Introduction
Melanoma, a lethal form of skin cancer, is an
aggressive malignancy derived from melanocytes. The incidence rate
of melanoma has doubled in the last two decades, and the mortality
rate is ~10% (1). Although melanoma
represents only 4% of skin cancer cases per year, it accounts for
74% of skin cancer mortalities (2).
The characteristics of melanoma include high rates of invasion and
metastasis, and it is difficult to treat. The early detection of
malignant melanoma is closely associated with survival rates of
≤90%. However, detection at late stages of the disease is
associated with survival rates of only 10% (3).
To date, there is no effective clinical treatment
for malignant melanoma as the mechanism underlying melanoma
development is unclear. Due to its propensity to metastasize,
early-stage melanoma is readily treatable but advanced metastatic
melanoma develops resistance to treatment (4). Consequently, available chemotherapeutic
approaches for melanoma often result in tolerance, a low response
rate (5) and high toxicity (6,7).
Radiotherapy is currently the one of the best and effective
treatments for melanoma. However, the acquisition of
radioresistance results in limited application of radiotherapy in
tumor tissue.
The transcriptional regulation of cytoprotective
genes serves a crucial role in the cellular defense against injury
by nuclear factor erythroid 2-related factor 2 (Nrf2) signaling
pathway in skin cells (8). Under
normal physiological conditions, the Nrf2-encoding gene is
constitutively expressed and forms an inactive complex by binding
with its negative regulator Kelch Like ECH Associated Protein 1
(Keap1) in the cytoplasm. Following endogenous or exogenous stress
stimulation, Nrf2 dissociates from Keap1 and translocates from the
cytoplasm into the nucleus and then binds to the antioxidant
response element (ARE) located in the nucleus (9,10). The ARE
is a cis-acting regulatory element that contributes to the
transcription and translation of downstream phase II antioxidant
enzymes, including NAD(P)H quinine oxidoreductase 1,
glutamate-cysteine ligase catalytic subunit and glutamate-cysteine
ligase modifier subunit (11). The
mutations that lead to the loss and gain of Keap1 and Nrf2 function
are responsible for the overexpression of Nrf2, which were detected
in various types of cancer cells, including lung, esophageal and
skin cancer (12–14). In previous studies, the activation of
Nrf2 was associated with the resistance of cancer cells to
radiation therapy (15), and Nrf2
regulated radiation-induced apoptosis via the Notch signaling
pathway (16).
However, whether the Nrf2/heme oxygenase 1 (HO-1)
signaling pathway is responsible for regulating changes in
radiation-induced cell proliferation and invasion remains to be
clarified. Therefore, the present study focused on the mechanism of
Nrf2/HO-1 in radiation-stimulated melanoma cells.
Materials and methods
Cell culture
B16-F10 melanoma cells were purchased from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal calf serum (Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml
streptomycin in a humidified atmosphere with 5% CO2 at
37°C. Confluent cells were used for the experiments between the 4th
and 6th passages. The cells were seeded at a density of
1×106 cells/ml.
Ionizing radiation treatment
Monolayer cells were exposed to ionizing radiation
at 37°C with a 6-MV X-ray beam produced by a radiotherapy Mark I
irradiator (JLS&A, San Fernando, CA, USA) at acute doses of 2,
4, 8, and 16 Gy with a dose rate of 200 cGy/min or were sham
irradiated as a control. Following irradiation, the cells were
cultured at room temperature for 2, 4, 6, 12, 24 and 48 h.
Transfection with small interfering
RNA (siRNA) against Nrf2
Nrf2 siRNAs were purchased from Santa Cruz
Biotechnology Inc., (Dallas, TX, USA), NRF2-siRNA,
5′-UGAAAGCACAGCAGAAUUTT-3′ and control-siRNA,
5′-GAGCGGCCGAGCAACGUCUAU-3′. According to the manufacturer's
protocol, the siRNAs (final siRNA concentration, 10 nM) were
transfected into B16-F10 cells by using the
Lipofectamine® RNAiMAX transfection reagent (Invitrogen;
Thermo Fisher Scientific Inc., Waltham, MA, USA). The cells were
seeded in 6-well culture plates and incubated with Nrf2 siRNA at 50
nM for 6 h in serum-free OPTI-MEM media (Invitrogen; Thermo Fisher
Scientific Inc.). Following incubation for 24 h at 37°C, the
transfected cells were used for subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cells were harvested to detect mRNA by RT-qPCR.
Total RNA was extracted from cultured cells by using
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific Inc.) according to the manufacturer's protocol, and
reverse transcription was performed using a PrimeScript RT Master
Mix (Takara Biotech Co., Ltd., Dalian, China) in a total volume of
20 µl followed by 30 cycles of 94°C for 20 sec, 55°C for 20 sec and
70°C for 40 sec; then final extension at 70°C for 5 min. The
primers were designed and synthesized by Hanghai Sangon Biological
Engineering Technology & Services (Shanghai, China). The
following primers were used for the PCR experiments: Nrf2 forward,
5′-AGCCCAGCACATCCAGTCA-3′ and reverse,
5′-TGCATGCAGTCATCAAAGTACAAAG-3′; and β-actin forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. SYBR-Green Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for RT-qPCR to
determine the relative levels of target mRNA. The reaction was
conducted on an FTC-3000 qPCR system (Shanghai Funglyn Biotech Co.,
Ltd. Shanghai, China), in the following thermocycling conditions:
94°C for 30 sec, 59°C for 30 sec and 72°C for 45 sec, for 40
cycles. The experiment was repeated 3 times. Relative expression
levels of Nrf2 were calculated according to β-actin (17).
Western blot analysis
The cells were collected to analyze the expression
of Nrf2 and HO-1. The cells were washed with cold PBS and lysed at
4°C with RIPA buffer (Beyotime Institute of Biotechnology, Haimen,
China) and harvested for 30 min on ice. Following centrifugation at
10,000 × g for 20 min at 4°C, the supernatant was used as total
cell lysate. The total protein concentrations were determined using
a Pierce BCA protein assay kit (Thermo Fisher Scientific Inc.)
according to the manufacturer's protocol. A total of 20 µg of
protein were separated onto 12% SDS-PAGE, and the proteins were
then transferred electrophoretically onto polyvinylidene fluoride
membranes (Roche Diagnostics, Basel, Switzerland). The membranes
were blocked with blocking solution [0.05% Tween and 5% bovine
serum albumin (BSA; BBI Life Sciences Corp., Shanghai, China)] in
Tris-buffered saline for 2 h at room temperature and incubated with
primary rabbit monoclonal antibodies against Nrf2 (cat. no.
ab62352; 1:500), primary mouse monoclonal antibodies against HO-1
(cat. no. ab13248; 1:500), primary rabbit polyclonal antibodies
against cleaved caspase-3 (cat. no. ab2302; 1:500), or primary
mouse monoclonal antibodies against β-actin (cat. no. ab8226;
1:1,000; all Abcam, Cambridge UK), overnight at 4°C. The membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies against Nrf2, HO-1, cleaved caspase-3 and β-actin
(anti-mouse IgG; dilution, 1:10,000; cat. no. ab97046; anti-rabbit
IgG; dilution, 1:10,000; cat. no. ab7090; Abcam) at room
temperature for 1 h, which were detected using an enhanced
chemiluminescence detection system (Amersham; GE Healthcare,
Chicago, IL, USA). The intensity of the bands was analyzed using
Image J software version 1.4.6 (National Institutes of Health,
Bethesda, MD, USA).
Cell viability
The viability of the cells was assessed by MTT
assay. The cells were seeded in a 96-well plate overnight. The
cells were treated with ionizing radiation and/or siRNA according
to the manufacturer's protocol. Subsequently, MTT solution was
added to each well and incubated for an additional 3 h at 37°C. The
media was discarded, and dimethyl sulfoxide was added to each well
to dissolve the formazan crystals. Absorbance at 540 nm was
measured using a microplate reader (EL-808; BioTek Instruments,
Inc., Winooski, VT, USA). The OD values of the 0 h or BF groups
were used as the controls.
HO-1 activity
HO-1 activity was determined at 12 h after treatment
with siRNA and ionizing radiation stimulation. As described in a
previous study (18), the cells were
collected by centrifugation for 25 min at 10,000 × g at 4°C. The
activity of the HO-1 enzyme was detected in a reaction mixture
containing microsomes, a cytosolic fraction of rat liver (a source
of biliverdin reductase) hemin and NADPH (18). The reaction mixture was incubated at
37°C for 1 h in the dark, and the bilirubin was extracted with 1 ml
chloroform by vigorous vortexing three times for 10 sec. The amount
of extracted bilirubin was measured at 464 and 530 nm of organic
phase. HO-1 activity was represented as pmol bilirubin/mg
protein/h.
Cell migration
The B16-F10 cell migration assay was performed using
a commercial Transwell insert (8-µm pore size; Corning
Incorporated, Corning, NY, USA). The migratory potential of the
cells was assessed at 24 h post-siRNA transfection, at 0, 2, 4, 6,
12 and 24 h after ionizing radiation in media containing 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.), followed by
serum starvation for an additional 24 h. Then, 2×105
serum-starved cells were seeded in the upper chamber of the
Transwell insert (cat. no. PIEP15R48; EMD Millipore, Billerica, MA,
USA). Dulbecco's modified Eagle's medium (Thermo Fisher Scientific,
Inc.) containing 10% FBS was added to the lower chamber as a
chemoattractant. The non-invading cells were removed from the upper
surface of the membrane in different groups after 24 h. The
migrated cells on the underside of the filter were first fixed with
100% methanol and then stained by 0.1% crystal violet solution for
10 min at room temperature. The cells were counted in five random
fields under an inverted microscope at magnification, ×40. The data
were calculated and expressed as fold increase vs. control
groups.
Cell invasion
Cell invasive ability was assessed using Matrigel
invasion chambers (BD Biosciences, Franklin Lakes, NJ, USA). The
invasive ability of the cells were detected at 24 h post-siRNA
transfection at 0, 2, 4, 6, 12 and 24 h after ionizing radiation,
in media containing 10% FBS followed by serum starvation for an
additional 12 h. Then, 1×105 cells in serum-free medium
containing 10% BSA were added to the upper chamber, while
serum-containing medium with 10% FBS was placed in the lower
chamber. After 24 h, the cells on the surface of the upper membrane
were removed. The cells that penetrated the insert and migrated to
the bottom chamber were stained with 0.1% crystal violet solution
for 10 min at room temperature and counted as previously described
for the cell migration assay.
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance was used to determine
significant differences among all groups followed by Fisher's least
significant difference comparison. P<0.05 was considered to
indicate a statistically significant difference.
Results
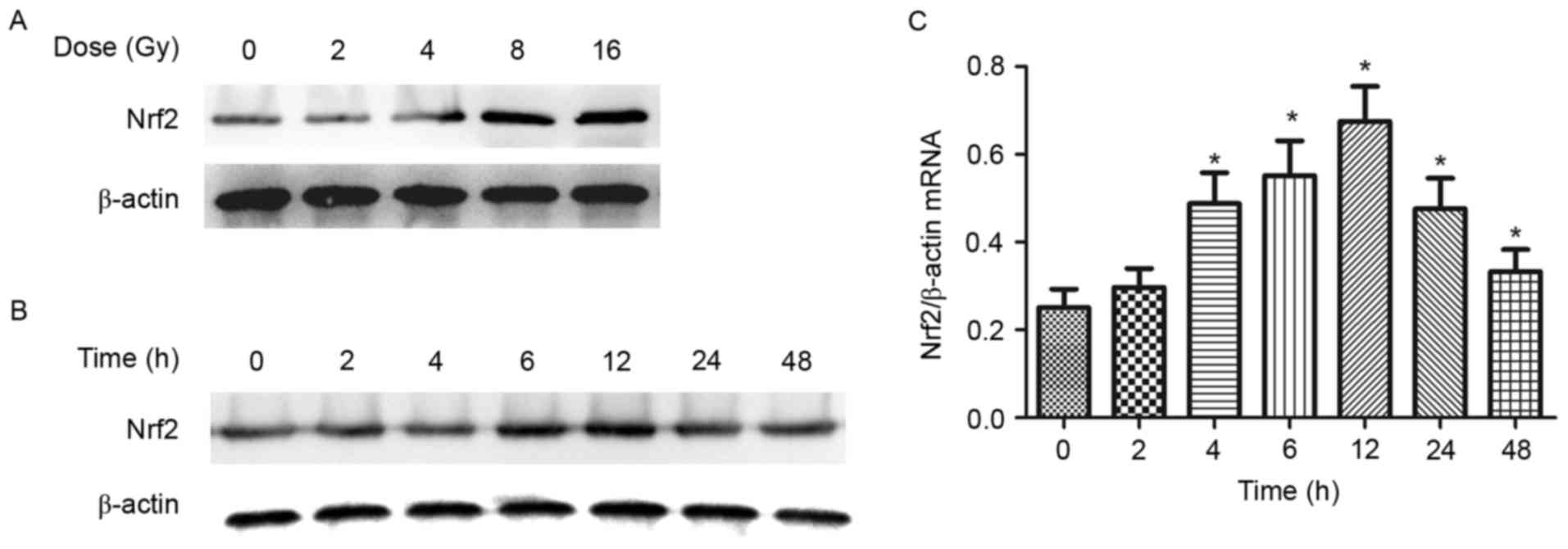
Ionizing radiation stimulates Nrf2
expression in B16-F10 mouse melanoma cells
Nrf2 serves an important role in a number of
skin-associated diseases (19) and
also protects melanocytes against harmful oxidative stress
(20). Previously, studies have
reported that Nrf2 is induced by a variety of stimuli, including
ionizing radiation, in bone tissue and lung cancer cells (16,21). To
clarify whether variations in Nrf2 expression are involved in
ionizing radiation-induced melanoma development, the effect of
ionizing radiation on Nrf2 expression was examined in the present
study. Following the treatment of different doses of ionizing
radiation (1-16 Gy) and for various time periods (0-48 h), Nrf2
protein expression was investigated by western blot analysis. The
results indicated that Nrf2 expression was induced in a
dose-dependent manner with peak expression induced by 8 Gy
(Fig. 1A). Nrf2 expression was
gradually increased from 0-48 h following exposure to 8 Gy ionizing
radiation with peak expression at 12 h (Fig. 1B and C). The changes in Nrf2 mRNA
expression in B16-F10 melanoma cells were similar to the pattern of
Nrf2 protein expression following exposure to ionizing
radiation.
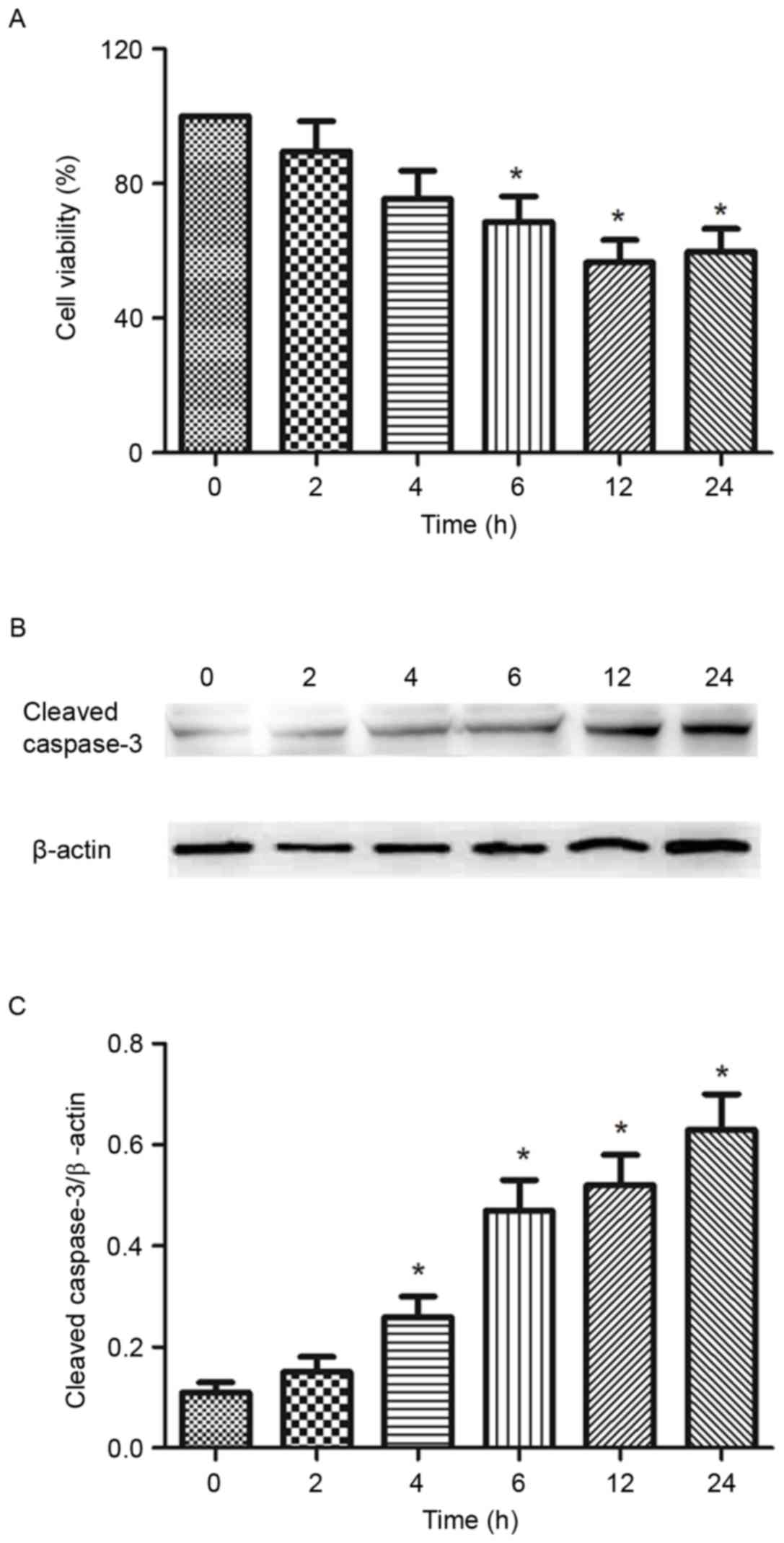
Effect of ionizing radiation on the
viability and apoptosis of B16-F10 mouse melanoma cells
Following the analysis of the results in Fig. 1, 8 Gy was selected to radiate the
melanoma cells in subsequent experiments. To investigate the
viability of B16-F10 cells following ionizing radiation, cell
viability and the levels of cleaved caspase-3 following the
exposure to 8 Gy ionizing radiation at 0-24 h were analyzed by MTT
assay and western blotting, respectively. The results of the cell
viability assay indicated a significant decrease in the number of
living cells from 2 to 24 h compared with 0 h, in a time-dependent
manner (Fig. 2A). Caspase-3 is
crucial mediator of apoptosis. Over the duration of the experiment
(24 h), the expression of cleaved caspase-3 was increasingly
elevated following exposure to 8 Gy ionizing radiation (Fig. 2B and C).
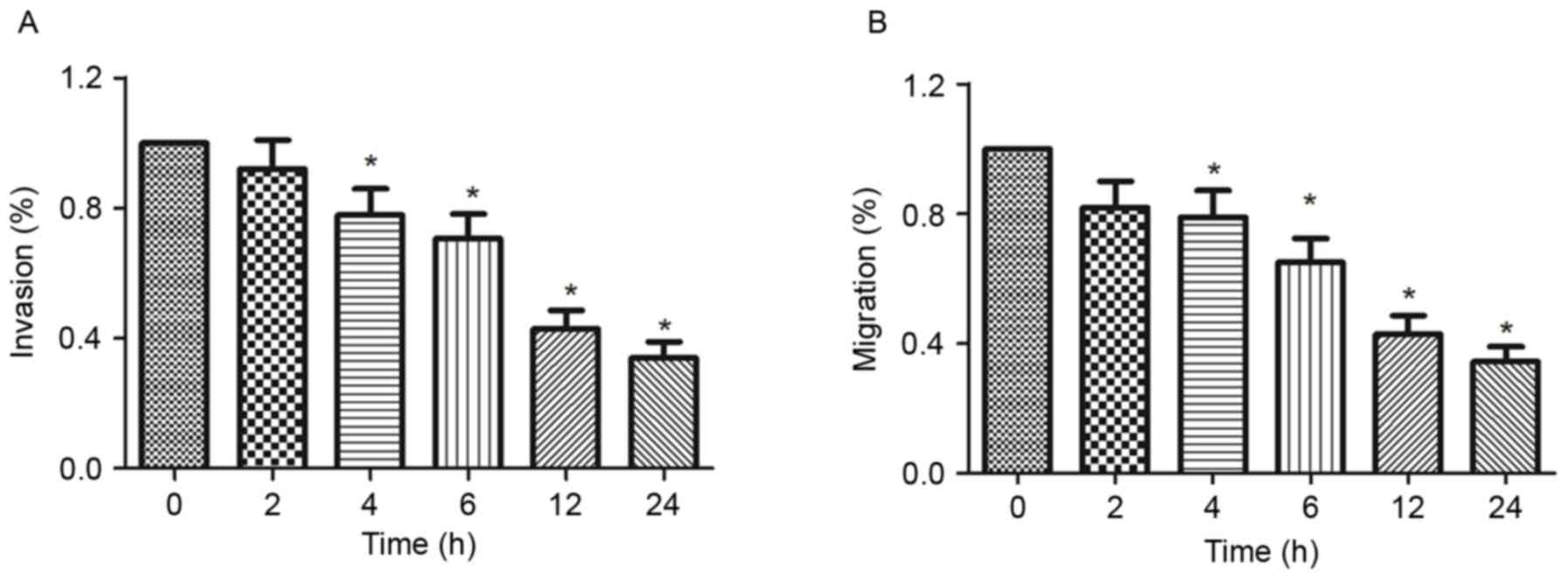
Ionizing radiation inhibits the
migration and invasion of melanoma cells
Cell migration and invasion serve an important role
in cancer metastasis. In the present study, the effect of ionizing
radiation on the migration and invasion of melanoma cells was
investigated by Transwell chamber and Matrigel invasion assays. The
results indicated that the invasive and migratory abilities of
B16-F10 cells were inhibited by 8 Gy ionizing radiation compared
with the 0 h group (Fig. 3A). The
results also indicated that exposure to 8 Gy ionizing radiation
inhibited the migration of B16-F10 cells in a time-dependent manner
compared with 0 h group (Fig.
3B).
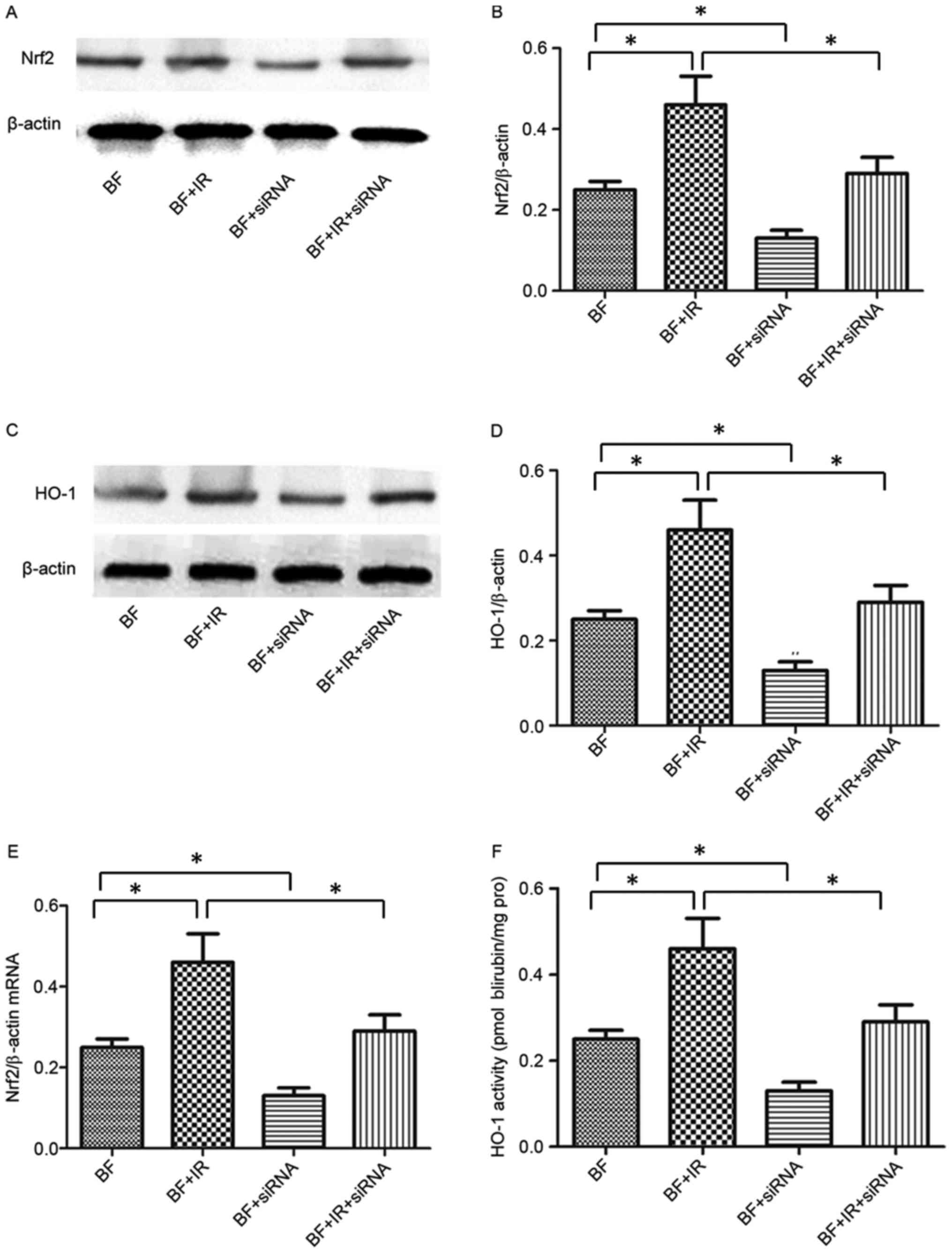
Transfection of Nrf2 siRNA decreases
Nrf2 expression and downstream HO-1 following ionizing radiation in
melanoma cells
To discuss the role of Nrf2 in melanoma cells
following exposure to ionizing radiation, Nrf2 siRNA was utilized
to inhibit Nrf2 expression in the present study. The results
identified that transfection with Nrf2 siRNA was able to inhibit
the expression of Nrf2 protein and mRNA compared with untreated
B16-F10 cells (Fig. 4). Furthermore,
the combined treatment with Nrf2 siRNA and ionizing radiation
significantly inhibited the expression of Nrf2 protein and mRNA in
the B16-F10 + IR + siRNA group compared with the B16-F10 + IR group
(Fig. 4). To further examine the
effect of Nrf2 on its downstream target gene, HO-1, mRNA levels
were detected following exposure to IR. Notably, the patterns of
HO-1 mRNA expression and changes in the activity of HO-1 were
similar to Nrf2 in the four treatment groups (Fig. 4C-F).
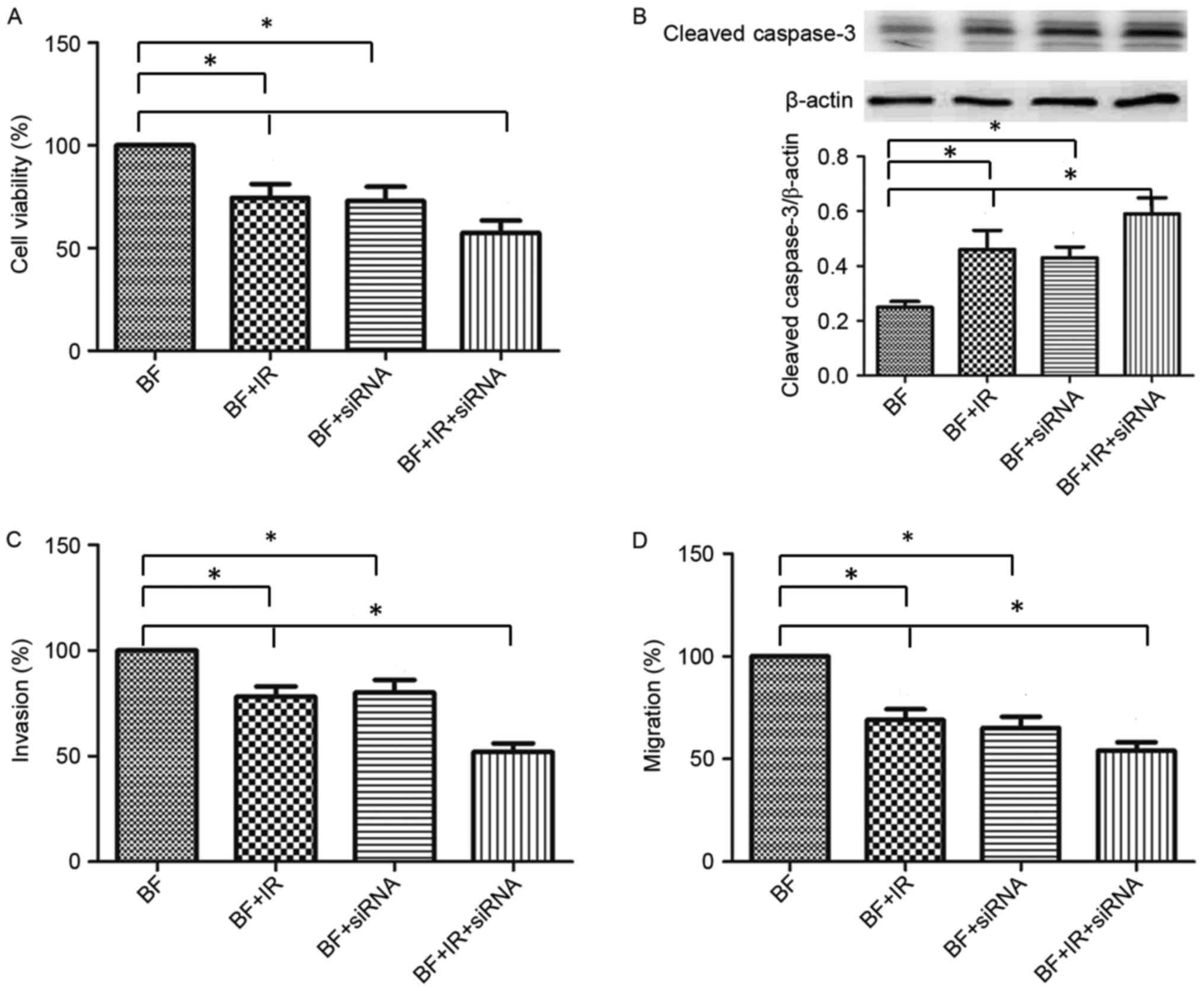
Effect of Nrf2 on cell viability and
the activity of caspase-3 in irradiated B16-F10 cells
Cell viability was analyzed by MTT assay. Compared
with the B16-F10 + IR group or B16-F10 + siRNA group, combined
treatment with Nrf2 siRNA and ionizing radiation markedly inhibited
cell viability in the B16-F10 + IR + siRNA group (Fig. 5A). A combination of exposure to
ionizing radiation and siRNA-induced Nrf2 inhibition increased the
cleaved caspase-3 expression compared with the untreated B16-F10
group (Fig. 5B). Compared with the
B16-F10 + IR group or B16-F10 + siRNA group, treatment with Nrf2
siRNA and ionizing radiation further elevated cleaved caspase-3
expression in the B16-F10 + IR + siRNA group (Fig. 5B). These results indicated that
treatment with ionizing radiation may inhibit cell viability and
promote apoptosis via Nrf2 expression in B16-F10 cells.
Inhibition of Nrf2 reduces the
migration and invasion of B16-F10 cells that are exposed to
ionizing radiation
Compared with the untreated B16-F10 cells, migration
and invasion were inhibited in the B16-F10 + IR and B16-F10 + siRNA
groups (Fig. 5C and D). Furthermore,
combined treatment with Nrf2 siRNA and ionizing radiation
significantly inhibited migration and invasion compared with the
B16-F10 + IR or B16-F10 + siRNA groups (Fig. 5D). These results demonstrated that
ionizing radiation may inhibit the migration and invasion of
B16-F10 cells via Nrf2 expression.
Discussion
The aim of the present study was to elucidate the
mechanisms and role of Nrf2 in the apoptosis, migration and
invasion of radiated melanoma cells. The results indicated that
ionizing radiation stimulated the expression of Nrf2 in B16-F10
melanoma cells. Furthermore, ionizing radiation decreased the cell
viability and increased cell apoptosis, while also inhibiting the
migration and invasion of melanoma cells. Transfection with Nrf2
siRNA decreased the expression and activity of Nrf2 and the
expression and activity of its downstream target, HO-1. Ionizing
radiation may exhibit a regulatory effect on the apoptosis,
migration and invasion via activation of the Nrf2 signaling pathway
in B16-F10 melanoma cells.
Metastasis is a primary cause of cancer-associated
mortalities, which accounts for >90%, and includes a multi-step
process of cell adhesion, migration and invasion (22). Inhibiting the metastasis of cancer
cells is a principal strategy for cancer therapy and research
(23). Melanoma, a type of skin
tumor, generally has a poor prognosis due to its invasive behavior.
The treatments for melanoma include surgery, radiotherapy and
chemotherapy (24). Particularly for
advanced malignant melanoma, there is currently no effective and
safe treatment. In the present research, it was identified that
exposure to 8 Gy radiation decreased cell viability and increased
the cleaved caspase-3 expression in B16-F10 melanoma cells.
Additional experiments verified that radiotherapy exerted an
inhibitory effect on the migration and invasion of melanoma cells.
These results indicated that radiotherapy exerts an inhibitory
effect on cancer metastasis, and therefore it has the potential to
improve the survival period of patients with melanoma.
Although Nrf2 exhibits a beneficial effect in liver
ischemia-reperfusion (25) and
macrophage inflammatory response (26), the effect of Nrf2 on melanoma is
currently unknown. Previous studies have suggested that Nrf2
functions as an oncogene in tumor progression, and it is regarded
as a pro-tumorigenic factor in a number of tumor types by
accelerating stress adaption, increasing drug resistance and
promoting oncogenesis (27). Types of
cancer that are often in a continuous state of oxidative stress
appear to have a constitutively activated Nrf2-ARE pathway,
resulting in enhanced tumor cell survival (28). In response to external stimuli, a
series of events leads to the stabilization of Nrf2 and its
translocation into the nucleus, where Nrf2 exerts its function and
controls the expression of antioxidants (29). In the present study, various doses of
ionizing radiation doses were used to investigate the changes in
Nrf2 expression following the treatment of B16-F10 melanoma cells.
The results indicated that ionizing radiation stimulated Nrf2
expression in a dose-dependent manner with the exception of 8 Gy
having the same effect on Nrf2 expression as 16 Gy. The effect of 8
Gy on Nrf2 expression following different time periods of exposure
to ionizing radiation was also investigated. It was indicated that
treatment with 8 Gy ionizing radiation gradually increased the
expression of Nrf2 from 0 to 12 h, and Nrf2 expression was
decreased from 12-24 h. Tsukimoto et al (30) reported that γ-radiation exhibited an
inducing effect on Nrf2 and that it increased the nuclear
accumulation of Nrf2 and HO-1 expression in the murine Raw 264.7
macrophage cell line. These results are consistent with those
obtained by the present study. Additionally, it was reported that
this highly robust Nrf2-ARE-mediated antioxidant response, which
was detected after 5 days, was radiation dose- and time-dependent.
The Nrf2-ARE-mediated antioxidant response was also associated with
delayed reactive oxygen species (ROS) production as measured by
fluorescent ROS-sensitive dyes (13).
To further investigate the effect of Nrf2 in
ionizing radiation-treated melanoma cells, Nrf2 expression was
inhibited by Nrf2 siRNA, and the levels of Nrf2 protein and its
downstream target were detected. In the present study, treatment
with Nrf2 siRNA was demonstrated to markedly inhibit Nrf2
expression. Furthermore, treatment with Nrf2 siRNA reversed the
increase in Nrf2 expression that was induced by ionizing radiation
compared with untreated melanoma cells.
Furthermore, the expression of HO-1 (a target of
Nrf2) and its activity were significantly inhibited by Nrf2 siRNA
treatment in melanoma cells. Meng et al (31) reported that Nrf2 and its target
protein HO-1 were involved with cell migration and vascular tube
formation in human microvascular endothelial cells, and Pan et
al (32) identified that Nrf2
exerted a regulatory effect on cell migration and invasion in
glioma cells. These results indicated that Nrf2 may participate in
the process of cell migration and invasion. Therefore, it was
hypothesized that Nrf2 was associated with cell migration and
invasion in melanoma cells that were treated with ionizing
radiation. The results of the present study revealed that
siRNA-induced Nrf2 inhibition decreased the migration and invasion
of melanoma cells, and Nrf2 siRNA was able to inhibit the cell
viability and augment caspase-3 activity in melanoma cells compared
with untreated melanoma cells. These results indicated that
ionizing radiation is able to decrease the migration and invasion
of melanoma cells and stimulate apoptosis and Nrf2 expression. The
knockdown of Nrf2 exerts a positive role in migration, invasion and
apoptosis.
In the present study, radiation stimulated Nrf2
expression and increased caspase-3 expression. Furthermore,
exposure to radiation reduced cell viability, migration and
invasion. Inhibition of Nrf2 expression induced by Nrf2 siRNA also
increased caspase-3 expression and reduced cell viability,
migration and invasion. Why is there a similar pattern of caspase-3
expression, cell viability, migration and invasion between Nrf2
overexpression and Nrf2 inhibition? Nrf2 exerts dual functions in
melanoma (33); radiation treatment
increased Nrf2 expression (13). We
hypothesize that the radiation treatment did not induce sufficient
Nrf2 expression to decrease caspase-3 expression, cell viability,
migration and invasion in the present study; therefore, the effect
of radiation was greater than Nrf2's ability to ameliorate its
effects.
To conclude, the present study identified the effect
of Nrf2/HO-1 on migration, invasion and apoptosis in melanoma cells
following ionizing radiation treatment. However the mechanisms by
which Nrf2 and its target genes regulate migration and invasion
remain to be elucidated. Further research is required in order to
investigate the prevention and treatment of melanoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
YG and ZZ designed the experiments. YG, XM and GF
performed the experiments. YG, ZZ and HC processed the data and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heideman DA, Lurkin I, Doeleman M, Smit
EF, Verheul HM, Meijer GA, Snijders PJ, Thunnissen E and Zwarthoff
EC: KRAS and BRAF mutation analysis in routine molecular
diagnostics: Comparison of three testing methods on formalin-fixed,
paraffin-embedded tumor-derived DNA. J Mol Diagn. 14:247–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanzler MH and Swetter SM: Malignant
melanoma. J Am Acad Dermatol. 48:780–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streicher KL, Zhu W, Lehmann KP,
Georgantas RW, Morehouse CA, Brohawn P, Carrasco RA, Xiao Z, Tice
DA, Higgs BW, et al: A novel oncogenic role for the miRNA-506-514
cluster in initiating melanocyte transformation and promoting
melanoma growth. Oncogene. 31:1558–1570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reintgen D, Cruse CW and Atkins M:
Cutaneous malignant melanoma. Clin Dermatol. 19:253–261. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serrone L, Zeuli M, Sega FM and Cognetti
F: Dacarbazine-based chemotherapy for metastatic melanoma:
Thirty-year experience overview. J Exp Clin Cancer Res. 19:21–34.
2000.PubMed/NCBI
|
|
6
|
Alwan LM, Grossmann K, Sageser D, Van Atta
J, Agarwal N and Gilreath JA: Comparison of acute toxicity and
mortality after two different dosing regimens of high-dose
interleukin-2 for patients with metastatic melanoma. Target Oncol.
9:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atkins MB, Lotze MT, Dutcher JP, Fisher
RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M,
et al: High-dose recombinant interleukin 2 therapy for patients
with metastatic melanoma: Analysis of 270 patients treated between
1985 and 1993. J Clin Oncol. 17:2105–2116. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li N, Alam J, Venkatesan MI,
Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen
E, Wang X, Huang A, et al: Nrf2 is a key transcription factor that
regulates antioxidant defense in macrophages and epithelial cells:
Protecting against the proinflammatory and oxidizing effects of
diesel exhaust chemicals. J Immunol. 173:3467–3481. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bütof R, Dubrovska A and Baumann M:
Clinical perspectives of cancer stem cell research in radiation
oncology. Radiother Oncol. 108:388–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baird L, Llères D, Swift S and
Dinkova-Kostova AT: Regulatory flexibility in the Nrf2-mediated
stress response is conferred by conformational cycling of the
Keap1-Nrf2 protein complex. Proc Natl Acad Sci USA.
110:15259–15264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canning P, Sorrell FJ and Bullock AN:
Structural basis of KEAP1 interactions with Nrf2. Free Radic Biol
Med. 91:101–107. 2015. View Article : Google Scholar
|
|
12
|
Lee S, Lim MJ, Kim MH, Yu CH, Yun YS, Ahn
J and Song JY: An effective strategy for increasing the
radiosensitivity of human lung cancer cells by blocking
Nrf2-dependent antioxidant responses. Free Radic Biol Med.
53:807–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mcdonald JT, Kim K, Norris AJ, Vlashi E,
Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky
L and McBride WH: Ionizing radiation activates the Nrf2 antioxidant
response. Cancer Res. 70:8886–8895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh A, Bodas M, Wakabayashi N, Bunz F
and Biswal S: Gain of Nrf2 function in non-small-cell lung cancer
cells confers radioresistance. Antioxid Redox Signal. 13:1627–1637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibata T, Ohta T, Tong KI, Kokubu A,
Odogawa R, Tsuta K, Asamura H, Yamamoto M and Hirohashi S: Cancer
related mutations in NRF2 impair its recognition by Keap1-Cu13 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Q, Mao A, Yan J, Sun C, Di C, Zhou X,
Li H, Guo R and Zhang H: Downregulation of Nrf2 promotes
radiation-induced apoptosis through Nrf2 mediated Notch signaling
in non-small cell lung cancer cells. Int J Oncol. 48:765–773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konrad FM, Zwergel C, Ngamsri KC and
Reutershan J: Anti-inflammatory effects of heme oxygenase-1 depend
on adenosine A2A- and A2B-receptor signaling in acute pulmonary
inflammation. Front Immunol. 8:18742017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gęgotek A and Skrzydlewska E: The role of
transcription factor Nrf2 in skin cells metabolism. Arch Dermatol
Res. 307:385–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeayeng S, Wongkajornsilp A, Slominski AT,
Jirawatnotai S, Sampattavanich S and Panich U: Nrf2 in
keratinocytes modulates UVB-induced DNA damage and apoptosis in
melanocytes through MAPK signaling. Free Radic Biol Med.
108:918–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rana T, Schultz MA, Freeman ML and Biswas
S: Loss of Nrf2 accelerates ionizing radiation-induced bone loss by
upregulating RANKL. Free Radic Biol Med. 53:2298–2307. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan N and Mukhtar H: Cancer and
metastasis: Prevention and treatment by green tea. Cancer
Metastasis Rev. 29:435–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu ZY, Lien JC, Huang YP, Liao CL, Lin JJ,
Fan MJ, Ko YC, Hsiao YP, Lu HF and Chung JG: Casticin inhibits
A375.S2 human melanoma cell migration/invasion through
downregulating NF-κB and matrix metalloproteinase-2 and −1.
Molecules. 21:3842016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Hu B, Huang H, Tsung A, Gaikwad NW,
Xu M, Jiang M, Ren S, Fan J, Billiar TR, et al: Estrogen
sulfotransferase is an oxidative stress responsive gene that
gender-specifically affects liver ischemia/reperfusion injury. J
Biol Chem. 290:14754–14764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi EH, Suzuki T, Funayama R,
Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi
H, Nakayama K and Yamamoto M: Nrf2 suppresses macrophage
inflammatory response by blocking proinflammatory cytokine
transcription. Nat Commun. 7:116242016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geismann C, Arlt A, Sebens S and Schäfer
H: Cytoprotection ‘gone astray’: Nrf2 and its role in cancer. Onco
Targets Ther. 7:1497–1518. 2014.PubMed/NCBI
|
|
28
|
Hayes JD and McMahon M: NRF2 and KEAP1
mutations: Permanent activation of an adaptive response in cancer.
Trends Biochem Sci. 34:176–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrándiz ML, Nacher-Juan J and Alcaraz
MJ: Nrf2 as a therapeutic target for rheumatic diseases. Biochem
Pharmacol. 13:338–346. 2018. View Article : Google Scholar
|
|
30
|
Tsukimoto M, Tamaishi N, Homma T and
Kojima S: Low-dose gamma-ray irradiation induces translocation of
Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res.
51:349–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meng D, Wang X, Chang Q, Hitron A, Zhang
Z, Xu M, Chen G, Luo J, Jiang B, Fang J and Shi X: Arsenic promotes
angiogenesis in vitro via a heme oxygenase-1-dependent mechanism.
Toxicol Appl Pharmacol. 244:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan H, Wang H, Zhu L, Mao L, Qiao L and Su
X: The role of Nrf2 in migration and invasion of human glioma cell
U251. World Neurosurg. 80:363–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Menegon S, Columbano A and Giordano S: The
dual roles of NRF2 in cancer. Trends Mol Med. 22:578–593. 2016.
View Article : Google Scholar : PubMed/NCBI
|