Introduction
Colorectal cancer refers to the cancer from the
dentate line to the rectosigmoid junction, which is one of the most
common malignant tumors of the digestive tract (1). With the fast-paced development of
society and the increase in life pressure, the incidence rate of
colorectal cancer has been increased year by year. Colorectal
cancer often occurs in patients aged above 46 years old, whose
incidence rate in young people has shown an increasing trend in
recent years (2,3). Colorectal cancer in early stage has no
obvious symptoms, so most of patients have been in the advanced
stage diagnosed with the survival rate below 28% because they do
not pay much attention to it in early stage (4).
At present, serum carcino-embryonic antigen (CEA),
carbohydrate antigen (CA) 19-9 and cyclooxygenase-2 (COX-2) are the
most commonly-used indexes in the clinical diagnosis of colorectal
cancer, but they are all non-specific antigens. The accuracy and
sensitivity of diagnosis based on a single indicator are usually
unsatisfactory. Therefore, combined detection is usually in
clinical studies to improve the diagnostic accuracy of the tumor.
Handy (5) reported that the
combination of CEA, CA199 and COX-2 can significantly improve the
accuracy of the diagnosis of gastric cancer with high accuracy.
Therefore, we assumethat the combination of CEA, CA199 and COX-2
may can also increase the diagnostic sensitivity and specificity
for colorectal cancer, which has not been reported by previous
studied. Therefore, our study aimed to investigate the diagnostic
value of the combination of CEA, CA199 and COX-2 for colorectal
cancer.
Materials and methods
Objects of study
A total of 50 patients with colorectal cancer
admitted to our hospital from August 2013 to August 2016 were
selected serve as cancer group. Those patients included 32 males
and 18 females, with an average of 52.8±1.8 years. According to the
guideline of staging of colorectal cancer in the United States in
2010, there were 12 cases in stage I, 15 cases in stage II, 13
cases in stage III and 10 cases in stage IV. As the same this 50
healthy people were also selected to serve as control group.
Control group included 31 males and 19 females, with an average of
51.3±2.7 years.
Inclusion and exclusion criteria
Inclusion criteria: Patients aged from 40 to 60
years; patients with colorectal cancer related pathological
conditions confirmed by pathological examination; patients received
no surgical operations, chemotherapy, hormones and other treatment
before admission; patients with complete clinical data. Exclusion
criteria: patients with other vital organs disease; patients with
inflammation; patients with a history of other types of tumors;
pregnant women; patients with autoimmune diseases; dipsopathy and
crapulent patients. The present study was approved by the Ethics
Committee of the Second People's Hospital of Shenzhen (Shenzhen,
China). All patients signed written informed consent.
Methods
A total of 2 ml fasting venous blood was drawn from
patients in each group using the pro-coagulation tube, placed at
room temperature for 1 h and centrifuged at 3,000 × g for 5 min
using a centrifugal machine. The supernatant was taken and divided
into two pieces. Serum CEA and CA19-9 in one piece were detected
and analysed using the full-automatic chemiluminiscence immunoassay
analyzer (Shanghai Honglian Medical Tech Co., Ltd., Shanghai,
China) and its supporting reagents; CEA >5 U/ml and CA19-9
>37 U/ml indicted the positive results. COX-2 in the other piece
was detected via enzyme-linked immunosorbent assay (ELISA) using
the ELISA kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and its supporting reagents. Patients with positive CEA, CA19-9 and
COX-2 were diagnosed as positive. According to Ng et al
(6), the cut-off level of COX-2 was
set as 52.00 ng/ml.
Statistical analyses
SPSS 22.0 software (IBM Corp., Armonk, NY USA) was
used to analyze the data. Count data were expressed as rate.
Measurement data were expressed as the mean ± standard deviation,
t-test was used to compare the data between groups, and analysis of
variance with a Student-Newman-Keuls post hoc text was used for
comparisons among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical data of patients
There was no significant difference (P>0.05)
between patients with colorectal cancer and healthy physical
examination patients in sex, age, smoking habit, alcoholism, sleep,
exercise, taste preference, residence and ethnic composition
(Table I).
 | Table I.Clinical data of patients with
colorectal cancer and those with benign lesions. |
Table I.
Clinical data of patients with
colorectal cancer and those with benign lesions.
| Characteristics | Patients with
colorectal cancer [n (%)] | Patient with benign
lesion [n (%)] | P-value |
|---|
| Sex |
|
| 0.446 |
| Male | 32 (64.0) | 31 (62.0) |
|
|
Female | 18 (36.0) | 19 (38.0) |
|
| Age, years |
|
| 0.328 |
|
<40 | 29 (58.0) | 30 (60.0) |
|
| ≥40 | 21 (42.0) | 20 (40.0) |
|
| Tumor size, mm |
|
| 0.201 |
|
<8 | 26 (52.0) | 17 (34.0) |
|
| ≥8 | 24 (48.0) | 33 (66.0) |
|
| Smoking |
|
| 0.285 |
| Yes | 28 (56.0) | 39 (78.0) |
|
| No | 16 (44.0) | 11 (22.0) |
|
| Drinking |
|
| 0.364 |
| Yes | 21 (42.0) | 17 (34.0) |
|
| No | 29 (58.0) | 33 (66.0) |
|
| Sleep |
|
| 0.276 |
|
Early | 26 (52.0) | 30 (60.0) |
|
| Late | 24 (48.0) | 20 (40.0) |
|
| Exercise |
|
| 0.288 |
| Yes | 22 (44.0) | 32 (64.0) |
|
| No | 28 (56.0) | 18 (36.0) |
|
| Taste preference |
|
| 0.316 |
|
Light | 18 (36.0) | 21 (42.0) |
|
|
Greasy | 32 (64.0) | 29 (58.0) |
|
| TNM staging |
|
| 0.168 |
| Stages I
and II | 39 (78.0) | 48 (96.0) |
|
| Stages
III and IV | 11 (22.0) | 2 (4.0) |
|
| Pathological
staging |
|
| 0.207 |
| Stages I
and II | 36 (0.72) | 50 (100.0) |
|
| Stages
III and IV | 14 (0.28) | 0 |
|
Expression levels of serum CEA, CA19-9
and COX-2
Levels of CEA, CA199 and COX-2 in cancer patients
were 36.44±12.26 (ng/ml), 51.73±21.81 (U/ml) and 47.06±11.06
(ng/ml), respectively. Levels of CEA, CA199 and COX-2 in healthy
controls were 2.13±0.76 (ng/ml), 12.91±8.03 (U/ml) and 7.87±5.19
(ng/ml), respectively. Significant differences were found between
two groups (P<0.05). Levels of CEA, CA199 and COX-2 were
increased with increased pathological stages. Significant
differences were found among stage I, II and III. No significant
differences were found between stage III and IV (Tables II and III).
 | Table II.Comparisons of expression levels of
serum CEA, CA19-9 and COX-2. |
Table II.
Comparisons of expression levels of
serum CEA, CA19-9 and COX-2.
| Group | Case (n) | CEA (ng/ml) | CA19-9 (U/ml) | COX-2 (ng/ml) |
|---|
| Group A | 50 |
36.44±12.26a |
51.73±21.81a |
47.06±11.06a |
| Control group | 50 | 2.13±0.76 | 12.91±8.03 | 7.87±5.19 |
| P-value | – | 0.019 | 0.032 | 0.012 |
 | Table III.Positive rates of serum CEA, CA19-9
and COX-2. |
Table III.
Positive rates of serum CEA, CA19-9
and COX-2.
| Group | Cases (n) | CEA [n (%)] | CA19-9 [n (%)] | COX-2 [n (%)] | Combined detection [n
(%)] |
|---|
| Group A | 50 | 28
(56.0)a, b | 32
(64.0)a, b | 21
(62.0)a, b | 44
(88.0)c |
| Group B | 50 | 5 (10.0) | 4 (8.0) | 3 (6.0) | 6 (12.0) |
| Control group | 50 | 2 (4.0) | 2 (4.0) | 0 (0.0) | 3 (6.0) |
| P-value | – | 0.023 | 0.012 | 0.028 | 0.036 |
Positive rates of serum CEA, CA19-9
and COX-2
The number of positive patients in serum CEA, CA199,
COX-2 and combined detection were 28, 32, 21 and 44, respectively.
Diagnostic coincidence rates were 56.0, 64.0, 62.0 and 88.0%,
respectively. In the healthy group, positive patients in serum CEA,
CA199, COX-2 and combined detection were 2, 2, 0 and 3,
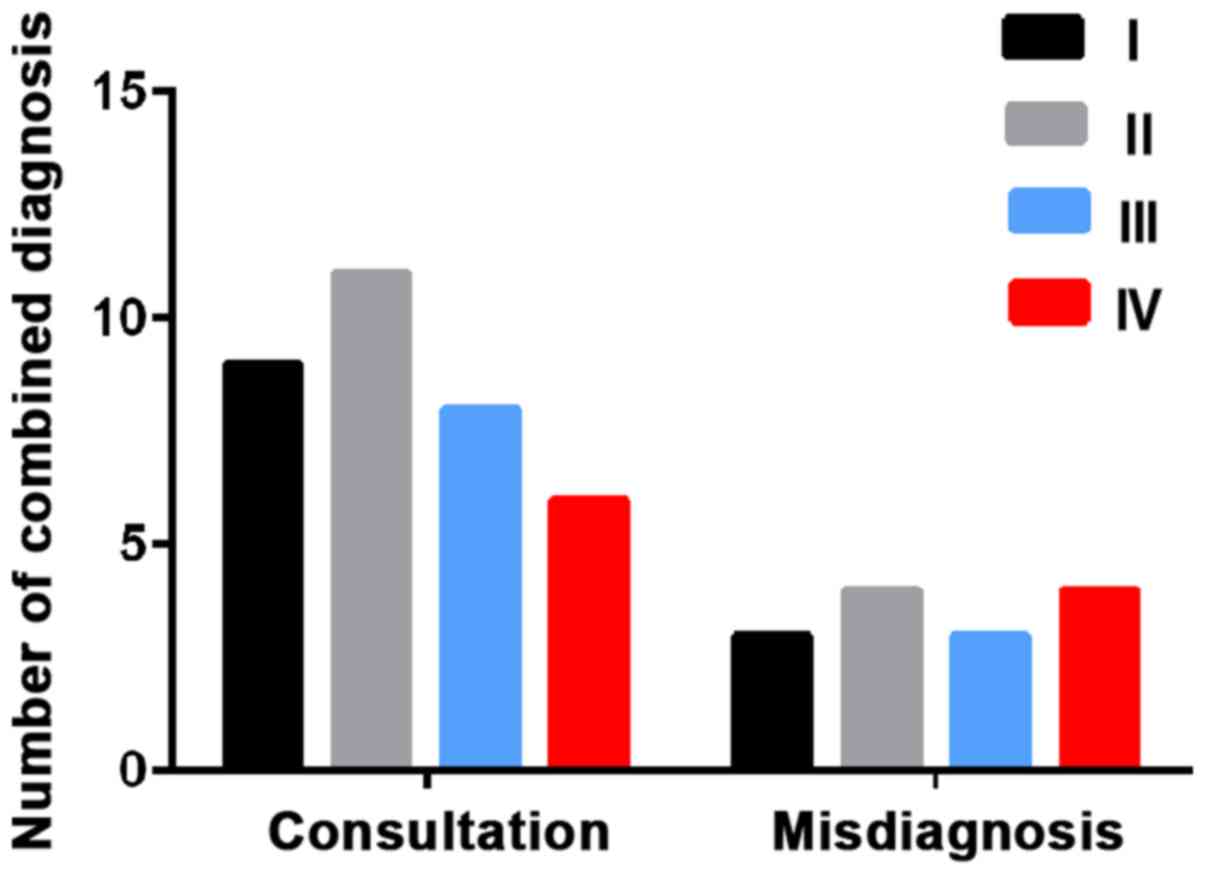
respectively. Combined detection identified 9 patients in stage I,
11 in stage II, 8 in stage III and 6 in stage IV. The diagnostic
coincidence rates were 75.0, 73.3, 72.7 and 60.0%, respectively
(Table IV and Fig. 1).
 | Table IV.Serum levels of CEA, CA19-9 and COX-2
in different pathological stages of tumor patients. |
Table IV.
Serum levels of CEA, CA19-9 and COX-2
in different pathological stages of tumor patients.
|
| Case | CEA | CA19-9 | COX-2 | Combined
detection |
|---|
| Group | (n) | [n (%)] | [n (%)] | [n (%)] | [n (%)] |
|---|
| Group A | 50 | 28
(56.0)a, b | 32
(64.0)a, b | 21
(62.0)a, b | 44
(88.0)a |
| Control group | 50 | 2 (4.0) | 2 (4.0) | 0 | 3 (6.0) |
| P-value | – | 0.023 | 0.012 | 0.028 | 0.036 |
Efficiency evaluation of serum CEA,
CA19-9 and COX-2 in diagnosis of colorectal cancer
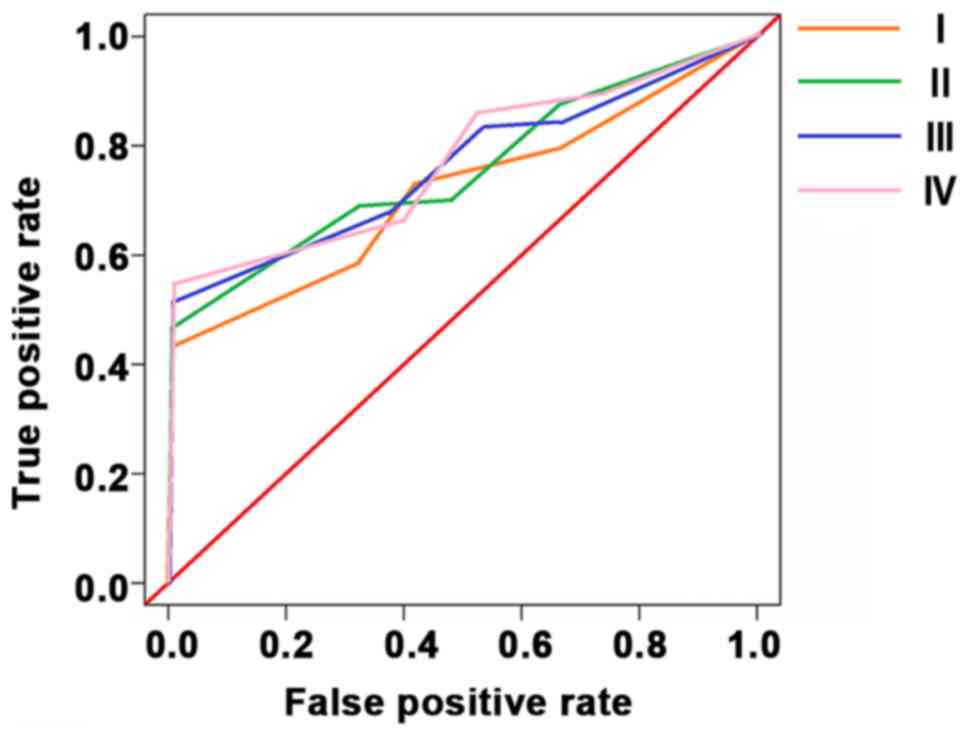
Sensitivities of CEA, CA199 and COX-2 in the
detection of colorectal cancer stage I, II, III and IV were 82.9,
85.3, 86.4 and 88.7%, respectively. The specificities were 65.3,
68.7, 57.8 and 58.6%, respectively. 95% confidence intervals were
0.48–0.93, 0.26–0.89, 1.04–1.77, 0.51–0.98 espectively (Table V and Fig.
2).
 | Table V.Efficiency of serum CEA, CA19-9 and
COX-2 in the diagnosis of rectal cancer. |
Table V.
Efficiency of serum CEA, CA19-9 and
COX-2 in the diagnosis of rectal cancer.
| Factor | Sensitivity
(%) | Specificity
(%) | Accuracy (%) |
|---|
| CEA | 41.8 | 60.7 | 80.3 |
| CA19-9 | 55.6 | 93.5 | 75.8 |
| COX-2 | 43.3 | 91.5 | 77.6 |
| Combined
detection | 90.1 | 89.9 | 92.3 |
| P-value | 0.015 | 0.072 | 0.043 |
Discussion
The incidence rate of colorectal cancer, one of the
most common malignant tumors in the digestive tract, has been
constantly increased in recent years. According to the report of
Siu et al (7), colorectal
cancer will take the place of lung cancer and gastric cancer and
become the malignant tumor with the highest incidence rate in the
world within the next three years. If there are timely detection
and treatment in the early stage of colorectal cancer, no great
damage will be caused to the patients. But the early symptoms are
very unobvious, so they will be ignored easily, and the treatment
will become increasingly more difficult with the proliferation and
metastasis of cancer cells (8). At
present, the colorectal cancer is often diagnosed using the high
expression and abnormality of CEA and other tumor markers combined
with medical imaging techniques (9).
This study aimed to study the value of combined detection of CEA,
CA19-9 and COX-2 in the diagnosis of colorectal cancer, so as to
provide a diagnostic method with higher accuracy and specificity
and simple detection means for the clinical treatment of colorectal
cancer in the future.
This study detected the expression of CEA, CA199 and
COX-2 in patients with colorectal cancer and healthy people, and
the expression levels of CEA, CA199 and COX-2 in patients with
colorectal cancer were significantly higher than those in healthy
people. Compared with diagnosis based on single indicator, the
combined detection significantly improved the accuracy. Compared
with diagnosis based on single indicator, sensitivity and
specificity of combined detection were increased for stage I and II
but reduced for stage III and IV, indicating that CEA, CA199 and
COX-2 can be used for the early diagnosis of colorectal cancer.
In this study, the expression levels and positive
rates of CEA, CA19-9 and COX-2 in patients with colorectal cancer
and benign lesions and healthy people were detected. The clinical
data were compared between patients with colorectal cancer and
benign lesion. The results showed that the patient's gender, age,
tumor size, smoking, drinking, sleep, exercise, taste preference,
TNM staging and pathological staging had no effects on the
detection of three indexes. The expression levels of CEA, CA19-9
and COX-2 in patients with colorectal cancer were significantly
higher than those in the other two groups. The combined detection
had a statistically significant difference compared with single
detection, indicating that the combined detection of CEA, CA19-9
and COX-2 can be clinically applied in the diagnosis of rectal
cancer. The comparisons of sensitivity, specificity and accuracy in
each group showed that there was no obvious difference in the
specificity between combined detection and single detection, but
the combined detection greatly improved the sensitivity and
accuracy, suggesting that the combined detection of CEA, CA19-9 and
COX-2 in the diagnosis of colorectal cancer can compensate for the
shortcomings of single detection and improve the diagnosis
accuracy.
In the early stage of tumor occurrence and
development, the accurate diagnosis via imaging is more difficult,
and the tumor markers are abnormally expressed in the blood in
different degrees, which is an index for the early detection of
tumor occurrence and development (10,11).
However, the abnormality of one single marker cannot provide highly
accurate information about the occurrence of tumor, so the combined
detection of two or more tumor markers is commonly applied in the
clinical diagnosis of the presence or abnormality of tumor
(12). CEA is a kind of cytoplasmic
glycoprotein that is highly expressed in most cancerous tissues, as
well as the most commonly-used tumor marker with a low specificity
(13,14). Therefore, the clinical detection with
CEA as a tumor marker is often combined with other tumor markers,
so as to improve the positive detection rate of cancer (15). CA19-9 is a kind of protein produced by
rectal cells that belongs to the oligosaccharide-associated
antigen, which is highly expressed in pancreatic cancer and
malignant tumors of digestive tract (16,17). COX
is divided into COX-1 structural type and COX-2 induced type. COX-1
is involved in a variety of pathological and physiological
functions, which is expressed stably in most tissues and cells
(18). COX-2 is seldom expressed in
normal tissues and cells, but its expression will be stimulated by
tumor promoters (19). Xiao et
al (20) studies showed that
COX-2 is involved in tumor formation and development through
inhibiting cell death and promoting cell growth. According to the
results of this study, COX-2 was highly expressed in 62.0% patients
with colorectal cancer, and 6.0% patients with benign lesions, but
it was not expressed in healthy subjects. The results indicated
that the high expression of COX-2 occurs in early stage of rectal
cancer, and participates in the development of cancer. According to
the study of Wang et al (21)
on the protein expression of COX-2 in colorectal cancer, combined
with the experimental results, it was found that COX-2 high
expression is significantly correlated with the malignant feature
of rectal cancer, which can be used as a new target for the
diagnosis, treatment and prevention of colorectal cancer in the
future. For colorectal cancer patients in stage III and IV, distant
tumor cells and lymph node metastasis can cause more significant
increase in levels of cancer markers. In this experiment, there was
no significant difference in the expression levels of CEA, CA199
and COX-2 between stage III and stage IV patients, suggesting that
the expression of cancer markers had reached the critical value, so
the increase was not significant, resulting in decreased decreased
and specificity of combined detection for colorectal cancer at
stages III and IV.
There are still some shortcomings in this experiment
due to the limited experimental conditions. For example, sample
size was small, and the expression of CEA, CA199 and COX-2 may be
affected by ages or genders. We will conduct a longer period of
follow-up investigation to further verify the conclusion.
In conclusion, serum CEA, CA199 and COX-2 were
highly expressed in colorectal cancer, and can be used as an
effective indicator for the early diagnosis of colorectal
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WY and QL conceived and designed the study. WY, YoL
and SH were responsible for the collection and analysis of the
patient data. YiL performed ELISA. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second People's Hospital of Shenzhen (Shenzhen,
China). Written informed consent was obtained from all
participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS,
Matsuda T, Ng SS, Lau JY, Zheng S, Adler S, et al: An updated Asia
Pacific Consensus Recommendations on colorectal cancer screening.
Gut. 64:121–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Zheng R, Zhang M, Zhang S, Sun X
and Chen W: Incidence and mortality of colorectal cancer in China,
2011. Chin J Cancer Res. 27:22–28. 2015.PubMed/NCBI
|
|
5
|
Handy B: The clinical utility of tumor
markers. Lab Med. 40:99–104. 2009. View Article : Google Scholar
|
|
6
|
Ng K, Meyerhardt JA, Chan AT, Sato K, Chan
JA, Niedzwiecki D, Saltz LB, Mayer RJ, Benson AB III, Schaefer PL,
et al: Aspirin and COX-2 inhibitor use in patients with stage III
colon cancer. J Natl Cancer Inst. 107:3452014.PubMed/NCBI
|
|
7
|
Siu AL; US Preventive Services Task Force
(USPSTF); Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW,
Ebell M, García FA, Gillman M, Herzstein J, et al: Screening for
colorectal cancer: US preventive services task force recommendation
statement. JAMA. 315:380–387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Xiang J, Li Z, Lu S, Hu J, Gao X,
Yu L, Wang L, Wang J, Wu Y, et al: A plasma microRNA panel for
early detection of colorectal cancer. Int J Cancer. 136:152–161.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castells A: Colorectal cancer screening.
Gastroenterol Hepatol. 38(Suppl 1): S64–S70. 2015. View Article : Google Scholar
|
|
10
|
de Rosa M, Pace U, Rega D, Costabile V,
Duraturo F, Izzo P and Delrio P: Genetics, diagnosis and management
of colorectal cancer (Review). Oncol Rep. 34:1087–1096. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schreuders EH, Ruco A, Rabeneck L, Schoen
RE, Sung JJ, Young GP and Kuipers EJ: Colorectal cancer screening:
A global overview of existing programmes. Gut. 64:1637–1649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bacac M, Fauti T, Sam J, Colombetti S,
Weinzierl T, Ouaret D, Bodmer W, Lehmann S, Hofer T, Hosse RJ, et
al: A novel carcinoembryonic antigen T-cell bispecific antibody
(CEA TCB) for the treatment of solid tumors. Clin Cancer Res.
22:3286–3297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Wang X, He H, Liu Z, Hu JF and Li
W: Combination of circulating tumor cells with serum
carcinoembryonic antigen enhances clinical prediction of non-small
cell lung cancer. PLoS One. 10:e01262762015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang C, Zhan T, Liu Y, Li Q, Wu H, Ji D
and Li Y: Glycomic profiling of carcinoembryonic antigen isolated
from human tumor tissue. Clin Proteomics. 12:172015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu XY and Huang XE: Clinical application
of serum tumor abnormal protein (TAP) in colorectal cancer
patients. Asian Pac J Cancer Prev. 16:3425–3428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J and Huang XE: Clinical application
of serum tumor abnormal protein from patients with gastric cancer.
Asian Pac J Cancer Prev. 16:4041–4044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar V, Al-Abbasi FA, Verma A, Mujeeb M
and Anwar F: Umbelliferone β-D-galactopyranoside exerts an
anti-inflammatory effect by attenuating COX-1 and COX-2. Toxicol
Res. 4:1072–1084. 2015. View Article : Google Scholar
|
|
19
|
Liu B, Qu L and Yan S: Cyclooxygenase-2
promotes tumor growth and suppresses tumor immunity. Cancer Cell
Int. 15:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao Y, Wang J, Qin Y, Xuan Y, Jia Y, Hu
W, Yu W, Dai M, Li Z, Yi C, et al: Ku80 cooperates with CBP to
promote COX-2 expression and tumor growth. Oncotarget. 6:8046–8061.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JY, Sun J, Huang MY, Wang YS, Hou MF,
Sun Y, He H, Krishna N, Chiu SJ, Lin S, et al: STIM1 overexpression
promotes colorectal cancer progression, cell motility and COX-2
expression. Oncogene. 34:4358–4367. 2015. View Article : Google Scholar : PubMed/NCBI
|