Introduction
Pancreatoduodenectomy (PD) is the main surgical
option for pancreatic neoplasms, duodenal neoplasms and other
lesions located in the pancreatic head and periampullary region
(1). Despite the prompt progress in
surgical technologies and the persistent innovation of
postoperative treatments over the last decades,
post-pancreaticoduodenectomy complications (PPC) remain around
30–60% (2–4), which may lead to several potential poor
outcomes, including prolonged hospital stays, increased medical
costs and mortality, all of which also affect pancreatic surgeons
and researchers. Therefore, assessing outcomes and quality of PD
has triggered interest in measuring and evaluating PPCs.
In most previous studies, various definitions and
classifications were applied to access specific PPCs. For example,
the International Study Group of Pancreatic Surgery (ISGPS) have
formulated a series of generally acceptable, objective definitions
and classifications for postoperative pancreatic fistula (POPF),
post-pancreatectomy hemorrhage (PPH), and delayed gastric emptying
(DGE) following PD from 2005 in order to facilitate objective and
accurate comparison among different surgical experiences and
diverse associated studies (5–7). These
classifications have been widely used and favored by numerous
studies (3,8,9). However,
there are a number of limitations to these classifications: i) They
are only focused on specific PPCs, including PPH, POPF and DGE; ii)
each system has complicated and unique assessment criteria for only
one type of PPC and therefore, one type of classification is not
applicable to others; and iii) synergistic efforts and risk factors
cannot assessed among these diverse classifications (10,11).
Owing to a lack of uniform classification, it is
difficult and unfeasible to uniformly interpret various PPCs. In
1992, Pierre Alain Clavien et al (12) established the Clavien-Dindo (C-D)
classification, a simple and feasible grading system for all types
of postoperative complications. The C-D classification system is
characterized by a consistent therapy-oriented, 4-level severity
grading, discriminating overall PPCs, and has been increasingly
applied to evaluate surgical practices (10,13,14).
However, to the best of our knowledge, this grading system has not
been used to evaluate PPCs in a large-sample cohort. Therefore, the
present study applied this system to retrospectively classify all
PPCs and systematically identify associated risk factors in our
high-volume pancreatic center.
Patients and methods
Patients
All patients who underwent PD at West China Hospital
(Sichuan, China) between January 2009 and December 2014 were
included, comprising 660 males (62.5%) and 396 females (37.5%) with
a mean age of 57.29±10.99 (range, 18–88) years. All related data,
including the patient characteristics, histopathology, surgical
factors, postoperative treatments and outcomes, were collected from
our database records and the electronic medical records of
individual patients.
Operative technique
Each surgical team performed either open PD or
laparoscopic PD in >20 cases annually according to the standards
of the National Comprehensive Cancer Network (15). As the standard requirement of PD, the
following organs were removed in the resection stage: Pylorus,
distal antrum of the stomach, duodenum, pancreatic head, distal
common bile duct, gallbladder and part of the jejunum. Vessel
reconstruction was performed in patients who were investigated for
segment or circumferential involvement of the superior
mesenteric/portal vein or short segment extension to the superior
mesenteric artery. Multivisceral resections were performed in
patients who were investigated for adjacent organ invasion,
including colon, liver, small bowel, spleen and kidney. In the
reconstruction stage, pancreatic remnant was uniformly
reconstructed by pancreatojejunostomy over an internal pancreatic
stent or external pancreatic drainage according to the preference
of the surgeon. Either classical Child type or isolated Roux-en-Y
type of reconstruction was selected according to patient features
during surgery.
Perioperative interventions and
treatments
Preoperative evaluation of general condition was
assessed by preoperative routine chest X-ray, electrocardiogram,
laboratory tests and respiratory function tests. All patients
routinely received perioperative antibiotic (Cefoxitin 1 g,
Cefmetazole 1 g or Cefuroxime 1 g; intravenous drip) prophylaxis 1
h prior to surgery and every 3 h during surgery. Routine
biochemical blood tests were measured every 2–3 days after surgery,
or more often in the presence of PPCs. Ultrasonography or computed
tomography scans were performed every 1–2 weeks according to
postoperative features. As POPF is considered to be a common PPC,
pancreatic amylase activity of abdominal drainage secretions was
routinely investigated every 2–3 days from the third postoperative
day (POD). Patients also underwent preventative postoperative
somatostatin injection (Stilamin, Merck Serono Corp.; 12 mg/day) if
they had complication-associated factors, including advanced age,
diabetes and soft pancreatic remnant.
Complications
The inpatient and outpatient medical records for
each included patient were reviewed to identify various PPCs from
the standard recovery, including the following local complications:
POPF, PPH, intra-abdominal infection, incision complication, DGE,
biliary fistula, intestinal obstruction, intestinal fistula,
chylous fistula and acute pancreatitis; and the following systemic
complications: Pulmonary complication, sepsis, cardiac complication
and deep venous thrombosis.
PPH and POPF were defined according to the ISGPS
criterion (4,7). DGE was defined according to the Johns
Hopkins definitions (16). Rare
complications were defined as those with an incidence of <1% of
all the included patients. All complications were defined according
to the Clavien-Dindo classification (12). Mortality was defined as any patients
who succumbed over a 60-day hospital stay, regardless of cause.
These were graded into overall complications (Grade I–V), severe
complications (Grade III–V) and mortality (Grade V) based on the
PPC-related intervention (e.g., medical treatment or an invasive
intervention) or mortality.
Statistical analysis
Table I presents the
descriptive statistics of basic characteristics and surgical
details. Table II presents the
inter-grade differences of each PPPC which were compared with rare
complications using the Ridit test. Table III presents univariate analysis of
risk factors of all C-D classification. Normally distributed
variables are reported as the mean and standard deviation and
compared using Student's t-tests. Non-normally distributed
variables are expressed as the median (range) and were compared by
Mann-Whitney U-tests. Categorical data were compared using
χ2 test, with Yates continuity correction in a two-way
contingency table, or Fisher's exact test. Additionally, Table IV presents multivariate analysis,
which included the potential factors with P≤0.05 in univariate
analysis. And it was analyzed by binary logistic regression with
conditional backward selection of potential factors. The results of
the multivariate logistic regression analysis are expressed using
P-values, odds ratios (ORs) and 95% confidence intervals (CIs). All
statistical analyses were performed using IBM SPSS Version 22 (IBM
Corp., Armonk, NY, USA). P≤0.05 was considered to indicate a
statistically significant difference.
 | Table I.Demographic characteristics, and
pathological and surgical details of all patients. |
Table I.
Demographic characteristics, and
pathological and surgical details of all patients.
| Variable | Value |
|---|
| Total | 1,056 (100) |
| Sex |
|
| Male | 660 (62.5) |
|
Female | 396 (37.5) |
| Age, years | 57.29±10.99 |
| Preoperative
factors |
|
|
Smoking | 380 (35.4) |
|
Diabetes | 223 (21.1) |
| Alcohol
abuse | 167 (15.6) |
| Chronic
pancreatitis | 127 (12.0) |
| Total
bilirubin, mmol/l | 125.00±122.09 |
|
Hemoglobin, g/l | 121.50±20.29 |
| Serum
albumin, g/l | 38.19±5.43 |
| CA19-9,
U/l | 247.65±327.40 |
|
Preoperative biliary
drainage | 122 (11.6) |
| ASA grade |
|
| I | 593 (56.2) |
| II | 318 (30.1) |
| III | 145 (13.7) |
| Histopathology |
|
|
Pancreatic ductal
adenocarcinoma | 347 (32.9) |
|
Periampullary
adenocarcinoma | 522 (49.4) |
| Chronic
pancreatitis | 45 (4.3) |
| Other
pancreatic neoplasms | 91 (8.6) |
|
Other | 51 (4.8) |
| Pancreas texture |
|
| Soft | 548 (51.9) |
| Firm | 508 (48.1) |
| Tumor size, cm | 3.31±1.77 |
| Size of pancreatic
duct, mm |
|
| ≤3 | 347 (32.9) |
|
<3 | 709 (67.1) |
| Intraoperative
transfusions | 1.56±18.51 |
| Intraoperative
transfused patients | 319 (29.7) |
| Total
transfusions | 3.49±22.79 |
| Laparoscopic PD | 40 (3.8) |
| Vessel
reconstruction | 105 (9.9) |
| Multivisceral
resection | 44 (4.2) |
|
Colon | 13 (1.2) |
|
Spleen | 4 (0.4) |
|
Liver | 11 (1.1) |
| Small
bowel | 9 (0.9) |
|
Kidney | 8 (0.8) |
| Postoperative
hospital stay, days | 16.22±10.87 |
 | Table II.Characteristics of Clavien-Dindo
classification of all post-pancreaticoduodenectomy
complications. |
Table II.
Characteristics of Clavien-Dindo
classification of all post-pancreaticoduodenectomy
complications.
|
| Total | Grade I | Grade II | Grade IIIa | Grade IIIb | Grade IVa | Grade IVb | Grade V |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Complications | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | P-value |
|---|
| Total | 475 | 45.0 | 185 | 17.5 | 128 | 12.1 | 50 | 4.7 | 25 | 2.4 | 35 | 3.3 | 19 | 1.8 | 33 | 3.1 |
|
| POPF | 264 | 25.0 | 143 | 54.2 | 46 | 17.4 | 30 | 11.4 | 7 | 2.7 | 17 | 6.4 | 10 | 3.8 | 11 | 4.2 | 0.000 |
| Intra-abdominal
infection | 88 | 8.3 | 6 | 6.8 | 27 | 30.7 | 27 | 30.7 | 3 | 3.4 | 12 | 13.6 | 8 | 9.1 | 5 | 5.7 | 0.000 |
| Incision
complication | 87 | 8.2 | 36 | 41.4 | 13 | 14.9 | 13 | 14.9 | 10 | 11.5 | 10 | 11.5 | 4 | 4.6 | 1 | 1.1 | 0.000 |
| Pulmonary
complication | 93 | 8.8 | 1 | 1.1 | 31 | 33.3 | 10 | 10.8 | 3 | 3.2 | 18 | 19.4 | 14 | 15.1 | 16 | 17.2 | 0.000 |
| PPH | 78 | 7.4 | 0 | 0.0 | 14 | 17.9 | 14 | 17.9 | 12 | 15.4 | 15 | 15.4 | 6 | 7.7 | 17 | 21.8 | 0.000 |
| Delayed gastric
emptying | 30 | 2.8 | 1 | 3.3 | 23 | 76.7 | 2 | 6.7 | 0 | 0.0 | 1 | 3.3 | 2 | 6.7 | 1 | 3.3 | 0.021 |
| Sepsis | 26 | 2.5 | 0 | 0.0 | 4 | 15.4 | 0 | 0.0 | 0 | 0.0 | 5 | 19.2 | 5 | 19.2 | 12 | 46.2 | 0.004 |
| Renal
complication | 22 | 2.1 | 0 | 0.0 | 2 | 9.1 | 0 | 0.0 | 1 | 4.5 | 0 | 0.0 | 3 | 13.6 | 16 | 72.7 | 0.014 |
| Heart
complication | 15 | 1.4 | 2 | 13.3 | 5 | 33.3 | 0 | 0.0 | 0 | 0.0 | 2 | 13.3 | 5 | 33.3 | 1 | 6.7 | 0.317 |
| Biliary
fistula | 9 | 0.9 | 4 | 44.4 | 3 | 33.3 | 2 | 22.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.000 |
| Intestinal
obstruction | 9 | 0.9 | 0 | 0.0 | 2 | 22.2 | 0 | 0.0 | 5 | 55.6 | 1 | 11.1 | 0 | 0.0 | 1 | 11.1 | 0.690 |
| Chylous
fistula | 7 | 0.7 | 4 | 57.1 | 2 | 28.6 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.831 |
| Deep venous
thrombosis | 5 | 0.5 | 0 | 0.0 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 2 | 40.0 | 0.925 |
| Intestinal
fistula | 2 | 0.2 | 0 | 0.0 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.609 |
| Acute
pancreatitis | 2 | 0.2 | 0 | 0.0 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.609 |
 | Table III.Univariate analysis of risk factors
in the Clavien-Dindo classification. |
Table III.
Univariate analysis of risk factors
in the Clavien-Dindo classification.
|
| Overall
complication (Grade I–V) | Severe complication
(Grade III–V) | Mortality (Grade
V) |
|---|
|
|
|
|
|
|---|
| Univariate
analysis | Value | P-value | Value | P-value | Value | P-value |
|---|
| Total | 475 (45.0) |
| 168 (15.9) |
| 33 (3.1) |
|
| Age, years |
| 0.129 |
| 0.035 |
| 0.000 |
|
<75 | 449 (94.5) |
| 155 (92.3) |
| 26 (78.8) |
|
|
≥75 | 26 (5.5) |
| 13 (7.7) |
| 7 (21.2) |
|
| Sex |
| 0.848 |
| 0.139 |
| 0.856 |
|
Male | 295 (62.1) |
| 114 (67.9) |
| 20 (60.6) |
|
|
Female | 180 (37.9) |
| 54 (32.1) |
| 13 (39.4) |
|
| Diabetes | 99 (20.8) | 0.880 | 23 (13.7) | 0.010 | 7 (21.2) | 1.000 |
| Chronic
pancreatitis | 45 (9.5) | 0.022 | 15 (8.9) | 0.197 | 3 (9.1) | 0.598 |
| Total bilirubin,
mmol/l | 123.66±130.03 | 0.102 | 160.29±142.48 | 0.008 | 173.40±119.13 | 0.019 |
| Serum albumin,
g/l | 34.90±5.49 | 0.048 | 34.43±5.32 | 0.034 | 33.06±4.88 | 0.019 |
| Hemoglobin,
g/l | 121.11±21.00 | 0.056 | 120.20±21.13 | 0.001 | 114.64±18.86 | 0.004 |
| CA19-9, U/l | 238.90±327.39 | 0.055 | 302.32±363.10 | 0.097 | 373.28±393.79 | 0.469 |
| Preoperative
biliary drainage | 58 (12.2) | 0.563 | 12 (7.1) | 0.640 | 3 (9.1) | 0.653 |
| Laparoscopic
PD | 26 (5.5) | 0.014 | 8 (6.4) | 0.507 | 1 (3.0) | 0.817 |
| Intraoperative
transfusions | 1.31±3.36 | 0.001 | 1.81±5.10 | 0.001 | 1.76±2.31 | 0.038 |
| Vessel
reconstruction | 43 (9.1) | 0.409 | 14 (8.3) | 0.573 | 4 (12.1) | 0.671 |
| Total multivisceral
resection | 23 (4.8) | 0.355 | 10 (6.0) | 0.208 | 3 (9.1) | 0.150 |
 | Table IV.Multivariate analysis of
characteristics in the Clavien-Dindo classification. |
Table IV.
Multivariate analysis of
characteristics in the Clavien-Dindo classification.
|
|
|
| 95% confidence
interval |
|---|
|
|
|
|
|
|---|
| Characteristic | P-value | Odds ratio | Lower | Upper |
|---|
| Overall
complications (Grade I–V) |
|
|
|
|
| Serum
albumin, g/l | 0.016 | 1.475 | 1.073 | 1.978 |
| Cancer
antigen 19-9, U/l | 0.055 | 0.745 | 0.577 | 1.063 |
|
Hemoglobin, g/l | 0.053 | 0.762 | 0.579 | 1.003 |
|
Intraoperative
transfusions | 0.067 | 0.611 | 0.225 | 1.119 |
| Severe
complications (Grade III–V) |
|
|
|
|
| Serum
albumin, g/l | 0.009 | 1.623 | 1.130 | 2.332 |
| Total
bilirubin, mmol/l | 0.048 | 1.443 | 1.004 | 2.073 |
|
Intraoperative
transfusions | 0.052 | 0.526 | 0.720 | 1.677 |
| Mortality (Grade
V) |
|
|
|
|
| Age ≥75
years | 0.000 | 5.860 | 2.321 | 14.979 |
| Serum
albumin, g/l | 0.034 | 2.191 | 1.063 | 4.516 |
| Total
bilirubin, mmol/l | 0.042 | 2.017 | 1.849 | 4.789 |
Results
Patient characteristics and surgical
details
All basic patient characteristics and surgical
details are summarized in Table I.
Patient preoperative conditions included the following: 380 (35.4%)
smokers, 223 (21.1%) with diabetes, 167 (15.6%) with alcohol abuse
and 127 (12.1%) with chronic pancreatitis. A total of 122 (11.6%)
patients underwent preoperative biliary drainage. According to the
pathological observations, periampullary adenocarcinoma was
identified as the major subtype (522 patients, 49.4%), followed by
pancreatic ductal adenocarcinoma with 32.9% (347 patients).
The majority of patients underwent open PD (1,016
patients, 96.2%) and 40 (3.8%) patients underwent laparoscopic PD.
During surgery, 319 (29.7%) patients were transfused with
1.56±18.52 units of blood on average. There were 105 (9.9%) vessel
reconstructions and 44 (4.2%) multivisceral resections, which
included colon (13 cases, 1.2%), liver (11 cases, 1.0%), small
bowel (9 cases, 0.9%), spleen (4 cases, 0.4%) and kidney (8 cases,
0.8%). The mean postoperative hospital stay of all patients was
16.22±10.87 days. Pancreatic texture, tumor size, size of
pancreatic duct and details of transfusion are presented in
Table I.
Postoperative complications
Of all the included patients, 475 (45.0%) developed
complications. Over time, the incidence of complications gradually
decreased from 47.4% in 2009 to 44.0% in 2014. All postoperative
complications are summarized in Table
II. POPF, a major complication, occurred in 264 (25.0%)
patients. Pulmonary complications, which occurred in 93 (8.8%)
patients, were identified as the major systemic complication. There
were 6 types of complication with rare incidences (<1% of the
included patients), including biliary fistula, intestinal
obstruction, chylous fistula, deep venous thrombosis, intestinal
fistula and acute pancreatitis.
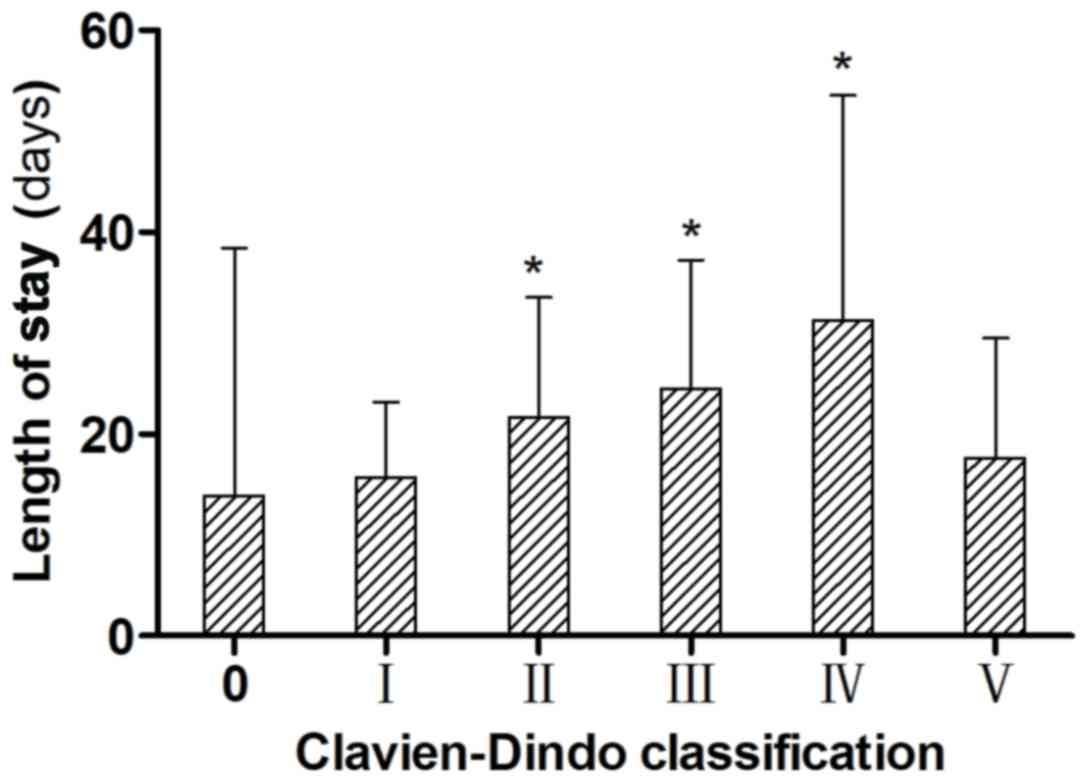
According to the C-D classification, all patients
with complications were identified as having the following grades
of disease: 185 (17.5%), grade I; 128 (12.1%), grade II; 50 (4.7%),
grade IIIa; 25 (2.4%), grade IIIb; 35 (3.3%), grade IVa; 19 (1.8%),
grade IVb; and 33 (3.1%), grade V. Compared with biliary fistula
(incidence, <1% of all the included patients), the C-D
classification identified the differences in severity, and the
differences between disease grades as being significant (P<0.05,
Table II). With regards to disease
grades, patients with grade II or higher disease, excluding grade V
disease, which resulted in patient mortality, had significantly
longer postoperative hospital stays than those with grade 0 or I
disease (P<0.05; Fig. 1).
Risk factors for complications
Univariate and multivariate analyses of risk factors
for PPCs were divided into three parts according to the severity of
the C-D grade: Overall complications (Grade I–V), severe
complications (Grade III–V) and mortality (Grade V; Tables III and IV).
The results of univariate analyses are presented in
Table III. The overall
complications (Grade I–V) were significantly associated with
chronic pancreatitis (P=0.022), serum albumin (P=0.048),
laparoscopic PD (P=0.014) and intraoperative transfusions
(P=0.001). Severe complications (Grade III–V) were revealed to be
associated with an age ≥75 years (P=0.035), diabetes (P=0.010),
total bilirubin (P=0.008), serum albumin (P=0.034), hemoglobin
(P=0.001) and intraoperative transfusions (P=0.001). Mortality
(Grade V), was associated with an age ≥75 years (P<0.001), total
bilirubin (P=0.019), serum albumin (P=0.019), hemoglobin (P=0.004)
and intraoperative transfusions (P=0.038).
Table IV demonstrates
the results of multivariate analyses. In terms of overall
complications (Grade I–V), the incidence was proven to be
associated with lower preoperative serum albumin (P=0.016; OR,
1.475; 95% CI, 1.073–1.978). Severe complications (Grade III–V)
were revealed to be associated with lower serum albumin (P=0.009;
OR, 1.623; 95% CI, 1.130–2.332) and higher total bilirubin
(P=0.048; OR, 1.443; 95% CI, 1.004–2.073). With regards to
mortality (Grade V), an age ≥75 years (P=0.000; OR, 5.860; 95% CI,
2.321–14.979), lower serum albumin (P=0.034; OR, 2.191; 95% CI,
1.063–4.516) and higher total bilirubin (P=0.042; OR, 2.017; 95%
CI, 1.849–4.789) were identified as independent risk factors.
Discussion
The present large sample cohort study was conducted
to retrospectively analyze PPCs at West China Hospital. The C-D
grading consistently classifies all types of PPCs with uniform and
feasible criteria, whether postoperative therapy is required and
which type of therapy is applied. Furthermore, these criteria have
been applied to evaluate postoperative complications in a number of
different surgical fields (17–19).
Therefore, its advantage is to respectively systematically analyze
all risk factors associated with PPCs and to affect the final
outcomes of PD. In the present study, certain independent risk
factors were confirmed on the basis of the PPC-related
intervention.
Due to the fact that serum albumin is produced in
the liver and normally forms ~50% of human plasma protein with
unique functions of oncotic pressure maintaining, transportation
and inflammatory reaction. Hypoproteinemia, a very common condition
in preoperative laboratory tests of PD, has been identified as a
reliable indicator of the nutritional status of a patient (20). However, this is easily ignored by
pancreatic surgeons, particularly modest hypoproteinemia (serum
albumin ≤30 and ≤35 mg/l). In the present study, preoperative
hypoproteinemia was correlated with PPCs, which was supported by a
previous American retrospective study in 108,898 patients who
underwent colorectal surgery (21).
The latter study evaluated the association between modest
hypoproteinemia and postoperative morbidity, and revealed that
patients with preoperative hypoproteinemia exhibited a higher
incidence of hospitalization for >30 days, unplanned intubation
and wound disruption (21).
Clinically, postoperative administration of human albumin is
routinely infused to patients with hypoproteinemia following major
abdominal surgery. However, persistent consumption, postoperative
fasting, surgical trauma and development of PPCs occlude the
extravascular deficiency of serum albumin and worsen the
nutritional status of the patient. Therefore, preoperative
hypoproteinemia requires consideration and necessary treatments to
be administered, which means not only raising the preoperative
serum albumin level, but also comprehensively improving the
preoperative nutritional status of the patients in order to improve
their surgical recovery.
Owing to cholestasis from biliary obstruction,
obstructive jaundice is a common clinical manifestation for
diseases at the pancreatic head and periampullary region.
Cholestasis also causes endotoxemia, impairs immune responses and
suppresses intravascular coagulation of blood cells, which was
believed to worsen the early outcomes of patients following
pancreatic surgery (22). These
conclusions were also supported by the results of the present
study, which demonstrated that jaundice increased the incidences of
severe complications (grade III–V) and mortality (grade V)
following PD. However, the outcomes of PD were not proven to
benefit from preoperative biliary drainage, a common palliative
intervention for jaundice, in the present study. This inconsistent
correlation was also reported by a multicenter prospective
randomized study in 202 patients with pancreatic cancer (23). It was explained that routine
preoperative biliary drainage is associated with a relatively high
incidence of technical failure and technical complications,
particularly drainage occlusion and cholangitis (23). Therefore, routine preoperative biliary
drainage does not benefit patients with obstructive jaundice who
will undergo surgery (23,24). In addition, the exact mechanisms by
which jaundice increases PPCs and the value of preoperative biliary
drainage require validation in additional studies (25).
With the gradually increasing number of older
patients being considered for PD, the correlation between advanced
age and PPCs has received attention from patients and surgeons. The
results of the present study demonstrated that older patients (age,
≥75 years) exhibited a higher rate of mortality. However, this
result requires confirmation. Older patients were reported to have
similar morbidity and mortality rates than younger patients by an
American retrospective study in 727 patients undergoing PD
(26). By contrast, another study
enrolled 3,736 patients to determine age-dependent short-term
outcomes following pancreatic resection by bivariate and
multivariate analyses (27). Older
patients exhibited a significantly higher in-hospital mortality
rate than younger patients (11.4 vs. 2.4%). They are also more
likely to require care at an inpatient nursing or acute care
facility at the time of discharge. Therefore, this result reminds
pancreatic surgeons to cautiously recommend PD for older patients
and to pay more attention to older patients with PPCs.
There are certain limitations to the present study.
To begin with, long-term outcomes of pancreaticoduodenectomy were
not evaluated in the present study. Additionally, the results of
the present study were limited by the inherent defects of
retrospective analysis, including information bias and selection
bias. In the present study, however, all types of PPCs were
evaluated as the uniform C-D classification. Univariate and
multivariate analyses were performed in order to obtain reliable
results. However, a further large-scaled, prospective study is
required to evaluate the results of the present study and to obtain
more valuable results.
A large retrospective study was performed in the
present study and PD is correlated with a high occurrence of PPCs.
The Clavien-Dindo system represents a widely applicable and
feasible system for evaluating PPCs in patients following PD. The
independent risk factors of PPCs that were identified in the
present study require further validation using the Clavien-Dindo
classification in further prospective studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the scientific
research fund of the Science and Technology Department of Chengdu
(grant no. 2014-HM01-00290-SF).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BLT designed the present study, YC and WGW conducted
the majority of the experimentation, SRB and LW collected the data
and edited the manuscript, WGW and YC analyzed data, and WGW
drafted the manuscript. BLT and HBH interpreted the results and
offered intellectual contribution to the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Senda Y, Shimizu Y, Natsume S, Ito S,
Komori K, Abe T, Matsuo K and Sano T: Randomized clinical trial of
duct-to-mucosa versus invagination pancreaticojejunostomy after
pancreatoduodenectomy. Br J Surg. 105:48–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balzano G, Zerbi A, Capretti G, Rocchetti
S, Capitanio V and Di Carlo V: Effect of hospital volume on outcome
of pancreaticoduodenectomy in Italy. Br J Surg. 95:357–362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Gaag NA, Harmsen K, Eshuis WJ,
Busch OR, van Gulik TM and Gouma DJ: Pancreatoduodenectomy
associated complications influence cancer recurrence and time
interval to death. Eur J Surg Oncol. 40:551–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Berge Henegouwen MI, Allema JH, van
Gulik TM, Verbeek PC, Obertop H and Gouma DJ: Delayed massive
haemorrhage after pancreatic and biliary surgery. Br J Surg.
82:1527–1531. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wente MN, Veit JA, Bassi C, Dervenis C,
Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr
MG, et al: Postpancreatectomy hemorrhage (PPH): An International
Study Group of Pancreatic Surgery (ISGPS) definition. Surgery.
142:20–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wente MN, Bassi C, Dervenis C, Fingerhut
A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG,
Traverso LW, et al: Delayed gastric emptying (DGE) after pancreatic
surgery: A suggested definition by the International Study Group of
Pancreatic Surgery (ISGPS). Surgery. 142:761–768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bassi C, Dervenis C, Butturini G,
Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W
and Buchler M; International Study Group on Pancreatic Fistula
Definition: Postoperative pancreatic fistula: An international
study group (ISGPF) definition. Surgery. 138:8–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alamo JM, Marín LM, Suarez G, Bernal C,
Serrano J, Barrera L, Gómez MA, Muntané J and Padillo FJ: Improving
outcomes in pancreatic cancer: Key points in perioperative
management. World J Gastroenterol. 20:14237–14245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braga M, Capretti G, Pecorelli N, Balzano
G, Doglioni C, Ariotti R and Di Carlo V: A prognostic score to
predict major complications after pancreaticoduodenectomy. Ann
Surg. 254:702–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeOliveira ML, Winter JM, Schafer M,
Cunningham SC, Cameron JL, Yeo CJ and Clavien PA: Assessment of
complications after pancreatic surgery: A novel grading system
applied to 633 patients undergoing pancreaticoduodenectomy. Ann
Surg. 244:931–939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clavien PA, Sanabria JR and Strasberg SM:
Proposed classification of complications of surgery with examples
of utility in cholecystectomy. Surgery. 111:518–526.
1992.PubMed/NCBI
|
|
13
|
Zhou J, Yu P, Shi Y, Tang B, Hao Y, Zhao Y
and Qian F: Evaluation of Clavien-Dindo classification in patients
undergoing total gastrectomy for gastric cancer. Med Oncol.
32:1202015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torrecilla C, Vicéns-Morton AJ, Meza IA,
Colom S, Etcheverry B, Vila H and Franco E: Complications of
percutaneous nephrolithotomy in the prone position according with
modified Clavien-Dindo grading system. Actas Urol Esp. 39:169–174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tempero MA, Arnoletti JP, Behrman S,
Ben-Josef E, Benson AB III, Berlin JD, Cameron JL, Casper ES, Cohen
SJ, Duff M, et al: Pancreatic adenocarcinoma. J Natl Compr Canc
Netw. 8:972–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeo CJ, Cameron JL, Maher MM, Sauter PK,
Zahurak ML, Talamini MA, Lillemoe KD and Pitt HA: A prospective
randomized trial of pancreaticogastrostomy versus
pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg.
222:580–592. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Monteiro E, Sklar MC, de Almeida Eskander
A Jr, Shrime M, Gullane P, Irish J, Gilbert R, Brown D, Higgins K,
et al: Assessment of the Clavien-Dindo classification system for
complications in head and neck surgery. Laryngoscope.
124:2726–2731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon PD, Chalasani V and Woo HH: Use of
Clavien-Dindo classification in reporting and grading complications
after urological surgical procedures: Analysis of 2010 to 2012. J
Urol. 190:1271–1274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panhofer P, Ferenc V, Schütz M, Gleiss A,
Dubsky P, Jakesz R, Gnant M and Fitzal F: Standardization of
morbidity assessment in breast cancer surgery using the Clavien
Dindo Classification. Int J Surg. 12:334–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martins Tde C, Duarte TC, Mosca ER,
Pinheiro Cde F, Marçola MA and De-Souza DA: Severe protein
malnutrition in a morbidly obese patient after bariatric surgery.
Nutrition. 31:535–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moghadamyeghaneh Z, Hwang G, Hanna MH,
Phelan MJ, Carmichael JC, Mills SD, Pigazzi A, Dolich MO and Stamos
MJ: Even modest hypoalbuminemia affects outcomes of colorectal
surgery patients. Am J Surg. 210:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baron TH and Kozarek RA: Preoperative
biliary stents in pancreatic cancer-proceed with caution. N Engl J
Med. 362:170–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Gaag NA, Rauws EA, van Eijck CH,
Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW,
Gerhards MF, de Hingh IH, et al: Preoperative biliary drainage for
cancer of the head of the pancreas. N Engl J Med. 362:129–137.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kozarek R: Role of preoperative palliation
of jaundice in pancreatic cancer. J Hepatobiliary Pancreat Sci.
20:567–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lassen K, Coolsen MM, Slim K, Carli F, de
Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN,
Demartines N, et al: Guidelines for perioperative care for
pancreaticoduodenectomy: Enhanced Recovery After Surgery
(ERAS®Society recommendations. Clin Nutr. 31:817–830.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sohn TA, Yeo CJ, Cameron JL, Lillemoe KD,
Talamini MA, Hruban RH, Sauter PK, Coleman J, Ord SE, Grochow LB,
et al: Should pancreaticoduodenectomy be performed in
octogenarians? J Gastrointest Surg. 2:207–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riall TS, Reddy DM, Nealon WH and Goodwin
JS: The effect of age on short-term outcomes after pancreatic
resection: A population-based study. Ann Surg. 248:459–467.
2008.PubMed/NCBI
|