Introduction
Colorectal cancer (CRC), the second-most diagnosed
cancer and the fourth-most frequent cause of cancer-associated
mortality (1), remains one of the
most serious health problems worldwide. In China, it ranks fifth in
the morbidity and mortality rates among all types of cancer, with
191,000 mortalities in 2015 (2).
Chemotherapy serves a vital role in the treatments
of CRC, particularly for patients with advanced CRC; it lessens the
number and severity of clinical symptoms, improves the quality of
lives and prolongs survival (3). Drug
resistance is a major obstacle in chemotherapy (4). Oxaliplatin (L-OHP), a third-generation
platinum (Pt) compound, is the first-line drug for CRC chemotherapy
(5). However, resistance to L-OHP
leads to treatment failure and relapse in patients with CRC
(4).
Reduced intracellular Pt accumulation has been
identified as a major mechanism of L-OHP resistance (6). Adequate accumulation of intracellular Pt
is essential for anticancer drugs to exert their cytotoxic effects
(7). Copper transporters serve
important roles in the cellular import and export of Pt drugs
(8). Human copper transporter 1
(hCTR1) and Copper-transporting p-type adenosine triphosphatases 1
(ATP7A) and 2 (ATP7B) have been identified as key copper
transporters (9). hCTR1 regulates the
influx of Pt drugs, while ATP7A and ATP7B regulate their efflux
(9). The upregulation of hCTR1 and
downregulation of ATP7A and ATP7B may be potential mechanisms of
L-OHP resistance (10).
Gambogic acid (GA), an active component of the
traditional Chinese medicine Garcinia hanburyi, exhibits
multi-target anti-tumour effects with few side effects (11). Previously, GA was identified to be
able to reverse resistance to anticancer drugs, including
resistance to 5-fluorouracil in CRC (12), to doxorubicin in breast (13) and ovarian cancer (14), and to docetaxel in gastric (15) and human epithelial cancer (16).
However, to the best of our knowledge, the ability
of GA to reverse L-OHP resistance in CRC cells has not been
investigated. Therefore, in the present study, using a step-wise
increasing concentration method, L-OHP-resistant LoVo/L-OHP and
L-OHP-sensitive LoVo/L-OHP/GA cell lines were successfully
established, and it was identified that GA may reverse L-OHP
resistance, potentially by increasing intracellular platinum
through increasing hCTP1 and decreasing ATP7A and ATP7B protein
levels. GA may represent a promising treatment agent for L-OHP
resistance.
Materials and methods
Materials
LoVo cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with 10% foetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. L-OHP was
purchased from Jiangsu Hengrui Pharmaceutical Co., Ltd. (cat no.
H20000337; Lianyungang, China). GA was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The Cell Counting
Kit-8 (CCK-8) was obtained from Beyotime Institute of Biotechnology
(Haimen, China). The Alexa Fluor®488 Annexin V/Dead Cell
Apoptosis kit was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. Antibodies against hCTR1 (cat. no. ab108481;
rabbit polyclonal), ATP7A (cat. no. ab42486; rabbit polyclonal),
ATP7B (cat. no. ab124973; rabbit monoclonal) and GAPDH (cat. no.
ab9485; rabbit polyclonal) were purchased from Abcam (Cambridge,
MA, USA).
Establishment of LoVo/L-OHP and
LoVo/L-OHP/GA cell lines
The L-OHP-resistant LoVo/L-OHP cell line was
established by exposing LoVo cells to increasing concentrations of
L-OHP (1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 25, 30, 35, 40, 45
and 50 µmol/l) for 48 h at each concentration as described
previously (17,18). LoVo/L-OHP cells were then cultured in
complete RPMI-1640 medium with 4 µmol/l L-OHP at 37°C with 5%
CO2. After 6 months, LoVo/L-OHP cells capable of growing
in 60 µmol/l L-OHP were obtained. To examine the effects of drug
intervention, the culture medium was changed to complete RPMI-1640
medium without L-OHP 1 week prior to experimentation.
The GA-reversed L-OHP-sensitive LoVo/L-OHP/GA cell
line was established by continuous exposure of LoVo/L-OHP cells to
GA.
Briefly, LoVo/L-OHP cells were cultured in complete
RPMI-1640 medium without L-OHP for 1 week, and then cultured in
complete RPMI-1640 medium with 0.5 µmol/l GA at 37°C with 5%
CO2 for 2 weeks. The culture medium was changed every 24
h. The LoVo/L-OHP/GA cells were then collected and stored for
subsequent experiments.
Morphological observations
The recovery established LoVo, LoVo/L-OHP or
LoVo/L-OHP/GA cells were cultured to ~80% confluency. Cells were
observed after 24 h using an inverted light microscope
(magnification, −800) in order to observe morphological
changes.
Cell viability assay
Cytotoxicity was determined by a CCK-8 assay.
Briefly, LoVo, LoVo/L-OHP or LoVo/L-OHP/GA cells (4×104
cells/ml) were cultured in 96-well plates overnight. A total of 100
µl of different concentrations of L-OHP (0, 5, 10, 15, 20, 25, 30,
35, 40, 45, 50, 55 and 60 µmol/l) were then added for at 37°C with
5% CO2 48 h. Next, 10 µl CCK-8 reagent was added for 2
h, and the absorbance at 450 nm was determined on a microplate
reader (iMark™; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Resistance index (RI)=half maximal inhibitory concentration
(IC50) of drug-resistant cells/IC50 of
drug-sensitive cells (10).
Assessment of cell apoptosis
Cells were harvested (0.25% trypsin was added for 30
sec to digest the cells, followed by centrifugation at 560 × g at
37°C for 5 min and the supernatant was then discarded) following
treatment with 20 µmol/l L-OHP for 6 h and re-suspended in
Annexin-binding buffer (Invitrogen; Thermo Fisher Scientific, Inc.)
to a concentration of 2×106/ml. Annexin V (solution in
25 mM HEPES, 140 mM NaCl, 1 mM EDTA, pH 7.4, 0.1% bovine serum
albumin) and propidium iodide working solutions (1 mg/ml) were then
added at room temperature for 15 min. Flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA) was then performed, and data
was analysed using FlowJo 7.6 software (FlowJo LLC, Ashland, OR,
USA).
Transwell matrix penetration
assay
Cells (LoVo, LoVo/L-OHP and LoVo/L-OHP/GA cells)
were cultured in RPMI-1640 medium without FBS for 24 h, following
which 2×104/ml cells suspended in 2 µmol/l L-OHP were
plated in the upper chamber of a polycarbonate Transwell filter in
BioCoat™ Invasion Chambers (BD Biosciences) and incubated for at
37°C with 5% CO2 for 24 h. RPMI-1640 medium with 10% FBS
was added to the lower chamber at 37°C with 5% CO2 for
24 h. Cells that migrated to the lower membrane were fixed with 1%
paraformaldehyde at 37°C for 10 min, stained with 1% haematoxylin
at 37°C for 10 min and counted by microscopy in 10 fields of view
using a light microscope (magnification, −400).
Intracellular accumulation of Pt
A total of 1×107 cells/ml of LoVo,
LoVo/L-OHP or LoVo/L-OHP/GA cells were seeded into 10 cm culture
dishes for 24 h. Then, 0, 0.5, 1, 2 or 4 µmol/l L-OHP was added for
4 h, or 2 µmol/l L-OHP for 1, 4, 12 or 24 h. Cells were harvested
(0.25% trypsin was added for 30 sec to digest the cells, followed
by centrifugation at 560 × g at 37°C for 5 min and the supernatant
was then discarded) following treatment, washed with PBS and lysed
with TRIzol® (Life Technologies; Thermo Fisher
Scientific, Inc.). Intracellular Pt was determined by inductively
coupled plasma mass spectrometry (ICP-MS; PerkinElmer, Inc.,
Waltham, MA, USA) as described previously (19).
Western blotting
Total protein from LoVo, LoVo/L-OHP and
LoVo/L-OHP/GA cells were extracted with SDS-PAGE Sample loading
buffer (cat no. P0015; Beyotime Institute of Biotechnology), and
proteins were determined using the Bradford method (14). A total of 50 g protein was resolved
using SDS-PAGE (10% separating glue and 4% concentrated glue) and
transferred on to polyvinylidene fluoride membranes. Subsequent to
blocking with 5% non-fat milk dissolved in Tris-buffered saline
with Tween-20 buffer (Tris-Hcl, NaCl and Tween-20) at 37°C for 1 h,
the membranes were incubated with anti-hCTR1 (1:1,000), anti-ATP7A
(1:1,000), anti-ATP7B (1:1,000) and anti-GAPDH (1:1,000) antibodies
at 4°C overnight. The membranes were then incubated with 15 ml
horseradish peroxidase-labelled secondary antibody (1:2,000; cat
no. 31490; Thermo Fisher Scientific, Inc.) at room temperature for
1 h. Signals were visualised with the SuperSignal West PICO
chemiluminescent detection system (Pierce; Thermo Fisher
Scientific, Inc.). Image J 1.48 (National Institutes of Health,
Bethesda, MD, USA) was used to perform the densitometric
analysis.
Statistical analysis
All data were analysed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Values are presented as mean ± standard
deviation. Differences were analysed using one-way analysis of
variance followed by a least significant difference post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
L-OHP inhibits the proliferation of
the LoVo, LoVo/L-OHP and LoVo/L-OHP/GA cells
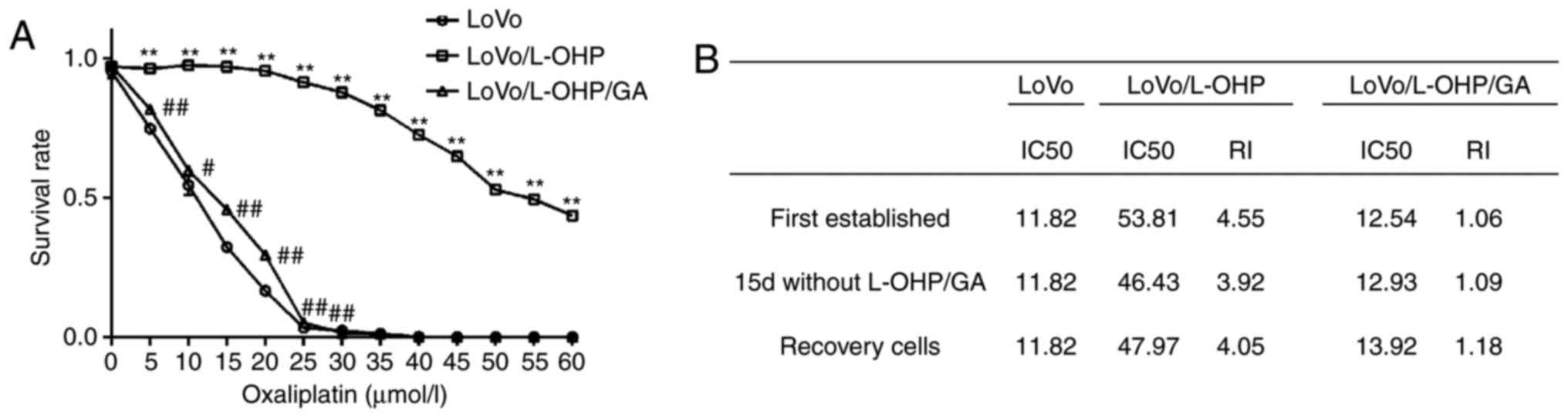
The cytotoxicity of L-OHP to LoVo, LoVo/L-OHP and
LoVo/L-OHP/GA cells was first analysed using a CCK-8 assay. As
demonstrated in Fig. 1A, as the
concentration of L-OHP increased, the survival rates of cells
decreased, indicating that L-OHP increased the levels of
cytotoxicity in a dose-dependent manner. The survival rate of the
LoVo/L-OHP cells was increased compared with those of the LoVo and
LoVo/L-OHP/GA cells (P<0.05). After 24 h treatment with 40
µmol/l L-OHP, the LoVo and LoVo/L-OHP/GA cells were almost entirely
killed. However, 72.53±3.06% LoVo/L-OHP cells survived.
The IC50 of L-OHP was then calculated
(Fig. 1B). The L-OHP IC50
for LoVo cells was 11.82 µmol/l, while that for the LoVo/L-OHP
cells was 53.81 µmol/l. The RI for the LoVo/L-OHP cells was 4.55.
The IC50 for the LoVo/L-OHP/GA cells was 12.54 µmol/l
and the RI was 1.06. The results demonstrated that the LoVo/L-OHP
cells were resistant to L-OHP, and that GA inhibited this
resistance.
Whether the established L-OHP-resistant cells and
sensitive cells were able to maintain their characteristics was
also assessed. LoVo/L-OHP cells were cultured in complete RPMI-1640
medium without L-OHP for 15 days, following which the
IC50 for the LoVo/L-OHP cells was 46.43 µmol/l, the RI
was 3.92 and resistance remained at 86.29% viable cells. Subsequent
to storage in liquid nitrogen (−196°C) for 2 months, recovered
LoVo/L-OHP cells were able to grow and proliferate. The
IC50 for the recovered LoVo/L-OHP cells was 47.97 µmol/l
and the RI was 4.05, indicating that the established LoVo/L-OHP
cells were able to maintain resistance. Regarding the LoVo/L-OHP/GA
cells, following culture incomplete RPMI-1640 medium without GA for
15 days, the IC50 was 12.93 µmol/l and the RI was 1.09.
The resistance was 27.85% of all viable LoVo/L-OHP cells, which was
higher compared with the first established cells (23.0% viable
cells). The LoVo/L-OHP/GA cells recovered from liquid nitrogen were
also able to grow and proliferate. The IC50 for the
recovered cells was 13.92 µmol/l and the RI was 1.18, suggesting
that GA was able to reverse L-OHP resistance, and that the
L-OHP-sensitive cells had been successfully established.
Morphological changes of LoVo/L-OHP
and LoVo/L-OHP/GA cells
The morphological changes of the established cells
were then observed through inverted light microscopy
(magnification, −800). As demonstrated in Fig. 2, the parent LoVo cells were adherent,
flat and polygonal, with numerous cell junctions.
LoVo/L-OHP-resistant cells were rounder and bigger, and the nuclei
were clearer. The LoVo/L-OHP/GA cells exhibited a similar
appearance to the recovered LoVo cells.
GA reverses the anti-apoptosis ability
of the LoVo/L-OHP cells
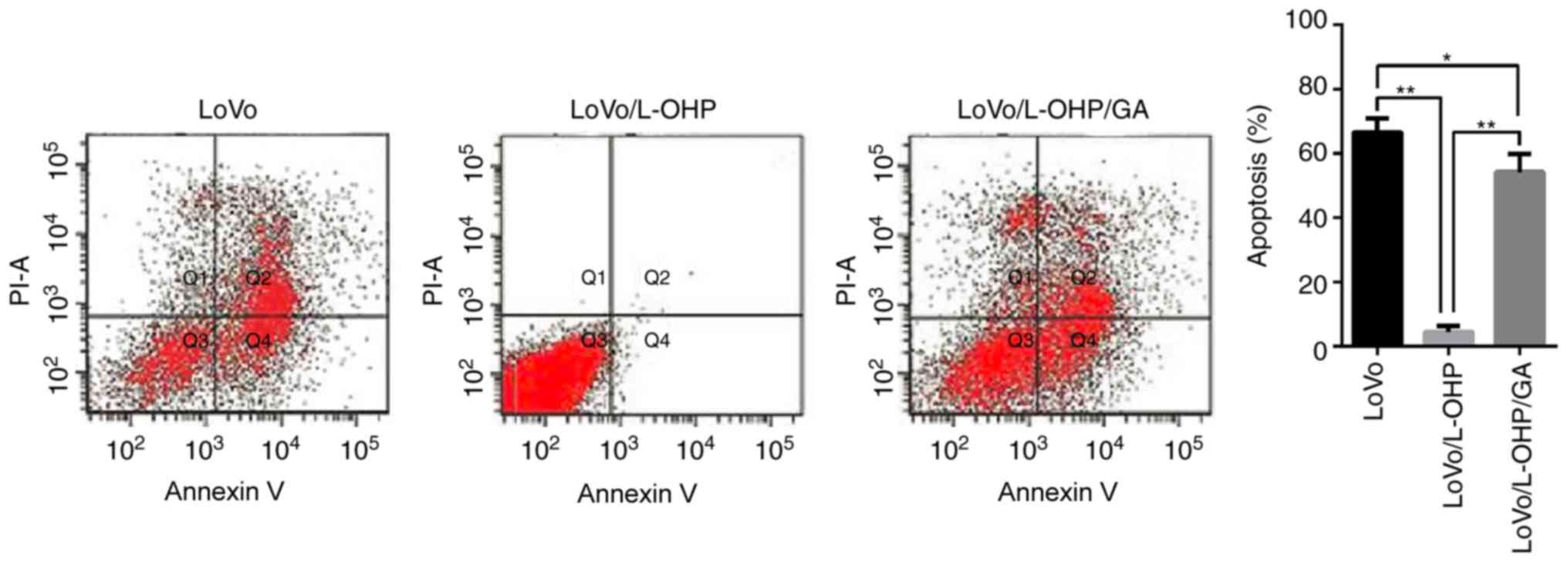
LoVo, LoVo/L-OHP and LoVo/L-OHP/GA cells were
treated with 20 µmol/l L-OHP for 6 h, following which the apoptosis
rates were determined by flow cytometry. As indicated in Fig. 3, the apoptosis induced by L-OHP in the
LoVo and LoVo/L-OHP/GA cells was 66.02±5.30 and 54.21±5.52%,
respectively. It is noteworthy that L-OHP only induced minimal
levels of apoptosis in the LoVo/L-OHP-resistant cells, with only
4.56±1.70% apoptosis. A comparison of apoptosis rates revealed that
the rate was decreased in the LoVo/L-OHP cells compared with the
LoVo and LoVo/L-OHP/GA cells (P<0.01), and that the apoptosis
rate in the LoVo/L-OHP/GA cells was decreased compared with that in
the LoVo cells (P<0.05). The results suggested that the
anti-apoptosis ability of the LOVO/L-OHP cells was increased
compared with the LoVo and LoVo/L-OHP/GA cells, and that GA
reversed these effects.
GA attenuates invasion in the
LoVo/L-OHP cells
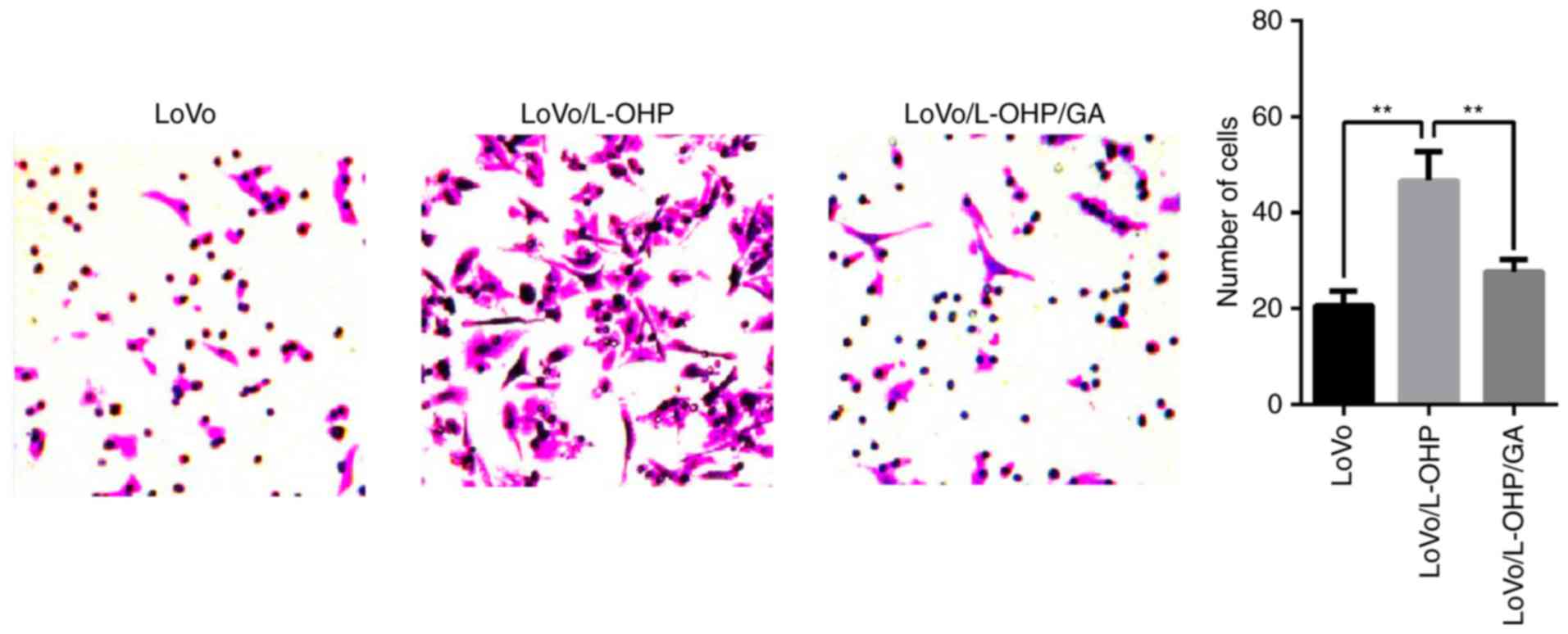
LoVo, LoVo/L-OHP and LoVo/L-OHP/GA cells were
treated with 2 µmol/l L-OHP for 24 h, following which the levels of
invasion were determined in Transwell assays. The numbers of
invasive LoVo, LoVo/L-OHP and LoVo/L-OHP/GA cells was 21±0.12,
46±0.15 and 17±0.09, respectively. As demonstrated in Fig. 4, a comparison of the numbers of
invading cells revealed that the LoVo/L-OHP cells yielded an
increased number of invasive cells compared with the LoVo and
LoVo/L-OHP/GA cells (P<0.01). Subsequent to treatment with
L-OHP, the invasive ability of the LoVo/L-OHP cells was increased
compared with that of the LoVo and LoVo/L-OHP/GA cells, suggesting
that the rates of invasion in LoVo/L-OHP-resistant cells increased,
and that GA was able to reverse and attenuate the invasion.
Determination of intracellular Pt
content
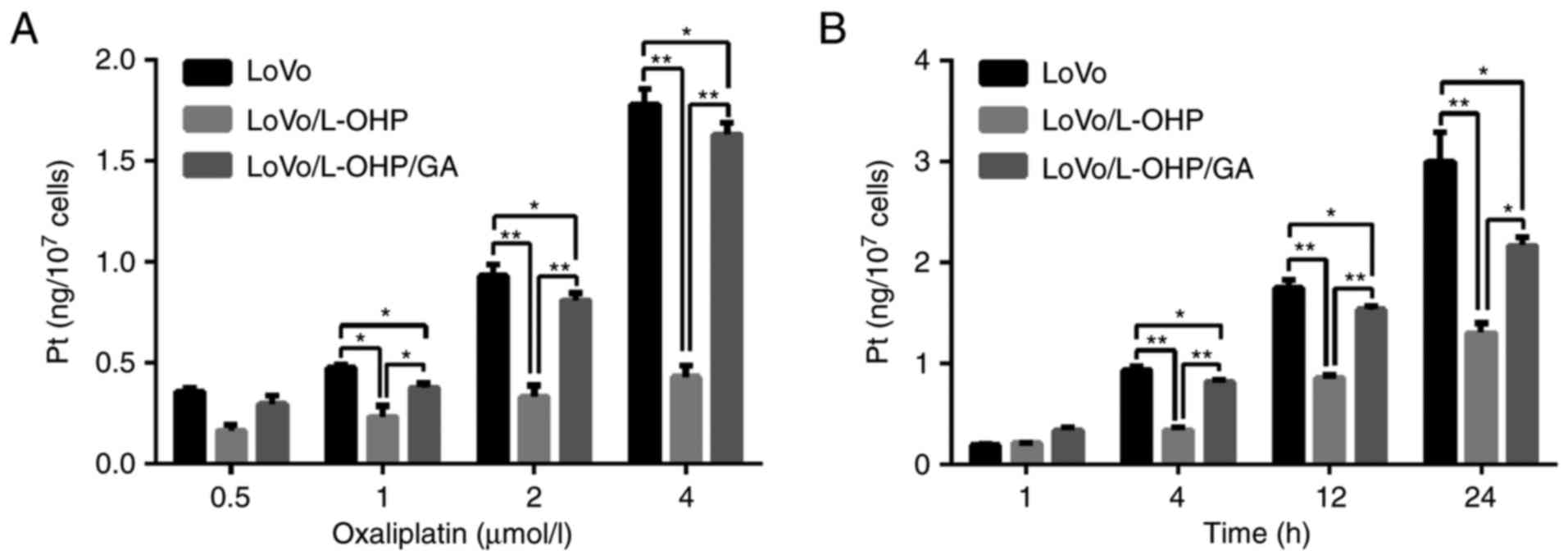
To explore the potential mechanisms by which GA
reversed resistance to L-OHP, the intracellular content of Pt was
detected. As demonstrated in Fig. 5A,
it was identified that intracellular Pt accumulated as the
concentration of L-OHP increased, indicating that L-OHP entered
into cells in a dose-dependent manner. Following 4 h treatment with
different concentrations (0, 0.5, 1, 2 or 4 µmol/l) of L-OHP, the
Pt content in LoVo and LoVo/L-OHP/GA cells was increased compared
with the LoVo/L-OHP cells (P<0.05). Intracellular Pt content was
highest in the LoVo cells, followed by LoVo/L-OHP/GA cells, and
then lowest in the LoVo/L-OHP cells.
The Pt content of cells then was detected following
treatment with 2 µmol/l L-OHP for different times (1, 4, 12 and 24
h). It was identified that intracellular Pt accumulated as
treatment time intervals increased, indicating a time-dependent
effect. There was no difference between the LoVo, LoVo/L-OHP and
LoVo/L-OHP/GA cells at 1 and 4 h (P>0.05). At 12 and 24 h, the
Pt content in the LoVo and LoVo/L-OHP/GA cells was increased
compared with the LoVo/L-OHP cells (P<0.05). Pt content in the
LoVo cells was increased compared with the LoVo/L-OHP/GA cells at
12 and 24 h (P<0.05; Fig. 5B).
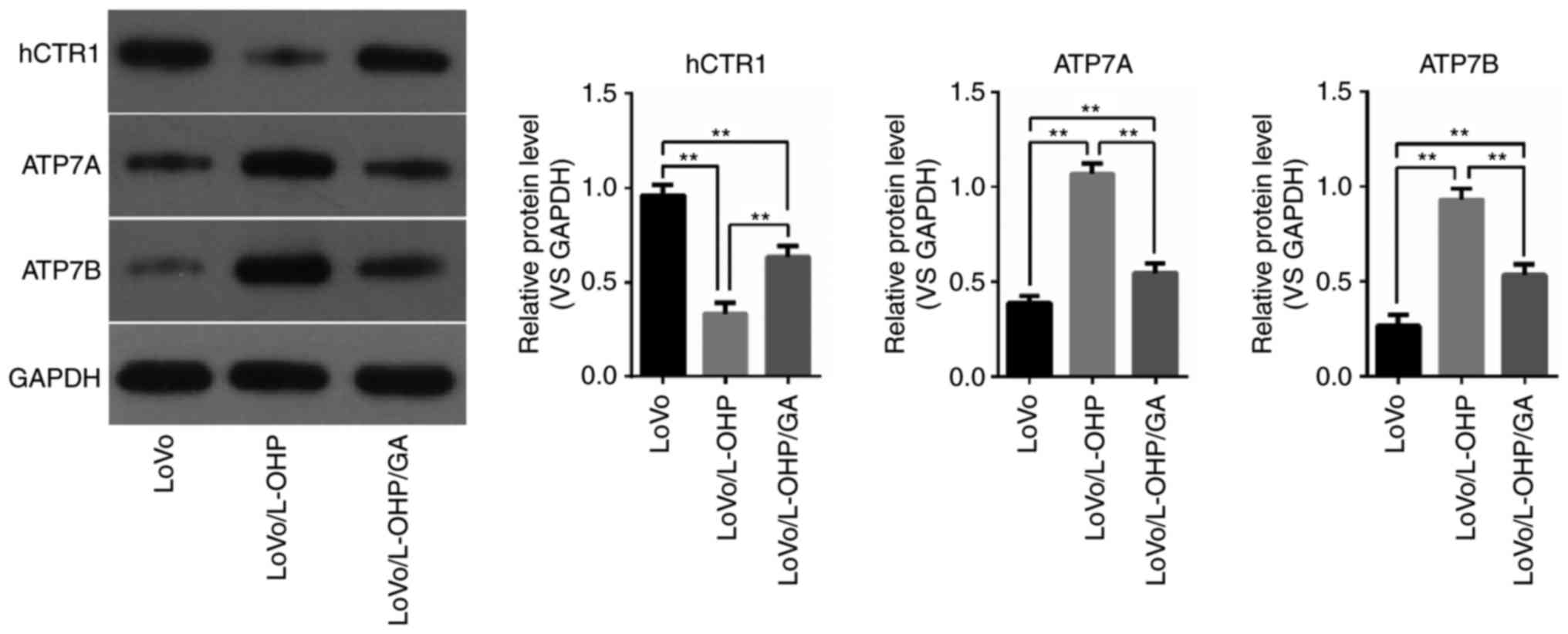
hCTR1, ATP7A and ATP7B protein
levels
In order to determine whether the changes in
intracellular Pt were associated with copper transporters, the
protein expressions of hCTR1, ATP7A and ATP7B in LoVo, LoVo/L-OHP
and LoVo/L-OHP/GA cells were examined. As demonstrated in Fig. 6, hCTR1 protein levels were decreased
in the LoVo/L-OHP cells compared with in the LoVo and LoVo/L-OHP/GA
cells (P<0.01), and decreased in the LoVo/L-OHP/GA cells
compared with the LoVo cells (P<0.01). The protein levels of
ATP7A and ATP7B were increased in the LoVo/L-OHP cells compared
with the LoVo and LoVo/L-OHP/GA cells (P<0.01), with levels
being increased in the LoVo/L-OHP/GA cells compared with the LoVo
cells (P<0.01). These results suggest that downregulated hCTP1
and upregulated ATP7A and ATP7B were associated with L-OHP
resistance, and that GA reversed the resistance by increasing the
levels of hCTR1 and decreasing ATP7A and ATP7B levels.
Discussion
Resistance to L-OHP remains one of the major
obstacles in chemotherapy for CRC, and it is essential to identify
novel drugs to overcome or reverse L-OHP resistance. GA is a
traditional Chinese medicine with multi-target anticancer effects,
including the inhibition of proliferation (20), induction of apoptosis (21), cell cycle arrest (22), and inhibition of angiogenesis
(23), invasion and metastasis
(24). GA was also identified to
exhibit inhibitory effects on resistance to anticancer drugs in CRC
(12), breast (13), ovarian (14), gastric (15) and human epithelial cancer (16).
Therefore, in the present study, the possibility of
using GA to reverse L-OHP resistance in CRC cells was evaluated. It
was identified that the LoVo/L-OHP cells were resistant to L-OHP,
and that GA reversed this resistance. Compared with the parent LoVo
cells, the anti-apoptosis and invasive abilities of resistant
LOVO/L-OHP cells were improved, and GA was able to reverse these
effects. Intracellular Pt content was highest in the LoVo cells,
followed by the LoVo/L-OHP/GA cells, and then lowest in the
LoVo/L-OHP cells. Decreased hCTP1 levels and increased ATP7A and
ATP7B levels were associated with L-OHP resistance, and GA reversed
this resistance by increasing hCTR1 and decreasing ATP7A and ATP7B
levels. These results indicated that GA exhibited the ability to
reverse L-OHP resistance in CRC cells, which was associated with an
increase in intracellular Pt content and a regulation of the
protein expression levels of copper transporters.
The cytotoxic effects of Pt drugs are directly
associated with intracellular Pt content, and the majority of
resistant cells exhibit decreased intracellular accumulation of
these drugs (25). Adequate
intracellular accumulation of Pt drugs is essential to exert their
anticancer effects (7). Intracellular
Pt content was directly associated with the content of L-OHP in
cells, while intracellular L-OHP content is positively associated
to the sensitivity of cells to L-OHP (7). In the present study, intracellular Pt
content was determined by ICP-MS, and it was identified that
intracellular L-OHP content increased in a dose- and time-dependent
manner. Intracellular L-OHP content was highest in the LoVo cells,
followed by the LoVo/L-OHP/GA cells, and then lowest in the
LoVo/L-OHP cells, suggesting that parent LoVo cells were relatively
sensitive to L-OHP, that LoVo/L-OHP cells were resistant to L-OHP,
and that GA was able to reverse this resistance.
The process of cellular import and export of Pt
drugs is primarily mediated by copper transporters (8). hCTR1, ATP7A and ATP7B are key copper
transporters involved in intracellular Pt accumulation (9). hCTR1 regulates the influx of Pt drugs,
while ATP7A and ATP7B regulate the efflux of these drugs (9). Previous studies have indicated that
copper transporters not only regulate the influx and efflux of Pt
drugs, but also affect cell cytotoxic sensitivity to Pt drugs:
Ishida et al (26) identified
that the downregulation of hCTR1 resulted in the reduced
accumulation of cisplatin and increased cisplatin resistance. Song
et al (27) also identified
that the upregulation of hCTR1 enhanced the accumulation of
oxaliplatin and carboplatin in small-cell lung cancer cells. Low
expression of hCTR1 was determined to be associated with poor
prognosis in patients with non-small cell lung cancer (NSCLC) and
ovarian cancer treated with Pt-based chemotherapy (28,29). hCTR1
is a potential biomarker for intracellular Pt accumulation and Pt
drug resistance. ATP7A serves an important role in Pt resistance by
transporting Pt drugs out of cells (30). The overexpression of ATP7A was
associated with Pt resistance in oesophageal squamous cell cancer
(31), NSCLC (32), CRC (33)
and ovarian cancer (34).
Overexpressed ATP7A was also identified to predict a poor prognosis
in patients with NSCLC receiving Pt-based chemotherapy (32). Similar to ATP7A, ATP7B facilitates the
efflux of Pt drugs, and also affects resistance to Pt drugs
(35). ATP7B silencing resulted in
improved cisplatin sensitivity in cisplatin-resistant ovarian cells
(36). The overexpression of ATP7B
was associated with Pt resistance in patients with CRC, and
predicted poor prognosis in patients following oxaliplatin-based
chemotherapy (37). In the present
study, it was identified that hCTR1 protein levels were decreased
in resistant LoVo/L-OHP cells compared with parent LoVo and
L-OHP-sensitive LoVo/L-OHP/GA cells, while ATP7A and ATP7B protein
levels were increased in resistant cells, indicating that
downregulated hCTP1 and upregulated ATP7A and ATP7B were associated
with L-OHP resistance, and that GA may reverse this resistance by
increasing hCTR1 and decreasing ATP7A and ATP7B levels.
Overall, the results of the present study
demonstrated that GA exhibits the potential ability to reverse
L-OHP resistance in CRC cells. The potential reversal mechanism may
involve an increase in intracellular Pt content and hCTR1 levels,
and a decrease in ATP7A and ATP7B levels, making it a potential
treatment agent for L-OHP resistance.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81272556),
Guangdong Planned Project of Science and Technology (grant nos.
2014A020212614 and 2017A020215009), Guangdong Science and Research
Project of Traditional Chinese Medicine Bureau (grant no. 20152039)
and Guangzhou Planned Project of Science and Technology (grant no.
2014Y2-00137).
Availability of data and materials
The datasets used and/or analysed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW, WL, JC, JW and CW designed the study. JW and CW
wrote the manuscript. TZ, DH, FW and FH performed the experiments.
WC, PY and SZ collected and analysed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GA
|
gambogic acid
|
|
L-OHP
|
oxaliplatin
|
|
CRC
|
colorectal cancer
|
|
Pt
|
platinum
|
|
hCTR1
|
human copper transporter 1
|
|
ATP7A
|
copper-transporting p-type adenosine
triphosphatases 1
|
|
ATP7B
|
copper-transporting p-type adenosine
triphosphatases 2
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
André T, Iveson T, Labianca R, Meyerhardt
JA, Souglakos I, Yoshino T, Paul J, Sobrero A, Taieb J, Shields AF,
et al: The IDEA (International Duration Evaluation of Adjuvant
Chemotherapy) collaboration: Prospective combined analysis of phase
III trials investigating duration of adjuvant therapy with the
FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months)
regimen for patients with stage III colon cancer: Trial design and
current status. Curr Colorectal Cancer Rep. 9:261–269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Zhu C, Yang K, Li J, Du N, Zong M,
Zhou J and He J: Poly(C)-binding protein 1 mediates drug resistance
in colorectal cancer. Oncotarget. 8:13312–13319. 2017.PubMed/NCBI
|
|
5
|
Alcindor T and Beauger N: Oxaliplatin: A
review in the era of molecularly targeted therapy. Cur Oncol.
18:18–25. 2011.
|
|
6
|
Martinez-Balibrea E, Martínez-Cardús A,
Ginés A, Ruiz de Porras V, Moutinho C, Layos L, Manzano JL, Bugés
C, Bystrup S, Esteller M and Abad A: Tumor-related molecular
mechanisms of oxaliplatin resistance. Mol Cancer Ther.
14:1767–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall MD, Okabe M, Shen DW, Liang XJ and
Gottesman MM: The role of cellular accumulation in determining
sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol
Toxicol. 48:495–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harrach S and Ciarimboli G: Role of
transporters in the distribution of platinum-based drugs. Front
Pharmacol. 6:852015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rabik CA, Maryon EB, Kasza K, Shafer JT,
Bartnik CM and Dolan ME: Role of copper transporters in resistance
to platinating agents. Cancer Chemother Pharmacol. 64:133–142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhang Q, Fan Z, Sun J, Liu X,
Cheng L, Li A and Xu J: A Chinese herbal formula, Chang-Wei-Qin,
synergistically enhances antitumor effect of oxaliplatin. Pathol
Oncol Res. 21:389–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi Q, You Q, Gu H, Zhao L, Liu W, Lu N and
Guo Q: Studies on the toxicity of gambogic acid in rats. J
Ethnopharmacol. 3:433–438. 2008. View Article : Google Scholar
|
|
12
|
Wen C, Huang L, Chen J, Lin M, Li W, Lu B,
Rutnam ZJ, Iwamoto A, Wang Z, Yang X and Liu H: Gambogic acid
inhibits growth, induces apoptosis, and overcomes drug resistance
in human colorectal cancer cells. Int J Oncol. 47:1663–1671. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Wang L, Chen M and Wang Y:
Gambogic acid sensitizes resistant breast cancer cells to
doxorubicin through inhibiting P-glycoprotein and suppressing
survivin expression. Chem Biol Interact. 235:76–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J and Yuan Z: Gambogic acid
sensitizes ovarian cancer cells to doxorubicin through ROS-mediated
apoptosis. Cell Biochem Biophys. 67:199–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Wei J, Qian X, Ding Y, Yu L and
Liu B: Gambogic acid, a potent inhibitor of survivin, reverses
docetaxel resistance in gastric cancer cells. Cancer Lett.
262:214–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Deng R, Lu Y, Xu Q, Yan M, Ye D
and Chen W: Gambogic acid as a non-competitive inhibitor of
ATP-binding cassette transporter B1 reverses the multidrug
resistance of human epithelial cancers by promoting ATP-binding
cassette transporter B1 protein degradation. Basic Clin Pharmacol
Toxicol. 112:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinez-Cardús A, Martinez-Balibrea E,
Bandrés E, Malumbres R, Ginés A, Manzano JL, Taron M,
Garcia-Foncillas J and Abad A: Pharmacogenomic approach for the
identification of novel determinants of acquired resistance to
oxaliplatin in colorectal cancer. Mol Cancer Ther. 8:194–202. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plasencia C, Martínez-Balibrea E,
Martinez-Cardús A, Quinn DI, Abad A and Neamati N: Expression
analysis of genes involved in oxaliplatin response and development
of oxaliplatinresistant HT29 colon cancer cells. Int J Oncol.
29:225–235. 2006.PubMed/NCBI
|
|
19
|
Nguyen TT, Ostergaard J, Stürup S and
Gammelgaard B: Determination of platinum drug release and liposome
stability in human plasma by CE-ICP-MS. Int J Pharm. 449:95–102.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Zhou M, Ouyang J, Wang J, Zhang Q,
Xu Y, Xu Y, Zhang Q, Xu X and Zeng H: Gambogic acid induces
mitochondria-dependent apoptosis by modulation of Bcl-2 and Bax in
mantle cell lymphoma JeKo-1 cells. Chin J Cancer Res. 2:183–191.
2013.
|
|
21
|
Duan D, Zhang B, Yao J, Liu Y, Sun J, Ge
C, Peng S and Fang J: Gambogic acid induces apoptosis in
hepatocellular carcinoma SMMC-7721 cells by targeting cytosolic
thioredoxin reductase. Free RadicBiol Med. 69:15–25. 2014.
View Article : Google Scholar
|
|
22
|
Zhao W, Zhou SF, Zhang ZP, Xu GP, Li XB
and Yan JL: Gambogic acid inhibits the growth of osteosarcoma cells
in vitro by inducing apoptosis and cell cycle arrest. Oncol Rep.
25:1289–1295. 2011.PubMed/NCBI
|
|
23
|
Lu N, Hui H, Yang H, Zhao K, Chen Y, You
QD and Guo QL: Gambogic acid inhibits angiogenesis through
inhibiting PHD2-VHL-HIF-1α pathway. Eur J Pharm Sci. 49:220–226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi Q, Lu N, Li C, Zhao J, Liu W, You Q and
Guo Q: Involvement of RECK in gambogic acid induced anti-invasive
effect in A549 human lung carcinoma cells. Mol Carcinog. 54(Suppl
1): E13–E25. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howell SB, Safaei R, Larson CA and Sailor
MJ: Copper transporters and the cellular pharmacology of the
platinum-containing cancer drugs. Mol Pharmacol. 77:887–894. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishida S, Lee J, Thiele DJ and Herskowitz
I: Uptake of the anticancer drug cisplatin mediated by the copper
transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA.
99:14298–14302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song IS, Savaraj N, Siddik ZH, Liu P, Wei
Y, Wu CJ and Kuo MT: Role of human copper transporter Ctr1 in the
transport of platinum-based antitumor agents in cisplatin-sensitive
and cisplatin-resistant cells. Mol Cancer Ther. 3:1543–1549.
2004.PubMed/NCBI
|
|
28
|
Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH,
Lai WW, Liu HS and Su WC: Predictive and prognostic value of human
copper transporter 1 (hCtr1) in patients with stage III
non-small-cell lung cancer receiving first-line platinum-based
doublet chemotherapy. Lung Cancer. 75:228–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishida S, McCormick F, Smith-McCune K and
Hanahan D: Enhancing tumor-specific uptake of the anticancer drug
cisplatin with a copper chelator. Cancer Cell. 17:574–583. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chisholm CL, Wang H, Wong AH,
Vazquez-Ortiz G, Chen W, Xu X and Deng CX: Ammonium
tetrathiomolybdate treatment targets the coppertransporter ATP7A
and enhances sensitivity of breast cancer tocisplatin. Oncotarget.
51:84439–84452. 2016.
|
|
31
|
Li ZH, Zheng R, Chen JT, Jia J and Qiu M:
The role of copper transporter ATP7A in platinum-resistance of
esophageal squamous cell cancer (ESCC). J Cancer. 7:2085–2092.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li ZH, Qiu MZ, Zeng ZL, Luo HY, Wu WJ,
Wang F, Wang ZQ, Zhang DS, Li YH and Xu RH: Copper-transporting
P-type adenosine triphosphatase (ATP7A) is associated with
platinum-resistance in non-small cell lung cancer (NSCLC). J Transl
Med. 10:212012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kitada N, Takara K, Minegaki T, Itoh C,
Tsujimoto M, Sakaeda T and Yokoyama T: Factors affecting
sensitivity to antitumor platinum derivatives of human colorectal
tumor cell lines. Cancer Chemother Pharmacol. 62:577–584. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mangala LS, Zuzel V, Schmandt R, Leshane
ES, Halder JB, Armaiz-Pena GN, Spannuth WA, Tanaka T, Shahzad MM,
Lin YG, et al: Therapeutic targeting of ATP7B in ovarian carcinoma.
Clin Cancer Res. 15:3770–3780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakagawa T, Inoue Y, Kodama H, Yamazaki H,
Kawai K, Suemizu H, Masuda R, Iwazaki M, Yamada S, Ueyama Y, et al:
Expression of copper-transporting P-type adenosine triphosphatase
(ATP7B) correlates with cisplatin resistance in human non-small
cell lung cancer xenografts. Oncol Rep. 20:265–270. 2008.PubMed/NCBI
|
|
36
|
Leonhardt K, Gebhardt R, Mössner J,
Lutsenko S and Huster D: Functional interactions of Cu-ATPase ATP7B
with cisplatin and the role of ATP7B in the resistance of cells to
the drug. J Biol Chem. 284:7793–802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinez-Balibrea E, Martínez-Cardús A,
Musulén E, Ginés A, Manzano JL, Aranda E, Plasencia C, Neamati N
and Abad A: Increasedlevels of copper efflux transporter ATP7B are
associated withpoor outcome in colorectal cancer patients receiving
oxaliplatin-based chemotherapy. Int J Cancer. 124:2905–2910. 2009.
View Article : Google Scholar : PubMed/NCBI
|