Introduction
Lacrimal apparatus includes lacrimal gland and
passage, and lacrimal passage includes lacrimal punctum, ductule,
sac and naso-lacrymal duct. Lacrimal gland exerts the secretion
function, while lacrimal passage exerts the excretory function.
Lacrimal gland mass is rare, and lacrimal gland mass in palpebral
part is even rarer than that in orbital part; but incidence of
tumors is the highest in lacrimal gland diseases. Inflammatory
lesions are common in lacrimal passage diseases, but malignant
tumors are rare. Color Doppler ultrasound applied in ophthalmic
diseases can not only clearly show the two-dimensional structures
of eyeballs and surrounding orbital wall tissues, but also display
the normal and abnormal blood flow conditions. Contrast-enhanced
ultrasound (CEUS) is a kind of imaging technique that the
ultrasonic contrast agent containing microbubbles is intravenously
injected to enhance the back-scattered blood flow signal in human
body, which can observe the microvascular perfusion in tissues in a
real-time and dynamic way, thereby providing more ultrasound
diagnostic information. At present, CEUS has been widely applied in
the diagnosis of liver tumors. However, the application of CEUS in
superficial organ tumors, especially eye tumors, is still in the
exploratory stage. Moreover, reports on CEUS applied in lacrimal
apparatus tumors are rare. This study investigated the application
values of two-dimensional, color Doppler ultrasound and CEUS in the
diagnosis of lacrimal apparatus tumors.
Patients and methods
Objects of study
A total of 48 patients pathologically and clinically
diagnosed with lacrimal apparatus tumors were treated in China
Meitan General Hospital (Beijing, China) from July 2016 to December
2017 underwent two-dimensional, color Doppler ultrasound and CEUS
examinations before operation, among which there were 18 males and
30 females with an average age of 38 years. This study was approved
by the Ethics Committee of China Meitan General Hospital (Beijing,
China) and written informed consents were signed by the patients or
their guardians.
Instruments and methods
MyLab 90 color Doppler ultrasound diagnostic
apparatus (Esaote SpA, Genoa, Italy) was used, and electronic
linear probe LA523 with a frequency of 4–13 MHz was used in
two-dimensional and color Doppler ultrasound. Real-time
contrast-tuned imaging (CnTI) technique was equipped in the
apparatus, and probe LA522 (3–9 MHz) was used in CEUS. SonoVue
(SF6) manufactured by Bracco (Milan, Italy) was used as ultrasonic
contrast agent. The average diameter of microbubbles was 2.5 µm,
and the contrast agent (59 µg) was used to make suspension (SF6
concentration, 5 mg/ml) with 5 ml normal saline. After intravenous
bolus injection of contrast agent, 5 ml normal saline was injected,
and patients were continuously observed for 3–5 min. Dynamic images
were saved for later analysis. Before contrast examination, the
patients signed an informed consent.
Under a supine position, patients closed both eyes
gently, and eyelids were coated with disinfectant coupling agent,
followed by axial and non-axial scanning to observe site, shape,
edge, size and echo of the mass, and color Doppler ultrasound was
used to observe the blood supply and other features of the mass.
Then the optimal section of the mass (the mass and some normal
choroid were shown in one section as far as possible) was selected,
and the contrast agent was injected under the contrast mode; at the
same time, the built-in timer of ultrasonic apparatus was started,
and the mass and surrounding tissues were observed in real-time for
~3-5 min; the whole process was videotaped.
According to CEUS features of 48 cases of masses,
enhancement degrees of masses were divided into three types: i)
Uniform enhancement: uniform filling of contrast agent in the mass;
ⅱ) non-uniform enhancement: Non-uniform filling of contrast agent
in the mass; non-filling area could be seen inside; and ⅲ) No
enhancement: No filling of contrast agent in the mass. There were
three types of enhancement modes: ⅰ) Overall enhancement: Overall
enhancement of filling of contrast agent in the mass; ⅱ)
centripetal enhancement: gradual enhancement of filling of contrast
agent in the mass from periphery area to inner area; and ⅲ)
circular enhancement: Circular enhancement of contrast agent only
around the mass. With the filling and extinction time of contrast
agent in normal retina and choroid near the mass as control, the
filling and extinction time of contrast agent in the mass was
observed: i) Rapid enhancement of the mass, named fast-developed:
The filling time of contrast agent in the mass is the same or
shorther than that in surrounding normal retina and choroid; ⅱ)
rapid extinction of the mass, named fast-extinct: The extinction
time of contrast agent in the mass is shorther than that in
surrounding normal retina and choroid; and ⅲ) slow extinction of
the mass, named slow-extinct: The extinction time of contrast agent
in the mass is longer or the same as that in surrounding normal
retina and choroid (1).
Results
The 48 pathologically diagnosed masses were 29 cases
with pleomorphic adenoma of lacrimal gland, 6 cases with adenoid
cystic carcinoma of lacrimal gland, 10 cases with lacrimal sac
cyst, and 3 cases with adenocarcinoma of lacrimal sac (Table I).
 | Table I.Analysis of two-dimensional ultrasound
and CEUS imaging of 29 cases of lacrimal gland pleomorphic adenoma
(n, %) (Fig. 1). |
Table I.
Analysis of two-dimensional ultrasound
and CEUS imaging of 29 cases of lacrimal gland pleomorphic adenoma
(n, %) (Fig. 1).
| Types | Cases |
|---|
| Male | 9 (31.03) |
| Female | 20 (68.97) |
| Age (years) |
|
|
<30 | 20 (68.97) |
|
30–50 | 3 (10.34) |
|
>50 | 22 (75.86) |
| Clear edge | 28 (96.55) |
| Unclear edge | 1 (3.45) |
| Internal echo dense
and uniform | 20 (68.97) |
| Inhomogeneous
calcification | 6 (20.69) |
| Echoic sheets | 3 (10.34) |
| Internal dotted blood
flow signal | 29 (100) |
| Contrast-enhanced
overall uniformity, fast forward and slow retreat | 20 (68.97) |
| High homogeneity of
centripetality, fast forward and slow retreat | 6 (20.69) |
| High inhomogeneity of
centripetality, fast forward and slow retreat | 3 (10.34) |
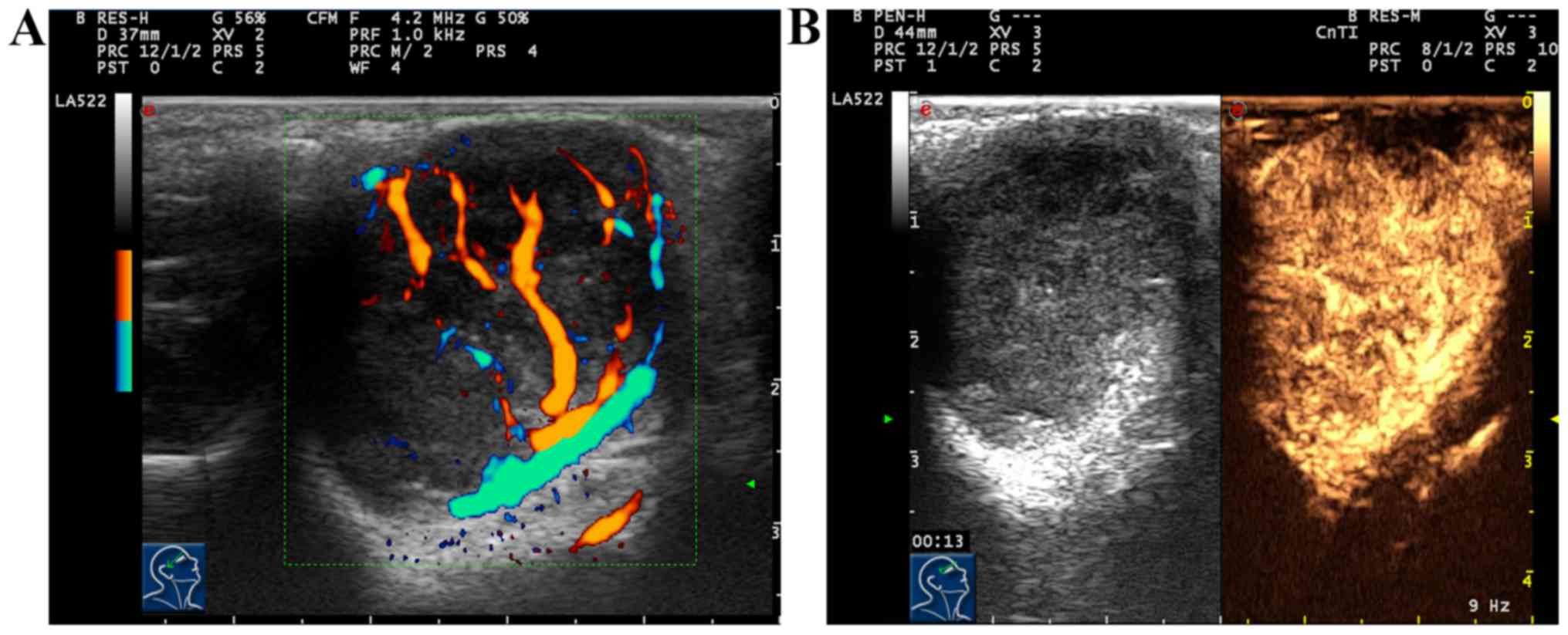
Results of conventional ultrasound of 29 cases with
pleomorphic adenoma of lacrimal gland showed moderate-hypoechogenic
solid masses in lacrimal gland. Twenty-eight cases (28/29, 96.55%)
had clear mass edge, while 1 case (1/29, 3.45%) had unclear mass
edge and irregular shape. Twenty cases (20/29, 68.97%) had dense
and uniform echo inside, 6 cases (6/29, 20.69%) had non-uniform
echo inside accompanied by scattered calcification, and 3 cases
(3/29, 10.34%) had echoic sheets. Results of color Doppler
ultrasound of blood flow displayed that 29 cases (29/29, 100%) had
dotted blood flow signal inside. After two-dimensional and color
Doppler ultrasound, CEUS was immediately performed for 29 cases
with pleomorphic adenoma of lacrimal gland. Results showed that 20
cases (20/29, 68.97%) had high and overall uniform enhancement in
masses; after enhancement, mass edge was clearer in regular shape,
and the contrast agent within the mass became extinct slowly; the
enhancement mode was ‘high, fast-developed slow-extinct and overall
uniform enhancement’. Nine cases (9/29, 31.03%) showed gradual
enhancement of masses from the periphery to the center, and 6 cases
showed uniform enhancement, and 3 cases showed non-uniform
enhancement; there was non-enhancement area inside, and after
enhancement, the mass edge was clear in regular shape, and retreat
of contrast agent within the mass became slowly extinct; the
enhancement mode was ‘high, fast-developed slow-extinct,
centripetal, uniform or non-uniform enhancement’ (Fig. 1).
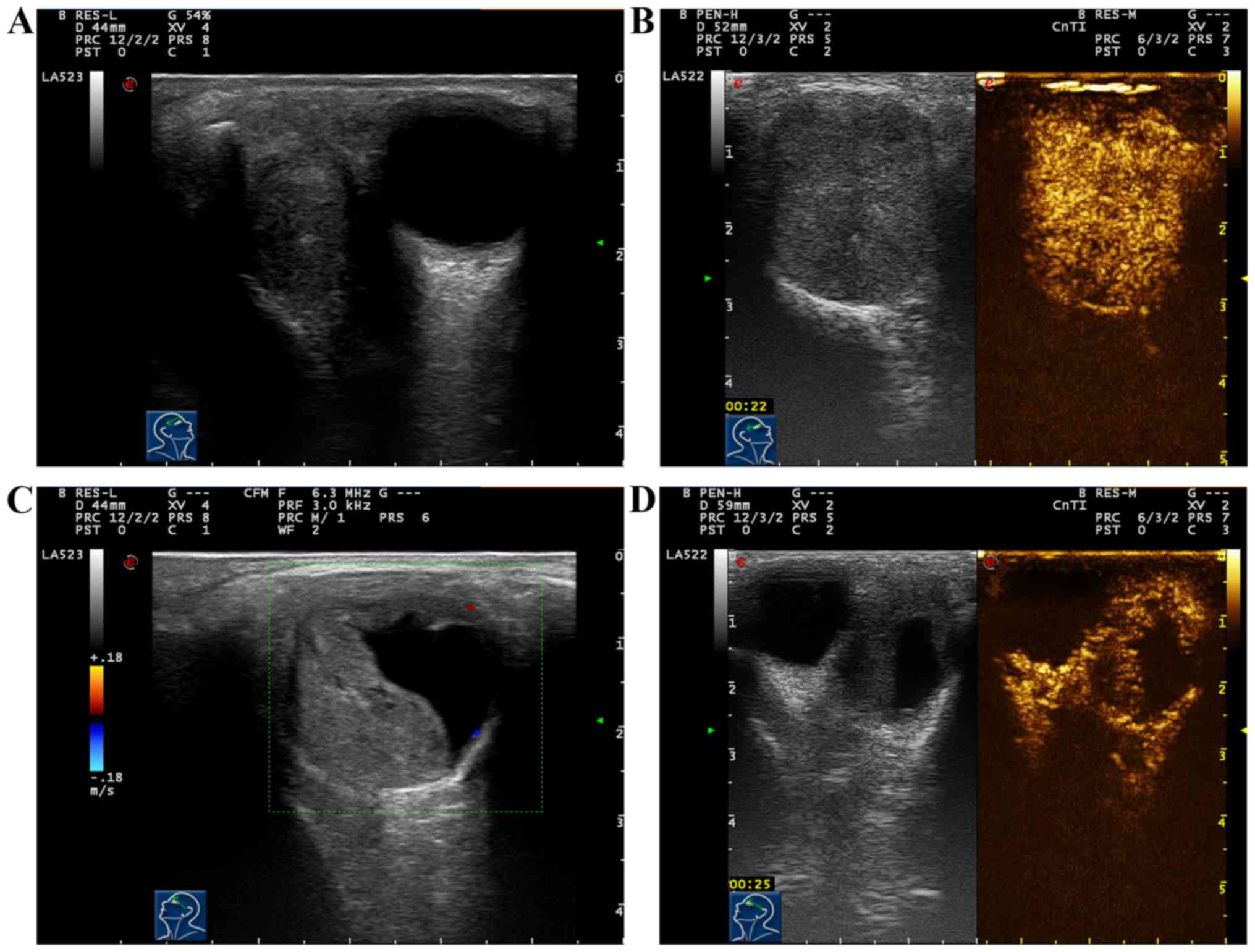
Results of conventional ultrasound of 6 cases with
adenoid cystic carcinoma of lacrimal gland showed hypoechogenic
solid masses with unclear edge, irregular form and non-uniform echo
inside the mass. Color Doppler ultrasound of blood flow displayed
abundant blood flow signals inside the mass, and 6 cases had thick
and tortuous vasa vasorum in the mass (Fig. 2A). After two-dimensional and color
Doppler ultrasound, CEUS was immediately performed for 6 cases with
adenoid cystic carcinoma of lacrimal gland. Results showed that 6
cases (6/6, 100%) had stong and overall uniform enhancement in
masses; in the early stage of enhancement, thick vasa vasorum could
be seen around and inside the mass; after enhancement, the mass was
larger than that in two-dimensional ultrasound, and retreat of
contrast agent within the mass became extinct fast; the enhancement
mode was ‘high, fast-developed fast-extinct, overall uniform
enhancement’ (Fig. 2B; Table II).
 | Table II.Analysis of two-dimensional ultrasound
and CEUS for 10 cases of lacrimal cysts (n, %) (Fig. 3A and B). |
Table II.
Analysis of two-dimensional ultrasound
and CEUS for 10 cases of lacrimal cysts (n, %) (Fig. 3A and B).
| Types | Cases |
|---|
| Clear edge | 10 (100) |
| Internal dot-strip
strong echo | 7 (70) |
| Capsule mixed
echo | 3 (30) |
| Rear enhancement | 10 (100) |
| Non-internal blood
flow signal | 10 (100) |
| Peripheral dotted
blood flow signal | 3 (30) |
| No enhancement in the
interior, mild enhancement in the peripheral capsule walls, and
circular enhancement in the enhancement | 10 (100) |
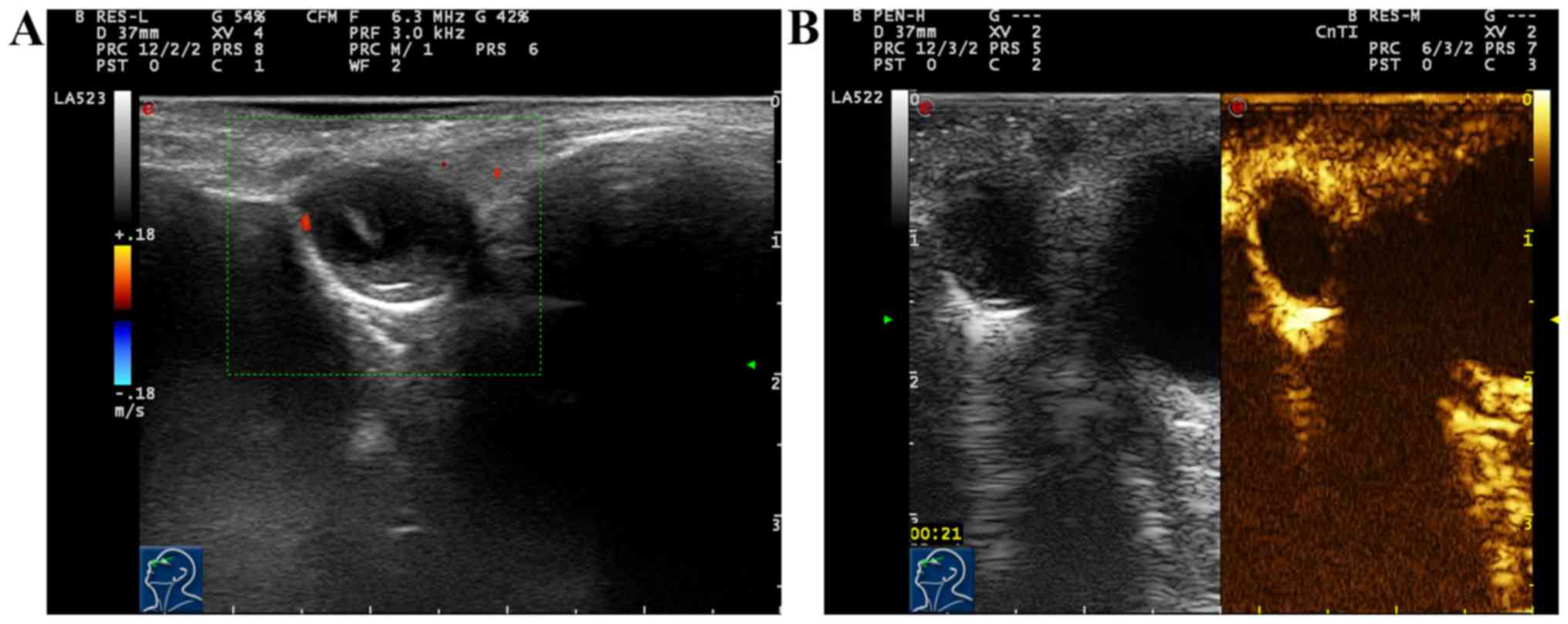
Ten cases with lacrimal sac cyst had clear mass
edge, among which 7 cases (7/10, 70%) showed moderate non-uniform
hypoechogenic and dot-strip strong echo inside, and 3 cases (3/10,
30%) showed cystic solid mixed echo and wavy shape at the junction
of mass and bone wall. Ten cases (10/10, 100%) had enhancement
behind the mass. Color Doppler ultrasound of blood flow displayed
that 10 cases (10/10, 100%) had no blood flow signal inside the
mass, and 3 cases (3/10, 30%) had dotted blood flow signal around
the mass. After two-dimensional and color Doppler ultrasound, CEUS
was immediately performed for 10 cases with lacrimal sac cyst.
Results showed that there was no enhancement in the mass, mild
enhancement could be seen around the mass, and the enhancement mode
was ‘circular enhancement’ (Fig. 3A and
B; Table III).
 | Table III.CEUS features of 48 cases of lacrimal
apparatus tumors. |
Table III.
CEUS features of 48 cases of lacrimal
apparatus tumors.
| Pathological
diagnosis | No. | Enhancement mode |
|---|
| Pleomorphic adenoma
of lacrimal gland | 20 | High, fast-developed
slow-extinct and overall uniform enhancement |
|
| 9 | High, fast-developed
slow-extinct, centripetal, uniform or non-uniform enhancement |
| Adenoid cystic
carcinoma of lacrimal gland | 6 | High, fast-developed
fast-extinct, overall uniform enhancement |
| Lacrimal sac
cyst | 10 | Circular enhancement,
no enhancement inside |
| Adenocarcinoma of
lacrimal sac | 3 | High, fast-developed
fast-extinct, overall uniform enhancement |
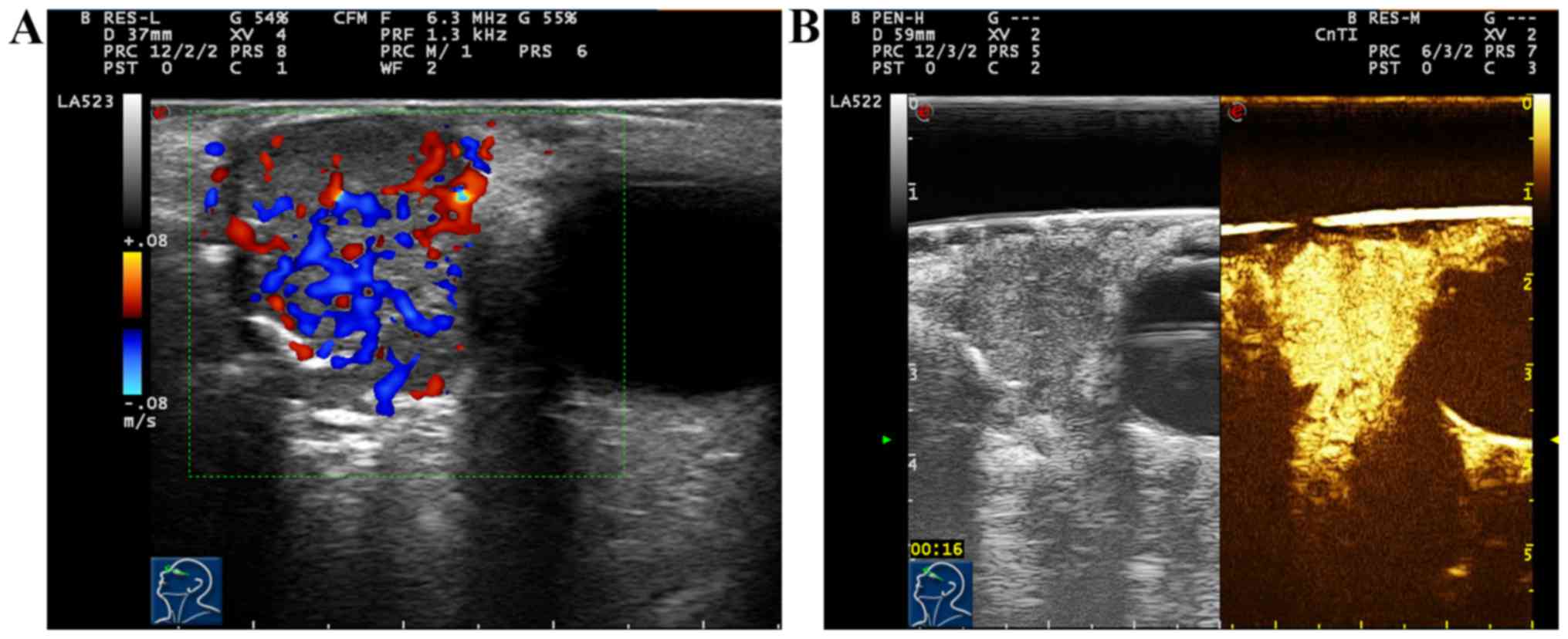
Results of conventional ultrasound of 3 cases with
adenocarcinoma of lacrimal sac showed hypoechogenic solid masses,
and superficial subcutaneous masses with unclear edge, irregular
form, non-uniform echo inside, scattered dotted strong echo and
incomplete periostea on the edge. Color Doppler ultrasound of blood
flow displayed abundant blood flow signals inside the mass. After
two-dimensional and color Doppler ultrasound, CEUS was immediately
performed for 3 cases with adenocarcinoma of lacrimal sac. Results
showed that the appearance of enhancement in mass was obviously
earlier than that in surrounding normal retina and choroid,
displaying overall uniform high enhancement; after enhancement,
mass was larger than that observed in two-dimensional ultrasound,
and retreat of contrast agent within the mass became extinct fast;
the enhancement mode was ‘high, fast-developed fast-extinct,
overall uniform enhancement’ (Fig. 4A and
B).
Discussion
Unrestricted growth of malignant tumors depends on
internal neovascularization. Ultrasound contrast agent is a kind of
intravascular contrast agent, which can display well the
distribution of microcirculation inside the tumor and reflect the
abundance of neovascularization inside the tumor. Thus, CEUS was
used in the evaluation of blood perfusion inside the tumor.
Moreover, the feature of CEUS also compensates the shortcomings of
traditional two-dimensional and color Doppler ultrasound. Color
Doppler flow imaging is sometimes affected by blood flow direction
within the lesion and the poor sensitivity of instrument to
low-speed blood flow, and alse-negative blood flow is also observed
in some lesions. Therefore, no blood flow signal in color Doppler
ultrasound does not indicate no blood supply lesion in the mass
(2). Application of CEUS imaging
technique makes ultrasound diagnostic instrument display clearer
microcirculation within lesion, providing a new method for the
diagnosis of ophthalmic space-occupying lesions (3,4).
Yang et al applied ultrasound contrast agent
to the human eye after relevant animal experiments, and confirmed
that its application in the eye did not cause local and systemic
side-effects, and also had no significant impact on retinal
function and structure (5).
Lacrimal apparatus tumor includes lacrimal gland and
passage tumor. The most common lacrimal gland tumor is the primary
epithelial lacrimal gland tumor, among which pleomorphic adenoma of
lacrimal gland is common in benign tumors, and adenoid cystic
carcinoma of lacrimal gland is common in malignant tumors (6).
Pleomorphic adenoma of lacrimal gland is the most
common lacrimal gland epithelial tumor, accounting for ~50%. It is
a kind of benign tumor composed of epithelial and interstitial
components. In the past, pleomorphic adenoma of lacrimal gland was
called benign mixed tumor. It occurs frequently in single eye in
adults and histopathological components mainly include epithelial
cells or interstitial components. Besides, it is composed of a
large number of tubular structures and cell nests in different
shapes formed by differentiated epithelial cells, with scattered
transparent myxoid and cartilage-like structure, diversified forms
and arrangements of tumor cells (7).
Ultrasound shows the round or oval solid mass above the orbit, and
the majority of masses have clear edge and dense and uniform echo
inside, but a small number of masses have unclear edge and
non-uniform echo inside with scattered calcification; the mass is
not compressed with a small number of blood flow signals. CEUS
shows rapid filling of contrast agent within the mass, most of
which show strong overall uniform enhancement, and a few of which
show concentric uniform or non-uniform enhancement; after
enhancement reaches the peak, retreat of contrast agent becomes
slowly extinct. In this study, it was found that the mass
containing more epithelial and mucinous tissues showed high and
uniform enhancement after CEUS, while the mass containing more
cartilage-like structures showed high and non-uniform enhancement
after CEUS, and flaky non-enhancement area could be seen within the
mass, and the proportion of cartilage-like structure was directly
proportional to the range of non-enhancement area within the mass.
Differential diagnosis of the disease is as follows: i)
Inflammatory pseudotumor of lacrimal gland: It often occurs in both
eyes, showing eyelid congestion and edema; hormone therapy is
effective but recurrence rate is high. Ultrasound examination shows
lacrimal gland enlargement in a flat or amygdaloidal shape, and it
can extend forward or towards the orbital apex, often accompanied
by adjacent extraocular muscle thickening. CEUS shows that after
enhancement, the mass edge is unclear, the mass is larger than that
observed in two-dimensional ultrasound, and the lesion generally
has no enhancement area; ⅱ) lymphoma of lacrimal gland: Orbital
lymphoma can often occur in the lacrimal gland. Ultrasound
examination shows the low and non-uniform echo in the lesion, and
scattered cord-like strong echo. Doppler detection shows that the
mass has abundant blood signal in a dendritic shape. Spectral
Doppler shows that the high-speed high-resistance arterial spectrum
is an imaging feature of orbital lymphoma, which is of significance
in the early diagnosis and timely treatment of lymphoma. Moreover,
CEUS shows that most tumors develop and disappear quickly, and show
high uniform enhancement; after enhancement, mass edge is unclear,
and the mass is larger than that observed in two-dimensional
ultrasound, and the posterior edge of lesion often shows an
‘inverted-triangle’ shape (1,8).
Adenoid cystic carcinoma of lacrimal gland is the
most common malignant epithelial tumor of lacrimal gland; the mass
is solid with no capsule and has a high degree of malignancy. It
often invades surrounding tissues, and relapse and metastasis occur
easily. This disease is considered as the most biologically
destructive and unpredictable tumor in the head and neck (8). Ultrasound shows solid hypoechogenic mass
in a spindle shape with unclear edge and irregular form without
capsule surrounding the mass; internal echo is not uniform, and
slightly attenuated in the rear; abundant blood flow signals and
multiple thick vasa vasorum can be detected in the mass (9,10). In
CEUS, the contrast agent in the mass is rapidly filled, showing
high overall uniform enhancement; after enhancement, the mass is
enlarged and retreat of contrast agent becomes extinct quickly
after enhancement reaches the peak, showing fast-developed
fast-extinct signs, which is different from pleomorphic adenoma and
tuberculosis of lacrimal gland (fast-developed slow-extinct).
However, the number of cases with such a sign is small, so it
cannot be used as a criterion to distinguish benign from malignant
lacrimal gland masses, but may provide references for the diagnosis
of malignant tumors. Differential diagnosis of the disease is the
same as that of pleomorphic adenoma of lacrimal gland; but it is
hard to distinguish it from inflammatory lesions from
two-dimensional ultrasound and CEUS, so the clinical
manifestations, signs and past medical history should be combined
for comprehensive analysis.
Stupp et al (11) performed ultrasound examination for 17
patients with lacrimal sac mass, and the results showed that
ultrasound could display the lacrimal sac mass well. The disease
should be distinguished from frontal and ethmoidal sinus cysts,
which are easily misdiagnosed as ophthalmic diseases.
Lacrimal passage tumor is rare, and the malignant
lacrimal passage tumor is even rarer, and the main pathological
types are squamous and transitional cell carcinoma, followed by
adenocarcinoma. Typical clinical manifestations of lacrimal passage
tumor are masses in inner canthus or lacrimal sac, and it only
displays the overflow of tears and unblocked lacrimal passage in
the early stage. Clinical diagnosis of this disease is difficult.
With the progression of disease, pain, overflow of lacrimal punctum
and bloody mucus, lacrimal sac area bulges will occur; in the late
stage, it may lead to epistaxis, protopsis and other complications.
Ultrasound of malignant lacrimal passage tumor shows no
specificity, and only shows the solid non-uniform mass in lacrimal
sac. In addition, different pathological types of tumors show
different strengths of blood flow signal; generally, blood flow
signal of squamous cell carcinoma is low, while
poorly-differentiated adenocarcinoma shows more abundant signals.
The sample size in this study was small, and its sensitivity,
specificity and accuracy were not discussed, so the results could
not be used as criteria to distinguish benign from malignant
lacrimal apparatus tumors. Therefore, future studies with larger
number of samples are needed to further confirm the conclusion.
In conclusion, traditional two-dimensional and color
Doppler ultrasound may be combined with CEUS in the determination
of mass size, edge and shape. This technology may facilitate the
identification of tumor scope and improve the diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YXL wrote the manuscript and treated the patients,
YL and JMX were responsible for the ultrasound diagnosis. QC and WX
helped with the statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
China Meitan General Hospital (Beijing, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q, Zhou G, Xiong W, Zhou Q and Yue
LX: Ultrasound and CEUS features of orbital and intraocular mass.
Shengxue Jishu. 30:466–470. 2011.(In Chinese).
|
|
2
|
Yang Q and Wei WB: CEUS and its clinical
application in ophthalmology. Int Rev Ophth. 31:145–148. 2007.
|
|
3
|
Tu Y, Jakobiec FA, Leung K and Freitag SK:
Distinguishing benign from malignant circumscribed orbital tumors
in children. Semin Ophthalmol. 33:116–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li HD and Fu P: Application of CEUS in
ophthalmology. Int Rev Ophth. 34:94–97. 2010.
|
|
5
|
Yang Q, Wei WB, Yang WL, Shi XH, Zhou D
and Liu Y: Experimental research on effects of SonoVue real-time
CEUS on choroidal retina structure and function. Ophthalmology.
17:193–197. 2008.
|
|
6
|
Alkatan HM, Al-Harkan DH, Al-Mutlaq M,
Maktabi A and Elkhamary SM: Epithelial lacrimal gland tumors: A
comprehensive clinicopathologic review of 26 lesions with
radiologic correlation. Saudi J Ophthalmol. 28:49–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tailor TD, Gupta D, Dalley RW, Keene CD
and Anzai Y: Orbital neoplasms in adults: Clinical, radiologic, and
pathologic review. Radiographics. 33:1739–1758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bischof M, Karagiozidis M, Krempien R,
Treiber M, Neuhof D, Debus J and Zierhut D: Radiotherapy for
orbital lymphoma: outcome and late effects. Strahlenther Onkol.
183:17–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng LL, Xian JF, Yan F, Fu L and Zhou HY:
Value of DCE-MRI and DWI in the differential diagnosis of
inflammatory pseudotumor and lymphoma in the lacrimal gland.
Zhonghua Yi Xue Za Zhi. 97:487–491. 2017.(In Chinese). PubMed/NCBI
|
|
10
|
Yan L, He G, Zhou X, Zheng Y, Zhu Y, Yang
J, Zhang M and Zhou Y: Contrast-enhanced ultrasound in the
diagnosis of orbital space-occupying lesions. Clin Radiol.
72:798.e1–798.e6. 2017. View Article : Google Scholar
|
|
11
|
Stupp T, Pavlidis M, Busse H and Thanos S:
Presurgical and postsurgical ultrasound assessment of lacrimal
drainage dysfunction. Am J Ophthalmol. 138:764–771. 2004.
View Article : Google Scholar : PubMed/NCBI
|