH,
USA). After 9 days of culture, cells were analyzed by the
WT1-specific IFN-γ ELISPOT assay and HLA tetramer assay (12).
WT1 peptide/HLA-A*2402 tetramer
assay
HLA tetramers (T-Select MHC Tetramer; MBL: Medical
and Biological Laboratories Co., Ltd., Nagoya, Japan) were used to
access WT1-specific CD8+ T-cells in peripheral blood
(21). Results were defined as
positive when CD3+, CD8+, and WT1/HLA-A24
tetramer-positive cells were detected. We could not detect
CD3+, CD8+, and HIV env/HLA-A24
tetramer-positive cells in the negative controls (12).
WT1-specific INF-γ ELISPOT assay
PBMCs were defined as sensitized to WT1 peptide when
the number of spots in response to the WT1 peptide was at least
twice that in response to HIV env peptide-pulsed stimulator cells
with the INF-γ ELISPOT assay (22).
Cell surface marker analysis for
phenotyping
PBMCs were incubated with each
fluorescent-conjugated antibody for 45 min at 4°C in the dark.
After washing with cell sorting buffer (phosphate-buffered saline
with 2% FBS), stabilizing fixative (BD Biosciences, San Jose, CA,
USA) was used to fix the cells and these were analyzed by flow
cytometry (Gallios; Beckman Coulter, Inc., Brea, CA, USA). FACS
data were analyzed by Kaluza software (Beckman Coulter, Inc.).
Statistical analysis
Statistical analyses were conducted using SPSS
software v21 (IBM Corporation, Armonk, NY, USA). The immune
response was analyzed by t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The patients' clinical characteristics are
summarized in Table I. From January
2012 to January 2014, 25 patients received HLA typing. Twelve
HLA-A*2402-positive patients were enrolled, 2 patients left the
study before the treatment protocol was started. One patient hoped
to undergo another treatment after providing consent. Another
patient dropped out of this study owing to deterioration in renal
function. The remaining 10 patients (2 with stage IV esophageal
squamous cell cancer and 8 with recurrence of esophageal squamous
cell cancer after surgery) had a median age of 60.5 years (range,
49–71 years). All patients had squamous cell carcinoma. Five
patients received only first-line chemotherapy; 1 patient,
second-line; 3 patients, third-line; and 1 patient, fourth-line
therapy. Seven patients had already received chemoradiotherapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Age (years) | Sex | Clinical stage | Site of
metastasis | PS | Pre-protocol
chemotherapy | Protocol DC
(times) | Post-protocol DC
(times) | Post-protocol
chemotherapy | Outcome | OS (month) |
|---|
| 1 | 55 | M | IVb | Liver/para-aortic
LN | 0 | 1st FP 2nd FP +
RT | 4 | 5 | None | PD | 4.6 |
| 2 | 70 | M | Recurrence | Bone/para-aortic
LN | 2 | 1st DCF (NAC :
FP) | 4 | 0 | FP + RT | PD | 7.8 |
| 3 | 55 | M | Recurrence |
Peritoneum/para-aorticLN | 1 | 1st FP | 4 | 3 | Ned + Doc | PD | 5.4 |
| 4 | 65 | M | Recurrence | PleuraLN/abdominal
wall | 0 | 1st DCF (NAC :
FP) | 4 | 1 | None | PD | 3.7 |
| 5 | 54 | M | Recurrence | Anastomosis
site | 0 | 1st DCF (NAC : FP +
RT) | 4 | 5 | None | PD | 5.3 |
| 6 | 61 | M | Recurrence | Lung/skin
Mediastinum LN | 0 | 1st 5FU+CDDP+RT 2nd
DCF 3rd PTX (NAC : FP) | 2 | 0 | None | PD | 1.9 |
| 7 | 49 | W | IVb | Lung/abdominal
LN | 0 | 1st FP + RT 2nd
PTX | 2 | 0 | None | SD | 1.1 |
| 8 | 73 | M | Recurrence | Lung | 1 | 1st FP + RT 2ndTS1
3rdDCF (NAC : FP) | 2 | 3 | None | PD | 11.6 |
| 9 | 71 | M | Recurrence | Mediastinum LN | 0 | 1st FP + RT 2nd DCF
3rd PTX (NAC : FP) | 6 | 0 | None | PD | 7.6 |
| 10 | 60 | M | Recurrence | Lung/mediastinum
LN | 0 | 1st FP + RT 2nd Ned
+ DOC 3rd DCF 4th PTX (NAC : FP) | 2 | 0 | None | PD | 4 |
The median overall survival was 5 months (range,
1.1–11.6). Only 1 patient completed the protocol therapy. Eight
patients terminated the protocol treatment because of rapid disease
progression. One patient (patient 7) died, probably from tumor
bleeding, during therapy. Primary esophageal cancer invaded the
aorta, and a large metastatic lymph node invaded the stomach wall
directly. However, the exact cause of death is not confirmed
because her family refused an autopsy. The remaining 5 patients
received post-protocol DC vaccination after termination or
completion of the protocol treatment. The median number of DC
vaccine administration was 5 times (range, 2–9 times).
Adverse events
All adverse events information within the protocol
treatment period is reported in Table
II. There were no adverse skin reactions at the site of
vaccination. Three patients had grade 4 neutropenia, and 1 patient
had grade 3 febrile neutropenia. Grade 5 hypoxia occurred in
patient 6, for whom we stopped the protocol therapy because of
disease progression after 1 course of therapy. One month after the
protocol therapy was stopped, patient 6 died due to tracheal
obstruction. Patient 7, who died during the protocol therapy as
mentioned above, was defined as grade 5 sudden death (not otherwise
specified). Patients 6 was died due to the disease progression,
however we could not deny the possibility of treatment-related
death of patient 7. We reported the independent data monitoring
committees of this trial about these cases and they decided to
continue this study with careful patient monitoring.
 | Table II.Adverse events. |
Table II.
Adverse events.
|
| Grade |
|---|
|
|
|
|---|
| Adverse event | 1 | 2 | 3 | 4 | 5 |
|---|
|
|---|
| A,
Hematotoxicity |
|---|
|
|---|
| Febrile | 0 | 0 | 1 | 0 | 0 |
|---|
| Neutropenia | 0 | 2 | 2 | 3 | 0 |
|---|
|
|---|
| B,
Non-hematotoxicity |
|---|
| Respiratory
disorders |
|
Pulmonary fistula | 0 | 1 | 0 | 0 | 0 |
|
Hypoxia | 0 | 0 | 0 | 0 | 1 |
| Pleural
effusion | 0 | 1 | 0 | 0 | 0 |
| Gastrointestinal
disorders |
|
Nausea | 1 | 0 | 0 | 0 | 0 |
|
Constipation | 2 | 0 | 0 | 0 | 0 |
|
Ascites | 1 | 0 | 0 | 0 | 0 |
|
Mucositis oral | 1 | 0 | 0 | 0 | 0 |
| Nervous system
disorders |
|
Peripheral sensory neuropathy
General disorders | 1 | 0 | 0 | 0 | 0 |
| Sudden
death not otherwise specified | 0 | 0 | 0 | 0 | 1 |
|
Fatigue | 2 | 1 | 0 | 0 | 0 |
|
Pain | 0 | 0 | 1 | 0 | 0 |
| Metabolism and
nutrition disorders |
|
Anorexia | 1 | 0 | 2 | 0 | 0 |
|
Hypercalcemia | 0 | 0 | 1 | 0 |
|
| Musculoskeletal
disorders |
|
Myalgia | 1 | 0 | 0 | 0 | 0 |
| Skin and
subcutaneous tissue disorders |
|
Alopecia | 0 | 1 | 0 | 0 | 0 |
Immunological monitoring and clinical
outcomes
Three patients (6, 8 and 10) terminated the protocol
therapy at the first cycle. Thus, we could not check their
immunological monitoring data after DC vaccination. Only patient 8
underwent an immunological monitoring blood test and CT scan, which
revealed disease progression the same day. Finally, we analyzed the
results of tetramer and ELISPOT assays for 8 patients and DTH for 7
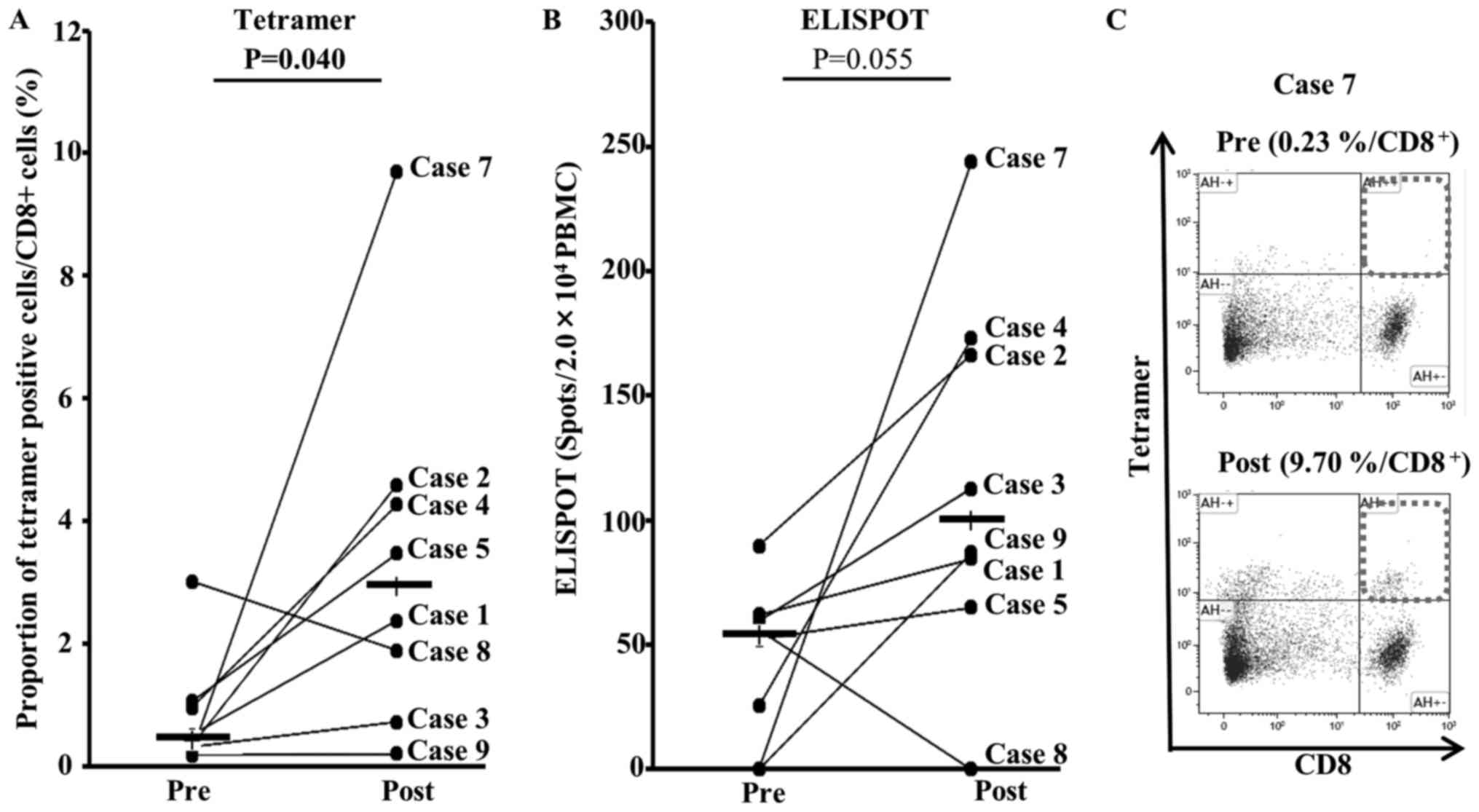
patients. DCDOC elicited a WT1-specific response in 5 of the 8
patients as indicated by the HLA/WT1-tetramer assay (Table III). The proportion of
HLA/WT1-tetramer-positive T-cells significantly increased after DC
vaccination (P=0.040; Fig. 2A).
Furthermore, the WT1-specific T-cell response was observed to be
enhanced in 5 of the 8 patients according to the ELISPOT assay
(Table III). The number of spots
after DC vaccination tended to increase (P=0.055; Fig. 2B).
 | Table III.Immunological monitoring data and
clinical outcome. |
Table III.
Immunological monitoring data and
clinical outcome.
| Case | LYMP (/ul) | NLR | CRP (mg/dl) | IL-6 (pg/ml) | IL-8 (pg/ml) | G-MDSC
(CD15+DR-CD11b+)/CD15+ | G-MDSC
(CD15+DR-CD66b+)/CD15+ | Minimum count of
LYMP (/µl) | DTH | ELISPOT (WT1) | HLA tetramer
(WT1) | Clinical
outcome | PFS/OS (month) |
|---|
| 1 | 700 | 1.43 | 0.13 | 1.55 | 5.17 | 10.49 | 11.40 | 510 | − | − | + | PD | 2/4.6 |
| 2 | 1,722 | 3.62 | 2.68 | 4.30 | 2.04 | 4.82 | 9.87 | 384 | + | + | + | PD | 2/7.8 |
| 3 | 1,310 | 2.65 | 0.17 | 1.66 | 3.38 | 6.50 | 6.64 | 610 | + | + | − | PD | 2/5.4 |
| 4 | 462 | 7.55 | 1.69 | 10.67 | 3.87 | 8.66 | 9.90 | 462 | + | + | + | PD | 2/3.7 |
| 5 | 1,221 | 3.04 | 1.46 | 6.59 | 8.42 | 5.63 | 7.01 | 390 | − | + | + | PD | 2/5.3 |
| 6 | 795 | 4.78 | 0.26 | 2.84 | 3.72 | 14.30 | 15.67 | 600 | ND | ND | ND | PD | 1/1.9 |
| 7 | 432 | 14.50 | 1.27 | 7.80 | 10.39 | 7.09 | 9.08 | 216 | − | + | + | SD | 1/1.1 |
| 8 | 2,057 | 1.37 | 0.06 | 0.83 | 2.99 | 9.45 | 17.32 | 713 | ND | − | − | PD | 2/11.6 |
| 9 | 776 | 4.36 | 0.04 | 0.77 | 4.99 | 16.28 | 23.26 | 693 | − | − | − | PD | 3/7.6 |
| 10 | 955 | 6.17 | 5.81 | 15.69 | 17.85 | 15.42 | 39.80 | 593 | ND | ND | ND | PD | 1/4.0 |
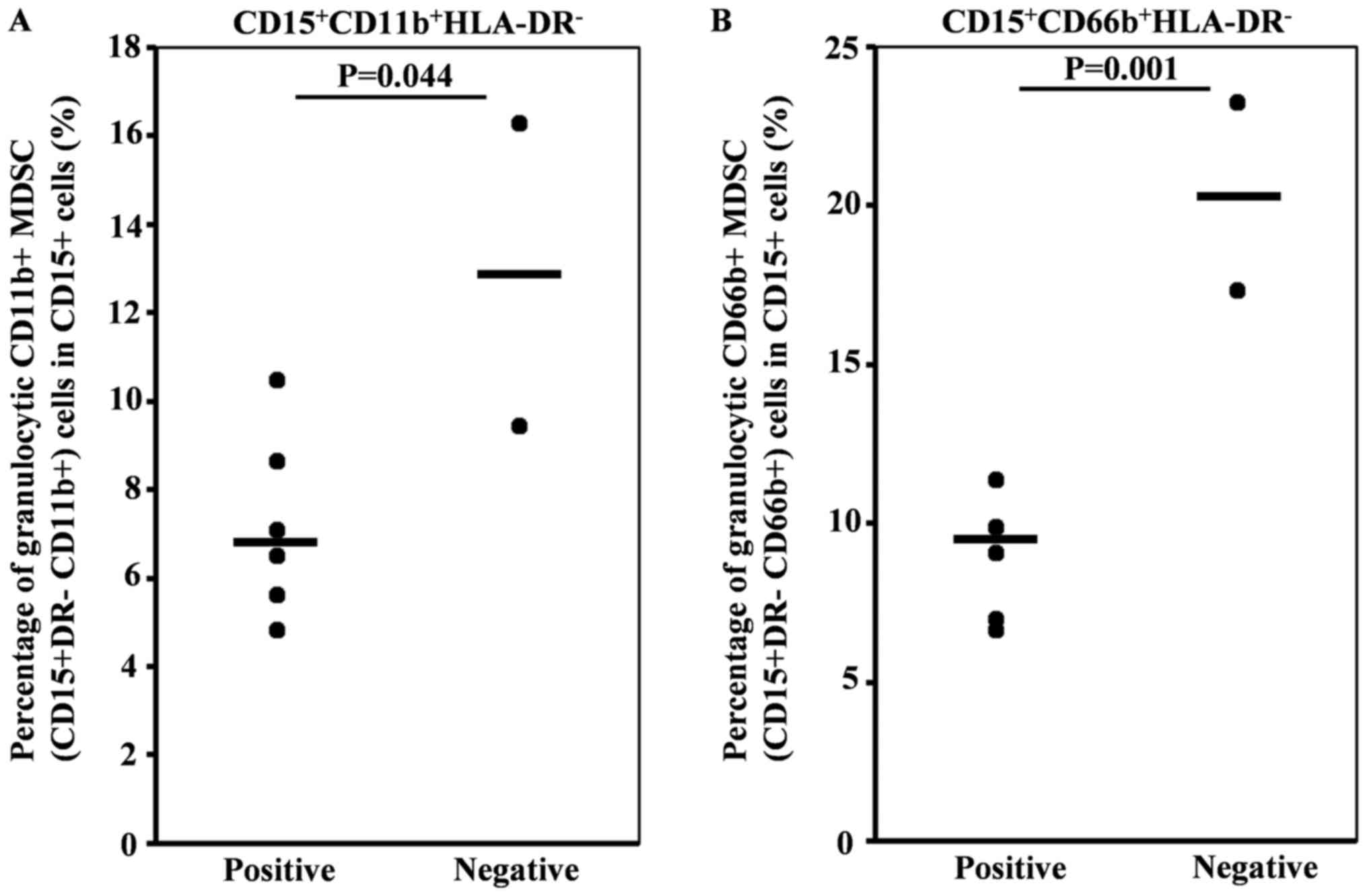
To identify predictive factors for the immune
response to DC vaccination, we checked the various cytokines and
immune cell subsets in pretreatment peripheral blood by flow
cytometry-based comprehensive leukocyte immunophenotyping. Positive
immune response (tetramer or ELISPOT) positivity significantly
correlated with a low percentage of CD11b+ and
CD66b+ granulocytic myeloid-derived suppressor cells
(MDSCs) in CD15+ cells (Fig.
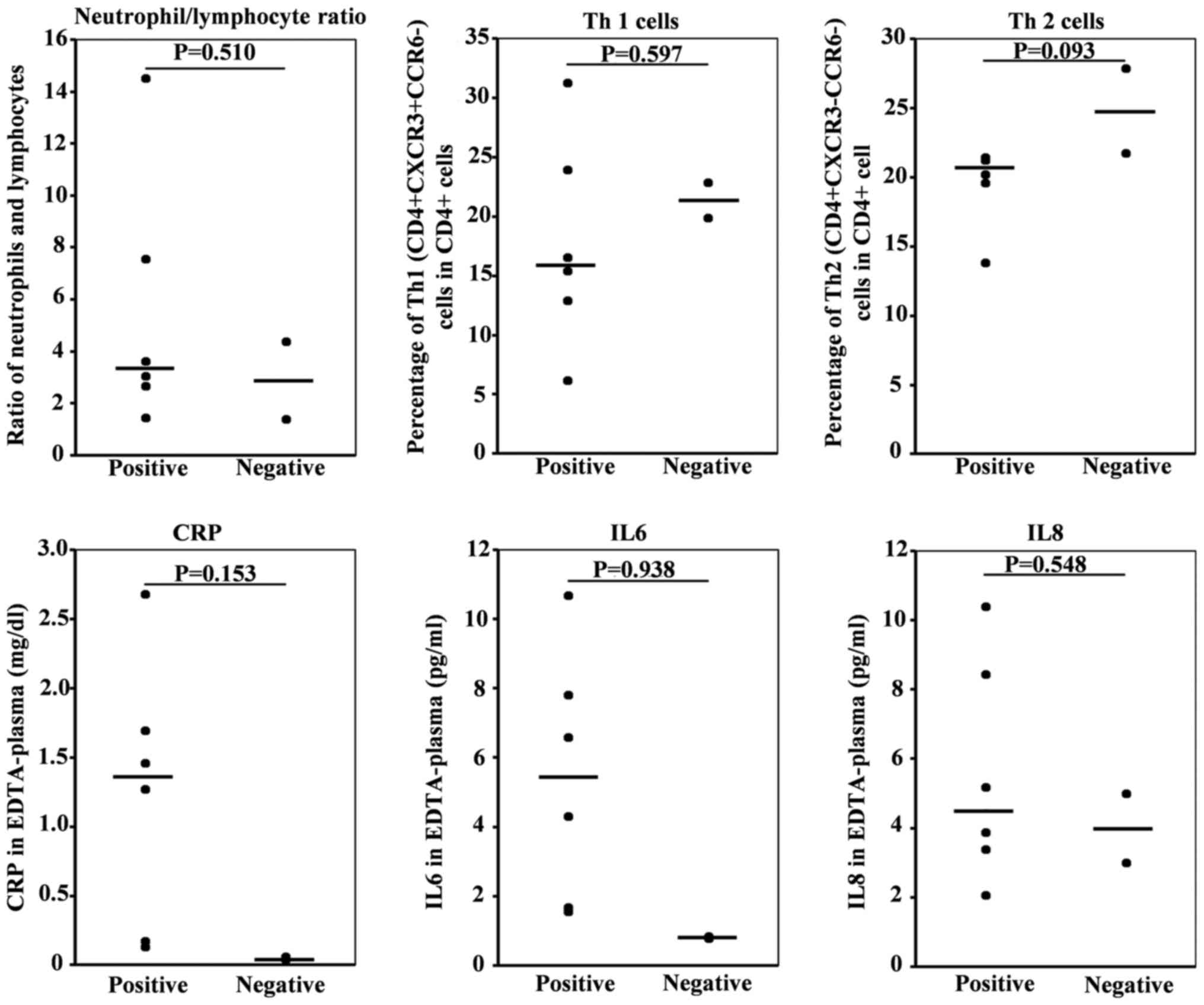
3). However, no differences in neutrophil lymphocyte ratio or
percentages of Th1
(CD4+CXCR3+CCR6−) or Th2
(CD4+CXCR3+CCR6−), C-reactive
protein (CRP), IL-6, and IL-8 were observed. (Fig. 4 and Table
III).
Discussion
Safety of WT1 peptide-pulsed
DCDOC
In this study, we could not evaluate the safety of
WT1 peptide-pulsed DCDOC therapy for esophageal squamous cell
cancer. Only 1 patient could complete the protocol therapy.
Five patients (50%) had grade 3 or 4 neutropenia,
and 1 patient had grade 3 febrile neutropenia. In an earlier report
on single-agent docetaxel (docetaxel 70 mg/m2 every 21
days) for patients with metastatic esophageal cancer, grade 3 or 4
neutropenia was noted in 43 of 49 patients (88%), and 9 of 49
patients (18%) developed febrile neutropenia (14). In our study, we enrolled patients who
had already received first-line chemotherapy or chemoradiotherapy;
therefore, we decreased the docetaxel dose to 50 mg/m2
every 28 days. The rate of neutropenia in this study seemed
tolerable. However, we could not determine if the docetaxel dose of
50 mg/m2 every 28 days was appropriate or not because,
owing to disease progression, the protocol study failed in most
patients.
Clinical effect of WT1 peptide-pulsed
DCDOC for esophageal cancer
We could not observe the clinical effect of DCDOC
therapy. Disease progression was observed in 9 patients, and 1
patient died during the second cycle of the protocol therapy. The
median overall survival was 5 months (range 1.1–11.6).
In a previous study on single-agent docetaxel for
patients with metastatic esophageal cancer, the response rates in
patients with and without prior chemotherapy were reported to be
16% (6 of 38) and 36% (4 of 11), respectively (14). Two reasons might explain the lack of a
clinical response to DCDOC therapy. First, all patients had already
received first-line chemotherapy (first-line chemotherapy, N=5;
second-line N=1; third-line N=3; fourth-line N=1). Second, 7 of 10
patients had already received chemotherapy including docetaxel,
which failed. We enrolled the patients who had already received
docetaxel, because we expected immune enhancement effect of
docetaxel in addition to the direct antitumor effect (15–17). Under
these severe conditions, the antitumor effect of DCDOC therapy
seems to be insufficient.
Immune response of esophageal cancer
patients receiving WT1 peptide-pulsed DCDOC
An emerging avenue of clinical research in solid
cancers is the use of immune-checkpoint inhibitors. Several
checkpoint inhibitors, targeting multiple different checkpoints,
have been developed; however, their efficacy is still not as
expected. At the same time, DC-based immunotherapy has been
receiving a lot of attention and is therefore being applied for
treating various cancers (23).
Nevertheless, only a few studies have reported on DC-based
immunotherapy for esophageal cancer, and none demonstrated a
clinical response (24–27). Narita et al (25), performed a phase I/II clinical trial
of monocyte-derived DCs pulsed with SART1 peptide in 7 patients
with advanced esophageal cancer. The effectiveness was not clearly
confirmed; however, in vitro studies revealed that DCs
generated for this therapy possessed a potent ability of inducing
SART1 peptide-specific CTLs (25).
The most important result in this study was that DCDOC elicited a
WT1-specific response in 6 of the 8 patients as detected by the
HLA/WT1-tetramer or ELISPOT assay, regardless of the
myelosuppression associated with docetaxel. To our knowledge, this
is the first report of immune response induced by DCDOC in
esophageal squamous cell cancer. We previously conducted a pilot
study of WT1 peptide (DCGEM) as first-line therapy in chemo-naive
pancreatic cancer patients with local advancement or metastasis
(12). In that study, the disease
control rate and median overall survival were 60% and 243 days,
respectively, which are promising. Furthermore, we observed fewer
adverse effects compared with gemcitabine in combination with
nab-paclitaxel or S-1. Koido et al reported that the
combined treatment of chemotherapy and DCs pulsed with a mixture of
3 types of WT1 peptides, including both MHC class I and
II-restricted epitopes, induced WT1-CTLs during long-term
vaccination and may be associated with disease stability in
advanced pancreatic cancer (28). On
the basis of these reports and our present results, we believe that
WT1 peptide-pulsed DC vaccination might be an effective therapy for
esophageal cancer, either alone or in combination with other
immunotherapy approaches, such as immune-checkpoint inhibitors.
Patient selection is important for effective
immunotherapy development. To this end, we evaluated various
immunological biomarkers in our patients before treatment. We found
that positive immune response had significant relevance to the low
percentage of CD11b+ and CD66b+ granulocytic
myeloid derived suppressor cells (MDSCs) in CD15+
cells.
One of the reasons why we failed to observe a
clinical effect was the huge tumor burden. Lee et al
described a phase I/IIa trial of adjuvant immunotherapy with tumor
antigen-pulsed DCs for patients with hepatocellular carcinoma. They
reported that the median time to progression was 11.8 months in the
control group and 36.6 months in the DC-vaccination group (29). In addition dysfunction of
vaccine-induced T cells might be another reason. The tetramer
staining results of some patients are not consistent with the
ELISPOT assay (Fig. 2). It indicates
that certain percentage of tetramer-positive T cells is not
functional. We would like to examine the correlation between
dysfunction of vaccine-induced T cells and PD1 expression level in
the future experiment. If high PD1 expression is correlated with
dysfunction of vaccine-induced T cells, PD-1 blockade therapy may
be combined to enhance the cytolytic activity. Nevertheless, we
believe that our protocol study had an antitumor effect towards
esophageal squamous cell carcinoma.
We report that DCDOC elicited a WT1-specific immune
response regardless of the myelosuppression associated with
docetaxel. In future studies, we plan to assess DCDOC as an
adjuvant therapy for esophageal cancer.
Acknowledgements
Not applicable.
Funding
This study was supported in part by Grants-in-Aid
for Scientific Research from the Japan Society for Promotion of
Science (26221005); a Project for Development of Innovative
Research on Cancer Therapeutics (P-Direct) and the Project for
Cancer Research And Therapeutic Evolution (P-CREATE) from Japan
Agency for Medical Research and Development (AMED); The Tokyo
Biochemical Research Foundation to Yutaka Kawakami; and tella Inc.,
Tokyo, to Yuko Kitagawa, Masato Okamoto, and Yutaka Kawakami. This
clinical trial was performed partly by the research fund from
Tella, Inc. However, Tella, Inc. was not involved in any of this
study, including the study design, patient treatement, clinical and
immunological evaluation, and paper writing. Tella, Inc, does not
have benefit from the results of this study.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HT, YKa, TM, SM, TS, TF, MO, MS and YKi planned the
project. HT, YKa, MS and YKi supervised the project. HT, TM, EB,
SM, HH, JT, YH, HT, HK and YKi enrolled the patients. TS, TF and MO
conducted the experiments. TM, EB, TS, TF and MO analyzed the data.
HT, YKa and TM drafted and wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The institutional review board of Keio University
approved the study (Approval number 2011153), and all patients
provided written informed consent. This clinical trial was
registered in the University Hospital Medical Information Network
(UMIN) Clinical Trials Registry, number UMIN000006704.
Consent for publication
All patients provided written informed consent for
the publication of any associated data.
Competing interests
Professor Masato Okamoto is a stockholder in tella
Inc. and a former Director of the Board for Science and Medicine.
Professor Yuko Kitagawa, Professor Masato Okamoto and Professor
Yutaka Kawakami received research funding from tella Inc.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paul S and Altorki N: Induction therapy
for esophageal cancer. Thorac Surg Clin. 23:499–507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sukari A, Nagasaka M, Al-Hadidi A and Lum
LG: Cancer Immunology and Immunotherapy. Anticancer Res.
36:5593–5606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheever MA, Allison JP, Ferris AS, Finn
OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL,
Weiner LM and Matrisian LM: The prioritization of cancer antigens:
A national cancer institute pilot project for the acceleration of
translational research. Clin Cancer Res. 15:5323–5337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oji Y, Yano M, Nakano Y, Abeno S,
Nakatsuka S, Ikeba A, Yasuda T, Fujiwara Y, Takiguchi S, Yamamoto
H, et al: Overexpression of the Wilms' tumor gene WT1 in esophageal
cancer. Anticancer Res. 24:3103–3108. 2004.PubMed/NCBI
|
|
9
|
Miyatake T, Ueda Y, Morimoto A, Enomoto T,
Nishida S, Shirakata T, Oka Y, Tsuboi A, Oji Y, Hosen N, et al: WT1
peptide immunotherapy for gynecologic malignancies resistant to
conventional therapies: A phase II trial. J Cancer Res Clin Oncol.
139:457–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohno S, Okuyama R, Aruga A, Sugiyama H and
Yamamoto M: Phase I trial of Wilms' tumor 1 (WT1) peptide vaccine
with GM-CSF or CpG in patients with solid malignancy. Anticancer
Res. 32:2263–2269. 2012.PubMed/NCBI
|
|
11
|
Tsuboi A, Oka Y, Udaka K, Murakami M,
Masuda T, Nakano A, Nakajima H, Yasukawa M, Hiraki A, Oji Y, et al:
Enhanced induction of human WT1-specific cytotoxic T lymphocytes
with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues.
Cancer Immunol Immunother. 51:614–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mayanagi S, Kitago M, Sakurai T, Matsuda
T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K, et
al: Phase I pilot study of Wilms tumor gene 1 peptide-pulsed
dendritic cell vaccination combined with gemcitabine in pancreatic
cancer. Cancer Sci. 106:397–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuwano H, Nishimura Y, Oyama T, Kato H,
Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al:
Guidelines for diagnosis and treatment of carcinoma of the
esophagus April 2012 edited by the Japan esophageal society.
Esophagus. 12:1–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin
K, Hyodo I, Fujita H, Takiyama W and Ohtsu T: A phase II study of
single-agent docetaxel in patients with metastatic esophageal
cancer. Ann Oncol. 15:955–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garnett CT, Schlom J and Hodge JW:
Combination of docetaxel and recombinant vaccine enhances T-cell
responses and antitumor activity: Effects of docetaxel on immune
enhancement. Clin Cancer Res. 14:3536–3544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sundstedt A, Celander M, Ohman MW,
Forsberg G and Hedlund G: Immunotherapy with tumor-targeted
superantigens (TTS) in combination with docetaxel results in
synergistic anti-tumor effects. Int Immunopharmacol. 9:1063–1070.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kodumudi KN, Woan K, Gilvary DL, Sahakian
E, Wei S and Djeu JY: A novel chemoimmunomodulating property of
docetaxel: Suppression of myeloid-derived suppressor cells in tumor
bearers. Clin Cancer Res. 16:4583–4594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura Y, Tsukada J, Tomoda T, Takahashi
H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S,
Koido S, et al: Clinical and immunologic evaluation of dendritic
cell-based immunotherapy in combination with gemcitabine and/or S-1
in patients with advanced pancreatic carcinoma. Pancreas.
41:195–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Figdor CG, de Vries IJ, Lesterhuis WJ and
Melief CJ: Dendritic cell immunotherapy: Mapping the way. Nat Med.
10:475–480. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohnishi M, Sakurai T, Heike Y, Yamazaki R,
Kanda Y, Takaue Y, Mizoguchi H and Kawakami Y: Evaluation of
cytomegalovirus-specific T-cell reconstitution in patients after
various allogeneic haematopoietic stem cell transplantation using
interferon-gamma-enzyme-linked immunospot and human leucocyte
antigen tetramer assays with an immunodominant T-cell epitope. Br J
Haematol. 131:472–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuwana M, Okazaki Y, Kaburaki J and Ikeda
Y: Detection of circulating B cells secreting platelet-specific
autoantibody is useful in the diagnosis of autoimmune
thrombocytopenia. Am J Med. 114:322–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Song N, Liu Y, Liu Y, Li J, Ding J
and Tong Z: Efficient induction of anti-tumor immune response in
esophageal squamous cell carcinoma via dendritic cells expressing
MAGE-A3 and CALR antigens. Cell Immunol. 295:77–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Narita M, Kanda T, Abe T, Uchiyama T,
Iwafuchi M, Zheng Z, Liu A, Kaifu T, Kosugi S, Minagawa M, et al:
Immune responses in patients with esophageal cancer treated with
SART1 peptide-pulsed dendritic cell vaccine. Int J Oncol.
46:1699–1709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forghanifard MM, Gholamin M, Moaven O,
Farshchian M, Ghahraman M, Aledavood A and Abbaszadegan MR:
Neoantigen in esophageal squamous cell carcinoma for dendritic
cell-based cancer vaccine development. Med Oncol. 31:1912014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujiwara S, Wada H, Miyata H, Kawada J,
Kawabata R, Nishikawa H, Gnjatic S, Sedrak C, Sato E, Nakamura Y,
et al: Clinical trial of the intratumoral administration of labeled
DC combined with systemic chemotherapy for esophageal cancer. J
Immunother. 35:513–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koido S, Homma S, Okamoto M, Takakura K,
Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et
al: Treatment with chemotherapy and dendritic cells pulsed with
multiple Wilms' tumor 1 (WT1)-specific MHC class I/II-restricted
epitopes for pancreatic cancer. Clin Cancer Res. 20:4228–4239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JH, Lee Y, Lee M, Heo MK, Song JS, Kim
KH, Lee H, Yi NJ, Lee KW, Suh KS, et al: A phase I/IIa study of
adjuvant immunotherapy with tumour antigen-pulsed dendritic cells
in patients with hepatocellular carcinoma. Br J Cancer.
113:1666–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|