Introduction
Osteosarcoma (OS) is the most common type of primary
malignant bone tumor in children and young adults, originating from
mesenchymal cells and is characterized by the production of
immature osteoid (1,2). Due to the combination of surgery and
multi-agent chemotherapy, the 5-year survival rate of
non-metastatic OS has risen from 25 to 60% in the last decade
(3). However, >20% patients with
OS exhibit lung metastases at initial diagnosis. Lung metastasis
occurs following surgery in ~80% patients with OS (4,5).
Therefore, the 5-year survival rate for metastatic OS is only 30%,
a figure which has remained constant for 30 years (6). Previous studies reported a variety of
genetic alterations in OS, however, the molecular mechanism
underlying OS development remains to be elucidated (7–9).
Therefore, there is an urgent requirement to characterize the
molecular mechanisms underlying OS development in order to identify
novel and effective targets for OS chemotherapy.
The Skp1-cullin-F-box (SCF) complex is a member of
the E3 ubiquitin ligase family, involved in substrate recognition,
ubiquitination recruitment and degradation in the ubiquitin
proteasome system (10). The F-box
family proteins belong to a critical subunit of the SCF complex,
characterized by their ~40 amino-acid motifs. F-box proteins
recognize and combine substrates in the SCF complex, and
participate in a series of cellular processes, including cell cycle
and immune responses (10–13). It has been reported that F-box protein
39 (FBXO39) was highly expressed in normal human testis and
abnormally expressed in a variety of cancer cell types (14,15).
Furthermore, it has been demonstrated that patients with colon
cancer exhibit a high level of serum FBXO39 expression
compared with healthy individuals (14,15).
In the present study, it was demonstrated that
FBXO39 was highly expressed in OS, and that FBXO39
knockdown inhibited cell proliferation and promoted apoptosis in
human osteosarcoma U-2OS cells.
Materials and methods
Cell culture
The human OS cell lines, HOS, SaOS-2 and U-2OS, were
purchased from the American Type Culture Collection (Manassas, VA,
USA). All cells were cultured in Dulbecco's Modified Eagle's Medium
(DMEM; Corning Incorporated, Corning, NY, USA) supplemented with
10% fetal bovine serum (FBS; Vian-saga, Shanghai, China) at 37°C in
a humidified atmosphere containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from HOS, Saos-2 and U-2OS
cells using TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Reverse transcription was performed using M-MLV
Reverse Transcriptase, RNase Inhibitor and dNTPs (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol. RT-qPCR was performed using SYBR Master mix (Takara
Biotechnology Co., Ltd., Dalian) and a Roche Light Cycler 480
Real-time PCR system (Roche Diagnostics, Basel, Switzerland). GAPDH
was used as an internal control gene. Quantification was performed
using the 2−ΔΔCq method (16). The primer sequences were as follows:
FBXO395 forward, 5′-GATGGGCAAACGCCTGGATTA-3′ and reverse,
5′-GGAGGGTGCTGGCATTCTCAC-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′, and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The thermal cycle included: 95°C for
15 sec, 45 cycles of 95°C for 5 sec, and 60°C for 30 sec.
Lentivirus-mediated short hairpin RNA
(shRNA) FBXO39 knockdown
The lentivirus-mediated shRNA vector system was
designed, constructed, packed and purified by Shanghai GeneChem Co,
Ltd. (Shanghai, China), and all procedures were performed according
to the manufacturer's protocol. Cells transfected with lentivirus
containing human FBXO39 shRNA were the experimental group, denoted
as shFBXO39 group in the subsequent experiments. Cells transfected
with lentivirus containing blank shRNA were used as a negative
control, denoted as shCtrl group. The multiplicity of infection was
10 (2×105 cells transfected per well and
2×106 TU lentivirus transfected per well in a 6-well
plate), and the infection was proceeded with the addition of DMEM
and 5 µg/ml Polybrene (Clontech Laboratories, Inc., Mountainview,
CA, USA) to the cells. The fluorescent microscopy (Olympus IX71;
Olympus Corporation, Tokyo, Japan) demonstrated that the
transfection efficiency of U-2OS cells transfected with shFBXO39
and shCtrl lentivirus was >80%. The knockdown efficiency of the
target gene was detected by RT-qPCR and western blotting 3 days
after transfection.
Western blot analysis
Western blotting was used to validate lentiviral
knockdown efficiency at the protein level in U-2OS cells. Proteins
were extracted from cells using a lysis buffer (2%
2-mercaptoethanol, 4% SDS and 20% glycerol, 100 mM Tris-HCl), and
the protein concentration was measured using a bicinchoninic acid
(BCA) Protein Assay kit (Beyotime Institute of Biotechnology,
Haimen, China). A total of 20 µg protein was separated by SDS-PAGE
(10% gels), and transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). The plasmid was constructed
with a flag epitope, and GAPDH was used as a loading control. The
membranes were blocked in Tris-buffered saline with Tween (TBST)
containing 5% non-fat milk overnight at 4°C. The membranes were
then incubated with the following primary antibodies: Flag
(dilution, 1:2,000; cat. no. F1804; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and GAPDH (dilution, 1:2,000; cat. no.
sc-32233; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. The membranes were then washed three times in
TBST. The membranes were incubated with a horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin secondary
antibody (dilution, 1:2,000; cat. no. sc-2005; Santa-Cruz
Biotechnology, Inc.) for 2 h at room temperature, then washed three
times in TBST. Bands were visualized using enhanced
chemiluminescence (ECL; Pierce; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol.
Celigo analysis
U-2OS cells were seeded at a density of
2×103 cells/well in a 96-well plate at 72 h
post-transfection (at 37°C in an atmosphere of 5% CO2).
After plating, Celigo® Image Cytometer (Nexcelom,
Lawrence, MA, USA) was used to evaluate the number of cells by
scanning green fluorescence daily for 5 days at room temperature
(17,18).
MTT assay
MTT assay was used to analyze the effect of
FBXO39 knockdown on cell proliferation (19). Cells transfected with shCtrl and
shFBXO39 were seeded in 96-well plates at a density of
2×103 cells/well at 72 h post-transfection. A total of
20 µl 5 mg/ml MTT was added to each well for 4 h at 37°C. Then, the
medium was discarded and 110 µl dimethyl sulfoxide (Shanghai Test
Chemical Reagent Co, Ltd., Shanghai. China) was added to each well
to dissolve the formazan crystals. The optical density (OD) was
detected at 490 nm using M2009PR Multifunctional Microplate Reader
(Tecan Group, Ltd., Mannedorf, Switzerland).
Caspase 3/7 activity analysis
Cells transfected with shCtrl or shFBXO39
were seeded in 96-well plates and incubated at 37°C for 5 days.
Caspase-Glo reaction solution was made up by mixing 10 ml
Caspase-Glo 3/7 buffer solution and Caspase-Glo 3/7 substrate
(Caspase-Glo® 3/7 Assay; G8091; Promega Corporation,
Madison, WI, USA). A total of 100 µl Caspase-Glo reaction solution
was added per well, each well containing 1×104 cells.
Following a 2-h incubation at room temperature, a microplate reader
was used to detect the luminescence intensity at 570 nm.
FACS analysis
Fluorescence-activated cell sorting (FACS) was used
to analyze cell apoptosis (20).
Cells transfected with shCtrl or shFBXO39 were plated in
6-cm dishes 5 days after transfection and grown to 70% confluence.
Following washing with binding buffer [from the Annexin
V-allophycocyanin (APC) Detection kit (cat. no. 88-8007;
eBioscience; Thermo Fisher Scientific, Inc.] once, cells were
stained with 200 µl binding buffer containing 10 µl APC Detection
kit for 10–15 min at room temperature in the dark. A flow cytometer
(Merck KGaA) and InCyte 3.1 (Merck KGaA) were then used to analyze
the cells.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp, Armonk, NY, USA). All data are presented as the
mean of ≥3 independent experiments. The error bars represent
standard deviation. Differences between 2 groups were determined
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
U-2OS cells express a high level of
FBXO39 compared with HOS and SaOS-2 cells
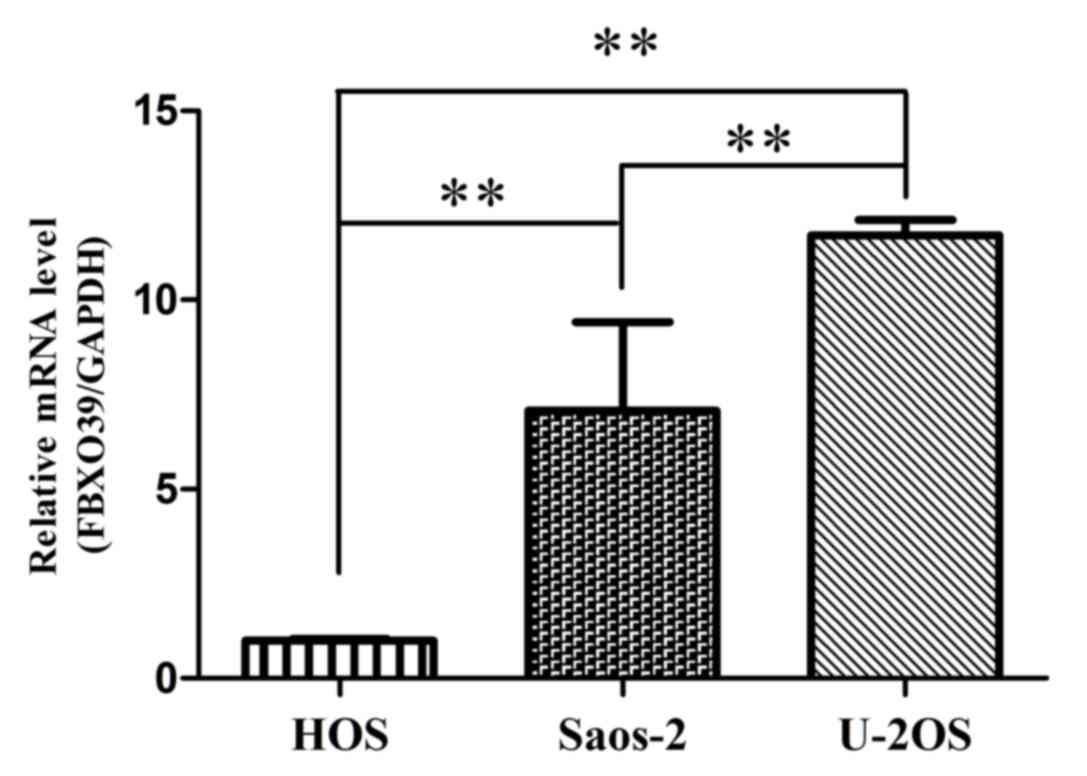
The expression level of FBX039 mRNA was
analyzed in OS cells lines using RT-qPCR (Fig. 1). It was demonstrated that the U-2OS
cell line exhibited the highest expression of FBXO39 among
the three cell lines. Based on this result, U-2OS cells were
selected for subsequent experiments.
FBXO39 knockdown in U-2OS cells by
lentiviral transfection
To investigate the role of FBXO39 on OS cell
proliferation and apoptosis, FBXO39 was knocked down using
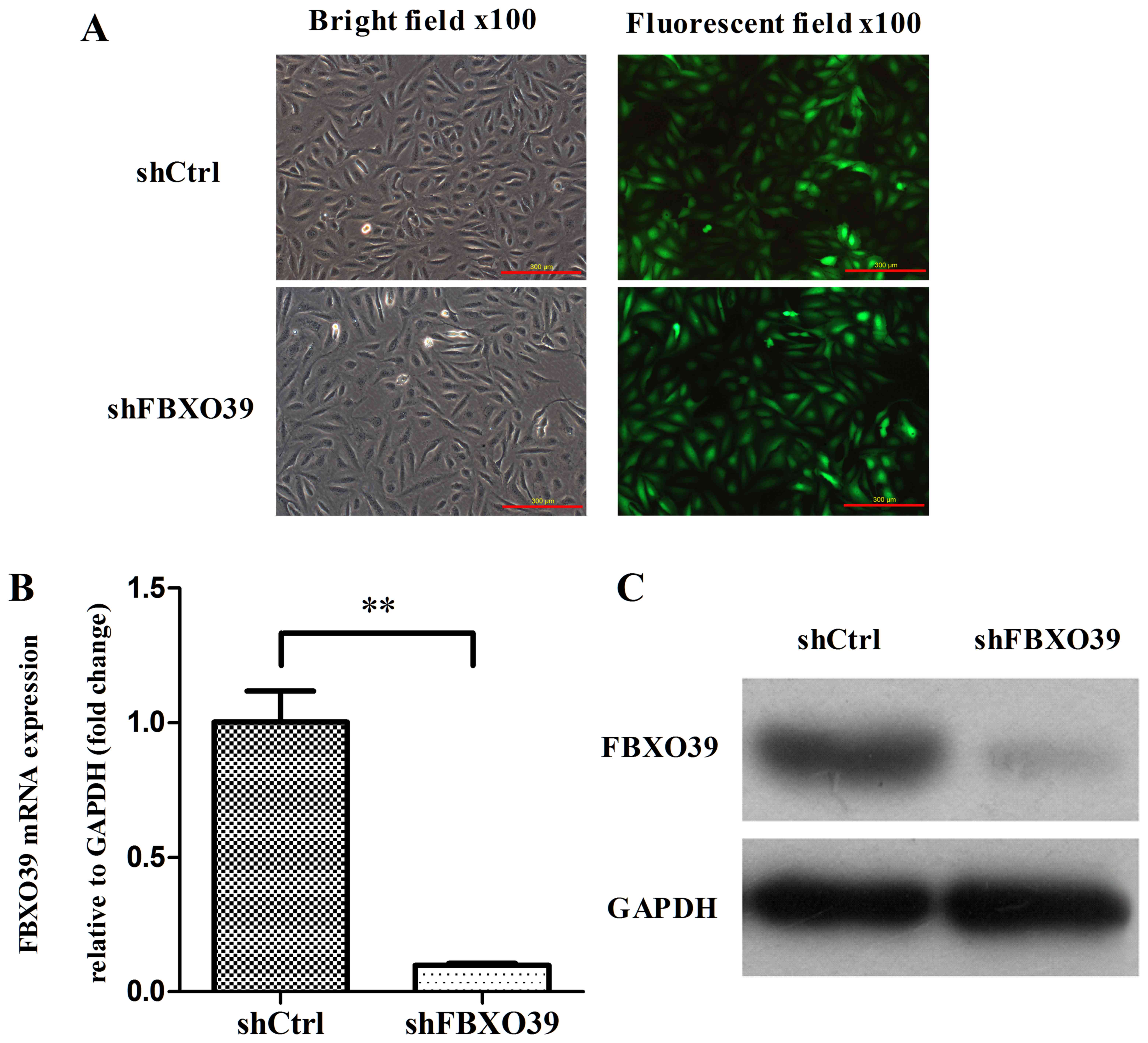
lentiviral vectors. Transfection efficiency was assessed by
fluorescence imaging 72 h after transfection (Fig. 2A). According to the results, >80%
cells transfected with lentivirus expressed green fluorescent
protein (data not shown). RT-qPCR and western blotting analyses
were conducted to assess the knockdown efficiency on the mRNA and
protein levels, respectively (Fig.
2A-C). The mRNA expression level of FBXO39 in U-2OS
cells transfected with shFBXO39 was significantly decreased
compared with that in cells transfected with shCtrl (P<0.01).
Similarly, the FBXO39 protein expression level was decreased
in U-2OS cells transfected with shFBXO39 compared with cells
transfected with shCtrl (Fig. 2C).
Thus, FBX039 knockdown reduced FBXO39 expression at
the mRNA and protein level.
FBXO39 knockdown inhibits the
proliferation of U-2OS cells
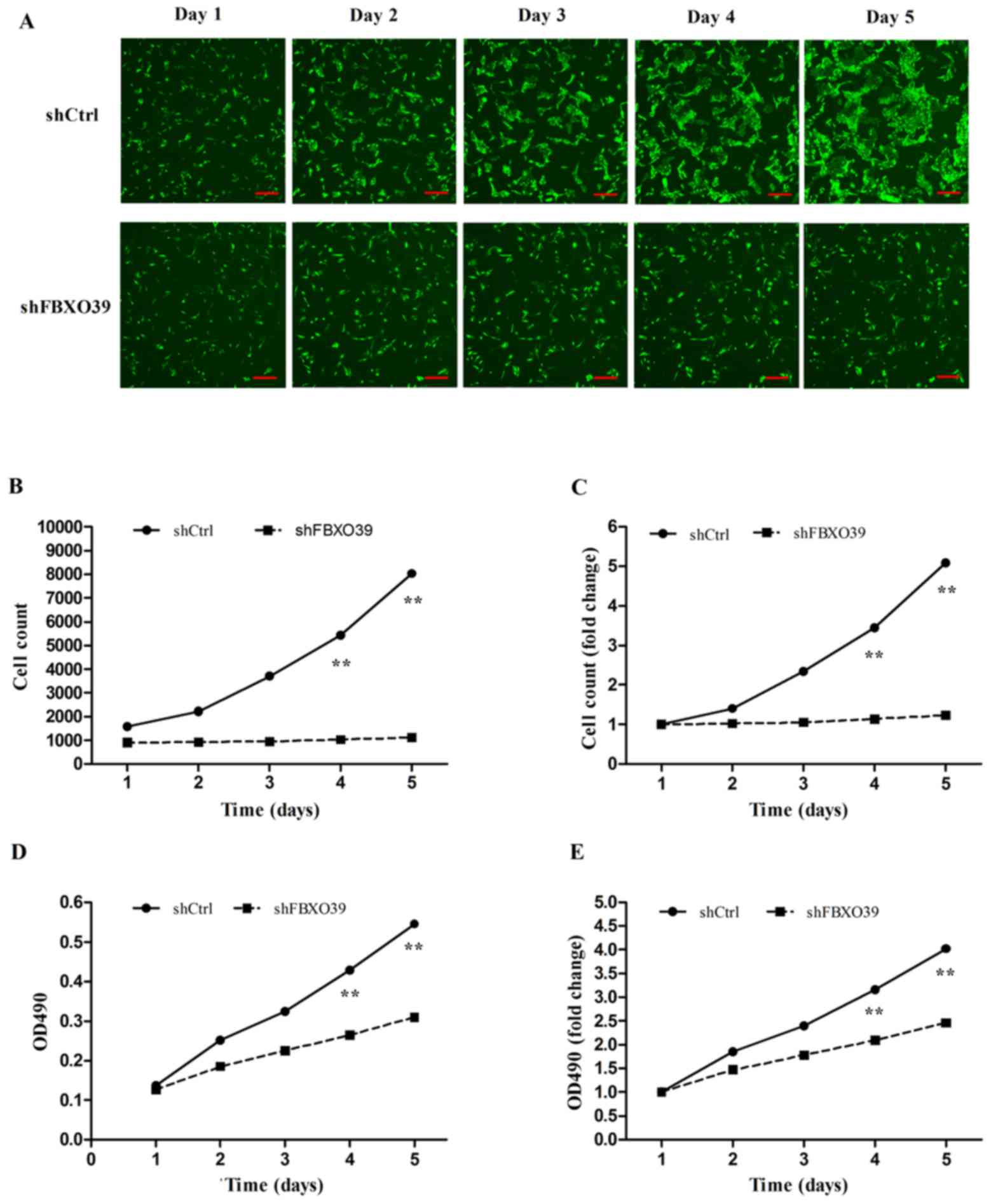
To investigate the effect of FBXO39 knockdown
on cell proliferation in U-2OS cells, Celigo analysis and MTT
assays were performed. Celigo analysis revealed that the
proliferation of cells transfected with shFBOX39 was
significantly inhibited 5 days after transfection compared with
control (P<0.01; Fig. 3A-C). On
day 5, the cell count of cells transfected with shCtrl was
5.08-fold greater compared with that on day 1, whereas the cell
count of cells transfected with shFBXO39 only increased by
1.23-fold (Fig. 3C). The MTT assay
also demonstrated that FBXO39 knockdown inhibited
proliferation compared with control (P<0.01; Fig. 3D and E). In conclusion, FBXO39
knockdown inhibited proliferation of U-2OS cells.
FBXO39 knockdown promotes apoptosis of
U-2OS cells
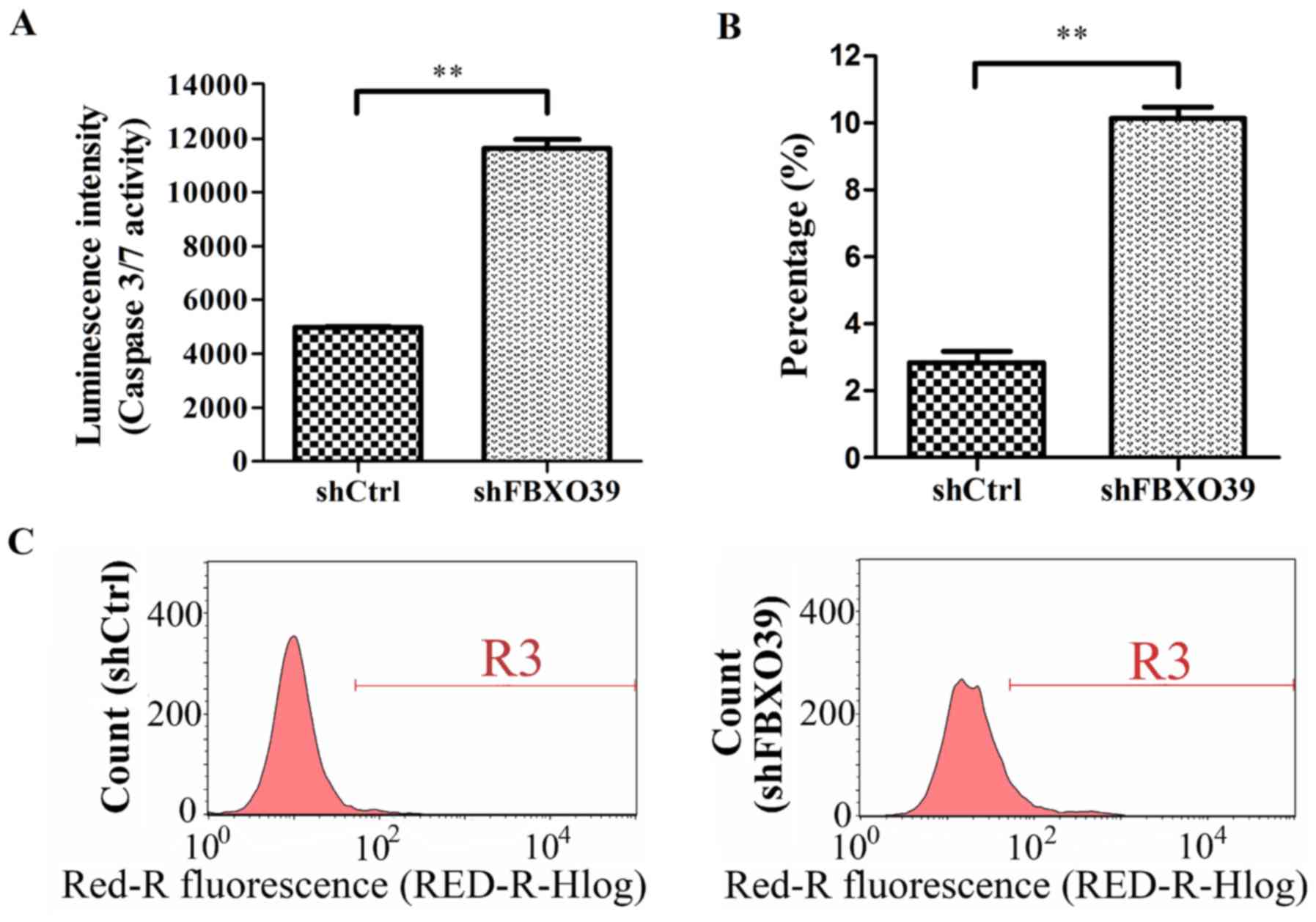
To investigate the effect of FBXO39 knockdown
on U-2OS cell apoptosis, cells transfected with shFBXO39 and
shCtrl were subjected to FACS analysis and caspase 3/7 activity
analysis (Fig. 4A-C). FACS analysis
revealed a significant increase in the apoptotic rate in
shFBXO39 cells compared with shCtrl cells 5 days after
transfection (P<0.01; Fig. 4B and
C). Cell apoptosis was further analyzed by detecting activated
caspase 3/7. In this assay, it was demonstrated that cells
transfected with shFBXO39 exhibited an increased level of
activated caspase 3/7 activity compared with that in cells
transfected with shCtrl (P<0.01; Fig.
4A). In conclusion, FBXO39 knockdown promoted apoptosis
in U-2OS cells.
Discussion
Despite efforts to improve the diagnosis and therapy
in patients with OS, the 5-year survival rate and prognosis of
patients with OS remains poor (9). An
improved understanding of OS progression is required for the
development of novel therapies. Previous studies have reported
several genes associated with OS, including MYC, FBJ murine
osteosarcoma viral oncogene homolog, Mouse double minute 2, RECQ
helicase, tumor protein P53, retinoblastoma 1, kruppel like factor
8 and collagen triple helix repeat containing 1
(21–24).
F-box proteins serve a key role in the regulation of
cellular functions, including proliferation, apoptosis and immune
responses, by detecting and recruiting substrates for
ubiquitination (10). F-box proteins
are associated with the carcinogenesis and progression of various
types of cancer. For example, S-phase kinase associated protein
2 has been demonstrated to be highly expressed in cancer cells,
and its downregulation may promote oncogenesis (25). In contrast, F-box and WD repeat
domain containing 7 (FBXW7) has been demonstrated to
function as a tumor-suppressor in several types of cancer, and
upregulation of FBXW7 has been indicated to reduce cancer
cell proliferation and migration (26–28).
FBXO39 has been demonstrated to be highly expressed in
normal testis tissue and several types of cancer (14,15). The
association between OS and FBXO39 was explored in U-2OS
cells in the present study.
The results of the present study revealed that U-2OS
cells exhibited the highest expression level of FBXO39 among
OS cell lines. Additionally, a significant decrease in
proliferation was observed in response to FBXO39
downregulation compared with control as assessed by Celigo and MTT
analysis. These results suggest that FBXO39 may promote
proliferation in OS cells. Furthermore, FACS and caspase 3/7
analysis revealed that FBXO39 knockdown in U-2OS cells
caused an increase in the rate of apoptosis compared with control.
These results demonstrate that FBXO39 knockdown inhibited
proliferation and promoted apoptosis of human OS cells.
In conclusion, the present study indicates that
FBXO39 serves an important role in OS carcinogenesis and
progression. Future studies that examine cellular function,
including migration and invasion, as well as signaling pathways and
in vivo experiments are required to elucidate the role of
FBXO39 in OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Sanming
Project of Medicine in Shenzhen; The National Natural Science
Foundation of China (grant no. 21602137) and the Shenzhen Health
and Family Planning Science Project (grant no. 201501014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and JZ conceived and designed the study. CZ, PW,
JC, XJ, ZZhu, ZZha and AG performed the experiments, and collected
and interpreted the data. SZ, JZ, WY, WL and JT analyzed the data
and edited the draft manuscript. TJ and WG analyzed the data and
revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript, and
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heare T, Hensley MA and Dell'Orfano S:
Bone tumors: Osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr.
21:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caudill JS and Arndt CA: Diagnosis and
management of bone malignancy in adolescence. Adolesc Med State Art
Rev. 18:62–78, ix. 2007.PubMed/NCBI
|
|
4
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bridge JA, Nelson M, McComb E, McGuire MH,
Rosenthal H, Vergara G, Maale GE, Spanier S and Neff JR:
Cytogenetic findings in 73 osteosarcoma specimens and a review of
the literature. Cancer Genet Cytogenet. 95:74–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Helman LJ and Meltzer P: Mechanisms of
sarcoma development. Nat Rev Cancer. 3:685–694. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kipreos ET and Pagano M: The F-box protein
family. Genome Biol. 1:REVIEWS3002. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Cardozo T, Lovering RC, Elledge SJ,
Pagano M and Harper JW: Systematic analysis and nomenclature of
mammalian F-box proteins. Genes Dev. 18:2573–2580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho MS, Ou C, Chan YR, Chien CT and Pi H:
The utility F-box for protein destruction. Cell Mol Life Sci.
65:1977–2000. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uddin S, Bhat AA, Krishnankutty R, Mir F,
Kulinski M and Mohammad RM: Involvement of F-BOX proteins in
progression and development of human malignancies. Semin Cancer
Biol. 36:18–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song MH, Ha JC, Lee SM, Park YM and Lee
SY: Identification of BCP-20 (FBXO39) as a cancer/testis antigen
from colon cancer patients by SEREX. Biochem Biophys Res Commun.
408:195–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seifi-Alan M, Shamsi R, Ghafouri-Fard S,
Mirfakhraie R, Zare-Abdollahi D, Movafagh A, Modarressi MH, Kazemi
G, Geranpayeh L and Najafi-Ashtiani M: Expression analysis of two
cancer-testis genes, FBXO39 and TDRD4, in breast cancer tissues and
cell lines. Asian Pac J Cancer Prev. 14:6625–6629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nabzdyk CS, Chun M, Pradhan L and Logerfo
FW: High throughput RNAi assay optimization using adherent cell
cytometry. J Transl Med. 9:482011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vinci M, Gowan S, Boxall F, Patterson L,
Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D and Eccles
SA: Advances in establishment and analysis of three-dimensional
tumor spheroid-based functional assays for target validation and
drug evaluation. BMC Biol. 10:292012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moodley S, Koorbanally NA, Moodley T,
Ramjugernath D and Pillay M: The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay is a rapid, cheap, screening test for the in vitro
anti-tuberculous activity of chalcones. J Microbiol Methods.
104:72–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan Y, Shan W, Fang H, Guo M, Nie Z, Huang
Y and Yao S: Sensitive and visible detection of apoptotic cells on
Annexin-V modified substrate using aminophenylboronic acid modified
gold nanoparticles (APBA-GNPs) labeling. Biosens Bioelectron.
52:62–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smida J, Baumhoer D, Rosemann M, Walch A,
Bielack S, Poremba C, Remberger K, Korsching E, Scheurlen W,
Dierkes C, et al: Genomic alterations and allelic imbalances are
strong prognostic predictors in osteosarcoma. Clin Cancer Res.
16:4256–4267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walkley CR, Qudsi R, Sankaran VG, Perry
JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, et
al: Conditional mouse osteosarcoma, dependent on p53 loss and
potentiated by loss of Rb, mimics the human disease. Genes Dev.
22:1662–1676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin F, Shen Z, Tang LN, Zheng SE, Sun YJ,
Min DL and Yao Y: KLF8 knockdown suppresses proliferation and
invasion in human osteosarcoma cells. Mol Med Rep. 9:1613–1617.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sang W, Zhu L, Ma J, Lu H and Wang C:
Lentivirus-mediated knockdown of CTHRC1 inhibits osteosarcoma cell
proliferation and migration. Cancer Biother Radiopharm. 31:91–98.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin HK, Chen Z, Wang G, Nardella C, Lee
SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al: Skp2
targeting suppresses tumorigenesis by Arf-p53-independent cellular
senescence. Nature. 464:374–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Welcker M and Clurman BE: FBW7 ubiquitin
ligase: A tumour suppressor at the crossroads of cell division,
growth and differentiation. Nat Rev Cancer. 8:83–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grim JE, Knoblaugh SE, Guthrie KA, Hagar
A, Swanger J, Hespelt J, Delrow JJ, Small T, Grady WM, Nakayama KI
and Clurman BE: Fbw7 and p53 cooperatively suppress advanced and
chromosomally unstable intestinal cancer. Mol Cell Biol.
32:2160–2167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crusio KM, King B, Reavie LB and Aifantis
I: The ubiquitous nature of cancer: The role of the SCF(Fbw7)
complex in development and transformation. Oncogene. 29:4865–4873.
2010. View Article : Google Scholar : PubMed/NCBI
|