Introduction
Adult T cell leukemia/lymphoma (ATL) is a
hematological malignancy derived primarily from cluster of
differentiation (CD)4+/CD25+/C-C Motif
Chemokine Receptor 4 (CCR4)+ T cells (1). Combination cytotoxic chemotherapy
results in 3-year overall survival (OS) rates of ~24% in medically
fit patients with ATL (2). Allogeneic
stem cell transplantation (allo-SCT) produces long-term remission,
but transplantation-associated toxicity remains a significant
concern (3). In addition, allo-SCT
confers limited benefits in elderly patients with ATL and those
patients with ATL and progressive disease status following
inductive chemotherapy (4). Recently,
treatment with an anti-CCR4 antibody (mogamulizumab) demonstrated
promising results for patients with relapsed/refractory ATL
(5); however, studies of long-term
outcomes and side effect profiles are required for an accurate
assessment of its efficacy and safety.
Human T lymphotropic virus (HTLV)-1 is a known
causative agent of ATL; thus, antiviral therapy using interferon α
(IFNα) and zidovudine [azidothymidine (AZT)] has been proposed as
an alternative therapy for ATL (6).
Despite the clinical efficacy of IFNα/AZT, the exact mechanism of
IFNα/AZT against ATL is currently unknown (7). Recently, combination therapy using
arsenic trioxide (ATO) and IFNα/AZT exhibited promising outcomes in
patients with chronic and untreated ATL (8). Furthermore, Dassouki et al
(9) recently reported that the
anti-ATL effect of ATO/IFNα is triggered by Tax, an HTLV-1-derived
oncoprotein that is degraded through the small ubiquitin-related
modifier 1 (SUMO)/protein PML (PML)/E3 ubiquitin-protein ligase
RNF4 (RNF-4) signaling pathway. However, Tax expression at the
transcript and protein levels is often undetectable at the leukemic
stage (10). In addition, certain ATL
cells have Tax mutations (10).
In the present study, the actions of ATO/IFNα/AZT in
ATL cell lines and primary ATL cells were assessed. It was
identified that IFNα/AZT induces apoptotic cell death via caspase
activation in the S1T cell line, a non-Tax-expressing ATL
patient-derived cell line, as well as in primary ATL cells obtained
from a patient with ATL. Combining ATO with IFNα/AZT produced a
synergistic anti-ATL effect. To the best of our knowledge, this is
the first in vitro evidence to demonstrate the cell
growth-inhibition effect of IFNα/AZT in a non-Tax-expressing ATL
patient-derived cell line and primary ATL cells. Although not all
HTLV-1 derived cell lines and primary ATL cells were killed
following treatment with IFNα/AZT, IFNα/AZT was able to produce
anti-ATL effects via pro-apoptotic signaling. These results
demonstrate that IFNα/AZT exhibits anti-ATL effects in
vitro; however, this effect may be limited to a subsection of
population of patients with ATL.
Materials and methods
Clinical samples
The subjects evaluated in this study included four
patients with ATL (two acute-type and two chronic-type; age, 52–66;
2 male and 2 female) who were treated from 2012–2014 at Kagoshima
University Hospital (Kagoshima, Japan). The subjects were examined
by standard serological testing for the presence of HTLV-1, and by
hematological/Southern blotting analysis for the diagnosis of ATL.
The classification of ATL was performed according to the Shimoyama
criteria (11). All subjects provided
written informed consent for participation in this study, their
medical records and a sample of peripheral blood for the isolation
of peripheral blood mononuclear cells (PBMCs). The study protocol
was reviewed and approved by the Medical Ethics Committee of
Kagoshima University Hospital. PBMCs were separated from the
peripheral blood samples by Ficoll/Hypaque (Pharmacia Biotech; GE
Healthcare Life Sciences, Uppsala, Sweden) density gradient
centrifugation.
Cell lines
The S1T cell line was derived from a patient with
acute-type ATL (12). The integration
site of the HTLV-1 provirus in S1T cells was identical to the ATL
leukemic clone found in the patient with ATL, and was confirmed by
inverse polymerase chain reaction (PCR) (13). Tax protein expression in S1T cells is
barely detectable by western blotting, but Tax mRNA expression is
detectable by reverse transcription-quantitative PCR (12). The MT2 cell line is an HTLV-1-infected
T cell line derived from normal human leukocytes transformed by
leukemic T cells obtained from a patient with ATL (14). Tax expression is detectable in MT2
cells by western blotting (15). S1T
cells, MT2 cells, and freshly isolated ATL cells were cultured in
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2
mM L-glutamine, and 10% heat-inactivated fetal calf serum (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 50 U/ml recombinant
human interleukin-2 (IL-2; PeproTech, Inc., Rocky Hill, NJ,
USA).
Reagents
Recombinant IFNα-2b (1,000 U/ml, Intron®
A; Merck KGaA, Darmstadt, Germany), 5 µM AZT (GlaxoSmithKline,
Brentford, UK) and 1 µM ATO (Trisenox®; Nippon Shinyaku
Co., Kyoto, Japan) were used for in vitro experiments,
unless otherwise specified. Caspase inhibitor Z-VAD-FMK was
purchased from Medical and Biological Laboratories Co., Ltd.
(Nagoya, Japan).
Primary antibodies against Poly (ADP-ribose)
polymerase (PARP), cleaved-PARP (#9542; 1:2,000 dilution),
caspase-9 (#9502; 1:2,000 dilution), caspase-8 (#4790; 1:2,000
dilution), cleaved caspase-8 (#9496; 1:2,000 dilution), inhibitor
of κ light polypeptide gene enhancer in B-cells (IκB) kinase β
(IKKβ) (#2370; 1:2,000 dilution), phospho-IKKα/β (#2697; 1:2,000
dilution), inhibitor of nuclear factor of κ light polypeptide gene
enhancer in B-cells inhibitor, α (IκBα) (#4814; 1:2,000 dilution),
phospho-IκBα (#2859; 1:2,000 dilution), nuclear factor κB (NFκB)
(#8242; 1:2,000 dilution), NFκB p65, phospho-NFκB p65 (#3033;
1:2,000 dilution), signal transducer and activator of transcription
1 (STAT1) (#9172; 1:2,000 dilution), phospho-STAT1 (#8826; 1:2,000
dilution), phospho-p53 (#9284; 1:2,000 dilution),
apoptosis-inducing factor (AIF) (#4642; 1:2,000 dilution), and
histone H3 (#4499; 1:2,000 dilution) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Primary antibodies
against p53 (sc-6243; 1:500 dilution) were obtained from Santa Cruz
Biotechnology (Dallas, TX, USA). Antibodies against cytochrome
c (S2207; 1:100 dilution) and cytochrome c oxidase 4
(COX4; S2050; 1:500 dilution) were obtained from Clontech (Mountain
View, CA, USA). Anti-β-actin antibodies (A5441; 1:5,000 dilution)
were obtained from Sigma-Aldrich (Merck KGaA).
Cell viability assay
The effects of ATO/IFNα/AZT on cell viability were
examined using a cell proliferation reagent and cell counting
reagent, according to the manufacturer's instruction (cell count
reagent SF; Nacalai Tesque, Inc., Kyoto, Japan). Briefly, 96-well
plates containing 1×104 cells/well were incubated at
37°C in the absence or presence of ATO/IFNα/AZT for 48 h. WST-8
reagent (Cell counting kit-8; Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA) was added to each well and the cells were
incubated for 4 h at 37°C in the dark, after which the absorbance
at 450 nm (A450) was determined using a Multiskan™ FC
microplate (Thermo Fisher Scientific, Inc.). The viability of the
treated cells was expressed relative to that of the untreated
control cells, which were considered to be 100% viable. RPMI-1640
medium without phenol-red was used for the WST-8 assay.
Analysis of combination effects
To determine the concentrations of the drugs to be
investigated in the combination study, dose-response curves were
generated for ATO and IFNα/AZT alone. Experiments with ATO and
IFNα/AZT in a fixed ratio combination (a total of 1,000 U/ml IFNα
and 5 µM AZT were defined as 1.0) were performed using a WST-8
assay. Possible drug interactions were calculated using an
IC50 plot (16).
Protein extraction and western blot
analysis
Whole-cell extracts were lysed in cell lysis buffer,
according to the manufacture's protocol (Cell Signaling Technology,
Inc.) with or without a protease inhibitor cocktail (Thermo Fisher
Scientific, Inc.) and a Halt Phosphatase Inhibitor Cocktail (Thermo
Fisher Scientific, Inc.). Mitochondrial fractions and nuclear
extracts were obtained using an ApoAlert Cell Fraction kit
(Clontech Laboratories, Inc.) and a Nuclear/Cytosol Fraction kit
(BioVision, Inc., Milpitas, CA, USA), respectively, according to
the manufacturer's protocols. The whole-cell, mitochondrial and
nuclear extracts were used immediately or stored at −80°C. Western
blotting was performed as follows: Cell extracts were subjected to
4–15% SDS-PAGE (Mini-Protean TGX gels; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), electroblotted onto Immun-Blot® PVDF
membranes (Bio-Rad Laboratories, Inc.), and analyzed for
immunoreactivity with the appropriate primary and secondary
antibodies (anti-rabbit IgG, HRP-linked antibody was used for all
the primary antibodies from Cell Signaling Technologies, Inc.; goat
anti-rabbit HRP Conjugate was used for anti-p53 and anti-cytochrome
c; goat anti-mouse IgG-HRP was used for anti-COX4 and anti-beta
actin) using Can Get Signal® Solution (Toyobo Co., Ltd.,
Osaka, Japan). The reaction products were visualized using an ECL
Advance Western Blotting Detection kit (GE Healthcare BioSciences,
Pittsburgh, PA, USA).
NFκB reporter assay
NFκB activity was evaluated using a firefly
luciferase reporter assay (E1910; Promega Corporation, Madison, WI,
USA). Reporter cell lines were established by transducing S1T cells
with a Cignal Lenti NFκB Reporter or a Cignal Lenti TK Renilla
Control (Puro; Qiagen N.V., Venlo, the Netherlands), according to
the manufacturer's protocols. Luciferase assays were performed with
firefly luciferase or Renilla luciferase assay systems (Promega
Corporation) on cell lysates in Renilla luciferase lysis buffer
(Promega Corporation). Relative NFκB activity was calculated as the
ratio of firefly luciferase activity to Renilla luciferase activity
in each of the samples, according to the manufacture's protocol
(Promega Corporation).
Statistical analysis
Data are expressed as the mean ± standard deviation.
For data analysis, two-tailed Student's t-test and the one-way
analysis of variance (ANOVA) were performed using EZR (17). ANOVA was used to determine whether
there are any statistically significant differences between the
means of three or more independent groups Dunnett's method was used
for multiple comparisons with a control group. Bonferroni's
correction was used for an adjustment made to P-values when several
dependent or independent statistical tests were being performed
simultaneously. In all tests, P<0.05 was considered to indicate
a statistically significant difference.
Results
ATO/IFNα/AZT inhibit the viability of
S1T cells by inducing apoptosis
In the first set of experiments, it was examined
whether ATO/IFNα/AZT affected the viability of S1T cells
(patient-derived non-Tax-expressing ATL cells) using WST-8 assays.
IFNα and ATO significantly inhibited the growth of S1T cells in a
dose-dependent manner, with IC50 values of 1,100 U/ml
and 1.68 µM, respectively. Treatment with AZT alone had little
observable effect on the proliferation of S1T cells (P=0.79);
however, the combination of IFNα and AZT was more effective than
IFNα alone (P<0.005). Of the examined treatments, the
combination of ATO, IFNα and AZT most effectively inhibited the
proliferation of S1T cells. Notably, ATO and IFNα/AZT demonstrated
a synergistic effect on S1T cell proliferation (Fig. 1A and B). IFNα/AZT had a marginal
effect on MT2 cells (data not presented), whereas ATO inhibited the
growth of MT2 cells in a dose-dependent manner.
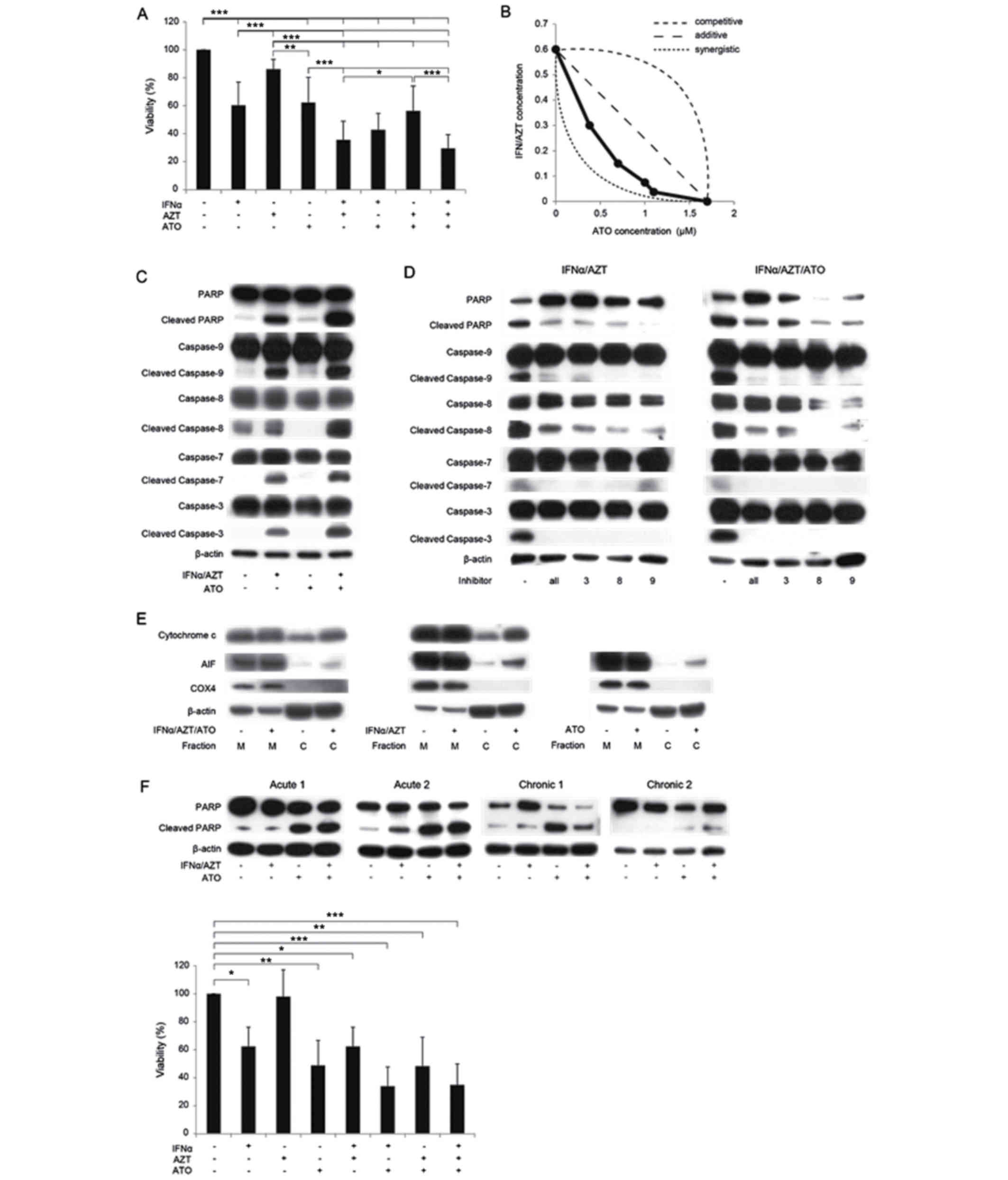 | Figure 1.In vitro inhibition of cell
proliferation by ATO/IFNα/AZT. (A) ATO/IFNα/AZT inhibits the
proliferation of S1T cells as determined by an WST-8 assay. Cells
were treated with 1 µM ATO, 1,000 U/ml IFNα or 5 µM AZT for 48 h.
*P<0.05, **P<0.01, ***P<0.005. (B) Effects of combination
treatment with ATO/IFNα/AZT. ATO/IFNα/AZT exhibited a synergistic
inhibitory effect on proliferation in S1T cells. Cells were treated
with ATO, IFNα or AZT for 48 h. A total of 1,000 U/ml IFNα and 5 µM
AZT were defined as 1.0 on their respective axes in this
experiment. (C) The expression levels of numerous intracellular
regulators of apoptosis were measured by western blotting. Cells
were treated with 1 µM ATO, 1,000 U/ml IFNα or 5 µM AZT for 48 h.
(D) IFNα/AZT-induced apoptosis was reversed by caspase inhibitors
(a pan-caspase inhibitor, a caspase-3 inhibitor, a caspase-8
inhibitor and a caspase-9 inhibitor; 50 µM each). Cells were
treated with 1 µM ATO, 1,000 U/ml IFNα or 5 µM AZT for 48 h. (E)
Increased cytosolic cytochrome c and AIF expression
following ATO/IFNα/AZT treatment. Cells were treated with 1 µM ATO,
1,000 U/ml IFNα or 5 µM AZT for 48 h. (F) The in vitro
anti-ATL effect of ATO/IFNα/AZT in primary ATL cells. Cells were
treated with 1 µM ATO, 1,000 U/ml IFNα or 5 µM AZT for 48 h. In ATL
cells derived from one patient (acute 2), IFNα/AZT treatment
increased cleaved PARP expression levels. IFNα/AZT inhibited the
proliferation of primary ATL cells from patient acute 2, as
determined by a WST-8 assay. *P<0.05, **P<0.01,
***P<0.005. M, mitochondrial fraction; C, cytosolic fraction;
ATO, arsenic trioxide; IFNα, interferon α; AZT,
zidovudine/azidothymidine; ATL adult T cell leukemia/lymphoma; AIF,
apoptosis inducing factor; PARP, poly (ADP-ribose) polymerase
1. |
To clarify the molecular mechanisms underlying the
ATO/IFNα/AZT-induced inhibition of S1T cell proliferation, the
expression levels of various intracellular regulators of apoptosis
were examined using western blotting. IFNα/AZT treatment increased
the expression levels of cleaved PARP and cleaved caspase-3,
caspase-7, caspase-8 and caspase-9, indicating that these cysteine
proteases are involved in the regulation of apoptosis in S1T cells
(Fig. 1C). The effect of IFNα/AZT
treatment on PARP and caspase cleavage was attenuated by inhibitors
of caspase-3, caspase-8 and caspase-9, as well as by a pan-caspase
inhibitor (Fig. 1D). Treatment with
ATO alone had a less marked effect on cleaved PARP expression than
treatment with IFNα/AZT, indicating that the inhibition of S1T cell
proliferation by ATO is not primarily due to pro-apoptotic
signaling.
To further dissect the mechanism of ATO/IFNα/AZT
induced apoptosis in S1T cells, the mitochondrial apoptosis
signaling pathway was examined. In this process, mitochondrial
protein AIF is released as a result of mitochondrial outer membrane
permeabilization, which leads to the release of pro-apoptotic
proteins from the mitochondrial intermembrane space and the
promotion of caspase-independent cell death (18). Cytosolic cytochrome c and
cytosolic AIF expression levels were markedly increased following
treatment with ATO/IFNα/AZT (Fig.
1E). The potential of ATO/IFNα/AZT to treat ATL was then
analyzed in vitro using cells from two patients with
acute-type ATL and two patients with chronic-type ATL. Increased
levels of cleaved PARP following treatment with IFNα/AZT were
observed only in ATL cells from a single patient (acute, patient 2;
Fig. 1F); the WST assay revealed the
anti-ATL effect of IFNα/AZT on cells obtained from this patient
(P<0.05, Dunnett contrast over untreated group). The ATL cells
collected from the other patients in the current study were only
identified to be sensitive to ATO.
Mediation of the anti-ATL effect of
ATO/IFNα/AZT by the NFκB pathway
The HTLV-1 derived protein Tax is a powerful
activator of the NFκB signaling pathway. ATO is recognized to
promote Tax degradation (19);
however, little is known about the effect of IFNα/AZT on
non-Tax-expressing cells such as the S1T line. In the present
experiment, IFNα/AZT treatment decreased phospho-IKK and
phospho-IκBα levels in the cytoplasm in the S1T cells (Fig. 2A). Concomitantly, nuclear NFκB protein
levels were decreased, indicating that the translocation of NFκB
from the cytoplasm to the nucleus was inhibited under the
conditions of IFNα/AZT-induced cell death. Regulation of nuclear
NFκB, phospho-IKK and phospho-IκBα expression was not observed when
MT2 cells were treated with IFNα/AZT. Furthermore, IFNα/AZT
decreased NFκB activity in S1T cells, as determined by a luciferase
reporter assay (P<0.001; Fig.
2B).
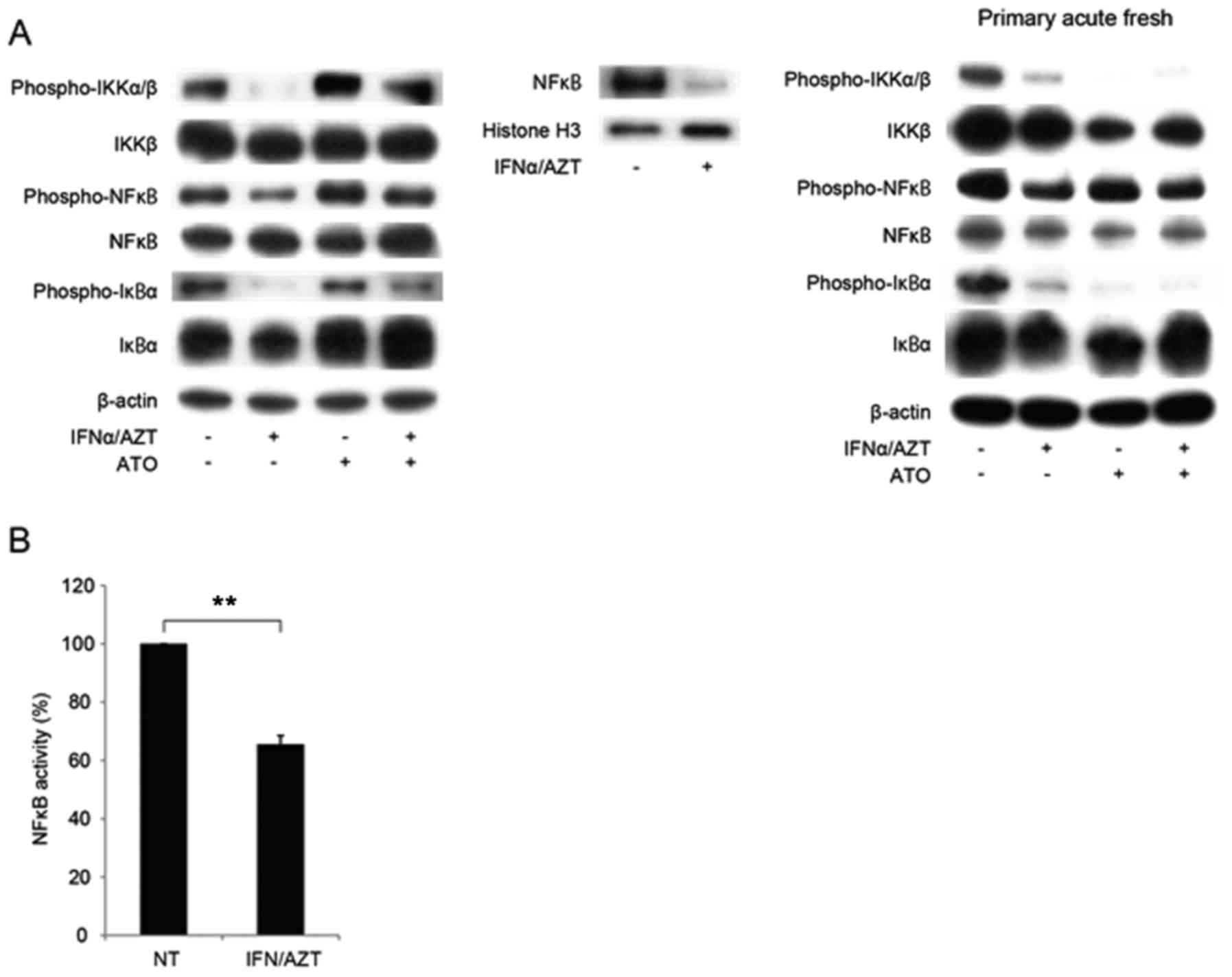 | Figure 2.IFNα/AZT induced cell death via the
NFκB signaling pathway. (A) Translocation of NFκB into the nucleus
was inhibited by IFNα/AZT through decreased levels of phospho-IKK
and phospho-IκB. Cells were treated with 1 µM ATO, 1,000 U/ml IFNα
or 5 µM AZT for 48 h. (B) The presence of histone H3 indicates the
nuclear fraction. The NFκB promoter assay demonstrated that NFκB
activity was decreased by IFNα/AZT treatment. Cells were treated
with 1 µM ATO, 1,000 U/ml IFNα or 5 µM AZT for 48 h. **P<0.01.
ATO, arsenic trioxide; IFNα, interferon α; AZT,
zidovudine/azidothymidine; NFκB, nuclear factor κB; IκB, inhibitor
of κ light polypeptide gene enhancer in B-cells; IKK, IκB kinase
β. |
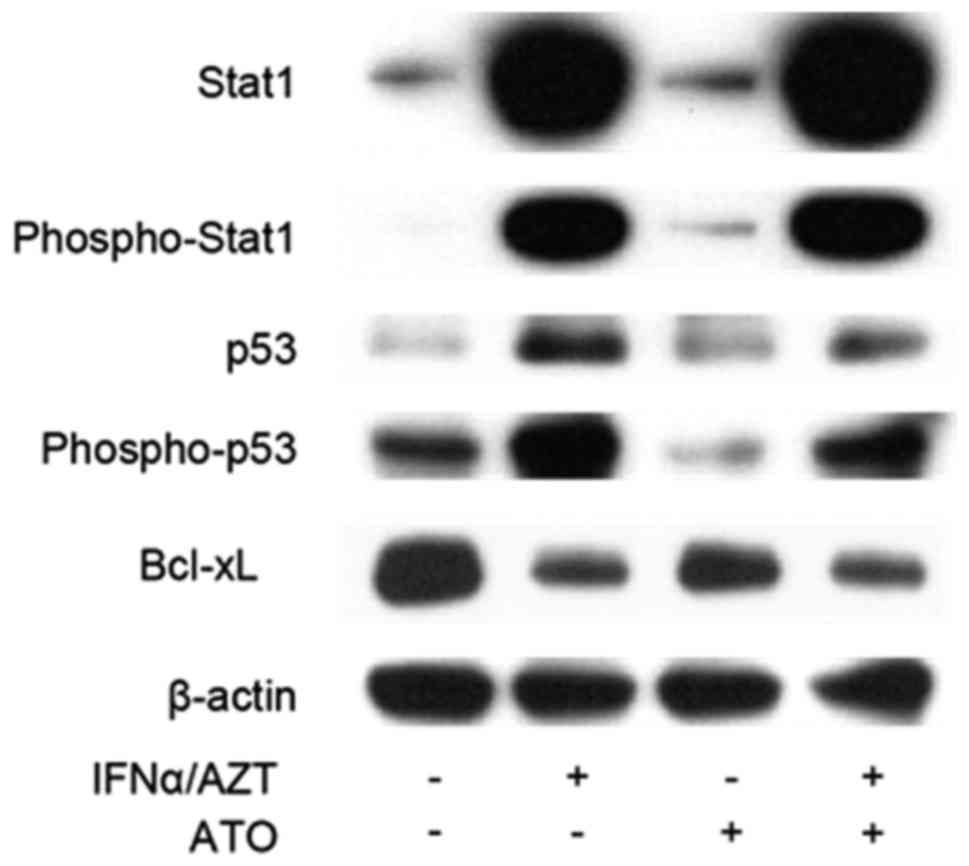
ATO/IFNα/AZT induces STAT1
activation
The STAT family of transcription factors regulates
cell fate and can promote apoptosis via interactions with p53
(20). STAT1 is activated by
phosphorylation, upon which it inhibits the transcription of
anti-apoptotic B-cell lymphoma 2 (Bcl-2) family genes, whereas p53,
when activated by DNA damage, is able to activate downstream
cysteine proteases, specifically caspase-8, which then enters the
mitochondria, triggering cytochrome c release and an apoptotic
signaling cascade via caspase-9 (21). STAT1 acts in conjunction with p53 via
protein-protein interactions (20).
The combined activity of phospho-STAT1 and p53 more efficiently
promotes apoptosis, as compared with the activity of each protein
alone. Ectopic treatment with IFNα activates STAT1 signaling
(22); therefore, STAT1 and
phospho-STAT1 expression was examined in the IFNα treated S1T
cells. Marked induction of STAT1 and phospho-STAT1 expression was
observed in IFNα-treated S1T cells (Fig.
3). Phospho-p53 was also upregulated in IFNα-treated S1T cells,
whereas anti-apoptotic Bcl-extra large (xL) expression was
downregulated, which may have led to apoptosis.
Discussion
In the present study, the anti-ATL effect of
IFN-α/AZT treatment was demonstrated in an ATL patient-derived cell
line and freshly isolated ATL cells via numerous apoptosis
signaling pathways, in addition to the synergistic effect of the
combination of ATO and IFNα/AZT. Bazarbachi et al (7) previously reported that the combination
of AZT and IFNα did not exhibit cytotoxic effects on HuT-102, MT2
or fresh ATL cells, despite inducing complete remission in
vivo in one patient treated with IFN-α/AZT. HuT-102 and MT2
cells constitutively express Tax, and the authors examined only the
anti-ATL effect of AZT/IFNα in PBMCs derived from one patient with
ATL who was treated with 100 U/ml IFNα and 5 µM AZT in the presence
of phytohemagglutinin (PHA) and IL-2. By contrast, in the current
study, an ATL patient-derived Tax-non-expressing ATL cell line
(S1T) and freshly isolated ATL cells from numerous patients, which
were cultured with a 10-fold higher concentration of IFNα in the
absence of PHA, were tested; however, little cytotoxic effect was
observed on MT2 cells. An in vitro anti-ATL effect of
IFNα/AZT was demonstrated for only one patient with ATL; the cells
from the other three patients with ATL were not identified to be
sensitive to IFNα/AZT exposure, similar to the results of previous
studies (7). Kinpara et al
(23) recently reported an in
vitro anti-ATL effect of IFNα/AZT in IL-2-dependent
HTLV-1-infected T cells derived from patients with ATL. In
concordance with the data from the current study, they demonstrated
that IFNα activates the p53 signaling pathway in cooperation with
AZT and the suppression of NFκB activity. Kinpara et al
(23) used IL-2-dependent
HTLV-1-infected T cells and did not specify whether the cells were
ATL clone-derived. IL-2-dependent HTLV-1-infected T cells express
Tax protein that is detectable using flow cytometry, though at a
level markedly lower than that present in HuT102 cells (19). In addition, the authors did not
demonstrate an in vitro anti-ATL effect of IFNα/AZT on
primary ATL cells. The results of the current study demonstrated
the following: The in vitro anti-ATL effects of IFNα/AZT in
a non-Tax-expressing ATL patient-derived cell line and in ATL
patient-derived primary ATL cells; and the apoptosis of ATL cells
that was associated with increased levels of phospho-p53, possibly
due to STAT1 activity directly induced by IFNα stimulation,
decreased expression levels of Bcl-xL and the suppression of NFκB.
The in vitro anti-ATL effect of IFNα was observed to be
enhanced by AZT. The mechanism underlying the synergistic effect of
the combination of AZT and IFNα remains to be elucidated, and the
S1T cell line may be a good model for this purpose.
The synergistic anti-ATL effect of ATO/IFNα has been
extensively examined in vitro (9,19,24–26). ATO
synergizes with IFNα to induce cell cycle arrest and apoptosis,
whereas ATO/IFNα leads to Tax degradation, suggesting that the
apoptosis of HTLV-1-derived cells may reflect targeting of the Tax
oncoprotein (19). In the present
study, a synergistic anti-ATL effect of ATO/IFNα/AZT in a
non-Tax-expressing cell line was demonstrated (Fig. 1B). In addition, the ATO/IFNα/AZT
combination was not synergistic, but was instead competitive in the
Tax-expressing MT2 cell line (data not presented). Primary ATL
cells from three patients with ATL were only sensitive to ATO
treatment in vitro (Fig. 1F),
and were not observed to be sensitive to IFNα/AZT, as was
previously reported (7). Dassouki
et al (9) recently
demonstrated that the ATL response to ATO/IFNα therapy is triggered
by SUMO/PML/RNF4-dependent Tax degradation. In this study, Tax
expression was only detectable at the mRNA level in the MT-1 cell
line, which was sensitive to ATO/IFNα/AZT treatment.
In MT-1 cells, silencing of Tax by short hairpin
(sh)RNA impairs cell survival (9).
This result suggests that even significantly low Tax expression
levels are sufficient to sustain HTLV-1 transformed cell survival.
Kinpara et al (23) also
demonstrated the anti-ATL effect of IFNα on low-Tax-expressing
cells with a notable further reduction in Tax expression. As
significantly low levels of Tax mRNA were identified in S1T cells
in the current study, we cannot exclude the possibility that
IFNα/AZT induces the degradation of Tax in S1T cells. The silencing
of Tax in S1T cells by Tax shRNA may facilitate the elucidation of
this mechanism. Takeda et al (10) reported that Tax mRNA could be detected
in only 14/41 freshly isolated ATL cases (34%) using RT-PCR. In
addition, Tax is significantly upregulated following overnight cell
culture, even without any stimulation. Even with this induction of
Tax expression, the in vitro treatment of cells with
IFNα/AZT did not previously demonstrate an anti-ATL effect, and
thus further studies were warranted in order to elucidate the
mechanism underlying cell sensitivity to IFNα/AZT in vitro.
Notably, S1T cells were observed to be sensitive to ATO/IFNα/AZT,
possibly due to a mechanism independent of Tax degradation.
Therefore, the Tax-independent mechanism underlying the anti-ATL
effect of ATO must also be clarified. These findings provide a
foundation for further studies aimed at revealing the underlying
molecular mechanisms of IFNα/AZT, and identifying the subgroup of
patients with ATL for whom this treatment could be effective.
Acknowledgements
The authors thank Ms. Aya Hamada, Division of
Hematology and Immunology, Center for Chronic Viral Diseases,
Graduate School of Medical and Dental Sciences, Kagoshima
University (Kagoshima, Japan) for technical assistance.
Funding
This study was supported in part by a Grant-in-Aid
for Scientific Research (grant no. JP25461427) from the Japan
Society for the Promotion of Science.
Availability of data and materials
All the datasets generated during the present study
are included in this manuscript.
Authors' contributions
MH and MY designed and performed the experiments,
analyzed the data and wrote the manuscript. CE and AK performed the
experiments; TK designed and performed the experiments. NA designed
and supervised the project and wrote the manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Medical Ethics Committee of Kagoshima University Hospital
(Kagoshima, Japan). All subjects provided written informed consent
for participation in the present study.
Consent for publication
Study participants provided their consent for the
publication of this data and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ishitsuka K and Tamura K: Human T-cell
leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet
Oncol. 15:e517–e526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsukasaki K, Utsunomiya A, Fukuda H,
Shibata T, Fukushima T, Takatsuka Y, Ikeda S, Masuda M, Nagoshi H,
Ueda R, et al: VCAP-AMP-VECP compared with biweekly CHOP for adult
T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study
JCOG9801. J Clin Oncol. 25:5458–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hishizawa M, Kanda J, Utsunomiya A,
Taniguchi S, Eto T, Moriuchi Y, Tanosaki R, Kawano F, Miyazaki Y,
Masuda M, et al: Transplantation of allogeneic hematopoietic stem
cells for adult T-cell leukemia: A nationwide retrospective study.
Blood. 116:1369–1376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shigematsu A, Kobayashi N, Yasui H, Shindo
M, Kakinoki Y, Koda K, Iyama S, Kuroda H, Tsutsumi Y, Imamura M and
Teshima T: High level of serum soluble interleukin-2 receptor at
transplantation predicts poor outcome of allogeneic stem cell
transplantation for adult T cell leukemia. Biol Blood Marrow
Transpl. 20:801–805. 2014. View Article : Google Scholar
|
|
5
|
Ishida T, Joh T, Uike N, Yamamoto K,
Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S,
Suzushima H, Tsukasaki K, et al: Defucosylated anti-CCR4 monoclonal
antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A
multicenter phase II study. J Clin Oncol. 30:837–842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bazarbachi A, Plumelle Y, Carlos Ramos J,
Tortevoye P, Otrock Z, Taylor G, Gessain A, Harrington W, Panelatti
G and Hermine O: Meta-analysis on the use of zidovudine and
interferon-alfa in adult T-cell leukemia/lymphoma showing improved
survival in the leukemic subtypes. J Clin Oncol. 28:4177–4183.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bazarbachi A, Nasr R, El-Sabban ME, Mahé
A, Mahieux R, Gessain A, Darwiche N, Dbaibo G, Kersual J, Zermati
Y, et al: Evidence against a direct cytotoxic effect of alpha
interferon and zidovudine in HTLV-I associated adult T cell
leukemia/lymphoma. Leukemia. 14:716–721. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kchour G, Tarhini M, Kooshyar MM, El Hajj
H, Wattel E, Mahmoudi M, Hatoum H, Rahimi H, Maleki M, Rafatpanah
H, et al: Phase 2 study of the efficacy and safety of the
combination of arsenic trioxide, interferon alpha, and zidovudine
in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL).
Blood. 113:6528–6532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dassouki Z, Sahin U, El Hajj H, Jollivet
F, Kfoury Y, Lallemand-Breitenbach V, Hermine O, de Thé H and
Bazarbachi A: ATL response to arsenic/interferon therapy is
triggered by SUMO/PML/RNF4-dependent Tax degradation. Blood.
125:474–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeda S, Maeda M, Morikawa S, Taniguchi
Y, Yasunaga J, Nosaka K, Tanaka Y and Matsuoka M: Genetic and
epigenetic inactivation of tax gene in adult T-cell leukemia cells.
Int J Cancer. 109:559–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimoyama M: Diagnostic criteria and
classification of clinical subtypes of adult T-cell
leukaemia-lymphoma. A report from the Lymphoma Study Group
(1984–87). Br J Haematol. 79:428–437. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arima N, Molitor JA, Smith MR, Kim JH,
Daitoku Y and Greene WC: Human T-cell leukemia virus type I Tax
induces expression of the Rel-related family of kappa B
enhancer-binding proteins: Evidence for a pretranslational
component of regulation. J Virol. 65:6892–6899. 1991.PubMed/NCBI
|
|
13
|
Takemoto S, Matsuoka M, Yamaguchi K and
Takatsuki K: A novel diagnostic method of adult T-cell leukemia:
Monoclonal integration of human T-cell lymphotropic virus type I
provirus DNA detected by inverse polymerase chain reaction. Blood.
84:3080–3085. 1994.PubMed/NCBI
|
|
14
|
Yoshida M, Miyoshi I and Hinuma Y: A
retrovirus from human leukemia cell lines: Its isolation,
characterization, and implication in human adult T-cell leukemia
(ATL). Princess Takamatsu Symp. 12:285–294. 1982.PubMed/NCBI
|
|
15
|
Lee B, Tanaka Y and Tozawa H: Monoclonal
antibody defining tax protein of human T-cell leukemia virus
type-I. Tohoku J Exp Med. 157:1–11. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szumiel I and Nias AH: Isobologram
analysis of the combined effects of anti-tumour platinum complexes
and ionizing radiation on mammalian cells. Br J Cancer. 42:292–296.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transpl. 48:452–458. 2013. View Article : Google Scholar
|
|
18
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Sabban ME, Nasr R, Dbaibo G, Hermine O,
Abboushi N, Quignon F, Ameisen JC, Bex F, de Thé H and Bazarbachi
A: Arsenic-interferon-alpha-triggered apoptosis in HTLV-I
transformed cells is associated with tax down-regulation and
reversal of NF-kappa B activation. Blood. 96:2849–2855.
2000.PubMed/NCBI
|
|
20
|
Townsend PA, Scarabelli TM, Davidson SM,
Knight RA, Latchman DS and Stephanou A: STAT-1 interacts with p53
to enhance DNA damage-induced apoptosis. J Biol Chem.
279:5811–5820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin J, Tang H, Jin X, Jia G and Hsieh JT:
p53 regulates Stat3 phosphorylation and DNA binding activity in
human prostate cancer cells expressing constitutively active Stat3.
Oncogene. 21:3082–3088. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramana CV, Chatterjee-Kishore M, Nguyen H
and Stark GR: Complex roles of Stat1 in regulating gene expression.
Oncogene. 19:2619–2627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinpara S, Kijiyama M, Takamori A,
Hasegawa A, Sasada A, Masuda T, Tanaka Y, Utsunomiya A and Kannagi
M: Interferon-alpha (IFN-alpha) suppresses HTLV-1 gene expression
and cell cycling, while IFN-alpha combined with zidovudine induces
p53 signaling and apoptosis in HTLV-1-infected cells.
Retrovirology. 10:522013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bazarbachi A, El-Sabban ME, Nasr R,
Quignon F, Awaraji C, Kersual J, Dianoux L, Zermati Y, Haidar JH,
Hermine O and de Thé H: Arsenic trioxide and interferon-alpha
synergize to induce cell cycle arrest and apoptosis in human T-cell
lymphotropic virus type I-transformed cells. Blood. 93:278–283.
1999.PubMed/NCBI
|
|
25
|
Nasr R, Rosenwald A, El-Sabban ME, Arnulf
B, Zalloua P, Lepelletier Y, Bex F, Hermine O, Staudt L, de Thé H
and Bazarbachi A: Arsenic/interferon specifically reverses 2
distinct gene networks critical for the survival of HTLV-1-infected
leukemic cells. Blood. 101:4576–4582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown M, Bellon M and Nicot C: Emodin and
DHA potently increase arsenic trioxide interferon-alpha-induced
cell death of HTLV-I-transformed cells by generation of reactive
oxygen species and inhibition of Akt and AP-1. Blood.
109:1653–1659. 2007. View Article : Google Scholar : PubMed/NCBI
|