Introduction
Lung cancer is a malignant tumor with the highest
rate of cancer-associated mortality globally (1). Non-small cell lung cancer (NSCLC) is the
most common form of lung cancer and accounts for >70% of all
lung cancer cases (1). According to a
recent survey undertaken by the World Health Organization, the
incidence of lung cancer is 33.5/100,000, including (45.9/10,000
males and 21.3/100,000 females), with the incidence in males being
markedly higher than that in females (2). With the increasing progress of surgery
and other therapeutic alternatives, treatment options have already
greatly improved the prognosis and quality of life of patients with
lung cancer (3). However, due to the
fact that there is a lack of in-depth knowledge regarding the
molecular mechanisms of lung cancer morbidity, early diagnosis
targets and anticancer treatments for patients with lung cancer are
limited (3). It has reported that, by
the time patients with NSCLC see a doctor, >50% of cases have
already developed into (at least) stage III disease, meaning that
surgical treatment is no longer an option (4). In recent years, chemotherapy is regarded
as one of the most effective method for curing more advanced NSCLC
(5). However, cancer is a highly
heterogeneous disease. In patients with the same pathological type
of lung at the point of diagnosis, there is a marked difference in
the sensitivity of platinum chemotherapeutics (1).
At present, it is widely believed that different
genotypes in patients with NSCLC are associated with different
degrees of sensitivity to platinum-based chemotherapeutics
(6). Platinum-based
chemotherapeutics, including cis-platinum and carboplatin, are
widely applied as anticancer chemotherapeutics. Furthermore, the
curative effects of these agents are the most efficient and they
are more cytotoxic (7). Once platinum
drugs enter into the nucleus and combine with DNA, they cause
irreversible damage to DNA and induce apoptosis by forming
platinum-DNA complexes, in order to induce their anti-carcinogenic
action (8). Due to the fact that
there are DNA damage repair mechanisms in cells, DNA damage caused
by platinum-based chemotherapeutics may be repaired by increasing
the abundance of factors associated with DNA damage repair
(9). Therefore, it is possible that
the expression and function of regulatory factors in the DNA repair
process serves an important role in the sensitivity of tumors to
platinum-based chemotherapeutics (10). The expression of these molecules and
the abnormal activation of their functions may impact the effects
of platinum-based chemotherapeutics on patients with NSCLC by
enhancing the tolerance of cancer cells to platinum-based
chemotherapeutics (11).
MicroRNAs (miRNAs/) are short-chain, non-coding
RNAs, which develop their biological functions through multiple
mechanisms in order to control protein expression. In a previous
study, the results of bioinformatics analysis have demonstrated
that every miRNA is able to control several hundreds of gene
targets and to participate in a conduction group of multiple gene
signaling paths (12). Therefore,
miRNAs control a series of biological functions, including cell
proliferation, differentiation and apoptosis (13). Altered expression of miRNAs may have a
huge influence on the functional activity of cells. Genomic
research of human cancer has revealed that miRNA expression is
varied (14). A lot of diagnosis and
prognosis-based biological information are associated with miRNA
expression (14). Therefore, miRNA
expression may predict the prognosis of NSCLC, as an imbalance in
miRNA expression frequently occurs in all types of tumor.
Therefore, these expression characteristics have potential value in
the diagnosis and prognosis of tumors (15).
AKT, also known as protein kinase B (PKB), is a
serine/threonine protein kinase and therefore, it is highly
homologous with protein kinase A (PKA) and PKC (16). AKT serves an important role in tumor
cell proliferation, differentiation, invasion and metabolism
(17). AKT2 is an important subtype
of AKT and, as an oncogene, may cause malignant changes to cells
(18). It has been revealed that AKT2
is overexpressed or has increased activity in ovarian cancer,
breast cancer, glioma and other types of tumor (18). Furthermore, AKT2 is associated with
tumor proliferation, invasion, metastasis and prognosis. At
present, studies regarding AKT2 expression in NSCLC are
insufficient. The present study demonstrated that microRNA-137
inhibits tumor growth and sensitizes tumor cells to cisplatin in
patients with non-small cell lung cancer through AKT2.
Materials and methods
Clinical specimens and cell
culture
NSCLC and adjacent non-cancerous tissues from 79
cisplatin-treated patients (age range, 65.5±8.5 years old, male)
were obtained during surgical resection in Jiangmen Central
Hospital (Jiangmen, China) between March 2015 and May 2015. The
experimental protocols were approved by the Ethics Committees of
Jiangmen Central Hospital and written informed consent was obtained
from all participants. The patients were followed up every month to
record the disease-free survival (DFS) and overall survival (OS)
rates. Human lung cancer A549 and H520 cell lines were maintained
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% fetal bovine serum
(Hyclone; Logan, UT, USA), 100 U/ml penicillin and 100 mg/ml
streptomycin in a humidified atmosphere of 5% CO2 and
95% O2 at 37°C.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Total RNA was reversely transcribed
with oligodT primers using PrimeScript RT Reagent kit (Vazyme,
Piscataway, NJ, USA) at 37°C for 60 min and at 85°C for 1 min. The
cDNA was amplified by RT-qPCR using SYBR® Premix Ex Taq
(Takara Biotechnology Co., Ltd., Dalian, China). PCR thermocycling
conditions were as follows: 5 min at 95°C, 40 cycles of 30 sec at
95°C, 60 sec at 60°C, 30 sec at 72°C. Experiments were performed in
triplicate. The primer sequences were as follows: microRNA-137
forward, 3′-GCTCCTCAGGTCGAACCTATTG-5′ and reverse,
3′-CCGACGCTATTGCTTAAGAATACG-5′; U6 Forward:
5′-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3′ and reverse:
5′-GCTTCACGAATTTGCGTGTCATCCTTGC-3′. The expression of microRNA-137
was calculated by relative quantification using the
2−ΔΔCq method (19).
Expression of microRNA-137 was considered to be low at <50% of
U6 expression, and high at 50-100% of U6 expression,
Transfection and treatment with the
AKT2 inhibitor, MK2206
Negative control (pLV3-control) and microRNA-137
mimic (pLV3-microRNA-137 mimic) were constructed by Sangon Biotech
Co., Ltd., (Shanghai, China). Negative control (100 ng) and
microRNA-137 mimic (100 ng) were transfected into A549 and H520
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C. Following transfection for 4 h, the
medium was refreshed and 1.25 µM cisplatin was added to the cells
at 37°C. Cells were treated with cisplatin at 12, 24 and 48 h for
MTT, and treated with cisplatin at 48 h for Caspase-3 activity and
western blot analysis. Next, 2.5 µM MK2206 and 1.25 µM cisplatin
were added to the cells following transfection for 4 h at 37°C.
Cell proliferation
A549 and H520 cells transfected with microRNA-137 or
anti-microRNA-137 plasmids were seeded onto 96-well plates at a
density of 4,000 cells/well and were treated with 1.25 µM cisplatin
for 12, 24 or 48 h. A total of 10 µl MTT (Invitrogen; Thermo Fisher
Scientific, Inc.) was added to each well, followed by incubated for
4 h in a humidified atmosphere of 5% CO2 and 95%
O2 at 37°C. A total of 200 µl dimethyl sulfoxide was
added to each well to dissolve the purple formazan, after the
supernatant had been discarded. The optical density (OD) was
detected at a wavelength of 490 nm using a POLARstar OPTIMA
multi-detection microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Caspase-3 activity
A549 and H520 cells transfected with microRNA-137 or
anti-microRNA-137 plasmids were seeded onto 6-well plates at a
density of 1×106 cells/well and were treated with 1.25
µM cisplatin for 24 h. Ac-DEVD-pNA was added to each well, followed
by incubation for 1 h in a humidified atmosphere of 5%
CO2 and 95% O2 at 37°C. The OD was detected
at a wavelength of 405 nm using a POLARstar OPTIMA multi-detection
microplate reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
A549 and H520 cells transfected with microRNA-137 or
anti-microRNA-137 plasmids were seeded onto 6-well plates at a
density of 1×106 cells/well and were treated with 1.25
µM cisplatin for 24 h. Cells were washed twice with
phosphate-buffered saline and were prepared using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China), supplemented with
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). The supernatants were collected and the protein
concentration was determined using a bicinchoninic acid assay
(Beyotime Institute of Biotechnology). Protein (60 µg) was
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Millipore). The membranes were blocked using
5% skimmed milk powder in TBST for 1 h at 37°C, and were incubated
with primary antibodies against Bax (cat no. sc-6236; 1:1,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Cyclin D1 (cat
no. sc-717; 1:1,000; Santa Cruz Biotechnology, Inc.), p-AKT2 (cat
no. sc-7985-R; 1:1,000; Santa Cruz Biotechnology, Inc.) and GAPDH
(cat no. sc-25778; 1:2,000; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The membranes were washed with TBST and incubated in a
solution containing a goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (cat no. sc-2004; 1:5,000;
Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. An enhanced
chemiluminescence detection system (Thermo Fisher Scientific, Inc.)
was used for signal detection and analyzed using ImageJ 3.0
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. A two-tailed Student's t-test or one-way analysis of
variance by Tukey's post-hoc test were used for statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between microRNA-137
expression and the DFS and OS of patients with NSCLC
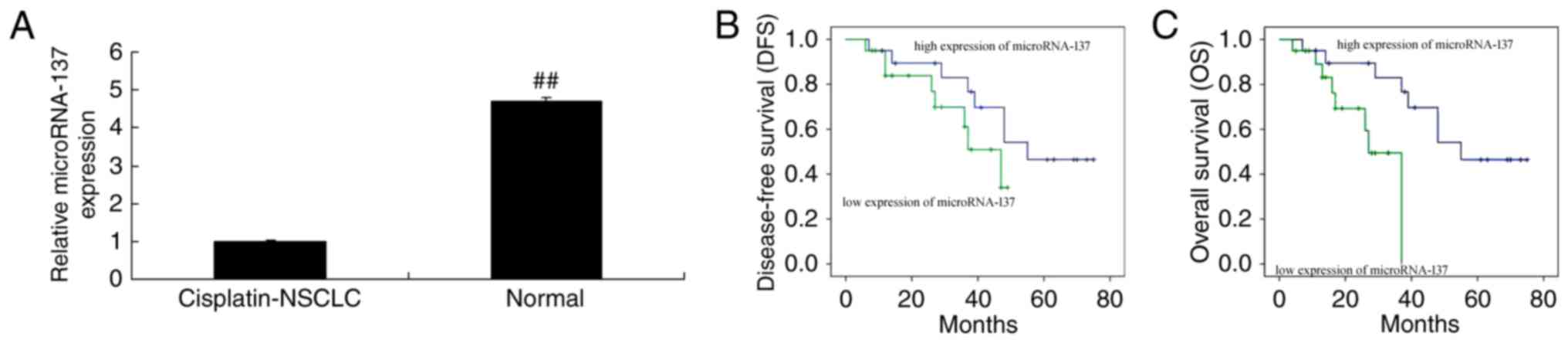
The microRNA-137 expression in cisplatin-treated
NSCLC patient tissue samples and adjacent healthy tissue samples
was measured, and it was revealed that the expression of
microRNA-137 in cisplatin-treated NSCLC patient tissue samples was
markedly lower than that in the adjacent healthy tissue samples
(Fig. 1A). Furthermore, the DFS and
OS rates in patients with NSCLC exhibiting a high expression of
microRNA-137 were lower compared with those in patients with NSCLC
exhibiting a low expression of microRNA-137 (Fig. 1B and C).
Effect of microRNA-137 overexpression
on cell proliferation and caspase-3 activity in cisplatin-treated
A549 and H520 cells
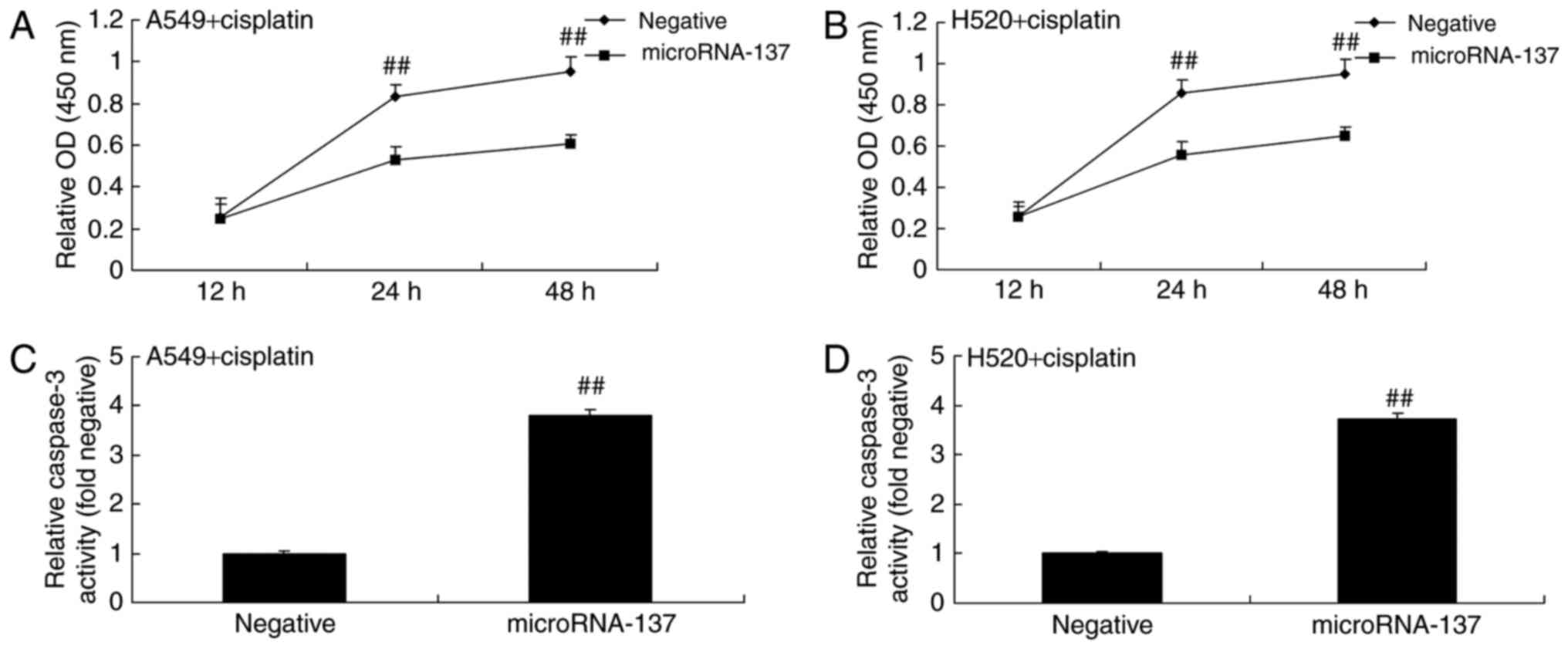
In order to determine the effect of microRNA-137 on
cell proliferation and caspase-3 activity in A549 and H520 cells
treated with cisplatin or transfected with microRNA-137 mimics (to
induce microRNA-137 overexpression). The results demonstrated that
overexpression of microRNA-137 significantly inhibited the
proliferation of A549 and H520 cells treated with cisplatin
compared with A549 cells expressing negative control (Fig. 2A and B). Overexpression of
microRNA-137 significantly increased caspase-3 activity in A549 and
H520 cells treated with cisplatin compared with those transfected
with microRNA-Negative (Fig. 2C and
D).
Effect of microRNA-137 overexpression
on Bax protein expression in A549 and H520 cells treated with
cisplatin
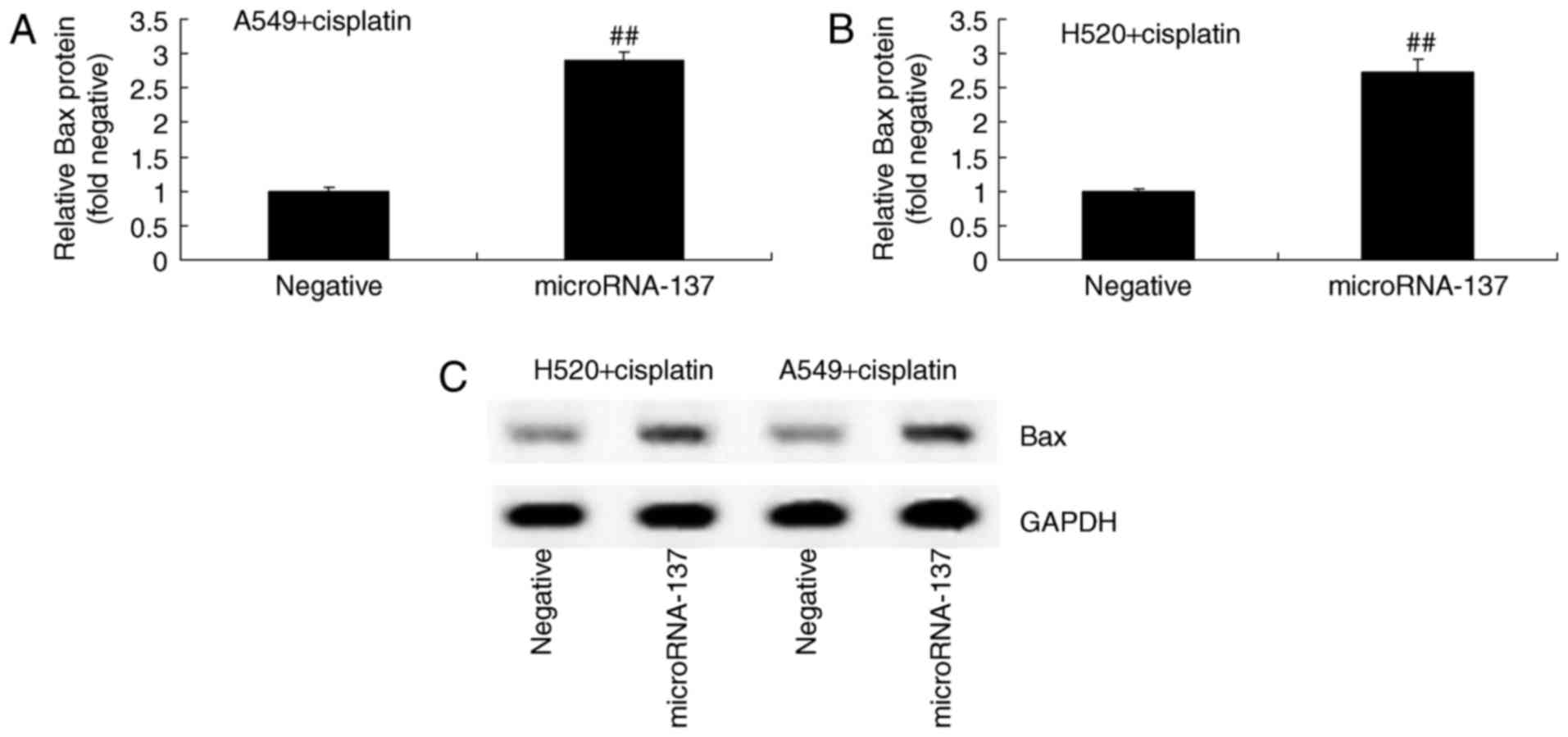
In order to investigate the effect of microRNA-137
on the apoptosis of A549 and H520 cells treated with cisplatin, Bax
protein expression was measured using western blot analysis
following overexpression of microRNA-137. The results demonstrated
that overexpression of microRNA-137 significantly increased the
expression of Bax protein in A549 and H520 cells treated with
cisplatin compared with those transfected with microRNA-Negative
(Fig. 3).
Effect of microRNA-137 overexpression
on Cyclin D1 protein expression in A549 and H520 cells treated with
cisplatin
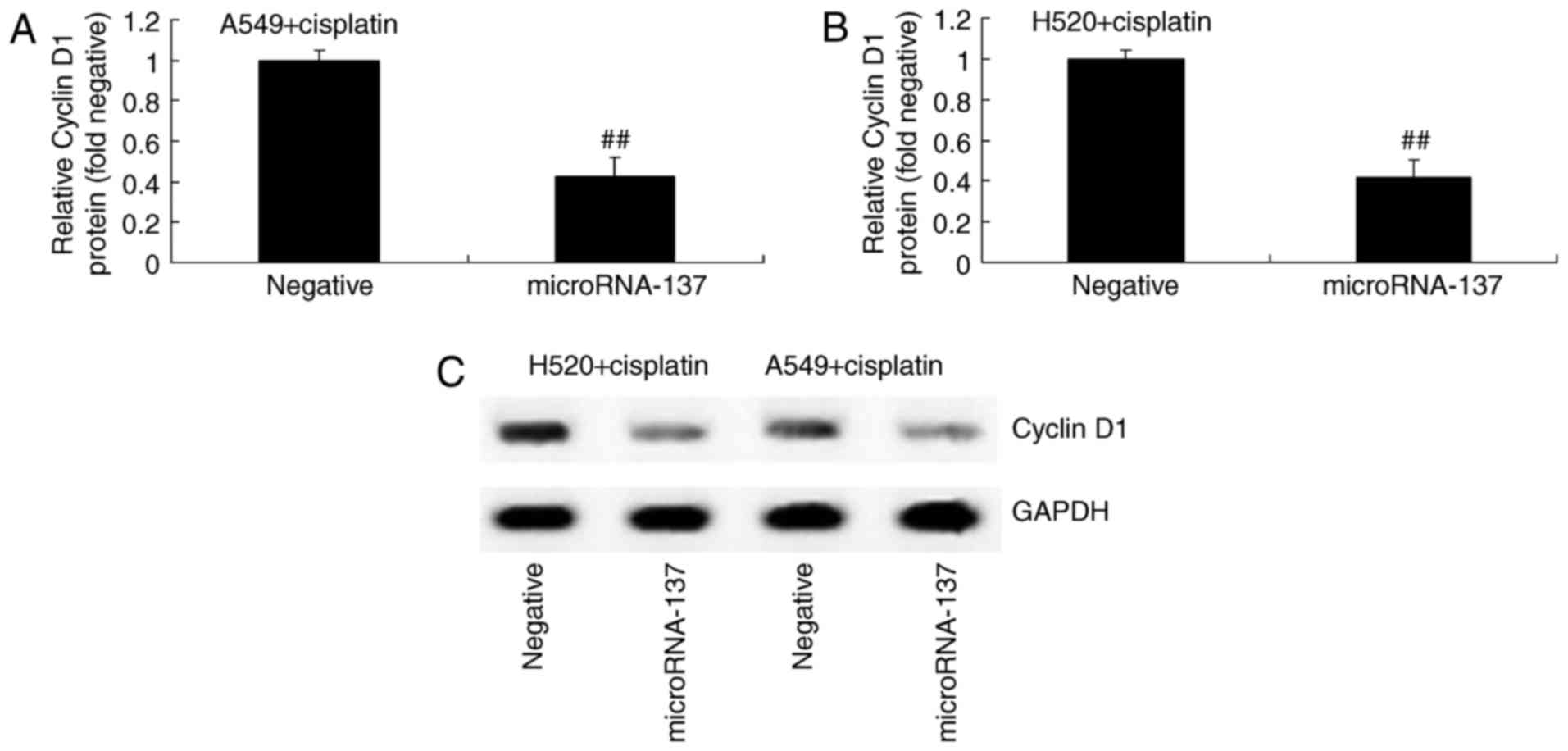
In order to determine the association between
microRNA-137 expression levels and Cyclin D1 expression in
cisplatin-treated patients with NSCLC, western blot analysis was
used to measure the protein expression of Cyclin D1 in A549 and
H520 cells treated with cisplatin. Compared with the cells
transfected with microRNA-Negative, Cyclin D1 protein expression
was significantly inhibited in cisplatin-treated A549 and H520
cells following overexpression of microRNA-137 (Fig. 4).
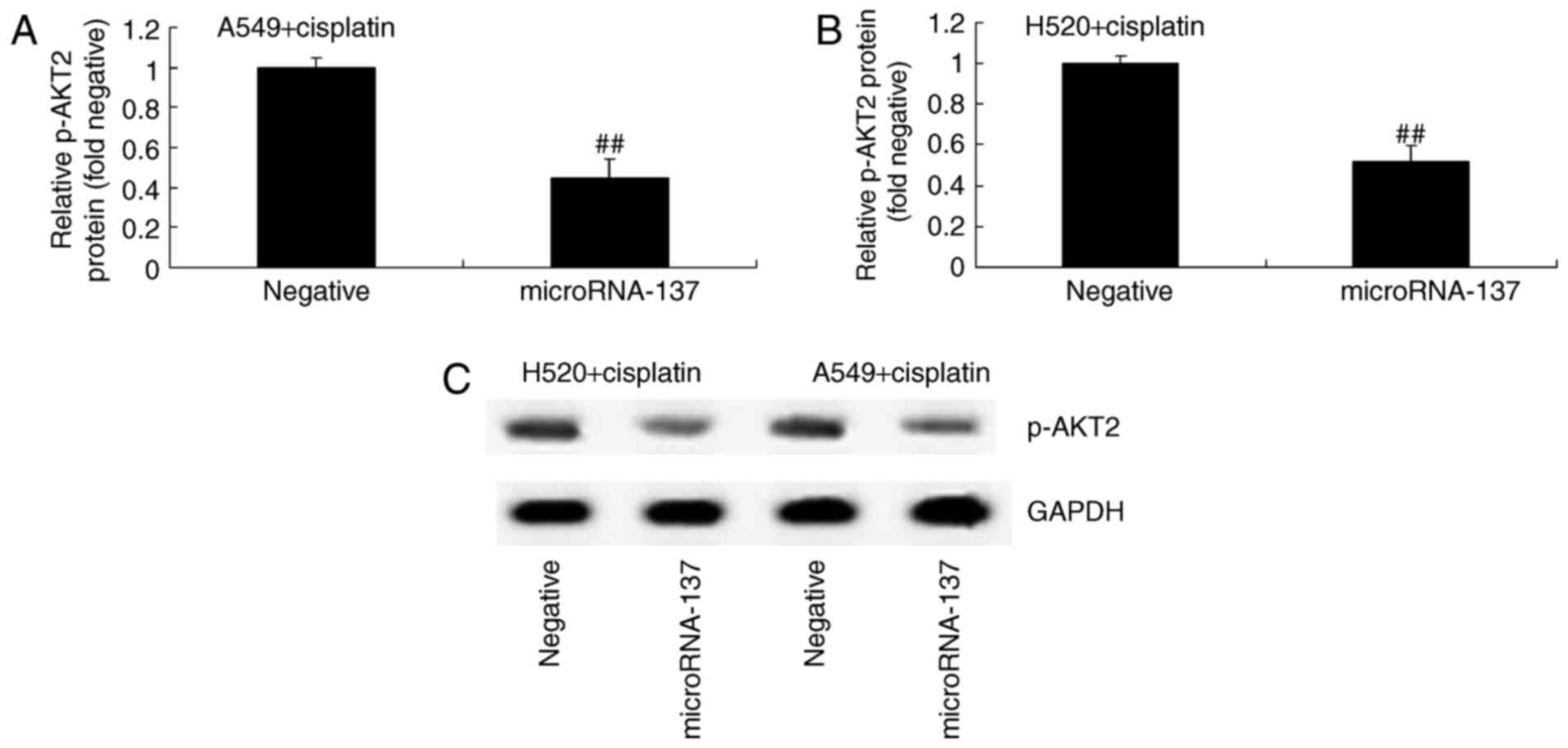
Effect of microRNA-137 overexpression
on AKT2 protein expression in A549 and H520 cells treated with
cisplatin
In order to identify any association between
microRNA-137 expression and p-AKT2 expression in cisplatin-treated
patients with NSCLC, p-AKT2 protein expression was determined using
western blot analysis. The results demonstrated that p-AKT2 protein
expression was significantly suppressed by overexpression of
microRNA-137 compared with transfection with microRNA-Negative
(Fig. 5).
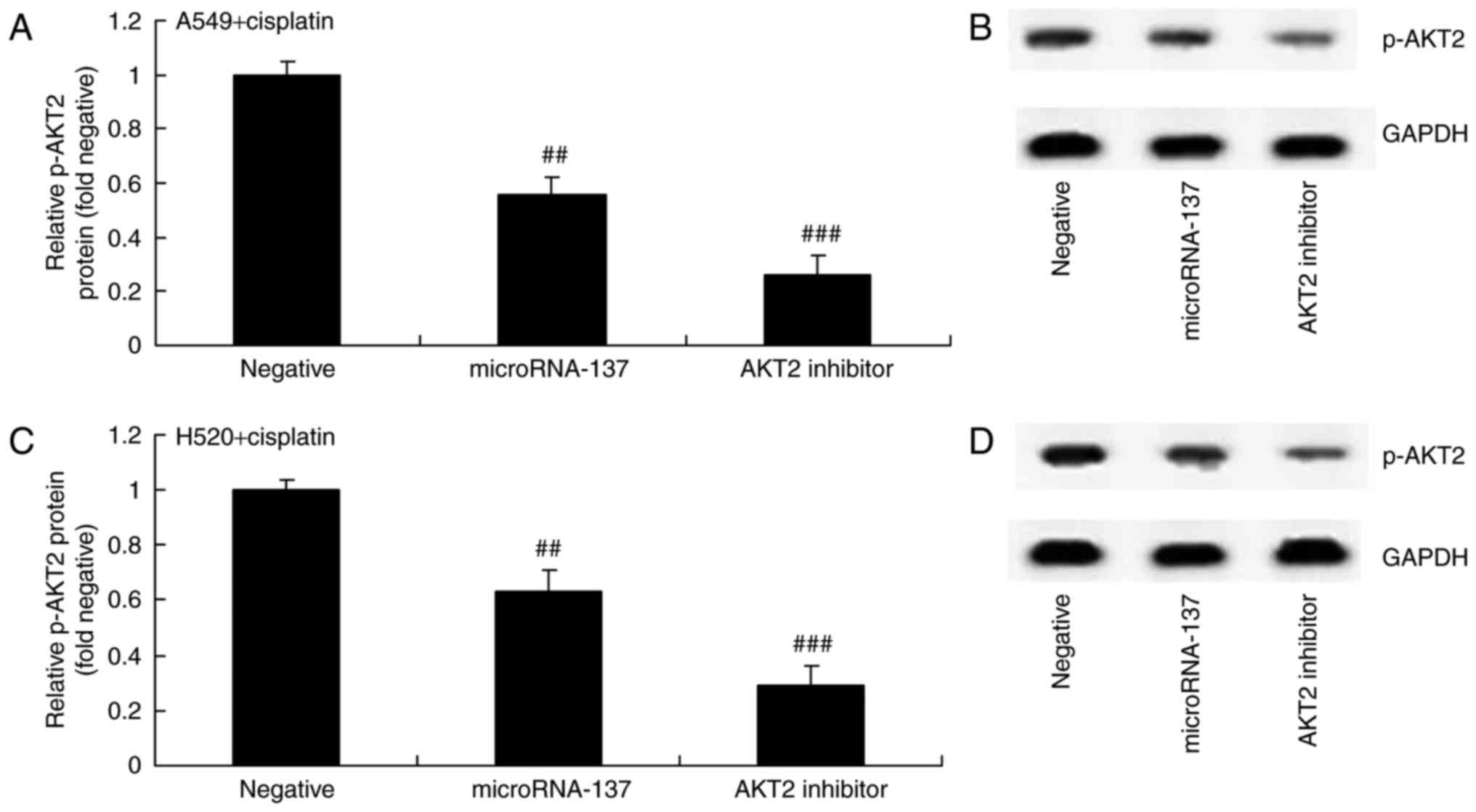
Effect of an AKT2 inhibitor on AKT2
protein expression in A549 and H520 cells treated with cisplatin
following downregulation of microRNA-137
Furthermore, the effect of suppression of AKT2
(MK2206) on AKT2 protein expression in A549 and H520 cells treated
with cisplatin following downregulation of microRNA-137. As
demonstrated in Fig. 6, MK2206, an
AKT2 inhibitor, inhibited p-AKT2 protein expression in A549 and
H520 cells treated by cisplatin following downregulation of
microRNA-137 compared with cells transfected with
microRNA-Negative.
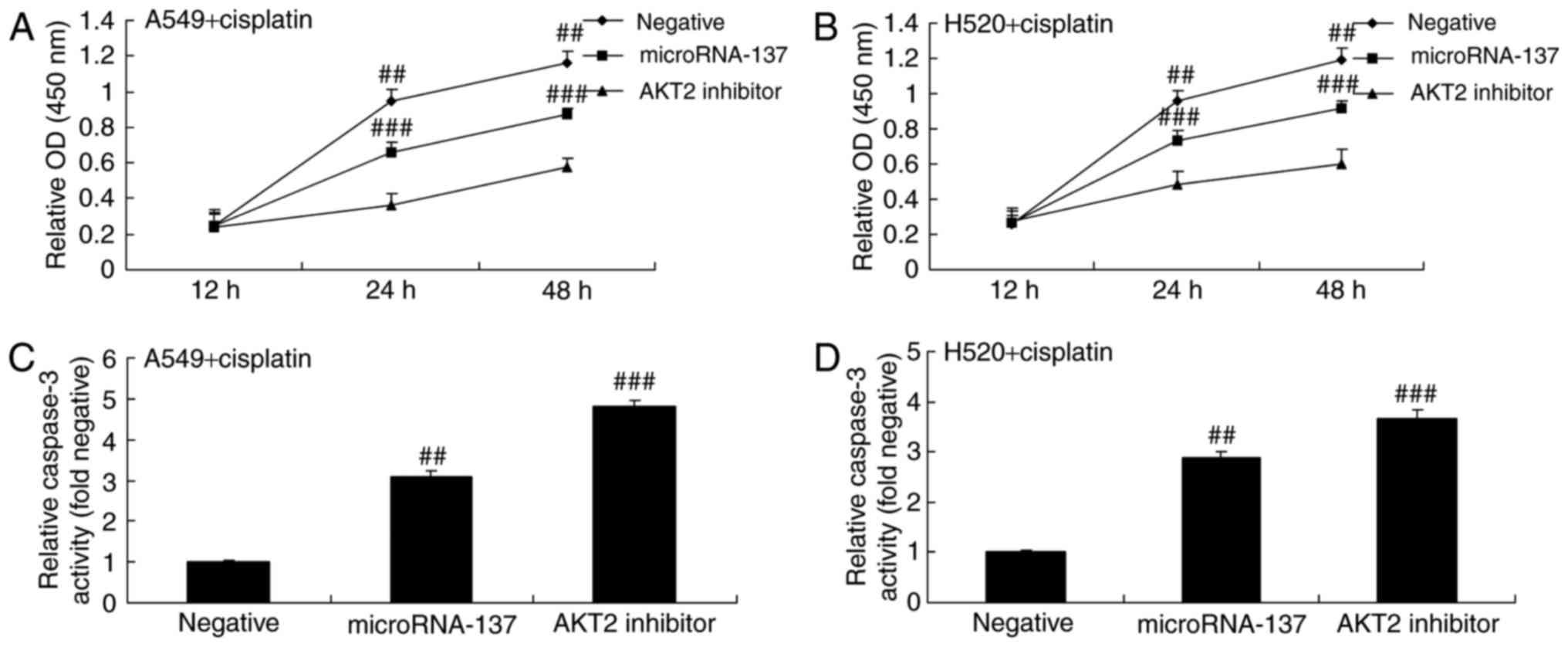
Effect of an AKT2 inhibitor on cell
proliferation and caspase-3 activity in A549 and H520 cells treated
with cisplatin following downregulation of microRNA-137
The present study investigated whether or not the
AKT2 inhibitor had an effect on cell proliferation and caspase-3
activity in A549 and H520 cell treated with cisplatin following
downregulation of microRNA-137. The AKT2 inhibitor significantly
suppressed the proliferation of A549 and H520 cells treated with
cisplatin following downregulation of microRNA-137 compared with
those transfected with microRNA-Negative (Fig. 7A and B). The suppression of AKT2
significantly increased caspase-3 activity in A549 and H520 cells
treated with cisplatin following downregulation of microRNA-137
compared with those transfected with microRNA-Negative (Fig. 7C and D).
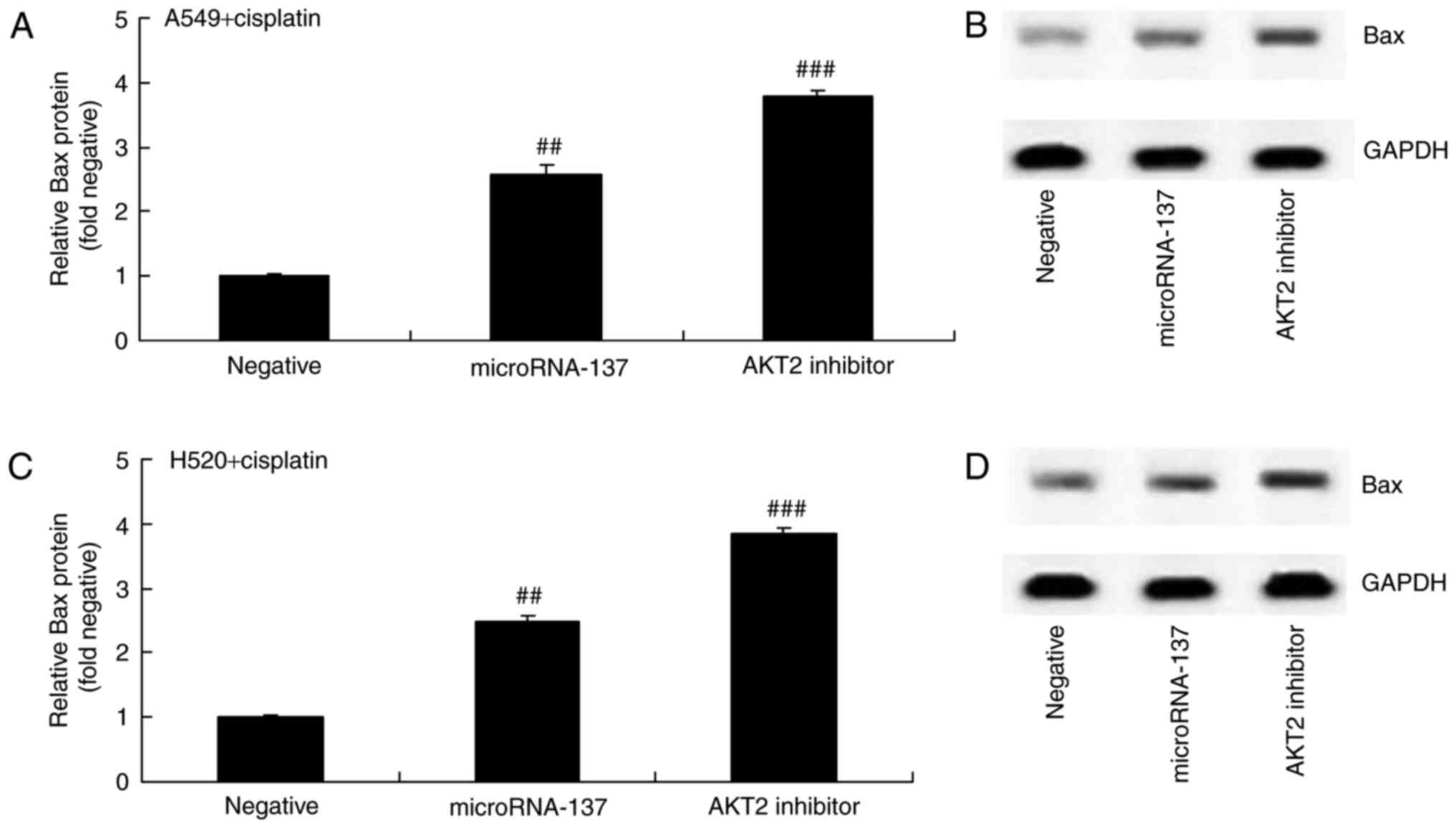
Effect of an AKT2 inhibitor on Bax
protein expression in A549 and H520 cells treated with cisplatin
following downregulation of microRNA-137
The present study investigated the effect of AKT2
inhibition on Bax protein expression in A549 and H520 cells treated
with cisplatin following downregulation of microRNA-137. The
results demonstrated that the suppression of AKT2 significantly
increased Bax protein expression in A549 and H520 cells treated
with cisplatin following downregulation of microRNA-137, compared
with cells transfected with microRNA-Negative (Fig. 8).
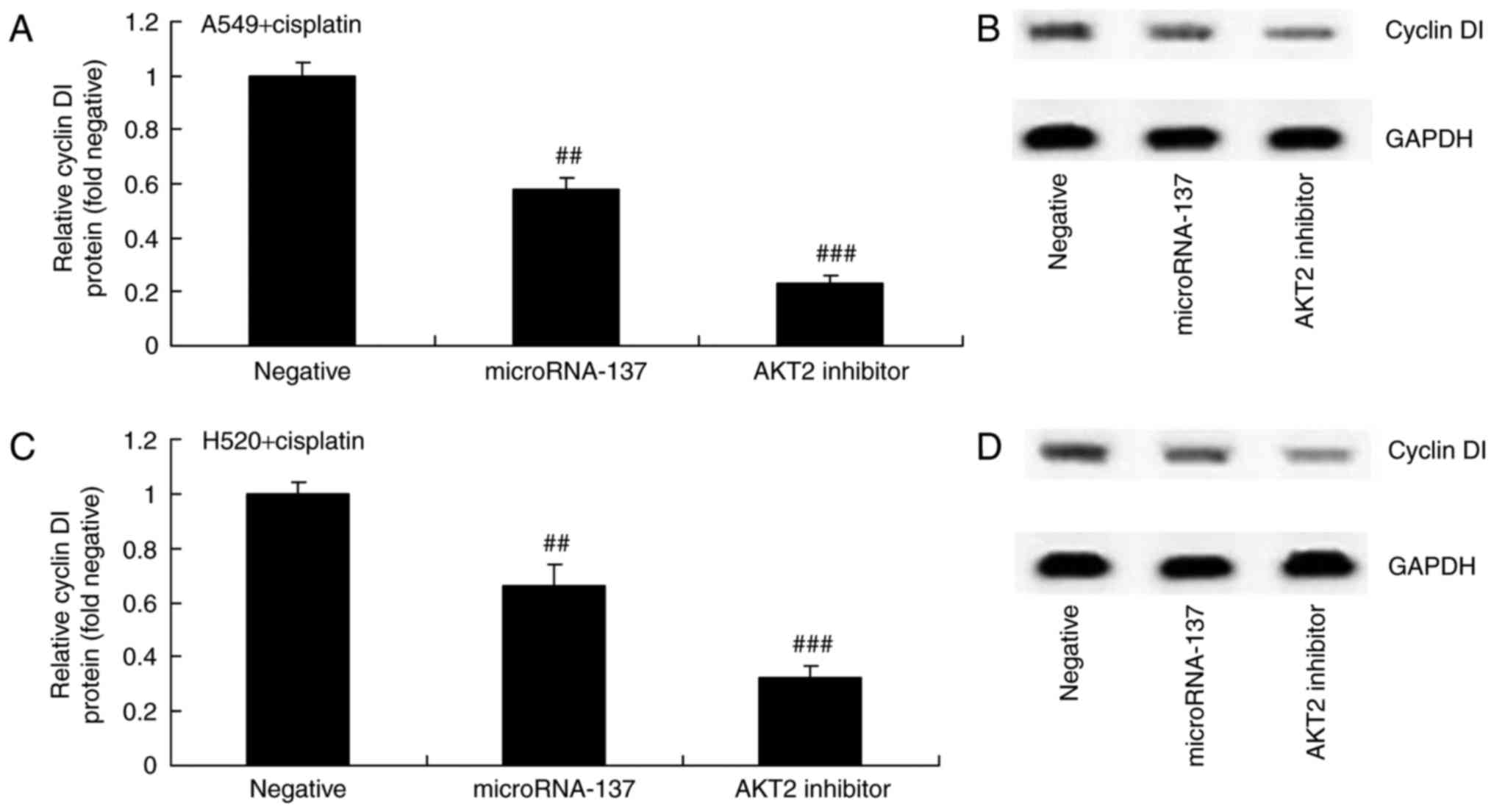
Effect of an AKT2 inhibitor on Cyclin
D1 protein expression in A549 and H520 cells treated with cisplatin
following downregulation of microRNA-137
The effect of AKT2 suppression on Cyclin D1 protein
expression in A549 and H520 cells treated with cisplatin following
downregulation of microRNA-137 was investigated. Downregulation of
microRNA-137 significantly suppressed the protein expression of
Cyclin D1 in A549 and H520 cells treated with cisplatin (Fig. 9).
Discussion
Lung cancer is regarded as a malignant tumor with
the highest rate of morbidity and mortality of all types of cancer.
NSCLC is the most common type of lung cancer, accounting for
>70% of all lung cancer cases (20). At present, a multidisciplinary
treatment strategy is employed for the treatment of NSCLC (4). Platinum-based chemotherapeutics,
including cis-platinum and carboplatin, are widely applied
anticancer chemotherapeutics (4).
Furthermore, these agents also exhibit the most efficient curative
effects and are more cytotoxic (5).
The present study revealed that the expression of microRNA-137 in
cisplatin-treated NSCLC patient tissue samples was significantly
lower than that of normal tissue samples.
Expression of microRNA-137 in adult brain glioma is
markedly reduced and may even participate in the morbidity of
schizophrenia by regulating the maturity of neurons in the nervous
system (21). microRNA-137 serves a
function in numerous diseases by participating in the regulation of
the cell cycle and cell differentiation (22). The results of the present study
demonstrated that the DFS and OS rates of patients with NSCLC
exhibiting a high expression of microRNA-137 were higher than those
of patients with NSCLC exhibiting a low expression of microRNA-137.
Additionally, it was also revealed that overexpression of
microRNA-137 significantly inhibited cell proliferation and
increased caspase-3 activity in A549 and H520 cells treated with
cisplatin.
Previous studies have demonstrated that the
development of lung cancer involves multiple factors, the formation
of numerous steps and the participation of various genes (23). The regulation of cell proliferation
and apoptosis serves an important role in the progression of
numerous stages in the development of NSCLC (24). Apoptosis is also known as programmed
cell death and is crucial in the development and stabilization of
multi-cellular organisms (25). In
particular, it serves an important role in embryonic development
modeling, the regulation of cells and the elimination of cells with
potential risks. Programmed cell death primarily includes two
pathways, namely the death receptor pathway (exogenous pathway) and
the mitochondrial pathway (endogenous pathway) (24). By virtue of a series of molecules and
biochemical pathways, the common ‘central processing unit
molecule’, namely excitation of Caspases belonging to two pathways,
induces degradation of associated substrates in the nucleus and
cytoplasm. Bax is an important regulatory factor of apoptosis. The
Bax gene belongs to the B cell lymphoma-2 family and serves a role
in the promotion of apoptosis (26).
The results of the present study demonstrated that the
overexpression of microRNA-137 significantly induced the expression
of Bax protein in A549 and H520 cells treated with cisplatin.
Research on cell strains demonstrated that p-AKT is
able to upregulate Cyclin D1 expression through multiple pathways
and that it promotes cell proliferation-it phosphorylates
downstream mTOR, which phosphorylates P70s6k (27). The activated P70s6k
mediates ribosomal protein. The cell cycle G1-S phases transform
the crucial translation of miRNAs (28). By phosphorylating GSK3 and resisting
the degradation of β-Catenin, β-Catenin accumulation in the
cytoplasm enters into the nucleus and lymphocyte enhancer to launch
the transcription of cytokines (LEF/TCF), as well as to upregulate
Cyclin D1 expression (29). In
conclusion, the results of the present study revealed that
overexpression of microRNA-137 significantly suppressed p-AKT2
protein expression and inhibited Cyclin D1 protein expression in
A549 and H520 cells treated with cisplatin.
A previous study on AKT revealed that AKT may
promote the proliferation of tumor cells by phosphorylating
multiple downstream substrates, restraining apoptosis, improving
the anoxia tolerance of cells, promoting angiogenesis, promoting
the invasion and metastasis of tumor cells, and promoting
resistance to chemotherapy and radiotherapy (17). In numerous tumor tissues, AKT is
overexpressed and exhibits increased activity (expressed as
phosphorylation). AKT2 is an important subtype of the AKT gene and
has already been confirmed to be an oncogene (30). In multiple types of tumor tissues
there is an overexpression or increased activity of the AKT2
protein. It has been proven that the PI3K/AKT pathway participates
in the generation and development of multiple types of tumors
(30). The results of the present
study revealed that the inhibition of AKT2 also increased caspase-3
activity and Bax protein expression, and suppressed Cyclin D1
protein expression in A549 and H520 cells treated with cisplatin
following overexpression of microRNA-137.
In conclusion, the results of the present study
revealed that the overexpression of microRNA-137 inhibited cell
proliferation and increased the caspase-3 activity of A549 and H520
cells treated with cisplatin through suppression of Cyclin D1 and
activation of the Bax pathway by targeting AKT2. Therefore,
microRNA-137-regulated AKT2 may be a potential therapeutic strategy
for the treatment of patients with NSCLC using cisplatin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZL designed the experiment. MW, SW, MY, ZL, TS and
CD performed the experiment. ZL and MW analyzed the data. ZL wrote
the manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committees of Jiangmen Central Hospital and written informed
consent was obtained from all participants.
Consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao Z, Liao H and Ju Y: Effect of
compound Kushen injection on T-cell subgroups and natural killer
cells in patients with locally advanced non-small-cell lung cancer
treated with concomitant radiochemotherapy. J Tradit Chin Med.
36:14–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Videtic GM, Hu C, Singh AK, Chang JY,
Parker W, Olivier KR, Schild SE, Komaki R, Urbanic JJ and Choy H: A
randomized phase 2 study comparing 2 stereotactic body radiation
therapy schedules for medically inoperable patients with stage I
peripheral non-small cell lung cancer: NRG oncology RTOG 0915
(NCCTG N0927). Int J Radiat Oncol Biol Phys. 93:757–764. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zinner RG, Obasaju CK, Spigel DR, Weaver
RW, Beck JT, Waterhouse DM, Modiano MR, Hrinczenko B, Nikolinakos
PG, Liu J, et al: PRONOUNCE: Randomized, open-label, phase III
study of first-line pemetrexed + carboplatin followed by
maintenance pemetrexed versus paclitaxel + carboplatin +
bevacizumab followed by maintenance bevacizumab in patients ith
advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol.
10:134–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semrau S, Zettl H, Hildebrandt G, Klautke
G and Fietkau R: Older patients with inoperable non-small cell lung
cancer: Long-term survival after concurrent chemoradiotherapy.
Strahlenther Onkol. 190:1125–1132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu W and Zhang Z: A phase II clinical
study of using nab-paclitaxel as second-line chemotherapy for
Chinese patients with advanced non-small cell lung cancer. Med
Oncol. 32:4982015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKenna E, Traganos F, Zhao H and
Darzynkiewicz Z: Persistent DNA damage caused by low levels of
mitomycin C induces irreversible cell senescence. Cell Cycle.
11:3132–3140. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Qi K, Zu L, Wang M, Wang Y and Zhou
Q: Anti-apoptotic brain and reproductive organ-expressed proteins
enhance cisplatin resistance in lung cancer cells via the protein
kinase B signaling pathway. Thorac Cancer. 7:190–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Paz-Ares LG, de Marinis F,
Molinier O, Sahoo TP, Laack E, John W, Zimmermann AH, Visseren-Grul
C and Gridelli C: PARAMOUNT: Descriptive subgroup analyses of final
overall survival for the phase III study of maintenance pemetrexed
versus placebo following induction treatment with pemetrexed plus
cisplatin for advanced nonsquamous non-small-cell lung cancer. J
Thorac Oncol. 9:205–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Yuan Y, Fu C, Xu X, Zhou J, Wang
S, Kong L, Li Z, Guo Q and Wei L: LZ-106, a novel analog of
enoxacin, inducing apoptosis via activation of ROS-dependent DNA
damage response in NSCLCs. Free Radic Biol Med. 95:155–168. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petrelli F and Barni S: Non-cancer-related
mortality after cisplatin-based adjuvant chemotherapy for non-small
cell lung cancer: A study-level meta-analysis of 16 randomized
trials. Med Oncol. 30:6412013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niho S, Kenmotsu H, Sekine I, Ishii G,
Ishikawa Y, Noguchi M, Oshita F, Watanabe S, Nakajima R, Tada H and
Nagai K: Combination chemotherapy with irinotecan and cisplatin for
large-cell neuroendocrine carcinoma of the lung: A multicenter
phase II study. J Thorac Oncol. 8:980–984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Yang Z, Chen M and Liu Y:
Downregulation of microRNA-196a enhances the sensitivity of
non-small cell lung cancer cells to cisplatin treatment. Int J Mol
Med. 37:1067–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu L, Pu X, Wang Q, Cao J, Xu F, Xu LI and
Li K: miR-96 induces cisplatin chemoresistance in non-small cell
lung cancer cells by downregulating SAMD9. Oncol Lett. 11:945–952.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Zhang L, Yin ZY, Fan XL, Hu B,
Wang LQ and Zhang D: miR-107 regulates cisplatin chemosensitivity
of A549 non small cell lung cancer cell line by targeting cyclin
dependent kinase 8. Int J Clin Exp Pathol. 7:7236–7241.
2014.PubMed/NCBI
|
|
15
|
Berghmans T, Ameye L, Lafitte JJ, Colinet
B, Cortot A, CsToth I, Holbrechts S, Lecomte J, Mascaux C, Meert
AP, et al: Prospective validation obtained in a similar group of
patients and with similar high throughput biological tests failed
to confirm signatures for prediction of response to chemotherapy
and survival in advanced NSCLC: A prospective study from the
european lung cancer working party. Front Oncol. 4:3862015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu W, Jin P and Liu W: Periostin
contributes to cisplatin resistance in human non-small cell lung
cancer A549 cells via activation of Stat3 and Akt and upregulation
of survivin. Cell Physiol Biochem. 38:1199–1208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu HH, Wu JY, Cheng YW, Chen CY, Lee MC,
Goan YG and Lee H: cIAP2 upregulated by E6 oncoprotein via
epidermal growth factor receptor/phosphatidylinositol 3-kinase/AKT
pathway confers resistance to cisplatin in human papillomavirus
16/18-infected lung cancer. Clin Cancer Res. 16:5200–5210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu B, Zhang Y, Jia L, Wu H, Fan C, Sun Y,
Ye C, Liao M and Zhou J: Binding of the pathogen receptor HSP90AA1
to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR
pathway. Autophagy. 11:503–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sebastian M, Papachristofilou A, Weiss C,
Früh M, Cathomas R, Hilbe W, Wehler T, Rippin G, Koch SD, Scheel B,
et al: Phase Ib study evaluating a self-adjuvanted mRNA cancer
vaccine (RNActive®) combined with local radiation as
consolidation and maintenance treatment for patients with stage IV
non-small cell lung cancer. BMC Cancer. 14:7482014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neault M, Mallette FA and Richard S:
miR-137 modulates a tumor suppressor Network-inducing senescence in
pancreatic cancer cells. Cell Rep. 14:1966–1978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin KK, Kim YS, Kim JY, Bae YC and Jung
JS: miR-137 controls proliferation and differentiation of human
adipose tissue stromal cells. Cell Physiol Biochem. 33:758–768.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen B, Wang X, Zhao W and Wu J: Klotho
inhibits growth and promotes apoptosis in human lung cancer cell
line A549. J Exp Clin Cancer Res. 29:992010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang C, Zhang J, Qi D, Fan X, Luo J, Liu L
and Tan Q: Evodiamine induces G2/M arrest and apoptosis via
mitochondrial and endoplasmic reticulum pathways in H446 and H1688
human small-cell lung cancer cells. PLoS One. 9:e1152042014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chougule M, Patel AR, Sachdeva P, Jackson
T and Singh M: Anticancer activity of Noscapine, an opioid alkaloid
in combination with Cisplatin in human non-small cell lung cancer.
Lung Cancer. 71:271–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng Y, Wang L, Qing Y, Li C, Ren T, Li Q,
Li M, Zhang S, Shan J, Wang G, et al: Polymorphisms of BCL2 and BAX
Genes associate with outcomes in advanced Non-small cell lung
cancer patients treated with platinum-based Chemotherapy. Sci Rep.
5:177662015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Żuryń A, Litwiniec A, Safiejko-Mroczka B,
Klimaszewska-Wiśniewska A, Gagat M, Krajewski A, Gackowska L and
Grzanka D: The effect of sulforaphane on the cell cycle, apoptosis
and expression of cyclin D1 and p21 in the A549 non-small cell lung
cancer cell line. Int J Oncol. 48:2521–2533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Cao Y, Yang D, Li K, Wang Z, Zhu
J, Bunjhoo H, Xiong S, Xu Y and Xiong W: Increase of the
therapeutic effect on non-small-cell lung cancer cells with
combination treatment of shRNA against Cyclin D1 and Bcl-xL in
vitro. Exp Ther Med. 3:255–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh T, Prasad R and Katiyar SK:
Inhibition of class I histone deacetylases in non-small cell lung
cancer by honokiol leads to suppression of cancer cell growth and
induction of cell death in vitro and in vivo. Epigenetics. 8:54–65.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong
SW, Li RL, Zhao MY, Zhen Y, Yu XL, et al: Alpha-enolase promotes
cell glycolysis, growth, migration, and invasion in non-small cell
lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol.
8:222015. View Article : Google Scholar : PubMed/NCBI
|