Introduction
Renal cell carcinoma (RCC), also called kidney
cancer, is a heterogeneous disease. RCC begins in the lining of the
proximal convoluted tubule, a component of the very small tubes in
the kidney which transport primary urine. RCC is the most common
kidneys malignancy in adults (1–4). The
incidence of RCC is rapidly growing, increasing on average 1.1%
over the last ten years (5). A total
of 66,466 new cases of kidney tumors were diagnosed in China in
2012. If diagnosed early, RCC can be cured by surgical treatment.
However, RCC is resistant to chemotherapy and radiotherapy. Since
2015, molecularly targeted therapy is the preferred first-line
treatment for clear-cell RCC (6).
MicroRNA (miRNA), is a small endogenous non-coding
RNA, which negatively regulates gene expression by inhibiting
translation of messenger RNA (mRNA) or targeting mRNAs for
degradation (7). Aberrant miRNA
expressions are involved in tumorigenesis of various cancers
(8–13).
MicroRNA-338-3p (miR-338-3p) functions as a tumor
suppressor in various cancers, including thyroid cancer (14), non-small-cell lung cancer (15,16),
hepatocellular carcinoma (17),
breast cancer (18), and colorectal
carcinoma (19).
However, the role of miR-338-3p in RCC remains
unknown. In the present study, we investigated the function of
miR-338-3p in RCC, and found that miR-338-3p is an inhibitor of
tumor growth. We anticipate that our data may provide a potential
molecular target for the treatment of RCC, but further
investigation is needed.
Materials and methods
Tissue samples
Twelve RCC tissues samples were collected from the
Department of Urology, West China Hospital of Sichuan University
(Chengdu, China). The use of human tissues in the present study was
evaluated and approved by the Ethics Committee of Sichuan
University. Written informed consent were obtained from all
patients enrolled in the presnet study and all specimens were
handled and made anonymous as required according to the legal
standards of China. All RCC tissues samples were evaluated and
confirmed by a senior pathologist at the Sichuan University Cancer
Center.
H&E staining
The twelve RCC tissues were processed in standard
protocol for H&E staining. Briefly, 4-µm-thick sections were
cut. After deparaffinization and hydration, the slides were stained
in hematoxylin for 3–5 min and washed in running water for 5 min.
After being differentiated in 1% acid alcohol, the slides were
stained in 1% Eosin Y for 10 min. Then these slides were dehydrated
in increasing concentrations of alcohol and cleared in xylene prior
to observation.
Cell culture
The RCC cell lines Caki-1 and 786-O cells, and human
proximal convoluted tubule epithelial cell line HK-2, were
purchased from the Cell bank of Sichuan University. All cells were
maintained cultured in DMEM medium supplemented with 10% fetal
bovine serum (Gibco BRL, Grand Island, NY, USA) in 6-well plate
(Shengong, Shanghai, China).
Detection of miR-338-3p in samples and
RCC cells
The expression of miR-338-3p in tissues samples and
cells were analyzed by RT-qPCR. Briefly, the total RNA from tissue
samples or cells were extracted using the TRIzol reagent according
to the manufacturers's protocol (Invitrogen, Carlsbad, CA, USA).
The level of miR-338-3p was determined by the TaqMan miRNA Assay
(Thermo-Fisher, Waltham, MA, USA). The U6 snRNA was used as an
internal loading control. RT-qPCR was performed on an ABI 7900HT
instrument (Applied Biosystems, Foster City, CA, USA). The primers
were synthesized and tested by the ShengRui Company (ShengRui,
Chengdu, China) (20–23).
Overexpression and downregulation of
miR-338-3p in RCC cells
The miR-338-3p levels in Caki-1 and 786-O cells was
increased and decreased by miR-338-3p mimics and miR-338-3p
antisense oligonucleotides (ASO), respectively. The miR-338-3p
mimics and miR-338-3p ASO were both purchased from JingHong
Biotechnoloy (Chengdu, China). Before transfection, the cells were
cultured overnight (1×106 per well). The cells
transfection was performed with Lipofectamine 2000 (Invitrogen), as
recommended by the manufacturer's instructions.
Cell proliferation assay
The cellular growth was analyzed by the MTT assay.
Briefly, cells were placed into 96-well plates at a density of
5×105/well for overnight. Then remove the medium and
replace it with 100 µl of fresh culture medium, and the MTT reagent
was added into the medium at a final concentration of 0.1 mg/ml.
Then the plates were incubated for 4 h at 37°C. Next medium from
each well was carefully removed and 100 µl DMSO were added and
incubated at 37°C for 15 min. OD was measured on a microplate
reader with a 570 nm filter (24).
Cell apoptosis analysis
Cells (5×105 cells/ml) were suspended in
the Annexin V-FITC (Abcam, Cambridge, UK) binding buffer. Then,
Annexin V-FITC was added and the suspension was incubated for 15
min at room temperature. Afterwards, propidium iodide (PI; Abcam)
was added to each sample. Next the samples were analyzed on a FACS
analyzer instrument using the 488 nm excitation line (Argon-ion
laser or solid state laser) and emission was detected at 530 nm
(green, FITC) and 575–610 nm (orange, PI).
Prediction of the putative targets of
miR-338-3p
The Targetscan software (http://www.targetscan.org/) was used to predict the
putative targets of miR-338-3p.
Dual luciferase reporter assays
Cells were seeded at 1×105 per well and
were serum-starved for 6 h pre-transfection. The 3′untranslated
region (3′UTR) of Akt3 and mutated controls were cloned and
inserted into the reporter plasmid (500 ng) and the pGL3-control
(100 ng; Promega, Madison, WI, USA). MiR-338-3p p mimics were then
transfected into the Caki-1 cells containing the wild-type or
mutant 3′UTR plasmids with Lipofectamine 2000 (Invitrogen). Cells
were harvested and luciferase activitity was measured after 24 h
using the Dual-Luciferase Reporter Assay System (Huijun Company,
Guangzhou, China). Mutant of Akt3 3′UTR were generated using the
Site-Directed Mutagenesis kit (Promega).
Western blot analysis
Cells were frozen and lysed in lysis buffer (150 mM
NaCl, 50 mM Tris-HCI, 1% Triton X-100 and 0.1% SDS) with the
protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and
phosphatase inhibitor cocktail. For Akt3 western blotting, an
anti-Akt3 antibody (Abcam) were used at a dilution of 1:1,000,
followed by detection with a peroxidase-linked antibody to rabbit
antibody IgG (1:2,000 dilution, Abcam). Proteins were detected with
the ECL Western Blotting Detection Reagents (GE Healthcare,
Chicago, IL, USA). Images were analyzed using Image J (NIH,
Bethesda, MD, USA).
Statistical analysis
All experiments were repeated three times. Data are
shown as mean ± SD. Two-tailed Student's t-test was used to analyze
the mean value between two groups; ANOVA was used to test the mean
value among three or more than three groups. P<0.05 was
considered to indicate a statistically significant difference. All
calculations were performed using SPSS software (version 16.0; SPSS
Inc., Chicago, IL, USA).
Results
Low levels of miR-338-3p in RCC
tissues samples
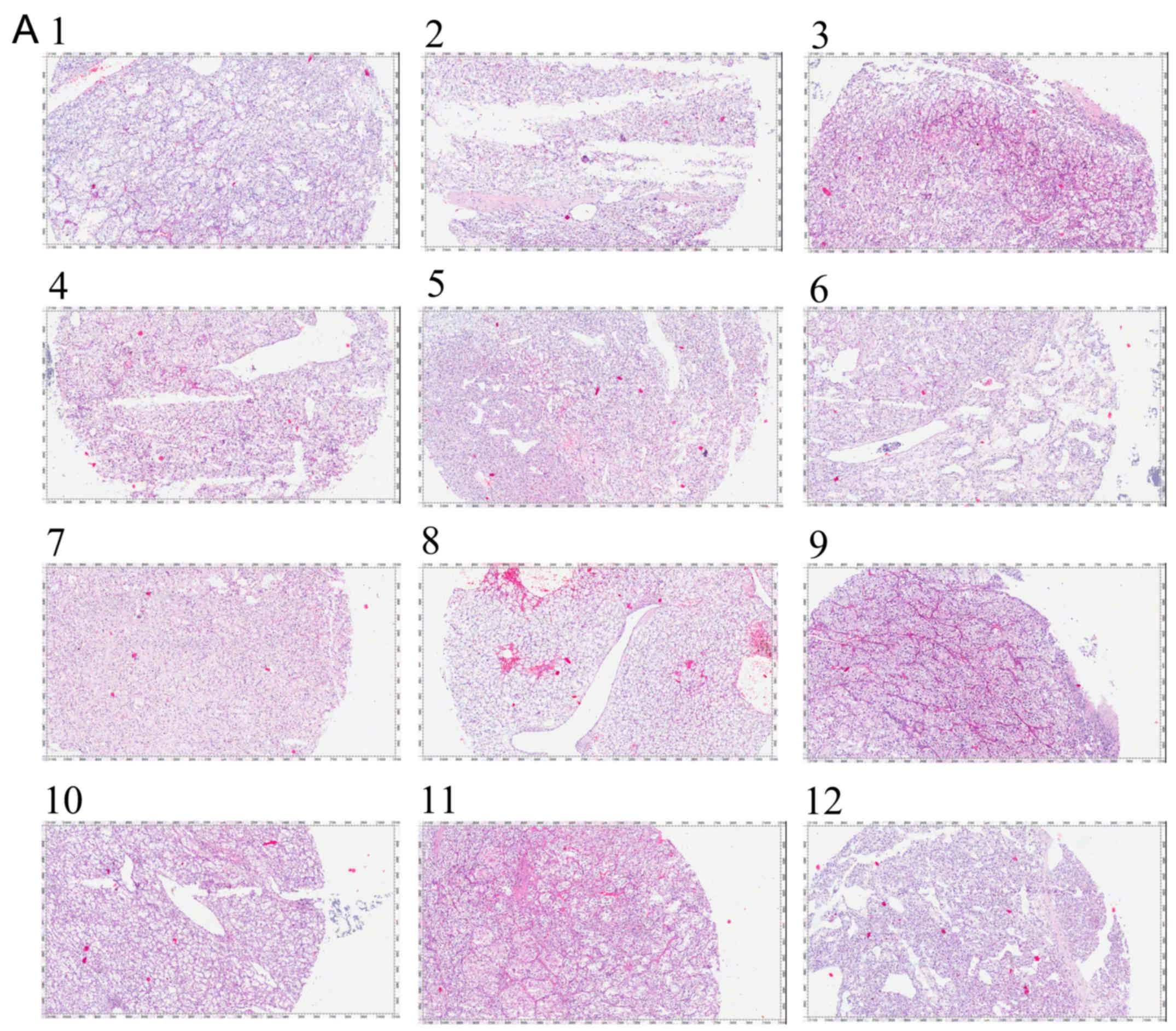
Initially, we collected 12 RCC tissues and the
pathological images are shown in Fig.
1A. Next, the 12 RCC tissues samples and their matched
tumor-adjacent samples were analyzed by RT-qPCR for miRNA-338-3p
expression. We found that in every tissue tissues sample from each
of the 12 RCC patients, the miRNA-338-3p level in the RCC sample
was lower than the level in the matched tumor-adjacent tissue
(Fig. 1B). In addition, we calculated
the mean value of the 12 RCC tissues samples and matched
tumor-adjacent tissues samples, and the results revealed that the
mean value of miR-338-3p in RCC tissues was lower than the mean
value detected in normal tissues (Fig.
1C).
Enforced expression of miR-338-3p
inhibits cellular growth and promoted cell apoptosis
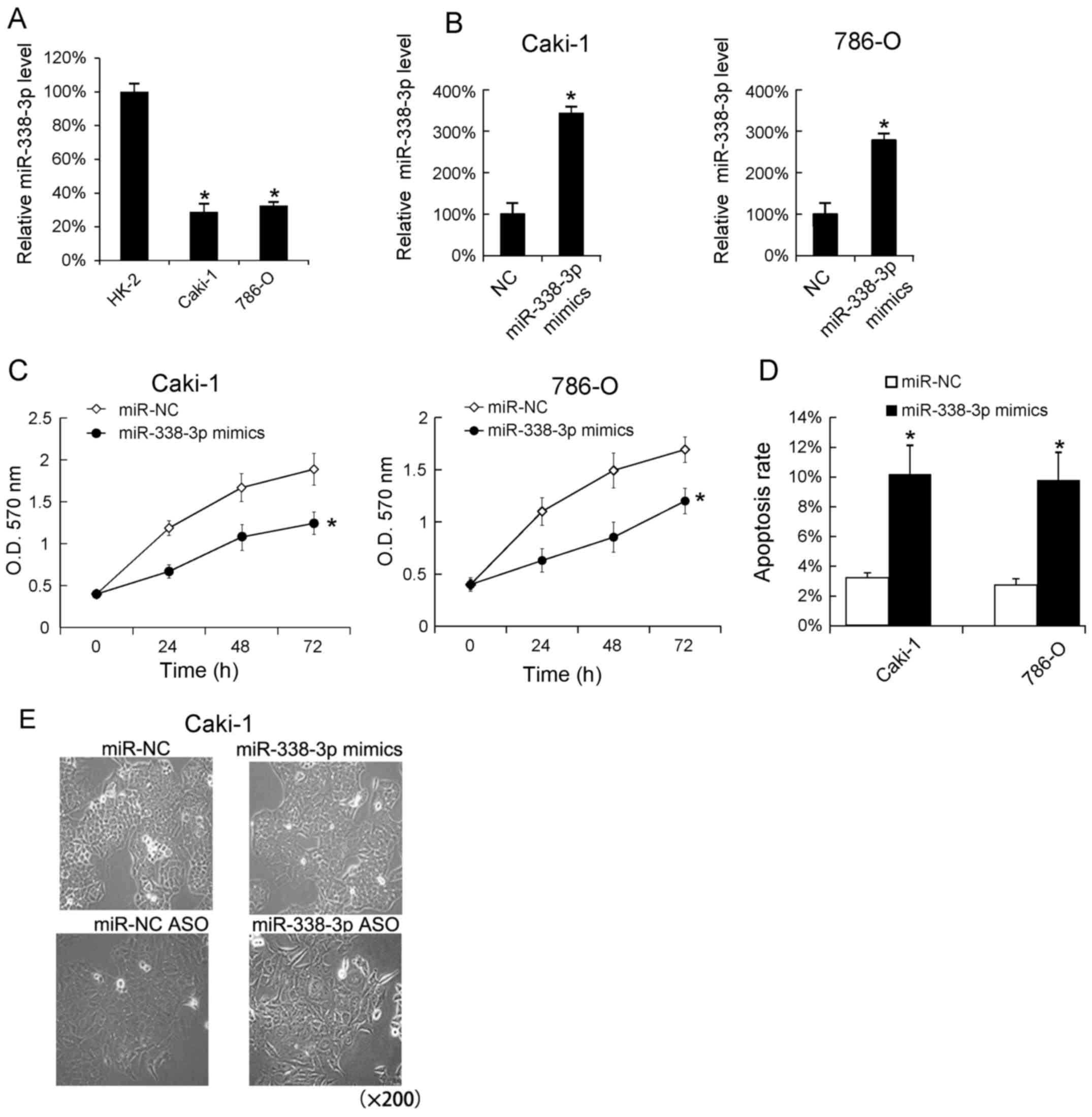
We assayed the miR-338-3p levels in RCC cell lines
(Caki-1 and 786-O) by RT-qPCR analysis. The human proximal
convoluted tubule epithelial cells, HK-2 were used as blank
control. We found that the miR-338-3p levels in Caki-1 and 786-O
cells was lower than in HK-2 cells (Fig.
2A). In addition, we transfected miR-338-3p mimics into Caki-1
and 786-O cells and found that miR-338-3p mimics very effectively
increased the miR-338-3p levels in Caki-1 and 786-O (Fig. 2B). Since miR-338-3p mimics could
increase the miR-338-3p levels in vitro, we analyzed
cellular proliferation following miR-338-3p mimics transfection,
and found that the increase miR-338-3p promoted the cells
proliferation in Caki-1 and 786-O cells (Fig. 2C). Additionally, we evaluated the
effect of miR-338-3p mimics on cells apoptosis, and found that
transfection of miR-338-3p mimics increased the apoptosis rate
(Fig. 2D). The Caki-1 cell
morphological changes following 24 h miR-338-3p mimics and
miR-338-3p ASO transfection were shown in Fig. 2E.
Downregulation of miR-338-3p promotes
cell growth
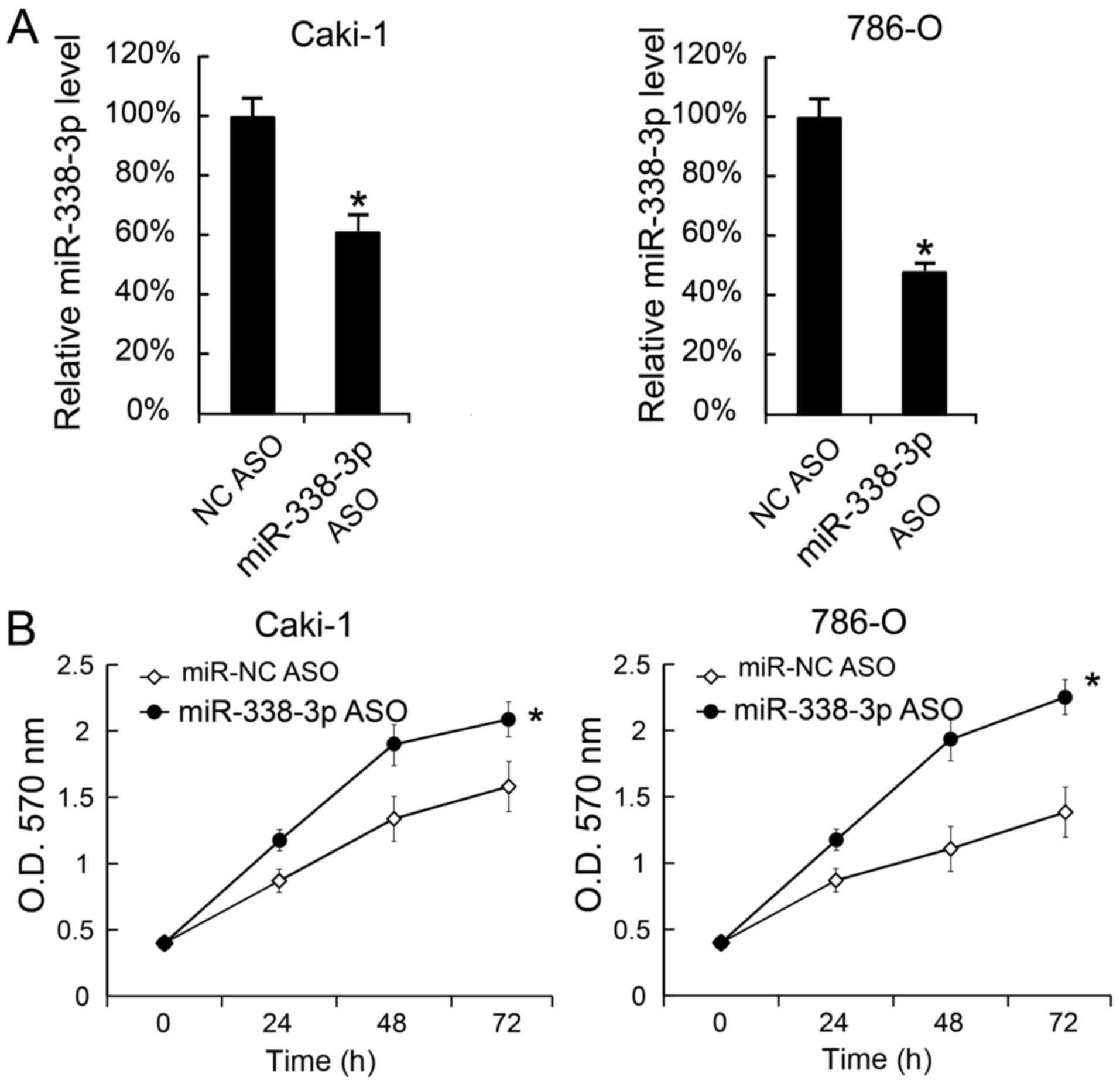
We also transfected miR-338-3p ASO into Caki-1 and
786-O cells to decrease the miR-338-3p levels. The miR-338-3p
levels in cells were measured 24 h after miR-338-3p ASO
transfection. We found that miR-338-3p was inhibited by miR-338-3p
ASO (Fig. 3A). Moreover, cellular
proliferation was assessed by MTT analysis, and the results
revealed that miR-338-3p transfection promoted cellular
proliferation (Fig. 3B).
AKT33 is a direct target of
miR-338-3p
To understand of the mechanisms whereby which
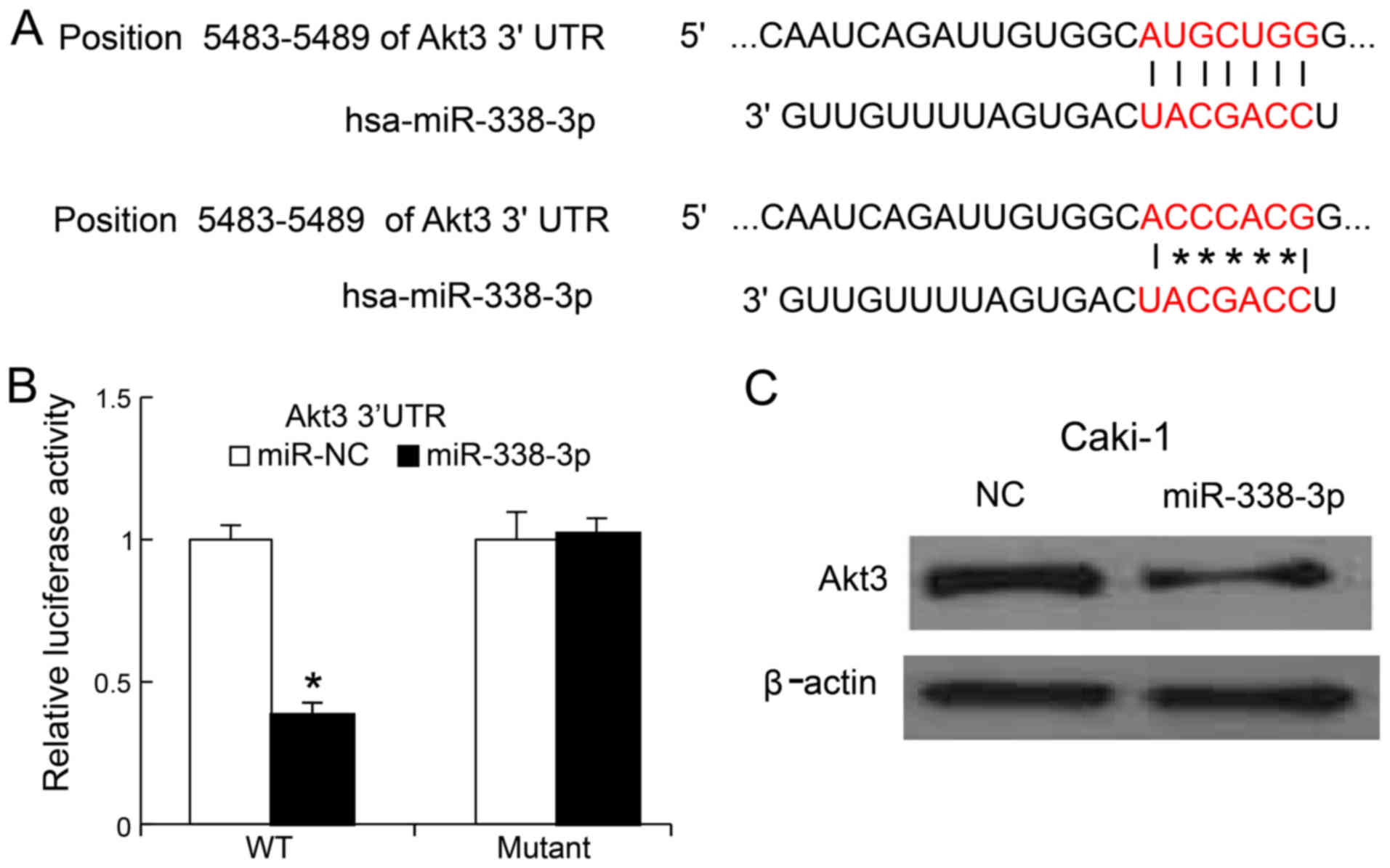
miR-338-3p suppressed RCC cells growth, we search the potential
targets of miR-338-3p by bioinformatics approach. We found that the
3′-UTR of AKT3 could bind to the seed region of miR-338-3p
(Fig. 4A). To confirm whether
miR-338-3p could target AKT3, we generated the mutant of
3′UTR of AKT3 and cloned the mutant and wild-type version
into luciferase reporter plasmids. MiR-338-3p mimics and mutant
version were co-transfected into Caki-1 cells. We found, 24 h post
transfection, that miR-338-3p mimics reduced the luciferase
activity of the 3′UTR of AKT3 (wild-type version), but not
that of mutated version of 3′UTR of AKT3 (Fig. 4B). We also transfected miR-338-3p
mimics into the Caki-1 cells, 48 h later, the AKT3 protein
levels were determined by western blot analysis, we found that
miR-338-3p mimics could inhibit Caki-1 the expression of AKT3 in
Caki-1 cells (Fig. 4C).
Discussion
In the present study, we examined the role of
miR-338-3p in RCC and found that miR-338-3p exhibited a tumor
suppressor activity in RCC. Additionally, we determined that the
direct target gene of miR-338-3p is AKT3.
Previous studies have shown that miR-338-3p
functions as a tumor suppressor in various cancers, including
thyroid cancer (14), non-small-cell
lung cancer (15,16), hepatocellular carcinoma (17), breast cancer (18), colorectal carcinoma (19). Our study elucidated the role of
miR-338-3p in RCC. In addition, we found that miR-338-3p inhibited
RCC cells growth by targeting AKT3.
AKT3 is a member of Akt kinase family, also called
PKB, serine/threonine protein kinase family. Akt kinases are
regulators of cell signaling in response to insulin and growth
factors. Akt kinases play a role in cell proliferation,
differentiation, apoptosis, and tumorgenesis (25–33). AKT
is an oncogene which is involved in the development and progression
of many cancers. Our data revealed that miR-338-3p could target
AKT3 in vitro. Previous studies showed that AKT3 may act in
two ways in the pathogenesis of cancer. One is that AKT3 is
significantly correlated with a 76-gene signature DNA revealed by
correlation analysis, and genomically amplified AKT3 activates the
DNA repair pathway and promotes glioma progression (34); The other is AKT3 knockdown induces
mitochondrial dysfunction in human cancer cells (35). However, the correlation between
miR-338-3p and AKT3 expression in RCC samples was not investigated
due to the limitation of RCC samples, and we will investigate this
and the precise role of AKT3 in RCC in next study.
In conclusion, our data revealed the suppressive
role in miR-338-3p in RCC. We hope our findings may provide a new
therapeutic target for further investigation.
Acknowledgements
The present study was supported by a grant from
Department of Sichuan Science and Technology (grant no.
2017FZ0057).
References
|
1
|
Perlman E, Grosfeld J, Togashi K and
Boccon-Gibod L: Pathology and genetics of tumors of the urinary
system and male genital organs. World Health Organization
Classification of Tumours. 1st edition. IARC Press; Lyon, France;
pp. 48–52. 2004
|
|
2
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gelb AB: Renal cell carcinoma: Current
prognostic factors. Union Internationale Contre le Cancer (UICC)
and the American Joint Committee on Cancer (AJCC). Cancer.
80:981–986. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo J, Ma J, Sun Y, Qin S, Ye D, Zhou F,
He Z, Sheng X, Bi F, Cao D, et al: Chinese guidelines on the
management of renal cell carcinoma (2015 edition). Ann Transl Med.
3:2792015.PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie B, Ding Q, Han H and Wu D: miRCancer:
A microRNA-cancer association database constructed by text mining
on literature. Bioinformatics. 29:638–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nugent M: microRNA and bone cancer. Adv
Exp Med Biol. 889:201–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Del Vescovo V and Denti MA: microRNA and
lung cancer. Adv Exp Med Biol. 889:153–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui GQ, Fei D, Guo F, Zhen X, Luo Q, Yin S
and Wang H: MicroRNA-338-3p inhibits thyroid cancer progression
through targeting AKT3. Am J Cancer Res. 7:1177–1187.
2017.PubMed/NCBI
|
|
15
|
Zhang G, Zheng H, Zhang G, Cheng R, Lu C,
Guo Y and Zhao G: MicroRNA-338-3p suppresses cell proliferation and
induces apoptosis of non-small-cell lung cancer by targeting
sphingosine kinase 2. Cancer Cell Int. 17:462017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang P, Shao G, Lin X, Liu Y and Yang Z:
MiR-338-3p inhibits the growth and invasion of non-small cell lung
cancer cells by targeting IRS2. Am J Cancer Res. 7:53–63.
2017.PubMed/NCBI
|
|
17
|
Zhang T, Liu W, Zeng XC, Jiang N, Fu BS,
Guo Y, Yi HM, Li H, Zhang Q, Chen WJ and Chen GH: Down-regulation
of microRNA-338-3p promoted angiogenesis in hepatocellular
carcinoma. Biomed Pharmacother. 84:583–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin Y, Zhao M, Xie Q, Zhang H, Wang Q and
Ma Q: MicroRNA-338-3p functions as tumor suppressor in breast
cancer by targeting SOX4. Int J Oncol. 47:1594–1602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue Q, Sun K, Deng HJ, Lei ST, Dong JQ and
Li GX: MicroRNA-338-3p inhibits colorectal carcinoma cell invasion
and migration by targeting smoothened. Jpn J Clin Oncol. 44:13–21.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li D, Liu X, Lin L, Hou J, Li N, Wang C,
Wang P, Zhang Q, Zhang P, Zhou W, et al: MicroRNA-99a inhibits
hepatocellular carcinoma growth and correlates with prognosis of
patients with hepatocellular carcinoma. J Biol Chem.
286:36677–36685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song B, Zhang C, Li G, Jin G and Liu C:
MiR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Q and Yu Y: Upregulated CDK16
expression in serous epithelial ovarian cancer cells. Med Sci
Monit. 21:3409–3414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Xu Y, Qiu W, Zhao D and Zhang Y:
Tissue miR-193b as a novel biomarker for patients with ovarian
cancer. Med Sci Monit. 21:3929–3934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gai D, Haan E, Scholar M, Nicholl J and Yu
S: Phenotypes of AKT3 deletion: A case report and literature
review. Am J Med Genet A. 167A:174–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm
C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H and
Zeiher AM: HMG-CoA reductase inhibitors (statins) increase
endothelial progenitor cells via the PI 3-kinase/Akt pathway. J
Clin Invest. 108:391–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stitt TN, Drujan D, Clarke BA, Panaro F,
Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD and Glass DJ: The
IGF-1/PI3K/Akt pathway prevents expression of muscle
atrophy-induced ubiquitin ligases by inhibiting FOXO transcription
factors. Mol Cell. 14:395–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manning BD, Tee AR, Logsdon MN, Blenis J
and Cantley LC: Identification of the tuberous sclerosis complex-2
tumor suppressor gene product tuberin as a target of the
phosphoinositide 3-kinase/akt pathway. Mol Cell. 10:151–162. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and-independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Turner KM, Sun Y, Ji P, Granberg KJ,
Bernard B, Hu L, Cogdell DE, Zhou X, Yli-Harja O, Nykter M, et al:
Genomically amplified Akt3 activates DNA repair pathway and
promotes glioma progression. Proc Natl Acad Sci USA. 112:3421–3426.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim M, Kim YY, Jee HJ, Bae SS, Jeong NY,
Um JH and Yun J: Akt3 knockdown induces mitochondrial dysfunction
in human cancer cells. Acta Biochim Biophys Sin (Shanghai).
48:447–453. 2016. View Article : Google Scholar : PubMed/NCBI
|