Introduction
Colorectal cancer (CRC) remains one of the most
common malignant diseases with approximately 400,000 new cases word
wide yearly (1). Close to 50% of
patients diagnosed with CRC develop metastatic disease (mCRC) with
the liver being the predominant organ affected (2). Surgical resection of liver metastases
results in long term survival for about 40% (3) but only a minority of patients are
eligible for resection. This has stimulated the interest in
non-surgical local treatments including radiofrequency ablation
(RFA) (4) and stereotactic body
radiotherapy (SBRT) (5), both of
which are established as standard of care.
Trans arterial chemo-embolization (TACE) was
introduced in the 1980's by Yamada (6) with the rationale of embolic induced
ischemia and necrosis and the delivery of chemotherapy with a
minimal of systemic exposure. This technique was further refined
with the introduction drug eluting beads (DEB) loaded with
irinotecan (DEBIRI), and the first exploratory trial on DEBIRI-TACE
for mCRC was published by Aliberti in 2006 (7). Today, the use of DEBIRI-TACE for mCRC is
supported by several observational studies and two published
randomized trials (8,9), yet several unclarified issues regarding
the optimal patient selection and technique still renders an
unsettled place for DEBIRI-TACE in the overall treatment landscape
for mCRC (10).
Cell-free DNA (cfDNA) constitutes all circulating
DNA fragments, both mutant and non-mutant alleles, and can be
detected and quantified in both healthy individuals, patients with
various medical disorders and the highest values are seen in cancer
patients (11). Since the first
report of circulating nuclear acids in the blood stream in 1948
(12), emphasis on cancer patients
was enhanced by Leon in 1977 (13).
During the past decades, there has been a growing interest in the
utility of cfDNA in various aspects of CRC management, both for
early stage CRC (14) and a recent
meta-analysis of 10 trials has shown a pronounced prognostic value
of cfDNA for patients receiving systemic palliative chemotherapy
(15).
In this study, we first report the results from a
single center phase II study of DEBIRI-TACE plus intensified
chemotherapy as first-line treatment for patients with liver
limited mCRC. Second, we describe results of total cfDNA measured
in plasma samples.
Materials and methods
Study design and ethics
This was an open-label phase II trial conducted at
Aarhus University Hospital in co-operation between the Departments
of Oncology, Radiology and Surgical Gastroenterology (Aarhus,
Denmark). The aim was to investigate the feasibility, toxicity and
efficacy of DEBIRI-TACE treatment with concomitant mFOLFOX6 as a
first-line treatment for patients with liver limited mCRC. Patients
were recruited from December 2012 to February 2014. The study
planned to include 50 patients, but was stopped prematurely
following the inclusion of 15 patients, by the decision of the
study board and sponsor.
All patients included received a thorough verbal and
written information and a signed consent was received prior to any
protocol treatment. The protocol was approved by the National Board
of Health (EudraCT no. 2012-000987-11), the Data Monitor Committee
(J.nr. 2012-41-0370) and the Regional Ethics Committee (no.
1-10-72-306-12).
Patients and eligibility
Patients eligible for this study were newly
diagnosed patients with liver limited mCRC not being candidates for
any standard local treatment e.g., liver resection, RFA or SBRT.
All potential candidates were systematically reviewed at a
multidisciplinary hepato-pancreatic tumor board with experienced
experts in radiology (including invasive radiology), oncology and
liver surgery. Patients judged potentially eligible for local
treatment in case of tumor shrinkage were allowed inclusion.
Key inclusion criteria were: Age >18 years, WHO
Performance Status (PS) of 0–1, verified metastatic colorectal
adenocarcinoma with no extra hepatic disease, less than 50% of the
liver parenchyma involved and satisfactory organ function. Key
exclusion criteria were: Presence of other malignant disease, prior
chemotherapy within 6 months, trombo-embolic disease and factors
not allowing for femoral artery access.
Treatments and procedures
The treatment scheme consisted of initially up to 4
DEBIRI-TACE treatments concomitant with systemic chemotherapy of
mFOLFOX6 (5-Fluororacil, leucoverin, Oxaliplatin) + bevacizumab 24
h after embolization. Each lobe was treated twice with a 4 weeks
interval, so patients with bi-lobar involvement received biweekly
treatment alternating the lobes, allowing for up to 4
embolizations. Subsequent to the 4 DEBIRI-TACE treatments patients
continued with systemic chemotherapy consisting of FLIRI
(5-fluorouracil, leocoverin, Irinotecan) + bevacizumab for up to a
total of 6 months treatment, which was the current standard of
care.
Patients were systematically evaluated by CT scan of
chest and abdomen at baseline and at weeks 8, 16 and 24 (end of
treatment). All imaging was reviewed at the multidisciplinary tumor
board and patients were considered for resection, RFA or SBRT if
eligible. After completion of treatment a follow-up schedule of CT
scan, clinical exam and CEA measurements were done every three
months. At time of progression, patients were considered for all
available standard treatment options.
All chemo-embolizations were done by experienced
invasive radiologist using a femoral approach catheterization of
the hepatic artery in local anesthesia. The vascular anatomy was
identified by angiogram prior to the delivery of the irinotecan
loaded beads of 70–150 µm in diameter. A microcatheter was placed
in the tumor feeding artery if possible. Otherwise in the left or
right hepatic artery, peripherally to extrahepatic branches. The
embolic procedure was given with a solution of 5 mg of irinotecan
pr ml in repeated doses until a maximum of 40 ml were administered
or until a decreased flow was recognized. Injection of solution was
monitored under fluoroscopy to ensure forward flow. Occlusion of
tumor feeding artery was avoided.
Patients were hospitalized during the DEBIRI-TACE
procedure, while standard systemic chemotherapy was given in
out-patient clinic. According to protocol, a regimen of supportive
care was applied consisting of anti-emetics (Ondanstron 8 mg,
emperal 20 mg, methylprednisolone 80 mg), antibiotics
(piperacillin/tazobactam 4/0.5 g 1 h prior to embolization),
analgesics (morphine 10 mg) and sedative (diazepam 10 mg). Patients
received hydration with saline and were monitored with
electrocardiogram, pulsoximetri and blood pressure
measurements.
Outcome
The primary endpoint of this study was response
rate, and secondary endpoints were survival, toxicity and
translational research. Response evaluation was done using the
Response Evaluation Criteria in Solid Tumors (RESCIST 1.1)
(16) classifying patients as either
a complete response (CR), partial response (PR), stable disease
(SD) or progressive disease (PD). The best obtained response
allowed for calculation of the response rate. Patients were
classified as No Evidence of Disease (NED) if no visible residual
disease was detected on a CT scan.
Toxicity and adverse events (AE) were registered
prospectively during the trial and recorded using the Common
Terminology Criteria for Adverse Events (CTC) v3.0. (17). Pain was further quantified by the
visual analogue score (VAS) (18)
ranging from 0–10.
Biomarkers
Blood samples for translational use were drawn at
baseline, prior to each DEBIRI-TACE, 24 h post-embolization
(concurrent with mFOLFOX6) and prior to each subsequent cycle of
protocol treatment.
Analysis of cfDNA was done using a fluorescent assay
for cfDNA quatificantion as originally described by Goldshtein
et al (19) and modified by
our group (20). In brief, 40 µl
plasma we used, adding SYBR® Gold Nucleic Acid Gel Stain
(1:8,000). The quantification of fluorescence was performed with a
96-well fluorometer (Infinite F200 PRO; Tecan Group, Ltd.,
Mannedorf, Switzerland) at an emission wavelength of 535 nm and an
excitation wavelength of 485 nm in a black 96-well plate (Bio-Plex
Pro Flat Bottom Plates; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
DNA standards were prepared from Human Control
Genomic DNA (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
diluted with a 10% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) solution. The concentrations of the
samples were calculated from a standard curve.
For determining the definitive concentration of
cfDNA in each sample, we calculated the median value of four
measurements, removing outliers according to Dixons q-test if the
standard deviation exceeded 10%.
Statistics
Descriptive statistics were applied reporting
categorical variables as counts and percentages and continuous
variables by median values and 95% confidence interval (95%
CI).
For analysis of survival we used the Kaplan-Meier
method and due to the small sample size (n=14) we used descriptive
statistics. Survival was calculated from the date of inclusion
until death or censoring at end of follow-up (September 2017).
Progression free survival (PFS) was defined as time until death,
progression or censoring. For analysis of the prognostic value of
the baseline cfDNA we stratified patients by the 75th quartile. For
analysis of the cfDNA change during the 4 DEBIRI-TACE procedures we
only included those patients who completed all 4 scheduled
treatments (n=11).
For analysis of contingency tables, we applied
Fischer's exact test due to the small sample size. Comparison of
continuous variables was done by linear regression analysis.
P<0.05 was considered to indicate a statistically significant
difference.
All calculations and data management were performed
using STATA version 14.1 (StataCorp LP, College Station, TX,
USA).
Results
Patients and treatment
Fifteen patients were included, following the
exclusion of one patients due to a protocol violation (9 days after
inclusion), 14 patients underwent treatment and a median follow-up
of 1.7 years (95% CI: 0.5–2.9 years). The study population
consisted of 8 males and the median age was 66 (range 43–74 years).
Nine patients had a colonic primary cancer and the median number of
liver metastases at baseline was 10 (range 1–60) with a median size
of 55 cm (95% CI: 39–92 cm). Details on baseline characteristics
are displayed in Table I. A total of
49 chemoembolization procedures were performed with a mean of 3.5
treatments pr patient. The median total dose of irinotecan
embolized pr patient was 345 mg (95% CI: 243–395 mg). Eleven
patients received the scheduled 4 DEBIRI/FOLFOX treatments while 7
patients completed the post-DEBIRI/FOLFOX planned systemic
chemotherapy with FLIRI + bevacizumab.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Number (%), n=14 | cfDNA ng/µl (95% CI)
baseline, median |
|---|
| Sex |
| Male | 8 (57) | 1.15 (1.04–1.37) |
|
Female | 6 (43) | 0.78 (0.64–1.11) |
| Age (years) |
|
Median | 66 |
|
|
Range | 43–74 |
|
| T stage |
| T1 | 0 (0) |
|
| T2 | 0 (0) |
|
| T3 | 4 (29) | 1.15 (1.13–1.16) |
| T4 | 8 (57) | 0.99 (0.65–1.30) |
| TX | 2 (14) | 0.97 (0.8–1.13) |
| N stage |
| N0 | 1 (7) | 1.01 (1.01–1.01) |
| N1 | 3 (21) | 1.13 (0.75–1.43) |
| N2 | 4 (29) | 1.06 (0.64–1.24) |
| NX | 6 (43) | 1.11 (0.65–1.15) |
| Site of primary |
|
Colon | 9 (64) | 1.10 (0.75–1.23) |
|
Rectal | 5 (36) | 1.07 (0.65–1.16) |
| Number of livermets
(baseline) |
|
Median | 10 |
|
|
Range | 1–60 |
|
| Size of largest
livermet (baseline, cm) |
|
|
| Median
(95% CI) | 55 (39–92) |
|
| Range
(cm) | 2.5–18 |
|
| RAS/BRAF
mutation |
|
Mutation | 4 | 0.88 (0.64–1.15) |
|
Wild-type | 9 | 1.13 (0.86–1.30) |
|
Unknown | 1 | 0.8 |
| Dose irinotecan
(mg) |
| Median
(95% CI) | 345 (243–395) |
|
| Debut of
metastases |
|
Synchronous | 3
(21) | 1.16
(1.01–1.43) |
|
Metachronous | 11 (79) | 1.03
(0.68–1.14) |
| Number of
embolization |
|
Median | 4 |
|
|
Range | 1–4 |
|
Outcome
Among the 14 patients in this cohort the best
obtained response during treatment was PR (n=7), and SD (n=4),
while the remaining patients (n=3) were not assessable for response
evaluation due to failure to complete the scheduled CT scans
(withdrawal of consent n=2, dead n=1). The corresponding response
rate according to RECIST 1.1 was 50%. Despite early response to
treatment 2 patients developed radiological PD during the treatment
course. Six patients were converted to eligibility for liver
resection and/or RFA and 2 patients achieved status of NED
(Table II).
 | Table II.Response obtained at the CT scan at 8
weeks (post-DEBIRI-TACE) and the best obtain response during the 6
months of scheduled treatment with CT scans at week 8, 16 and
24. |
Table II.
Response obtained at the CT scan at 8
weeks (post-DEBIRI-TACE) and the best obtain response during the 6
months of scheduled treatment with CT scans at week 8, 16 and
24.
|
| CT at week 8 (post
DEBIRI-TACE) | Best obtained
response |
|---|
| PD | 0 | 0 |
| SD | 9 | 4 |
| PR | 2 | 7 |
| CR | 0 | 0 |
| NA | 3 | 3 |
| Conversion to
eligibility for RFA/resection | 0 | 6 |
| Obtained status of
NED | 0 | 2 |
The median progression free survival (PFS) and
overall survival (OS) of all patients (n=14) were 240 days (95% CI:
161–357) and 522 days (95% CI: 174–1054) respectively, and the
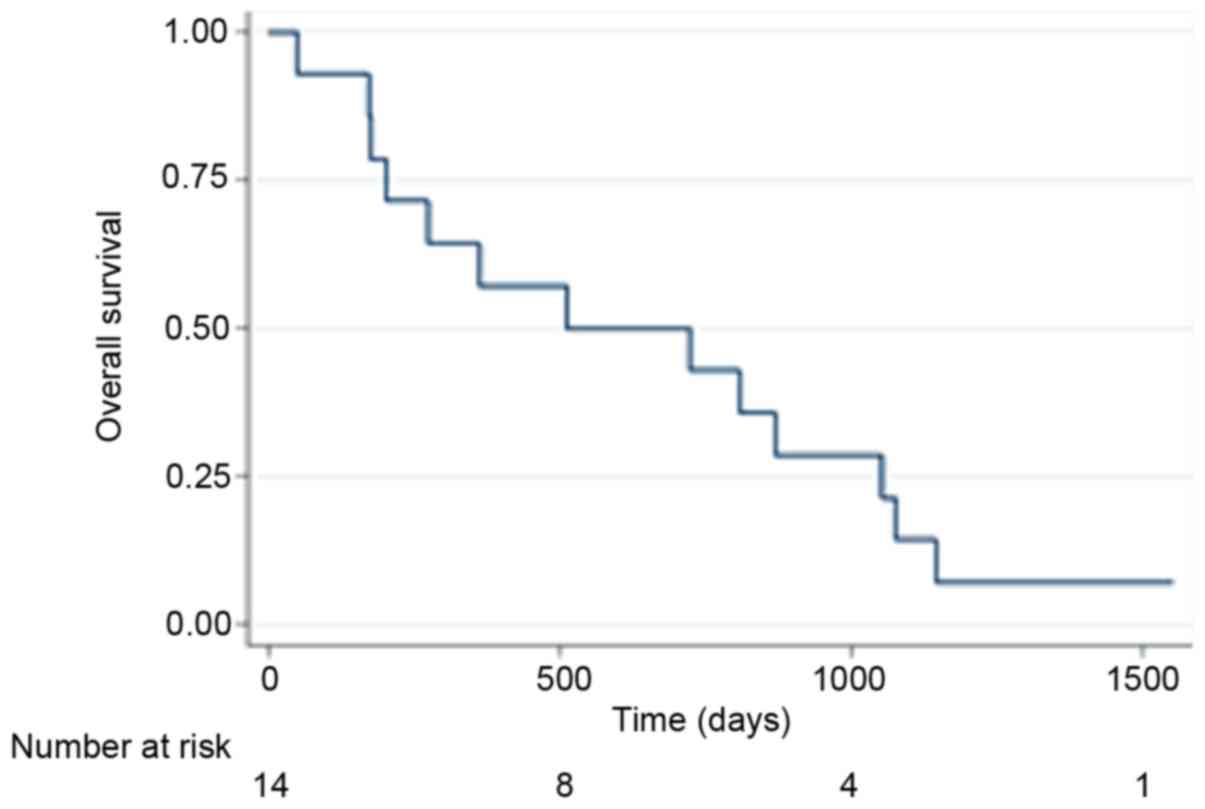
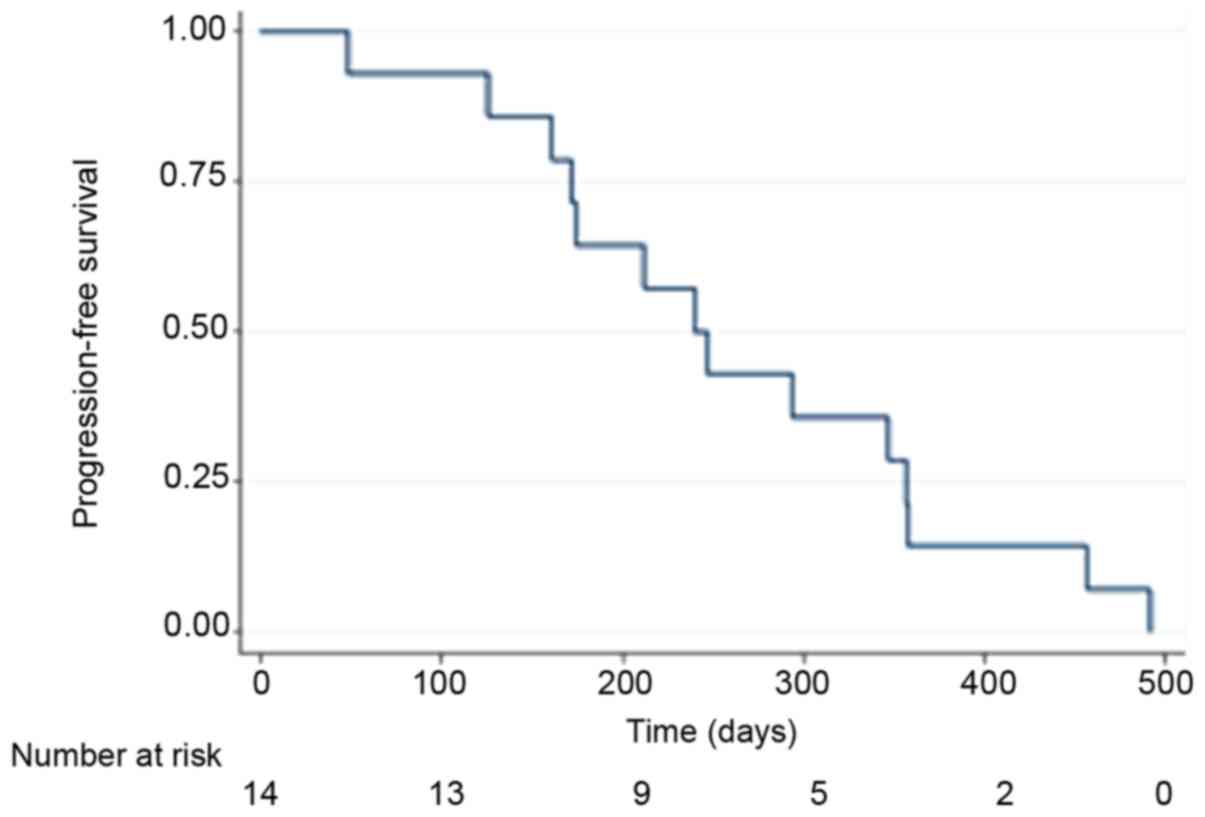
Kaplan-Meier curves are displayed for OS in Fig. 1 and PFS in Fig. 2. By the end of follow-up (September 1,
2017) 13 patients had died and the patient remaining alive was
censored.
Toxicity
A total of 187 AE (all grades) were documented and
25 AE of grade 3 or higher (Table
III). Twelve patients (86%) experienced a minimum of grade 3
toxicity. The most common grade 3 or higher toxicities were
nausea/vomiting, fatigue, diarrhea and pain. Three patients
experienced severe organ specific complications (pancreatitis n=1,
bowel perforation n=1 and kidney failure n=1). One treatment
related death was recorded (kidney failure).
 | Table III.Toxicity of grade 3 or higher. |
Table III.
Toxicity of grade 3 or higher.
| Toxicity | Number of patients
developing a grade 3–5 toxicity N (%) |
|---|
| Vomiting | 3 (21) |
| Fatigue | 8 (57) |
| Nausea | 3 (21) |
| Pain | 4 (29) |
| Diarhea | 4 (28) |
| Bowel
perforation | 1 (7) |
| Kidney failure | 1 (7) |
| Infection (non
neutropenic) | 1 (7) |
The median VAS score for patients was 7.5 (95% CI:
3.7–9.0). The median dose of supplemented extra orally administered
morphine was 45 mg (95% CI: 20–180 mg) for all completed DEBIRI
TACE treatments with a wide range of 0 to 450 mg.
Biomarkers
The baseline values of CEA, LDH and cfDNA were 228
µg/l (95% CI: 54–1,131 µg/l), 296 U/l (95% CI: 189–454 U/l) and
1.10 ng/µl (95% CI: 0.77–1.16 ng/µl), with one missing value for
the latter. A regression analysis between cfDNA and CEA
respectively LDH demonstrate a trend for correlation in linear
regression analysis for LDH (P=0.06) and CEA (P=0.17) with one
outlier removed from the dataset.
Separating patients by the 75th quartile of the
baseline cfDNA showed a median OS of 512 days (95% CI: 172–870)
(n=11) for patients with a baseline below the 75th quartile, and
274 days (95% CI: 274-NA) (n=2) for patients above the 75th
quartile.
Examining the change in cfDNA during the 8 weeks of
DEBIRI-TACE, 6 patients with a decline in cfDNA above the median
value had an OS of 870 (95% CI: 172-NA) days, while those with a
cfDNA decline below the median (n=5) has an OS of 362 days (95% CI:
201-NA).
Handling the response rate as a categorical variable
as PR/CR vs. not PR/CR in a 2×2 Table with the median cfDNA at
baseline did not show significance between response and baseline
cfDNA (P=0.9).
Changes in the concentration of cfDNA during the
treatment course were seen for all patients. All, except one, had a
change in cfDNA level in the blood sample 24 h after the first
chemo-embolization. Across all patients, the dynamic of cfDNA was
more pronounced following the first chemo-embolization than the
second. Later in the treatment course, the data are limited by
missing blood samples post-DEBIRI and post-progression. Only six
patients had blood samples available beyond four months of
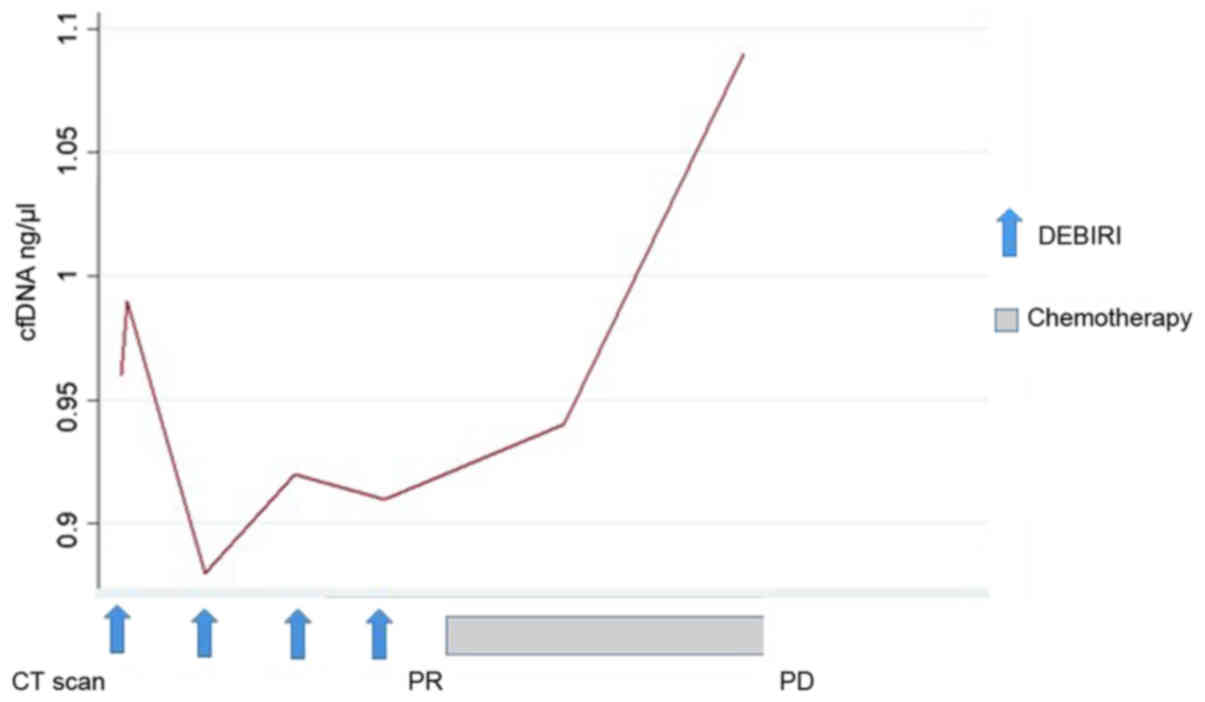
treatment, where one patient had an increase in cfDNA preceding the
radiologic evidence of PD (patient no. 1, Fig. 3). For the remaining five patients we
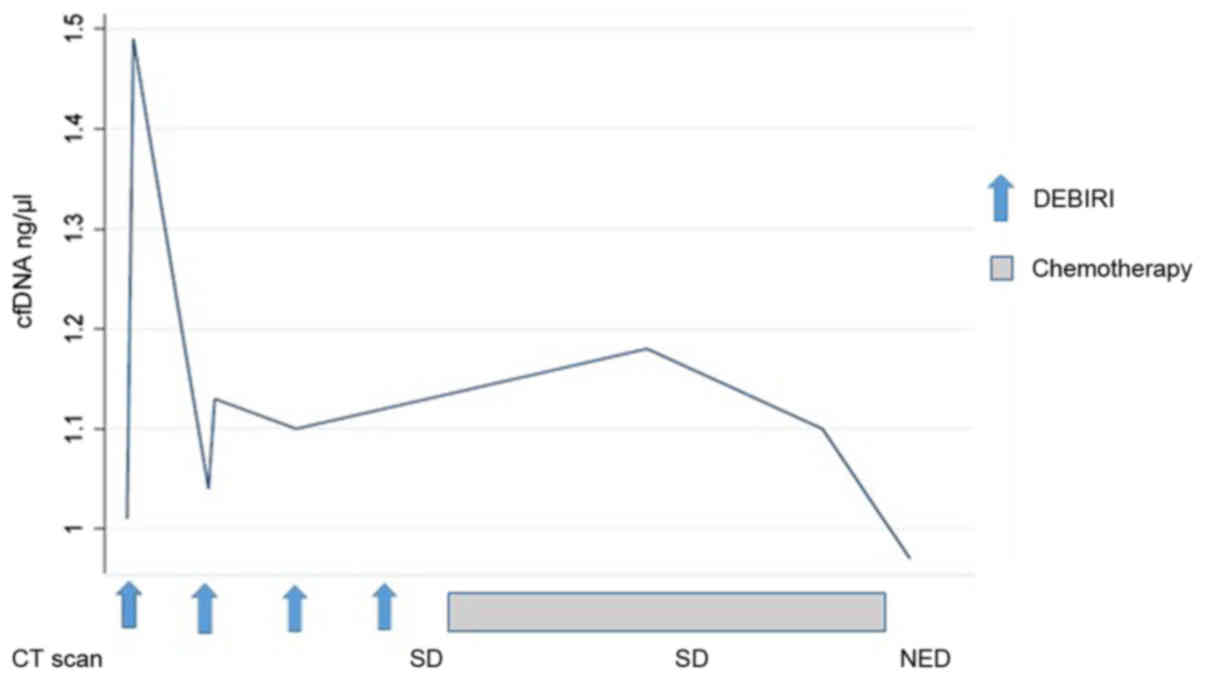
report a very heterogeneous pattern, although a tendency for a drop
in cfDNA for patients with SD/PR. This is illustrated (Fig. 4) by the disease course for patient no.
8 where a decline in cfDNA preceded the status of NED.
Discussion
In this study we report the feasibility, efficacy
and toxicity of DEBIRI-TACE/mFOLFOX6 bevacizumab as a first-line
treatment for newly diagnosed mCRC. In brief, we found a response
rate of 50%, a median PFS of 240 days (8 months) and a median OS of
522 days (17 months), which are outcome measures comparable to
other studies of DEBIRI-TACE in the first line setting (7,8,21,22). Six
patients were converted to eligibility for liver resection and/or
RFA and 2 patients achieved status of NED, but relapsed post-study.
Among them, the only patient who was still alive at end of
follow-up.
In this study, the interpretation of both the
clinical outcome and the corresponding translational research with
cfDNA measurements are limited by the small sample size due to the
early closure after inclusion only 15 of the planned 50 patients.
During the trial, we saw an unexpected high level of toxicity,
complications and failure to complete the scheduled treatment
course of 6 months. These difficulties are not reported to the same
degree in the aforementioned studies (7,8,21,22) where
DEBIRI-TACE is reported as a safe and well tolerated treatment.
It is a hypothesis, that the challenges encountered
in our study is due to the intense treatment of combining
DEBIRI-TACE with systemic combinations chemotherapy of mFOLFOX6 +
bevacizumab, which in itself can be a toxic treatment. This
combination was also examined by Martin et al (9) who randomized 60 patients between
DEBIRI-FOLFOX-bevacizumab vs. DEBIRI alone, and found an increased
response rate and PFS in the first group. Furthermore, they found
toxicity of grade 3 or higher for 80% of the patients in the
combination arm and for 60% in the DEBIRI only-arm. It should be
noted, that the treatment scheme in this study was different with
one week of separation between DEBIRI and FOLFOX, hence FOLFOX on
days 0 and 14, and DEBIRI-TACE on days 7 and 21. The intensity of
DEBIRI-TACE and adjacent FOLFOX 24 h after have not been reported
previously. The most frequent grade 3/4 toxicities encounter in our
study were nausea, vomiting and diarrhea, which could be attributed
to the systemic exposure.
A growing body of evidence support an intense
first-line treatment for mCRC, inspired by the promising results of
the Tribe study (23) using a purely
systemic triplet combination of 5-fluorouracil, oxaliplatin,
irinotecan (FOLFOXIRI) + bevacizumab yielding an OS of 31 months in
the experimental arm. Comparison of survival between studies should
be done with caution, although it is notable, with the short median
survival of 17 months in this study, with the exposure to the same
cytotoxic agents as in the Tribe study. In contrast to Tribe, the
patients in our study all had liver limited disease, yet with a
high burden of disease by number and size of metastases and
baseline CEA.
Using purely descriptive statistics, we report a
tendency for a shorter OS for patients with a baseline level of
cfDNA above the 75th quartile. With the small sample size, we are
not able to show any statistical significance, but the observed
tendency is in concordance with what has previously been shown for
cfDNA and survival for patients with mCRC (15). We saw a similar survival tendency for
patients with a large decline in cfDNA level during the 4
DEBIRI-TACE treatments, having a numerical longer survival than
patients with only a minor change in cfDNA.
We have analyzed the concentration of the total
level of circulating DNA fragments, thereby not limiting our
analysis to patients with detectable mutations in the blood (e.g.,
K or N-RAS). The advantage of total cfDNA quantification is a more
feasible laboratory investigation in contrast to the traditional
quantitative polymerase chain reaction (qPCR) techniques also
requiring DNA purification. By this newly developed direct
fluorescent assay, a quantitative measurement for all patients can
be obtained, including for patients where the circulating DNA do
not habour specific mutations. The limitations of this approach is
a potential lack further molecular detail, e.g., emerging of a new
RAS mutated clone would not be recognized by this methodology.
By plotting the changes in cfDNA concentration on a
time series with the corresponding response evaluation by a CT
scan, it is evident that the dynamics of cfNDA warrants further
investigations. Interestingly, all patients, but one, had a
detectable change in cfDNA level 24 h after the first DEBIRI-TACE.
Due to our limited sample size and missing values, we are unable to
demonstrate any statistical correlation between an early peak of
cfDNA and the clinical outcome. However, illustrating the cfDNA
course introduces the concept of molecular lead time, with a rising
cfDNA preceding the radiologic diagnosis of PD (Fig. 3; patient no. 1) and a decrease in
cfDNA prior to achieving status of NED on imaging (Fig. 4; patient no. 8). Early changes in
circulating DNA has been describe by Tie et al (24) who examined 53 patients receiving
first-line chemotherapy and found the early changes in the tumor
specific cfDNA could predict later response on imaging. A potential
utility of cfDNA, either as tumor specific or as a total quantity,
could be the introduction of molecular lead time in diagnosis of
response or progression, as also suggested by Reinert et al
(25) using a more detailed molecular
analysis of tumor specific DNA.
Modern treatment of mCRC is based on a
multidisciplinary approach including with improvements in the
systemic therapy integrating antiangiogenic treatment, classical
chemotherapeutic agents and anti-EGFR antibodies into a combined
modality with radiotherapy, surgery and invasive loco-regional
therapies. DEBIR-TACE, with or without concurrent chemotherapy,
constitutes a part of the loco-regional toolbox, endorsed by the
ESMO guidelines (10). We propose at
potential role of DEBIR-TACE as a therapy option for highly
selected patients within the framework of a clinical trial, but the
intense combination of DEBIRI-TACE with FOLFOX 24 h after
embolization does not seem as a feasible design for future
studies.
In conclusion, we report the clinical outcomes using
DEBIRI-TACE + mFOLFOX6 bevacizumab as a first-line treatment for
liver limited mCRC with a response rate of 50% and a median OS of
522 days (17 months). The study was impaired by an unexpected high
profile of toxicity and early closure. cfDNA analysis should be
explored as biomarker in larger cohorts of patients undergoing
local therapy for mCRC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Danish Cancer
Society, Novo Nordisk Foundation and Agnes og Ejnar Danielssens
Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MJ, AR, FVM and DTN collaborated in the study design
and conception. AKB, MJ, FVM, DTN, AR, BS and KLS were involved in
the acquisition, analysis or interpretation of data. AKB, MJ, FVM,
DTN, AR, BS and KLS drafted the paper or revised it critically.
AKB, MJ, FVM, DTN, AR, BS and KLS approved the final
manuscript.
Ethics approval and consent to
participate
The Regional Ethics Committee approved the study
protocol and all patients consented to inclusion.
Consent for publication
All patients included in this study have consented
to publication at the time of inclusion into the protocol.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: CCancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E389. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weiss L, Grundmann E, Torhorst I, Hartveit
F, Moberg I, Ider M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez
MJ, et al: Haematogenous metastatic patterns in colonic carcinoma:
an analysis of 1541 necropsies. J Pathol. 150:195–203. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopetz S, Chang GJ, Overman MJ, Eng C,
Sargent DJ, Larsson DW, Grothey A, Vauthey JN, Nagorney DM and
McWilliams RR: Improved survival in metastatic colorectal cancer is
associated with adoption of hepatic resection and improved
chemotherapy. J Clin Oncol. 27:3677–3683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillams AR and Lees WR: Five-year survival
in 309 patients with colorectal liver metastases treated with
radiofrequency ablation. Eur Radiol. 19:1206–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fode MM and Høyer M: Survival and
prognostic factors in 321 patients treated with stereotactic body
radiotherapy for oligo-metastases. Radiother Oncol. 114:155–160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamada R, Sato M, Kawabata K, Nakatsuka H,
Nakamura K and Takashima S: Hepatic artery embolization in 120
patients with unresectable hepatoma. Radiology. 148:397–401. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aliberti C, Tilli M, Benea G and
Fiorentini G: Trans-arterial chemoembolization (TACE) of liver
metastases from colorectal cancer using irinotecan-eluting beads:
Preliminary results. Anticancer Res. 26:3793–3795. 2006.PubMed/NCBI
|
|
8
|
Fiorentini G, Aliberti C, Tilli M,
Mulazzani L, Graziano F, Giodani P, Mambrini A, Montagnani F,
Alessandroni P, Catalano V and Coschiera P: IIntra-arterial
infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus
intravenous therapy (FOLFIRI) for hepatic metastases from
colorectal cancer: Final results of a phase III study. Anticancer
Res. 32:1387–1395. 2012.PubMed/NCBI
|
|
9
|
Martin RC II, Scoggins CR, Schreeder M,
Rilling WS, Laing CJ, Tatum CM, Kelly LR, Garcia-Monaco RD, Sharma
VR, Crocenzi TS and Strasberg SM: Randomized controlled trial of
irinotecan drug-eluting beads with simultaneous FOLFOX and
bevacizumab for patients with unresectable colorectal liver-limited
metastasis. Cancer. 121:3649–3658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Cutsem E, Cervantes A, Adam R, Sobrero
A, van Krieken JH, Aderka D, Aguilar Aranda E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spindler KL, Appelt A, Pallisgaard N,
Andersen RF, Brandslund I and Jakobsen A: Cell-free DNA in healthy
individuals, noncancerous disease and strong prognostic value in
colorectal cancer. Int J Cancer. 135:2984–2991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandel P and Metasis P: Les acides
nucléiques du plasma sanguin chez l'homme. C R Seances Soc Biol
Fil. 142:241–243. 1948.(In Undetermined Language). PubMed/NCBI
|
|
13
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of cancer patients and the effect of
therapy. Cancer Res. 37:646–650. 1977.PubMed/NCBI
|
|
14
|
Boysen AK, Wettergren Y, Sorensen BS,
Taflin H, Gustavson B and Spindler KG: Cell-free DNA levels and
correlation to stage and outcome following treatment of locally
advanced rectal cancer. Tumour Biol. 39:10104283177309762017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spindler KG, Boysen AK, Pallisgård N,
Johansen JS, Tabernero J, Sørensen MM, Jensen BV, Hansen TF,
Sefrioui D, Andersen RF, et al: Cell-free DNA in metastatic
colorectal cancer: A systematic review and meta-analysis.
Oncologist. 22:1049–1055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargant D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Therapy Evaluation Program, Common
Terminology Criteria for Adverse Events, . Version 3.0. DCTD, NCI,
NIH, DHHS March 31. 2003.http://ctep.cancer.govAugust 9–2006
|
|
18
|
Price DD, McGrath PA, Rafii A and
Buckingham B: The validation of visual analogue scales as ratio
scale measures for chronic and experimental pain. Pain. 17:45–56.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldshtein H, Hausmann MJ and Douvdevani
A: A rapid direct fluorescent assay for cell-free DNA
quantification in biological fluids. Ann Clin Biochem. 46:488–494.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schou JV, Larsen FO, Sørensen BS, Abrates
R, Boysen AK, Johansen JS, Jensen BV, Nielsen DL and Spindler KL:
Circulating cell-free DNA as predictor of treatment failure after
neoadjuvant chemo-radiotherapy before surgery in patients with
locally advanced rectal cancer. Ann Oncol. 29:610–615. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eichler K, Zangus S, Mack MG, Hammerstring
R, Gruber-Rouh T, Galluc C and Vogl TJ: First human study in
treatment of unresectable liver metastases from colorectal cancer
with irinotecan-loaded beads (DEBIRI). Int J Oncol. 41:1213–1220.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fiorentini G, Aliberti C, Turrisi G, del
Conte A, Rossi S, Benea G and Giovanis P: IIntraarterial hepatic
chemoembolization of liver metastases from colorectal cancer
adopting irinotecan-eluting beads: Results of a phase II clinical
study. In Vivo. 21:1085–1091. 2007.PubMed/NCBI
|
|
23
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tie J, Kinde I, Wang Y, Wong HL, Roebort
J, Christie M, Tacey M, Wong R, Singh M, Karapetis CS, et al:
Circulating tumor DNA as an early marker of therapeutic response in
patients with metastatic colorectal cancer. Ann Oncol.
26:1715–1722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reinert T, Schøler L, Thomsen R, Tobiasen
H, Vang S, Nordentoft I, Lamy P, Kannerup AS, Mortensen FV,
Stribolt K, et al: Analysis of circulating tumour DNA to monitor
disease burden following colorectal cancer surgery. Gut.
65:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|