Introduction
Gastric cancer, the fourth most common type of
cancer worldwide, remains the second leading cause of cancer
mortality. The incidence of gastric cancer is highest in Asia and
certain parts of South America, particularly in China, Korea, and
Japan (1,2). Over 90% of patients with gastric cancer
in China were diagnosed in the advanced stage (3). Gastric cancer is characterized by high
rates of incidence, metastasis, and mortality, and low rates of
early diagnosis, radical resection, and 5-year survival (3).
Upper gastrointestinal imaging and gastroscopy are
the most widely used methods for screening and diagnosing of
gastric cancer; however, these methods have limited utility in
evaluating the depth of invasion and the presence of extra-stomach
metastases (1). Currently, the most
commonly methods for imaging gastric cancer are computed tomography
(CT) scanning and ultrasonic endoscopy (1). Ultrasonic endoscopy has been utilized
widely to measure the depth of gastric wall invasion; however, this
procedure appears to be less effective than CT when used to detect
lymphatic and distant metastasis (4).
Although CT is an indirect method for evaluating the presence
gastric cancer and is not specific for early-stage gastric cancer,
with advances in CT scanning and post-reconstruction technologies,
CT has become an important method for the pre-operative staging and
treatment efficacy evaluation in patients with gastric cancer who
have already been diagnosed via electronic gastroscope (5–8). During CT
examination, the thickness of the focal wall is one of the imaging
measurements, as gastric thickening is one of the major
manifestations of an advanced gastric cancer (1). The thickness of the focal gastric wall
is closely associated with the depth of cancer invasion and
Tumor-Node-Metastasis (TNM) stages (9), which assist the clinics to select an
optimal treatment for early-stage or advanced gastric cancer. For
example, if the tumor has invaded the serosa layer in a patient
with gastric cancer, neoadjuvant chemotherapy may be recommended
prior to surgery. Furthermore, the thickness of a gastric tumor
could reflect the biological behaviors of tumor in a certain degree
(10). Previous studies have
indicated that the Response Evaluation Criteria in Solid Tumors are
not applicable for gastrointestinal tumors, including gastric
cancer, and that measuring the tumor volume and positron emission
tomography/CT examination could reflect the treatment efficacy more
accurately (11–13). However, according to our clinical
experience, effective non-surgical treatments can lead to reduced
gastric cancer thickness. Therefore, measuring the thickness of
gastric cancer could have potential significance for clinical
staging, clinical assessment and treatment efficacy evaluation.
During CT imaging, gastric filling is often performed to ensure
adequate distention, which is essential to ensure that areas of
disease are not overlooked and to prevent the collapsed gastric
wall from mimicking disease (14).
Liquid, often water, is commonly used, but an alternative method is
to introduce gas into the stomach (2,15). The
present study used anaerogenic powder to produce carbon dioxide gas
for gastric filling (1). However, it
remains unclear whether the measurement of gastric cancer thickness
is affected by the degree of gastric filling. The accuracy and
reliability of gastric cancer thickness measurements are rarely
reported. Therefore, the clinical relevance of gastric cancer
thickness remains uncertain; it is therefore necessary to
understand whether the thickness of gastric cancer is affected by
the degree of gastric filling, and to investigate the accuracy and
reliability of the measurements.
The present study aimed to investigate the influence
of gastric filling degree on the measurement of gastric cancer
thickness.
Patients and methods
Subjects
Data from patients with gastric cancer that
underwent enhanced abdominal CT scanning in the Department of MR of
the Fourth Hospital of Hebei Medical University (Shijiazhuang,
China) between July and September 2016 were prospectively included
in the present study.
The inclusion criteria of the patients were as
follows: i) Treatment-naïve and confirmed with gastric cancer by
gastroscopic biopsy, but had not received any antitumor therapies,
or were patients with gastric cancer that were undergoing
re-examination following non-surgical antitumor treatments (such as
radiotherapy, chemotherapy and oral targeting drugs); ii) without
contraindications for the use of anisodamine hydrochloride,
including glaucoma and urinary retention; iii) without
contraindications for the use of iodine contrast medium; iv)
without symptoms of gastrointestinal perforation; v) without
contraindications for aerogenic powder; and vi) with advanced
gastric cancer according to imaging staging, which had a thickness
that was 50% higher than the thickness of adjacent normal gastric
wall following filling.
The exclusion criteria were as follows: i) The
patients had pyloric obstruction, abiding food was found in the
abdominal cavity following food and water fasting, and the
abdominal cavity was in dilation; and ii) the abdominal cavity was
not well filled following oral intake of aerogenic powder, as
evidenced by the duplicature continuing to gather together.
A total of 45 patients completed the examination.
There were four patients with residual food in the abdominal cavity
or in a state of dilation during plain scanning, and three patients
in a sub-optimal filling state following the oral intake of
aerogenic powder, who were excluded. Therefore, a final total of 38
patients (30 males, 8 females; mean age, 63.2±7.7 years; age range,
37–76 years) were included in the present study.
The non-conventional clinical examination procedures
used in the present study were approved by the Ethics Committee of
the Fourth Hospital of Hebei Medical University. All the patients
provided written informed consent.
CT scanning
A second-generation dual-source CT scanner (SOMATOM
Definition Flash; Siemens Healthcare, Erlangen, Germany) was used
to perform CT scanning. The patients were fasted from food and
water for 6 h before the scan. During CT scanning, the patients
were placed in a supine position and plain scanning was performed
when the abdominal cavity was in the natural state of emptiness.
The plain scanning covered the area between the diaphragmatic dome
and pelvic floor. The parameters for the plain scanning were as
follows: Scanning voltage, 120 Kv; current, 210 mA; collimation
width, 128×0.6 mm; and screw pitch, 0.9. To reduce the tension of
the gastric wall, the abdominal cavity was fully dilated, and to
avoid gastrointestinal peristalsis artifacts, an intramuscular
injection of anisodamine hydrochloride (10 mg) was administered
once the plain scanning was completed. Once dry mouth symptoms
appeared, which indicated that the stomach muscle tension-lowering
drugs were taking effect, 6 g aerogenic powder was administered
orally. The patients were asked not to belch or talk, and then
enhanced scanning was performed immediately. A high-pressure
injector (CT-D; Medtron AG, Saarbrücken, Germany) was used to
inject the contrast agent (iohexol; 300 mgI/ml, dose of 2 ml/kg)
into the cubital fossa vein at a velocity of 3.0 ml/sec. Next,
dual-energy arterial phase and venous phase scanning were performed
at 25 and 70 sec after the injection of contrast agent, during
which the patients were asked to hold the breath. The enhanced
scanning covered the area between the diaphragmatic dome and lower
gastric edge, and between the diaphragmatic dome and pelvic floor.
The parameters for the enhanced scanning were as follows: Tubal
voltages, 100 kV and Sn140 kV, with Care Dose 4D turned on;
reference currents, 230 and 178 mA; collimation width, 32×0.6 mm;
and screw pitch, 0.55.
The aforementioned protocol consisted of two steps.
In the first step, the empty stomach in the natural state was
scanned. In the second step, the stomach was filled at a
low-tension state and then images were captured by enhancement
scanning. The procedure was feasible and easy to operate without
the increase of radiation dose. In the present study, the protocol
was only utilized to investigate the association between the
thickness of gastric lesions and the filling level.
Image analysis
The images obtained by plain scanning and in the
venous phase were reconstructed. The plain scanning images were
reconstructed to be 1.0 mm in thickness using the B10f algorithm
(Siemens Healthineers, Erlangen, Germany); the fusion images at
venous phase were reconstructed to be 1.0 mm in layer thickness
with the B30f algorithm. The reconstructed images were input into a
picture archive and retrieval system, and an investigator with 13
years of experience of imaging diagnosis was asked to measure the
thickness and perform the staging classification.
The axial and multiplanar reconstruction images were
used for imaging staging, according to the TNM staging system (7th
edition) issued by the American Joint Committee on Cancer (AJCC)
(16–18).
The different gastric areas were determined
according to the ‘three-area method’ (19) prior to thickness measurement, which
was in consistent with surgical stomach partition method. In brief,
the cardia was present at the joint between stomach and esophagus;
the antrum was the area between the angular incisure and the
pylorus; and the gastric body was the area between cardia and
antrum, which included the lesser and greater curvature sides. The
thickness was measured as follows. i) The length-measuring tool
(Syngo MMWP VE36A; Siemens Healthineers) was used for the
measurement, with the assistance of adjusting window position and
width, as well as local zoom in. The axial images in the venous
phase were observed consecutively, and the layer with the highest
thickness of gastric cancer following gastric filling was selected
for measurement. For the patients with ulcerated cancer, the
thickness of annular dikes was also measured. The areas adhering to
the perigastric lymph nodes and the perigastric adipose tissues,
with invasion involvement were not included in the measurement. ii)
According to the position used in the measurement during the venous
phase, the anatomical landmarks, including the cardia, pylorus, and
ulcer floor, were used as the references to enable selection of the
same position in the plain scanning images to measure the thickness
prior to filling. The same method was used to measure the thickness
of the gastric wall in the non-cancerous normal areas, of which the
area was at least 3 cm to the margin of the gastric cancer. The
thickness of the residual normal gastric wall at the cancerous area
was not measured. For each patient, the thickness of the gastric
cancer was measured again 1 month later, using the same imaging
data. An interval of 1 month, was chosen for the following reasons:
i) This length of time could minimize the influence of remembering
details of the first measurement; and ii) re-examination of the
gastric cancer patients who underwent a neoadjuvant therapy was
generally following 2 cycles of chemotherapy and therefore, 1 month
after the first examination.
The distance between the normal gastric wall and the
cancer margin was at least 3 cm, and the thickness of the gastric
wall in the area of the cancer was not measured. For instance, the
thickness of the normal wall of lesser gastric curvature was not
measured for the patients with cancer of the lesser gastric
curvature.
Statistical analysis
SPSS 21.0 (IBM Corp., Armonk, NY, USA) and MedCalc
16.2 (MedCalc Software bvba, Ostend, Belgium) were used for the
statistical analysis. P<0.05 was considered statistically
significant. The quantitative data in normal distribution are
depicted as the mean ± standard deviation; a paired Student's
t-test was used for the comparisons between two groups for
quantitative date with equal variances. Quantitative data in
non-normal distributions are depicted as medians and inter-quartile
ranges, and compared with Wilcoxon signed ranks test. Qualitative
data were described with frequencies and percentages, and compared
with Fisher's exact test or non-parametric test following rank
conversion. Consistency of the two measurements by the same
investigator was evaluated by Bland and Altman plotting.
Results
General characteristics of the
subjects
None of the patients experienced adverse effects,
such as allergy and perforation, or complications. Certain patients
reported abdominal fullness following the intake of the aerogenic
powder, which was tolerable and disappeared following the
spontaneous exhaust that occurred once the scanning was completed.
Among these patients, 21 were newly diagnosed, and the other 17
were being re-examined following treatments (Table I). The clinical and demographic
characteristics were similar between the two groups.
 | Table I.Clinical and demographic
characteristics of the 38 patients. |
Table I.
Clinical and demographic
characteristics of the 38 patients.
| Characteristic | Total, n (%) | Newly diagnosed, n
(%) | Re-examination, n
(%) | P-value (Newly
diagnosed vs. re-examination) |
|---|
| Sex |
|
|
|
|
| Male | 30 (78.95) | 15 (71.43) | 15 (88.24) | 0.257 |
|
Female | 8
(21.05) | 6
(28.57) | 2
(11.76) |
|
| Age (years) |
|
|
|
|
| Mean ± standard
deviation | 63.2±7.7 | 64.5±7.0 | 61.7±8.4 | 0.171 |
| Anatomical
location |
|
|
|
|
|
Cardia/corpus/antrum | 2 (5.26) | 0 | 2
(11.76) | 0.661a |
|
Cardia/orpus | 13 (34.21) | 8
(38.10) | 5
(29.41) |
|
|
Corpus | 4
(10.53) | 4
(19.05) | 0 |
|
|
Antrum | 6
(15.79) | 4
(19.05) | 2
(11.76) |
|
|
Cardia | 9
(23.68) | 3
(14.29) | 6
(35.29) |
|
|
Corpus/Antrum | 4
(10.53) | 2 (9.52) | 2
(11.76) |
|
| Stagea |
|
|
|
|
| T3 | 5
(13.16) | 3
(14.29) | 2
(11.76) | 1.000a |
| T4a | 28 (73.68) | 15 (71.43) | 13 (76.47) |
|
| T4b | 5
(13.16) | 3
(14.29) | 2
(11.76) |
|
| N0 | 8
(21.05) | 4
(19.05) | 4
(23.53) | 0.701a |
| N1 | 11 (28.95) | 6
(28.57) | 5
(29.41) |
|
| N2 | 14 (36.84) | 8
(38.10) | 6
(35.29) |
|
| N3 | 5
(13.16) | 3
(14.29) | 2
(11.76) |
|
| M0 | 26 (68.42) | 16 (76.19) | 10 (58.82) | 0.307 |
| M1 | 12 (31.58) | 5
(23.81) | 7
(41.18) |
|
| Treatment |
|
|
|
|
|
Surgical resection | 8
(21.05) | 8
(38.10) | 0 | 0.373a |
|
Chemotherapy | 22 (57.89) | 7
(33.335) | 15 (88.24) |
|
|
Radiotherapy | 1 (2.63) | 0 | 1 (5.88) |
|
|
Others | 7
(18.42) | 6
(28.57) | 1 (5.88) |
|
| Pathological
type |
|
|
|
|
|
Adenocarcinoma | 34 (89.47) | 18 (85.71) | 16 (94.12) | 0.383a |
|
Well
differentiated | 0 | 0 | 0 |
|
|
Moderately
differentiated | 5
(14.71) | 5
(27.78) | 0 |
|
|
Poorly
differentiated | 15 (44.12) | 7
(38.89) | 8
(50.00) |
|
|
Unknown | 14 (41.18) | 6
(33.33) | 8
(50.00) |
|
| Signet
ring cell carcinoma | 2 (5.26) | 1 (4.76) | 1 (5.88) |
|
|
Mucinous adenocarcinoma | 2 (5.26) | 2 (9.52) | 0 |
|
The stages of the 8 newly diagnosed patients that
underwent surgical treatments were determined by postoperative
pathological examination, whereas staging of the other patients was
conducting using imaging. Among the 17 patients for re-examination,
the treatments were as follows: Chemotherapy for 15 patients
(including 4 using the S-1/oxaliplatin strategy, 3 using the
oxaliplatin and capecitabine strategy, 3 used TO strategy, 1 using
the 5-fluorouracil, folinic acid and oxaliplatin strategy, 1 using
the ocetaxel and cisplatin strategy, 1 using oral intake of a
tegafur-gimeracil-oteracil potassium capsule and 1 using an unknown
chemotherapy regimen), radiotherapy for 1 patient, and
interventional embolization for 1 patient. For the 21 newly
diagnosed patients, 8 underwent surgical resection, 7 received
chemotherapy, and the other 6 were not treated in The Fourth
Hospital of Hebei Medical University. The consequent treatments for
the last 6 patients were also unknown. The pathological types of
the carcinoma in the 8 patients that underwent surgical resection
were determined by postoperative pathological examination, whereas
the pathological types of the others were pathological types of the
biopsies.
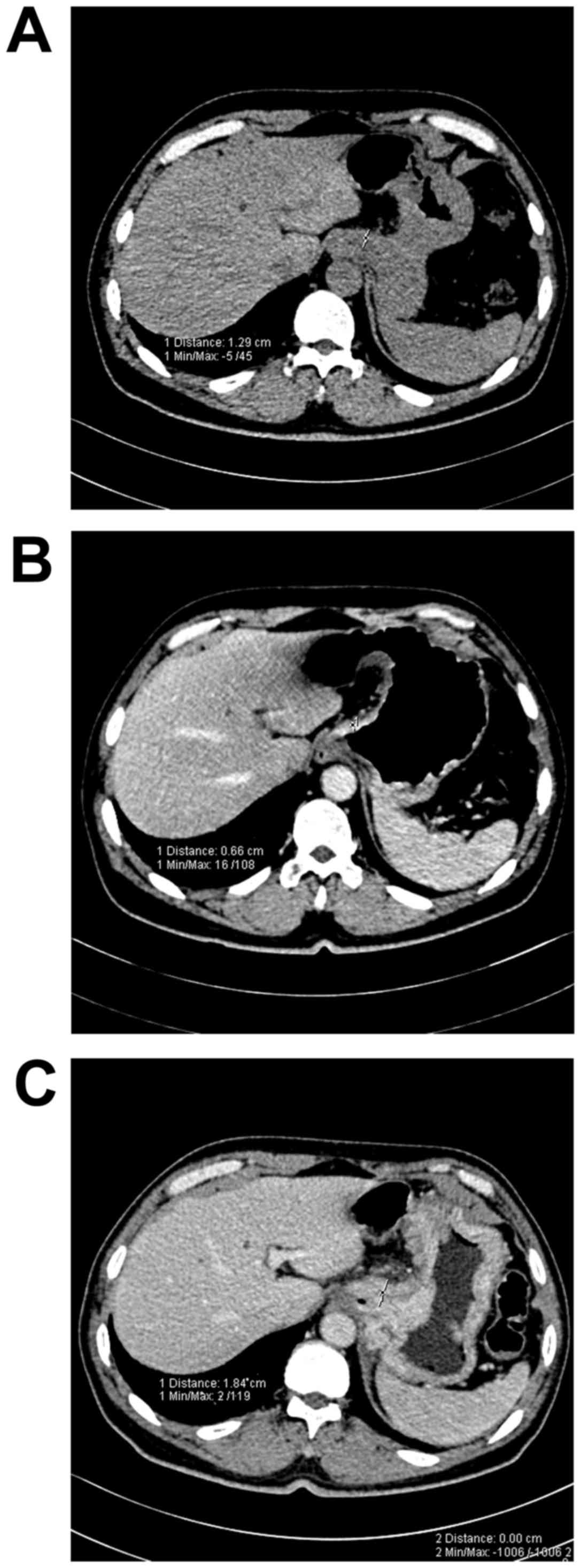
Measurement thickness of the normal
gastric walls prior to and following filling
The thickness of the normal gastric wall in 88 areas
was obtained from the 38 patients with gastric cancer. The
thickness of the normal gastric wall in each area prior to and
following filling was significantly different (P<0.05). The
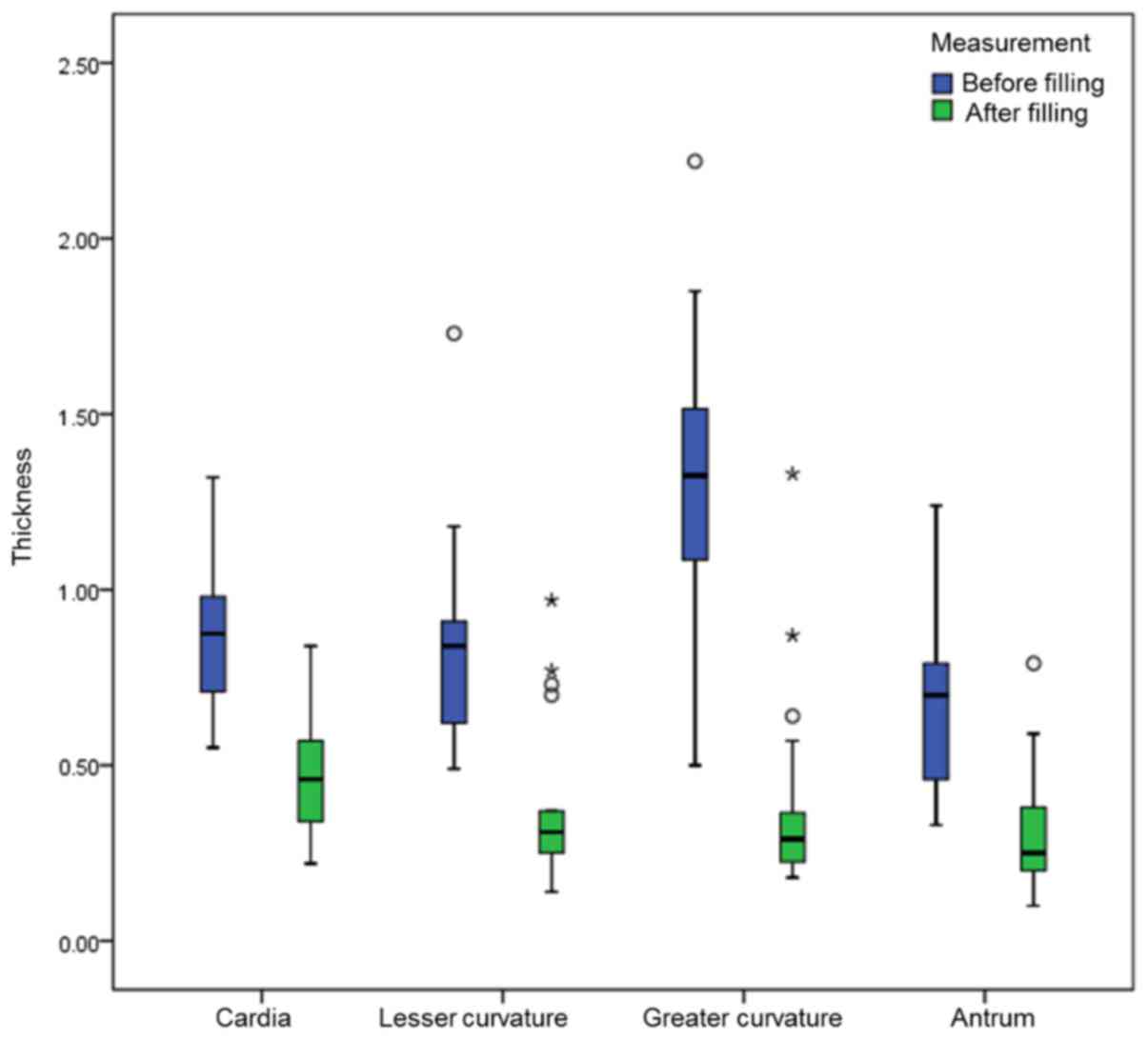
change was greatest at the greater curvature (Table I; Fig.
1).
Measurement thickness of the gastric
cancer prior to and following filling
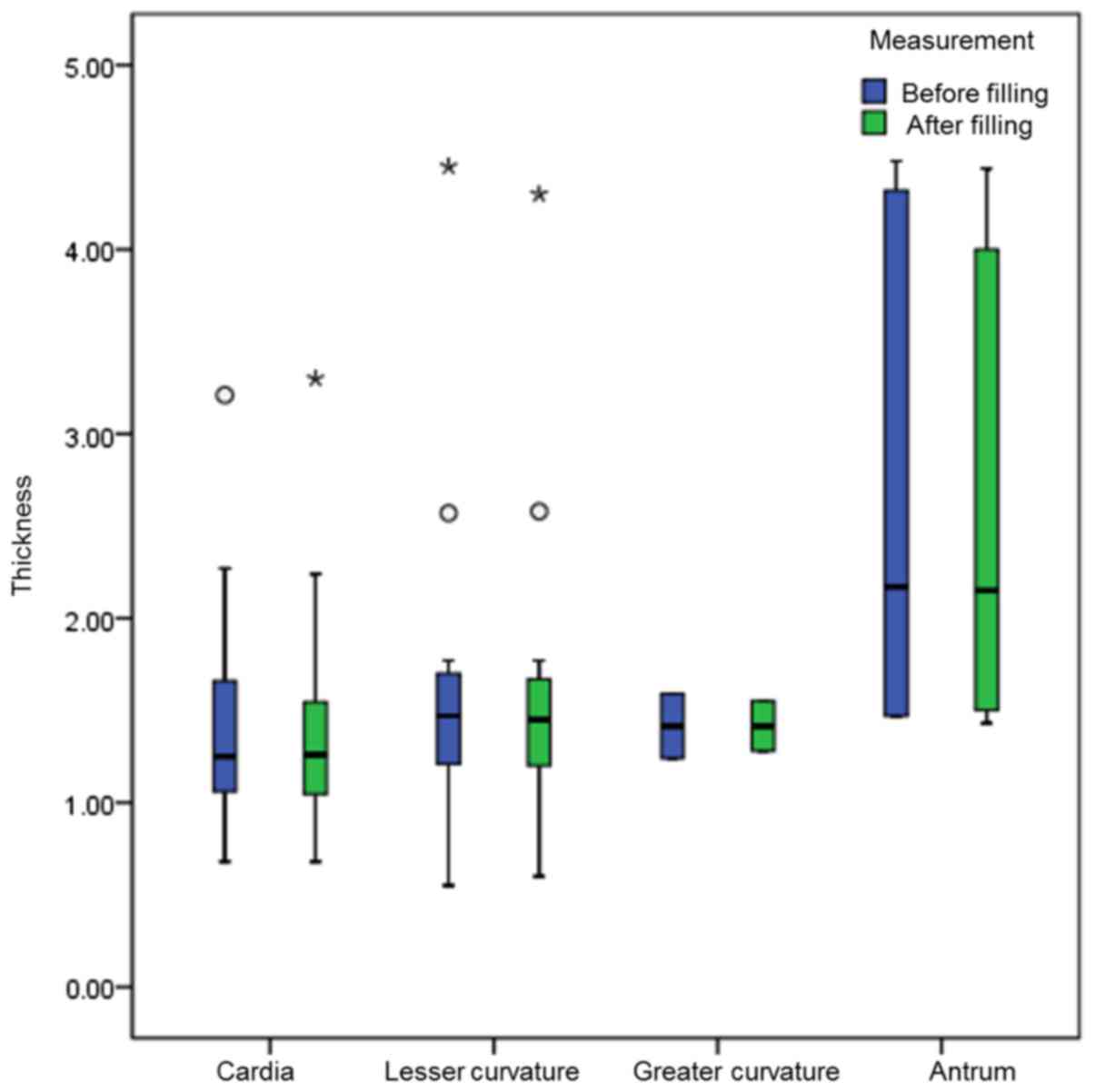
A total of 32 measurements of the gastric cancer
thicknesses were obtained from the 21 newly diagnosed patients. The
difference in the thickness of the gastric cancer at different
areas (except for those at the greater curvature, which were too
few to provide enough data for statistical analysis) prior to and
following filling was not statistically significant (P>0.05;
Table II; Figs. 2 and 3).
 | Table II.Comparison of the measurement
thickness of the normal gastric walls prior to and following
filling. |
Table II.
Comparison of the measurement
thickness of the normal gastric walls prior to and following
filling.
| Area | Patients, n | Thickness prior to
filling, cma | Thickness
post-filling, cma | Z- or t-value | P-value |
|---|
| Cardia | 14 | 0.89±0.23 | 0.47±0.03 | 9.751(t) | <0.001 |
| Lesser
curvature | 17 | 0.84
(0.60,1.01) | 0.31
(0.23,0.54) | −3.622(Z) | <0.001 |
| Greater
curvature | 32 | 1.33
(1.08,1.52) | 0.29
(0.22,0.37) | −4.937(Z) | <0.001 |
| Antrum | 25 | 0.70
(0.46,0.81) | 0.25
(0.20,0.39) | −4.286(Z) | <0.001 |
| Total | 88 | 0.88
(0.65,1.23) | 0.30
(0.23,0.42) | −8.101(Z) | <0.001 |
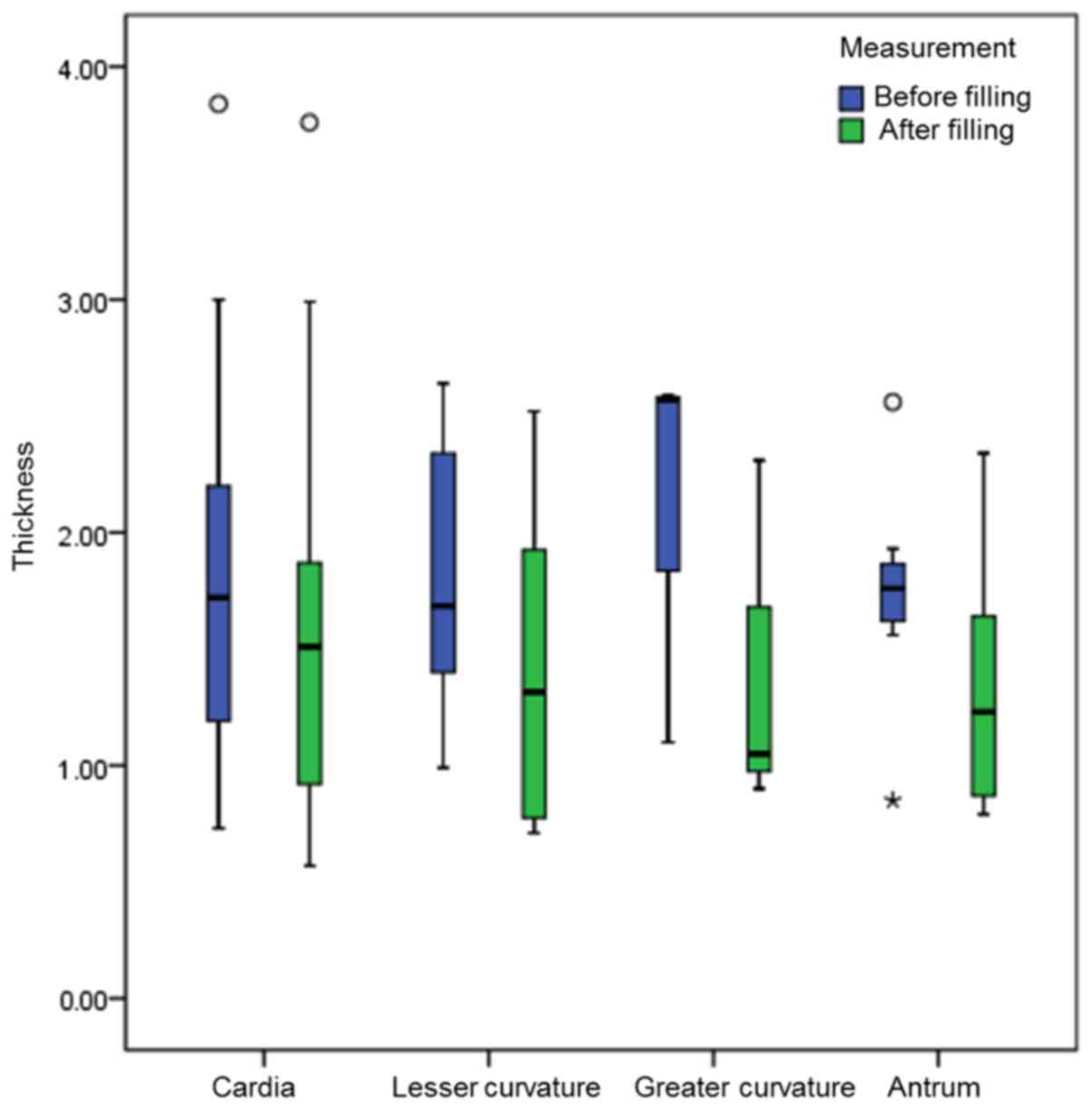
For the 17 patients who were being re-examined
following treatment, 31 measurements of the thickness of gastric
cancer at different areas were obtained. The thickness of the
gastric cancer at different areas (except for those at the greater
curvature, which were too few to provide enough data for
statistical analysis) prior to and following filling was
statistically significant (P<0.05; Table III; Figs.
4–6).
 | Table III.Comparison of the measured thickness
of the gastric carcinomas prior to and post-filling. |
Table III.
Comparison of the measured thickness
of the gastric carcinomas prior to and post-filling.
| Area | Patients, n | Thickness prior to
filling, cma | Thickness
post-filling, cma | Z-value | P-value |
|---|
| Newly
diagnosed |
|
|
|
|
|
|
Total | 32 | 1.47
(1.20,1.76) | 1.50
(1.18,1.75) | −1.660 |
0.097 |
|
Cardia | 11 | 1.25
(1.06,1.73) | 1.26
(1.01,1.58) | −0.868 |
0.386 |
| Lesser
curvature | 13 | 1.47
(0.94,1.74) | 1.45
(0.98,1.72) | −0.654 |
0.513 |
| Greater
curvature | 2 | 1.42
(1.24,1.59) | 1.42
(1.28,1.55) | – | −b |
|
Antrum | 6 | 2.17
(1.47,4.36) | 2.15
(1.48,4.11) | −1.577 |
0.115 |
| Re-examination |
|
|
|
|
|
|
Total | 31 | 1.76
(1.20,2.31) | 1.23
(0.87,1.87) | −4.861 | <0.001 |
|
Cardia | 13 | 1.86±0.87 | 1.58±0.96 |
2.591 |
0.024 |
| Lesser
curvature | 8 | 1.81±0.35 | 1.41±0.70 |
2.999 |
0.020 |
| Greater
curvature | 3 | 2.57
(1.10,2.58) | 1.05
(0.90,1.68) | – | −b |
|
Antrum | 7 | 1.73±0.51 | 1.34±0.57 |
3.224 |
0.018 |
Consistency of measuring the gastric
cancer thickness
The thicknesses of the gastric cancer at the 63
areas of the 38 patients were measured again 1 month later by the
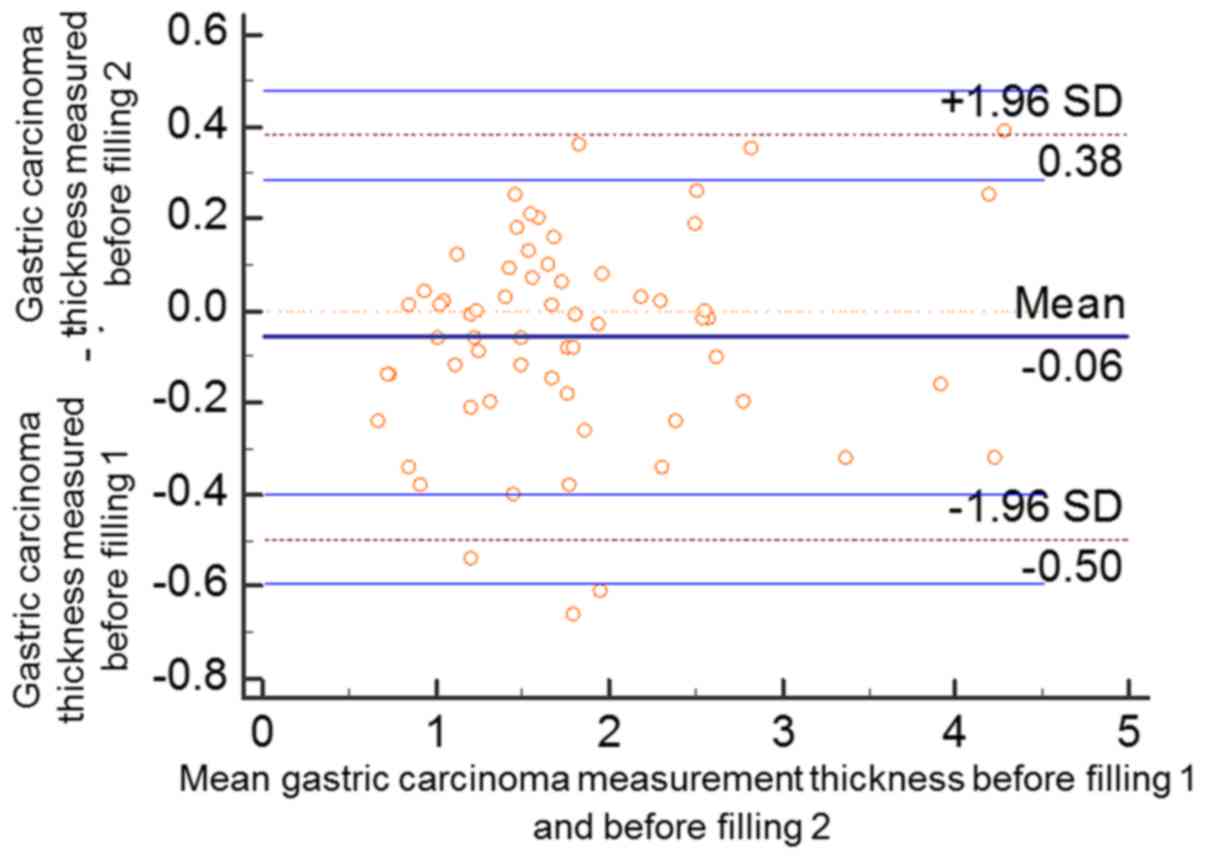
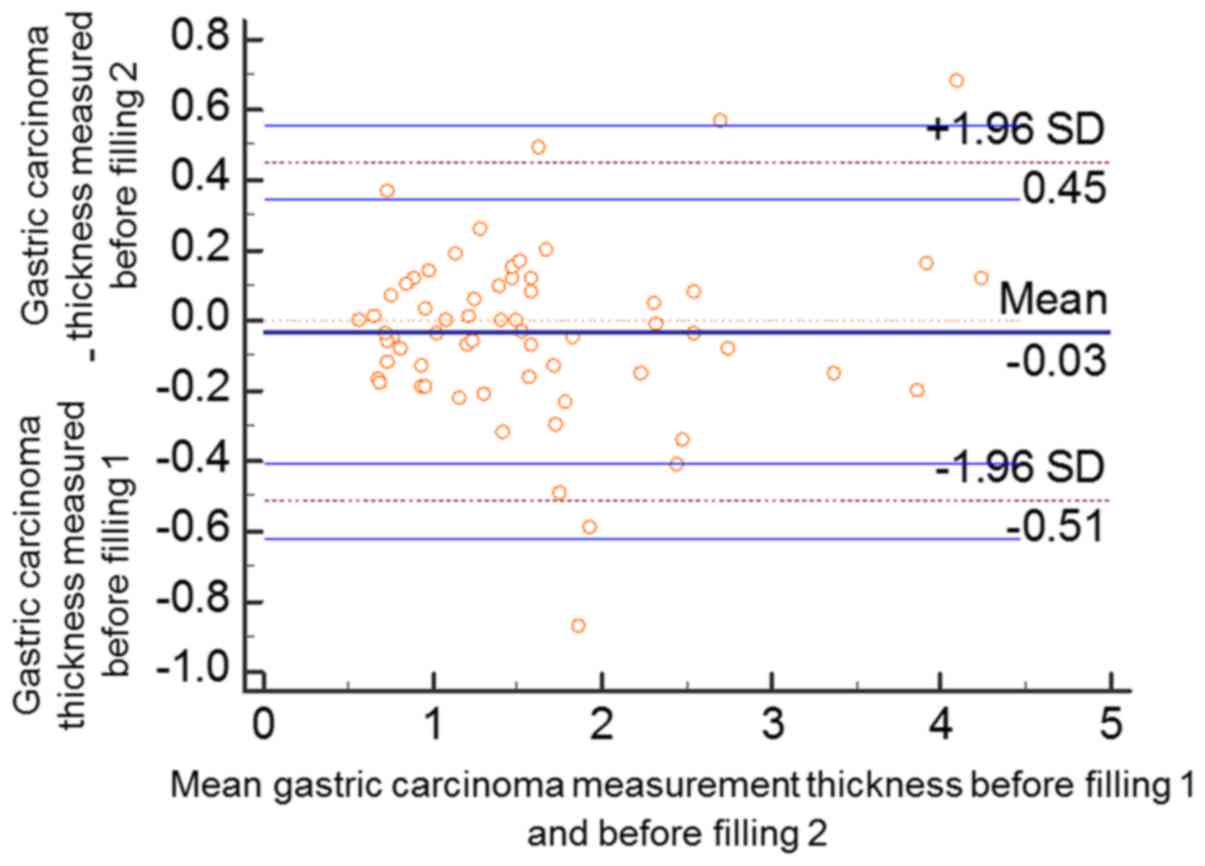
same investigator. According to Bland and Altman plots, two data
points prior to the gastric filling (2/63, 3.17%) were outside the
95% confidential interval (CI) of limits of agreement (LoA). The
mean value of the differences between the two measurements of the
gastric cancer thicknesses was −0.06 (95% CI of the LoA, −0.5943,
0.4784). A total of three data points following gastric filling
(3/63, 4.76%) were outside the 95% CI of LoA. The mean value of the
differences between the two measurements of the gastric cancer
thicknesses was −0.03 (95% CI of the LoA, −0.6194, 0.5566). Over
95% of all the data points prior to and following filling were in
the 95% CI of LoA, and the two measurements demonstrated high
consistency (Figs. 7 and 8).
Discussion
The present study aimed to assess the influence of
the degree of gastric filling on the measurement of gastric cancer
thickness. The results revealed that the gastric wall thickness
measurements prior to and following filling were significantly
different, and the change was greatest at the greater curvature.
The measurement of cancer thickness prior to and following gastric
filling was similar in newly diagnosed patients, but significantly
different in patients undergoing re-examination. However, two
thickness measurements, a month apart, in the same patients were
consistent. To the best of our knowledge, the present study is the
first to assess the differences in the CT measurement of cancer
thickness with or without gastric filling.
The stomach is a hollow organ, and anatomical
studies (20,21) undertaken long before CT scanning was
used to examine the stomach revealed that the thickness of the
normal gastric wall is associated with the degree of filling of the
abdominal cavity, which is concordant with the findings of the
present study. The thickness of the normal gastric wall was >5
mm when the stomach was empty, among which the thickness at the
greater curvature was >10 mm, as the duplicatures at this area
are thick and shrinking. However, once the abdominal cavity was
full, the thickness of the gastric wall at each area was <5 mm.
In addition, the effects of gastric filling degree on the thickness
of the gastric wall at the greater curvature were the highest.
However, the effects of filling on the thickness of gastric wall at
cardia and antrum were relatively low, which could be associated
with the thicker duplicatures at the greater curvature, whereas the
muscular layer is thinner. Therefore, the duplicatures gather
together when the stomach is empty, and are stretched following
filling. By contrast, the muscular layers at the cardia and antrum
are relatively thick, and thus their extensibility is limited.
Therefore, a criterium stating that ‘the thickness of gastric wall
>5 mm is considered as gastric wall thickening’ is not
necessarily applicable for all the gastric areas in clinical
imaging evaluation (22,23). The filling degree of the stomach and
the gastric area to be evaluated should therefore be considered
when using the criteria.
In the present study, to reduce the effects of
changes to the tumors on the measurements, and to avoid increasing
irradiation doses for patients, plain CT scanning was adopted when
the stomach was empty, and enhanced scanning was adopted subsequent
to filling. Therefore, the measurements prior to and following
filling could be obtained in one examination. Although the scanning
parameters were different in the two measurements, the density of
the soft tissues of gastric cancer was evidently different from
that of the intra-gastric gas and extra-gastric adipose tissue,
thus plain CT scanning could clearly measure the boundaries. In
addition, the methods of gastric cancer thickness measurement were
determined: For instance, the layer with the highest gastric cancer
thickness was selected, the regions adhered to perigastric lymph
nodes were avoided, and perigastric adipose tissues with evidence
of invasion were not included in the measurements. According to the
locations of the measurements in the venous phase, the same
locations were selected in the plain scanning images using
anatomical landmarks of the gastric wall, including the cardia,
pylorus and ulcer floor, rather than adjacent organs or selected
layers. Therefore, the thicknesses measured by these two different
scanning procedures were comparable. To avoid measurement errors in
CT assessment of the depth of gastric cancer invasion, as it is
widely accepted that CT is unable to measure either the depth of
early-stage gastric cancer or the effects of the rest of normal
tissue situated in deep layer on the measurement of the gastric
cancer wall depth, all patients enrolled in the present study had
advanced gastric cancer with tumor invasion through the whole
gastric wall under CT. The thickness of gastric cancer was 50%
higher than the thickness of the adjacent normal gastric wall
following filling. Therefore, the technique used in the present
study could not be applied to all patients with gastric cancer.
However, the technique was suitable for gastric cancer in any
region of the stomach, which met the inclusion criteria. The
present study included cases of cardia cancer.
The findings of the present study revealed that the
thickness of a gastric cancer is not affected by the degree of
gastric filling in newly diagnosed patients. However, for the
patients who were undergoing re-examination following non-surgical
treatment, the gastric cancer thickness could change with the
degree of filling. These changes could be associated with the more
evident muscular layer involvement, deeper gastric wall invasion
and denser cancerous tissues in newly diagnosed advanced gastric
cancer patients. However, necrosis, fibrosis, and shallower gastric
wall invasion were observed in the patients who had received
non-surgical treatments. Therefore, we hypothesized that the
thickness of advanced gastric cancer remains relatively unchanged
upon gastric filling prior to treatment, and could therefore be
used as the baseline measurement. However, the degree of filling of
the stomach should be consistent between the initial examination
and re-examination, which could aid the accurate assessment of the
treatment efficacy. The compliance of patients with gastric cancer
with the oral intake of a large volume of liquid filling agent is
lower than that of the general population. Thus, the dose of oral
liquid filling agent used in the two examinations among different
patients, and even in the same patient, could differ, as it is
difficult to maintain an identical degree of gastric filling
between the two measurements. As with liquid, gas has also been
widely used in clinical practices as a negative filling agent, and
its use has been reported in several previous studies (1,24–29). When mixed with liquid, aerogenic
powder generates carbon dioxide, which is non-toxic and
non-hazardous. In the present study, two packs of aerogenic powder
(6 g) were used for each patient, which released 800–1,000 ml gas,
which was similar to the dose of oral liquid filling agent.
Compared with the liquid filling agent, a gas filling agent has
several advantages. i) Prior to CT scanning, the degree of gastric
filling could be determined according to the positioning image. For
patients with a sub-optimal filling state, the filling agent could
be added. ii) The compliance and tolerability of patients was
superior when using a gas filling agent. Abdominal fullness
occurred in certain patients, which could be alleviated by belching
or exhausting. Compared with a liquid filling agent, a gas filling
agent is more easily excreted, and no complications, including
perforation, have been reported. iii) gas can be more homogeneously
distributed than liquid, and the dilation of gastric wall is more
even, thus the position of patients did not require changing during
the examination. iv) The volume of gas generated by single unit of
aerogenic powder is relatively stable, which aided control of the
degree of filling of the abdominal cavity. Therefore, it is easier
for clinicians to maintain the filling degree of the abdominal
cavity at the same level, and thus aid evaluation of treatment
efficacy. v) It could aid the performance of CT virtual endoscopy
and thus facilitate the early detection and display of a gastric
cancer. Therefore, when performing gastric CT scanning,
particularly for patients with gastric cancer who have undergone
non-surgical treatments, fixed-dose gas filling should be applied
to aid the consequent evaluation of treatment efficacy.
While performing evaluations of treatment efficacy
in clinical practices, the same investigator is generally asked to
conduct the measurements prior to and following treatments.
Therefore, in the present study the same investigator repeated the
measurements 1 month later to assess the reproducibility of
measuring gastric cancer thickness, following which Bland and
Altman plots were produced to assess consistency. The findings of
this study showed that >95% of all data points prior to and
following filling were in the 95% CI of LoA, indicating that the
two measurements were highly consistent. Therefore, measuring the
thickness of gastric lesions has relatively high reproducibility
and could be used as one of the parameters for the imaging
measurement.
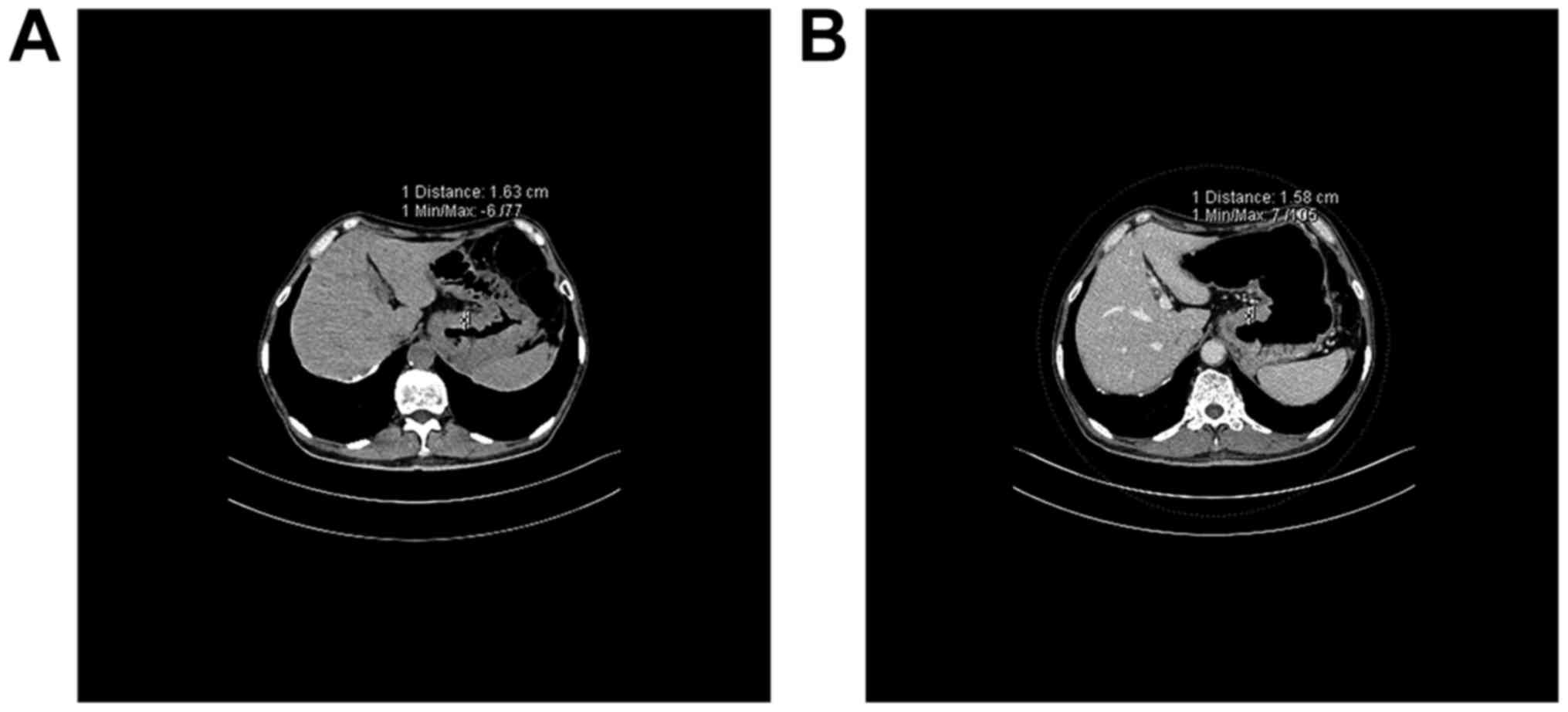
The CT images of the patients were also
retrospectively reviewed prior to treatment, and it was found that
for the patients that the gastric cancer thickness was greatly
affected by the filling degree, the thickness generally decreased
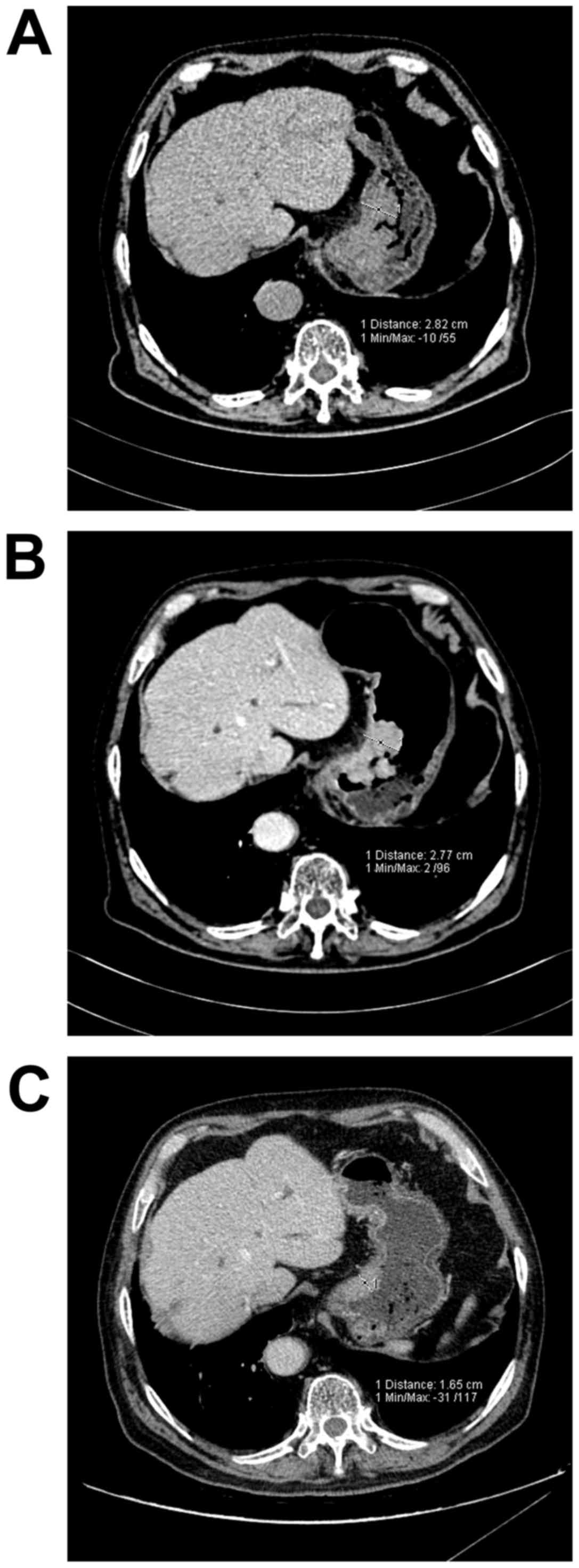
following the treatment (Fig. 5). For
the patients for whom the gastric cancer thickness was slightly
affected by the filling degree, the thickness generally remained
unchanged or even increased following the treatment (Fig. 6). As certain patients underwent a
liquid filling method at their initial examination, the filling
degree of the abdominal cavity could differ, thus the second
measurement was not obtained for these patients and the consequent
statistical analyses were not performed. These findings indicated
that the differences in gastric cancer thickness prior to and
following treatment could be associated with the treatment
efficacy.
There are several limitations to the present study.
First, because the method requires the tumors to be recognized in
plain CT images prior to gastric filling, the patients included in
the present study generally exhibited high gastric cancer thickness
and advanced disease stage, and there was a lack of T2-stage
patients. Second, the present study only assessed the effects of
gastric filling degree on the measurement of the thickness of
gastric cancer; the effects on the area or the longest diameter of
the cancer were not investigated. Third, the effects of gastric
filling degree on different pathological types of gastric cancer
were not compared. Finally, certain patients undergoing
re-examination used liquid filling prior to treatment, and the
treatment efficacies were not confirmed by pathological results.
Therefore, more studies are required to investigate the clinical
significance of measuring gastric cancer thickness on treatment
efficacy evaluation.
In conclusion, the measured thickness of gastric
cancer in newly diagnosed patients was relatively stable and may be
used as an indicator in baseline CT examination. Maintaining a
similar degree of gastric filling during re-examination may help to
accurately evaluate treatment efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the research. JL and GW collected
clinical data and performed computed tomography scans. LY and GS
performed the experiments. TZ and JP analyzed the data. LY, YL and
GS wrote the manuscript.
Ethics approval and consent to
participate
Application Form of Ethical Review from The Fourth
Hospital of Hebei Medical University (approval no. 2016
MEC111).
Consent for publication
Patients, parents or guardians provided written
informed consent for the publication of the present study.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Hallinan JT and Venkatesh SK: Gastric
carcinoma: Imaging diagnosis, staging and assessment of treatment
response. Cancer Imaging. 13:212–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carl-Mcgrath S, Ebert M and Röcken C:
Gastric adenocarcinoma: Epidemiology, pathology and pathogenesis.
Cancer Therapy. 6:877–893. 2008.
|
|
3
|
Zuo T, Zheng R, Zeng H, Zhang S and Chen
W: Epidemiology of stomach cancer in China. Chin J Clin Oncol.
44:52–58. 2017.
|
|
4
|
Ghiţă D, Glavici A, Pleşea IE, Săftoiu A,
Dumitrescu D and Ciurea T: Invasion assessment in gastric
carcinoma-imagistic and histopathologic combined study. Rom J
Morphol Embryol. 52(Suppl 1): S349–S361. 2011.
|
|
5
|
Moschetta M, Stabile Ianora AA, Anglani A,
Marzullo A, Scardapane A and Angelelli G: Preoperative T staging of
gastric carcinoma obtained by MDCT vessel probe reconstructions and
correlations with histological findings. Eur Radiol. 20:138–145.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Eun HW, Hong SS, Kim YJ, Han JK
and Choi BI: Gastric cancer detection using MDCT compared with 2D
axial CT: Diagnostic accuracy of three different reconstruction
techniques. Abdom Imaging. 37:541–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhandari S, Shim CS, Kim JH, Jung IS, Cho
JY, Lee JS, Lee MS and Kim BS: Usefulness of three-dimensional,
multidetector row CT (virtual gastroscopy and multiplanar
reconstruction) in the evaluation of gastric cancer: A comparison
with conventional endoscopy, EUS, and histopathology. Gastrointest
Endosc. 59:619–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furukawa K, Miyahara R, Itoh A, Ohmiya N,
Hirooka Y, Mori K and Goto H: Diagnosis of the invasion depth of
gastric cancer using MDCT with virtual gastroscopy: Comparison with
staging with endoscopic ultrasound. AJR Am J Roentgenol.
197:867–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Washington K: 7th Edition of the AJCC
Cancer Staging Manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DI Cicilia R, Mordenti P, Anselmi E,
Paties C, Carella E and Cavanna L: HER2-positive gastric cancer
showing marked thickening of the gastric wall on ultrasonographic
and computed tomographic scans. A chance phenomenon or a specific
behaviour of this cancer type? Report of three cases. Anticancer
Res. 34:5083–5086. 2014.PubMed/NCBI
|
|
11
|
Lee SM, Kim SH, Lee JM, Im SA, Bang YJ,
Kim WH, Kim MA, Yang HK, Lee HJ, Kang WJ, et al: Usefulness of CT
volumetry for primary gastric lesions in predicting pathologic
response to neoadjuvant chemotherapy in advanced gastric cancer.
Abdom Imaging. 34:430–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beer AJ, Wieder HA, Lordick F, Ott K,
Fischer M, Becker K, Stollfuss J and Rummeny EJ: Adenocarcinomas of
esophagogastric junction: Multi-detector row CT to evaluate early
response to neoadjuvant chemotherapy. Radiology. 239:472–480. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wieder HA, Beer AJ, Lordick F, Ott K,
Fischer M, Rummeny EJ, Ziegler S, Siewer JR, Schwaiger M and Weber
WA: Comparison of changes in tumor metabolic activity and tumor
size during chemotherapy of adenocarcinomas of the esophagogastric
junction. J Nucl Med. 46:2029–2034. 2005.PubMed/NCBI
|
|
14
|
Horton KM and Fishman EK: Current role of
CT in imaging of the stomach. Radiographics. 23:75–87. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gligorievski A: CT evaluation of gastric
lymphoma. Prilozi. 30:125–138. 2009.PubMed/NCBI
|
|
16
|
Giuliani A, Caporale A, Di Bari M, Demoro
M, Gozzo P, Corona M, Miccini M, Ricciardulli T and Tocchi A:
Maximum gastric cancer diameter as a prognostic indicator:
Univariate and multivariate analysis. J Exp Clin Cancer Res.
22:531–538. 2003.PubMed/NCBI
|
|
17
|
Ahn HS, Kim SH, Kodera Y and Yang HK:
Gastric cancer staging with radiologic imaging modalities and UICC
staging system. Dig Surg. 30:142–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JW, Shin SS, Heo SH, Choi YD, Lim HS,
Park YK, Park CH, Jeong YY and Kang HK: Diagnostic performance of
64-section CT using CT gastrography in preoperative T staging of
gastric cancer according to 7th edition of AJCC cancer staging
manual. Eur Radiol. 22:654–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kajitani T: The general rules for gastric
cancer study in surgery and pathology. Part I. Clinical
classification. Jpn J Surg. 11:127–139. 1981.
|
|
20
|
Miederer SE and Schepp W: The mucosal
barrier of the stomach. Anatomic structure and function. Dtsch Med
Wochenschr. 110:852–856. 1985.(In German). View Article : Google Scholar
|
|
21
|
Lim JH and Jeong YM: Sonography of the
stomach: An in vitro study to determine the anatomic cause of inner
hyperechoic and hypoechoic layers of the gastric wall. AJR Am J
Roentgenol. 162:335–338. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim
PN, Lee MG and Ha HK: Gastric cancer staging at multi-detector row
CT gastrography: Comparison of transverse and volumetric CT
scanning. Radiology. 236:879–885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukuya T, Honda H, Kaneko K, Kuroiwa T,
Yoshimitsu K, Irie H, Maehara Y and Masuda K: Efficacy of helical
CT in T-staging of gastric cancer. J Comput Assist Tomogr.
21:73–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang L, Li ZY, Li ZW, Zhang XP, Li YL, Li
XT, Wang ZL, Ji JF and Sun YS: Evaluating the response of gastric
carcinomas to neoadjuvant chemotherapy using iodine concentration
on spectral CT: A comparison with pathological regression. Clin
Radiol. 70:1198–1204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumano S, Okada M, Shimono T, Kuwabara M,
Yagyu Y, Imaoka I, Ashikaga R, Ishii K and Murakami T: T-staging of
gastric cancer of air-filling multidetector-row CT: Comparison with
hydro-multidetector-row CT. Eur J Radiol. 81:2953–2960. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moschetta M, Scardapane A, Telegrafo M,
Lorusso V, Angelelli G and Stabile Ianora AA: Differential
diagnosis between benign and malignant ulcers: 320-row CT virtual
gastroscopy. Abdom Imaging. 37:1066–1073. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yen PP and Stevenson G: Two- and
three-dimensional examination of the stomach (virtual gastroscopy):
Technical note. Can Assoc Radiol J. 61:41–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okten RS, Kacar S, Kucukay F, Sasmaz N and
Cumhur T: Gastric subepithelial masses: Evaluation of multidetector
CT (multiplanar reconstruction and virtual gastroscopy) versus
endoscopic ultrasonography. Abdom Imaging. 37:519–530. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi S, Hirayama M, Kuroiwa G, Kawano
Y, Takada K, Sato T, Miyanishi K, Sato Y, Takimoto R, Kobune M and
Kato J: Diagnostic validity of CT gastrography versus gastroscopy
for primary lesions in gastric cancer: Evaluating the response to
chemotherapy, a retrospective analysis. Gastric Cancer. 16:543–548.
2013. View Article : Google Scholar : PubMed/NCBI
|