Introduction
Osteosarcoma (OS) is the most common type of primary
bone tumor in adolescents and young adults with an incidence of
4–5/1,000,000 and a 5-year overall survival rate of 60–70%
(1–3).
A number of factors have been demonstrated to affect the prognosis,
including the axial localization of the primary tumor, the tumor
diameter and the histological response to preoperative chemotherapy
(4). Although extensive advancements
in diagnostic methods and surgical techniques have been developed,
the molecular etiology of osteosarcoma has not been fully
elucidated (5).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs of
20–25 nucleotides, which have been demonstrated to function in
various biological processes, including cancer initiation, growth
and progression, by targeting genes for post-transcriptional
degradation via their 3′untranslated region (UTR) (6,7).
miR-214, located within the sequence of the long
non-coding Dmn3os transcript, has been reported not only to be
highly dysregulated but also highly variable in its expression
level in multiple types of cancer (8,9). This
suggests that miR-214 may function as a tumor suppressor and may
have a tumorigenic role. In previous studies, researchers have
demonstrated that miR-214 was upregulated in osteosarcoma and
associated with tumor progression and poor prognosis (10,11).
However, little progress has been achieved in terms of elucidating
the molecular mechanisms of miR-214-3p-mediated tumorigenesis in
OS.
In the present study, it was demonstrated that
miR-214-3p directly targeted the 3′-UTR of cell adhesion molecule 1
(CADM1) and therefore suppressing the expression of CADM1. The
transfection of miR-214-3p mimic was able to promote the
proliferation, migration and invasion of OS cells in vitro
by activating the P44/42 mitogen activated kinase (MAPK) signaling
pathway.
Materials and methods
Cell culture
Human OS cell lines, MNNG/HOS Cl#5 and U2OS, and
normal human 293T cells were obtained from The Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (Shanghai, China)
and cultured in Dulbecco's modified Eagle's medium (DMEM),
supplemented with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin-streptomycin (all from HyClone; GE Healthcare, Chicago,
IL, USA). The cells were cultured at 37°C in a humidified incubator
containing 5% CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of MNNG/HOS Cl#5 and U2OS was isolated
using TRIzol (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. Reverse transcription of
mature miRNAs was performed using 1 µg RNA, M-MLV Reverse
Transcriptase (catalog no. M1701), Recombinant RNasin®
Ribonuclease inhibitor (catalog no. N2511) and dNTP (catalog. no
U1515) (all from Promega Corporation, Madison, WI, USA) as
described in a previous study (12).
The stem-loop primer sequences used are as follows: miR-214-3p,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACTGCCTG-3′ and U6,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. Reverse transcription of CADM1 was
performed using the ReverTra Ace® PCR-qRT kit (catalog
no. FSQ-101; Toyobo Life Science, Osaka, Japan) according to the
manufacturer's protocol.
The expression of CADM1 mRNA and mature miR-214-3p
was determined by RT-qPCR using a KAPA SYBR® FAST
Universal qPCR kit (catalog no. KK4601; Kapa Biosystems, Inc.,
Wilmington, MA, USA), according to the manufacturer's protocol. In
brief, 20 µl mixture was heated at 95°C for 3 min for enzyme
activation, then the 20 µl reaction mixture were incubated as
follows: 95°C for 3 sec and 60°C for 20 sec for 40 cycles. 18S and
U6 were used as internal controls for CADM1 and miR-214-3p,
respectively. The primer sequences (Beijing Genomics Institute,
Shenzhen, Guangdong, China) used are as follows: CADM1, forward,
5′-GCAAATCGGAGGTGGAAGA-3′, and reverse,
5′-GCACTTGAGGCTTATACTGTACTT-3′; 18S, forward,
5′-CAGCCACCCGAGATTGAGCA-3′, and reverse,
5′-TAGTAGCGACGGGCGGTGTG-3′; miR-214-3p, forward,
5′-ACACTCCAGCTGGGACAGCAGGCACAGACA-3′, and reverse,
5′-TGGTGTCGTGGAGTCG-3′, and U6, forward,
5′-CTCGCTTCGGCAGCACATATACT-3′, and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. The 2−∆∆Cq method was used
to quantify the expression of miR-214-3p and CADM1, and each
experiment was performed in triplicate (13).
Cell transfection
A total of 2×105 MNNG/HOS Cl#5 or U2OS
cells were seeded per well into 6-well plates and transfected
transiently with 50 nM miR-214-3p mimic/inhibitor and NC/inhibitor
NC (Shanghai GenePharma Co., Ltd., Shanghai, China) or CADM1 siRNA
and siNC (Biotend, Shanghai, China) using 3 µl
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The sequences of miRNA
mimics, inhibitor, NC and CAM1 siRNAs 1–3 used in assays are
presented in Table I. During
transfection, DMEM without FBS was used. For the cell function
assay, cells were collected 12 h after transfection. For RT-qPCR
and western blotting, cells were collected 24 and 48 h after
transfection. For the dual luciferase assay, cells were collected
48 h after transfection.
 | Table I.Sequences of miRNA mimics, inhibitor,
NC and CAM1 siRNAs 1–3. |
Table I.
Sequences of miRNA mimics, inhibitor,
NC and CAM1 siRNAs 1–3.
| Name | Sense/antisense | Sequence |
|---|
| miR-214-3p
mimics | Sense |
5′-ACAGCAGGCACAGACAGGCAGU-3′ |
|
| Antisense |
5′-UGCCUGUCUGUGCCUGCUGUUU-3′ |
| miRNA NC | Sense |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| Antisense |
5′-ACGUGACACGUUCGGAGAATT-3′ |
| miR-214
inhibitor |
|
5′-ACUGCCUGUCUGUGCCUGCUGU-3′ |
| miRNA inhibitor
NC |
|
5′-CAGUACUUUUGUGUAGUACAA-3′ |
| CADM1-siRNA-1 | Sense |
5′-GGUGGAAGGUGAGGAGAUUdTdT-3′ |
|
| Antisense |
5′-AAUCUCCUCACCUUCCACCdTdT-3′ |
| CADM1-siRNA-2 | Sense |
5′-UCAGGUGGUUCAAAGGGAAdTdT-3′ |
|
| Antisense |
5′-UUCCCUUUGAACCACCUGAdTdT-3′ |
| CADM1-siRNA-3 | Sense |
5′-CCAACCUGUUCAUCAAUAAdTdT-3′ |
|
| Antisense |
5′-UUAUUGAUGAACAGGUUGGdTdT-3′ |
| siNC | Sense |
5′-UUCUCCGAACGUGUCACGUdTdT-3′ |
|
| Antisense |
5′-ACGUGACACGUUCGGAGAAdTdT-3′ |
MTT assay
Transfected MNNG/HOS Cl#5 or U2OS cells were seeded
into 96-well plates at 3×103 cells/well. A total of 20
µl MTT (5 mg/ml; Sangon Biotech Co., Ltd., Shanghai, China) was
added to each well, and incubated with the cells for 4 h at 37°C.
Supernatant fractions were discarded, and 150 µl dimethyl sulfoxide
was added to each well to dissolve the crystals. Absorbance values
were obtained at 490 nm in quintuplicate using a spectrophotometric
plate reader (Infinite® 200 PRO; Tecan Group, Ltd.,
Mannedorf, Switzerland).
Scratch assay
The transfected MNNG/HOS Cl#5 or U2OS cells were
seeded into 12-well plates to form adherent monolayers. A 200-µl
pipette tip was used to make a scratch, and then the plates were
washed twice in PBS to remove the resultant debris and floating
cells. The cell culture medium was immediately replaced with DMEM
containing 1% FBS. The images of the scratch were taken at
different time points (0, 12, 24 and 48 h) using a Leica
Microsystems DMI3000B light microscope (Leica Microsystems GmbH,
Wetzlar, Germany). The assays were performed in triplicate.
Transwell assay
For the migration assay, transfected cells were
suspended in FBS-free DMEM medium containing 0.1% bovine serum
albumin (Bioworld Technology, Inc., St. Louis Park, MN, USA) and
5×104 cells/200 µl were seeded into the upper chamber.
For the invasion assay, Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) was diluted 1:4 with serum-free DMEM medium and used to
coat the Transwell inserts (pore size, 8-µm; EMD Millipore,
Billerica, MA, USA) to form a matrix barrier. Transfected cells
were suspended in FBS-free DMEM medium containing 0.1% bovine serum
albumin, and 5×104 cells/200 µl were seeded into the
upper chamber. A total of 600 µl medium containing 15% FBS was
added to the lower chamber. The cells were incubated at 37°C for
different durations. Then, the cells that had migrated or invaded
through the membrane were fixed with 95% ethyl alcohol for 15 min
at room temperature and stained with 0.1% crystal violet for 10 min
at room temperature. The images of the cells on the lower surface
were captured and counted in 5 random fields of view.
Dual luciferase assay
The human CADM1 3′UTR seed region was amplified by
PCR using the following primers: CADM1 3′UTR forward,
5′-GGCCTCGAGGGAACTTGCGAGAAATTCGTGT-3′, and reverse,
5′-TTAAGCGGCCGCAATGCGAATGGGAACATATGGA-3′. The transcript was then
cloned into a psiCHECK-2 vector (Promega Corporation), downstream
of the Renilla luciferase gene. The vector also contained the
Firefly luciferase gene. A total of 4×105 293T cells
were seeded per well into 6-well plates and co-transfected with
either 50 nM miR-214-3p mimics or miRNA NC and 2 µg plasmid vector
using Lipofectamine® 2000, according to the
manufacturer's protocol. The cells were lysed and assayed for
luciferase activity at 48 h post-transfection using a
Dual-Luciferase Assay kit (catalog no. E1910; Promega Corporation).
The assays were independently repeated ≥3 times. The firefly
luciferase was used as a reference for normalization.
Western blotting
The cells were lysed using radioimmunoprecipitation
assay buffer (catalog no. P00138; Beyotime Institute of
Biotechnology, Haimen, China) supplemented with protease inhibitors
(Complete™ Protease Inhibitor Cocktail; catalog no. 04693116001;
Roche Diagnostics, Basel, Switzerland; PMSF; catalog no. ST505;
Beyotime Institute of Biotechnology) and phosphatase inhibitors
(catalog no. 04906845001PhosSTOP™, Roche Diagnostics). The
supernatant was collected, and the protein concentration was
quantified using a BCA kit and a plate reader (Infinite®
M200 PRO, Group, Ltd., Mannedorf, Switzerland). A total of 30 µg
protein was subjected to 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes for 120 min at 200 mA. Then, the
blots were blocked with 5% fat free milk at room temperature for 1
h and incubated overnight at 4°C with anti-P44/42 MAPK (dilution,
1:1,000; catalog no. 4695; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-phospho-P44/42 MAPK (dilution, 1:1,000;
catalog no. 4370; Cell Signaling Technology), anti-CADM1 (dilution,
1:1,000; catalog no. 14335-1-AP, ProteinTech Group, Inc., Chicago,
IL, USA) and anti-GAPDH (AP0063, Bioworld Technology, Inc., St.
Louis Park, MN, USA). Following washing three times with PBST
(1×PBS with 1% Tween-20) for 5 min, the blots was incubated with
horseradish peroxidase-conjugated goat anti-rabbit (dilution,
1:5,000; catalog no. 4412; Cell Signaling Technology) at room
temperature for 1 h, and then washed with PBST for 5 min three
times. The proteins were visualized using Pierce ECL Western
Blotting substrate (catalog no. 32209; Invitrogen; Thermo Fisher
Scientific, Inc.) and a Tanon 5200 Multi system (Tanon Science and
Technology Co., Ltd., Shanghai, China). The grayscale value was
measured using ImageJ software (version no. 2006.02.01; National
Institutes of Health, Bethesda, MD, USA).
Bioinformatics analysis
Potential target genes of miR-214-3p were predicted
using TargetScan Human 7.1 (http://www.targetscan.org/vert_71/) and mirTarBase
(http://mirtarbase.mbc.nctu.edu.tw/php/index.php), and
miR-214-3p was used as a search term. The Kyoto Encyclopedia of
Genes and Genomes pathways of the potential target genes were
predicted using GeneCoDis (http://genecodis.cnb.csic.es/) and the target genes of
miR-214-3p were used as search terms. All the sites were accessed
on May 20th, 2015.
Statistical analysis
All statistical analyses were performed using SPSS
(version 21.0; IBM Corp., Armonk, NY, USA). Comparisons between 2
different groups were performed using Student's t-test. Comparisons
between ≥3 independent groups were performed using one-way analysis
of variance (ANOVA) followed by Scheffé's post hoc test was used.
Multivariate ANOVA was used for the comparison of multiple groups
at different time points. All data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-214-3p affects the proliferation,
migration and invasion of OS cells
In a previous study by the authors, miR-214-3p
expression was detected in cancerous and noncancerous bone tissues
from 92 children treated for primary osteosarcoma (12). The previous study revealed that
upregulated expression of miR-214 may be associated with tumor
progression and adverse prognosis in pediatric osteosarcoma
(13,14). To investigate the effect of miR-214-3p
on OS in vitro, miR-214-3p mimics and miR-NC were
transfected into U2OS and MNNG/HOS Cl#5 cells in the present study
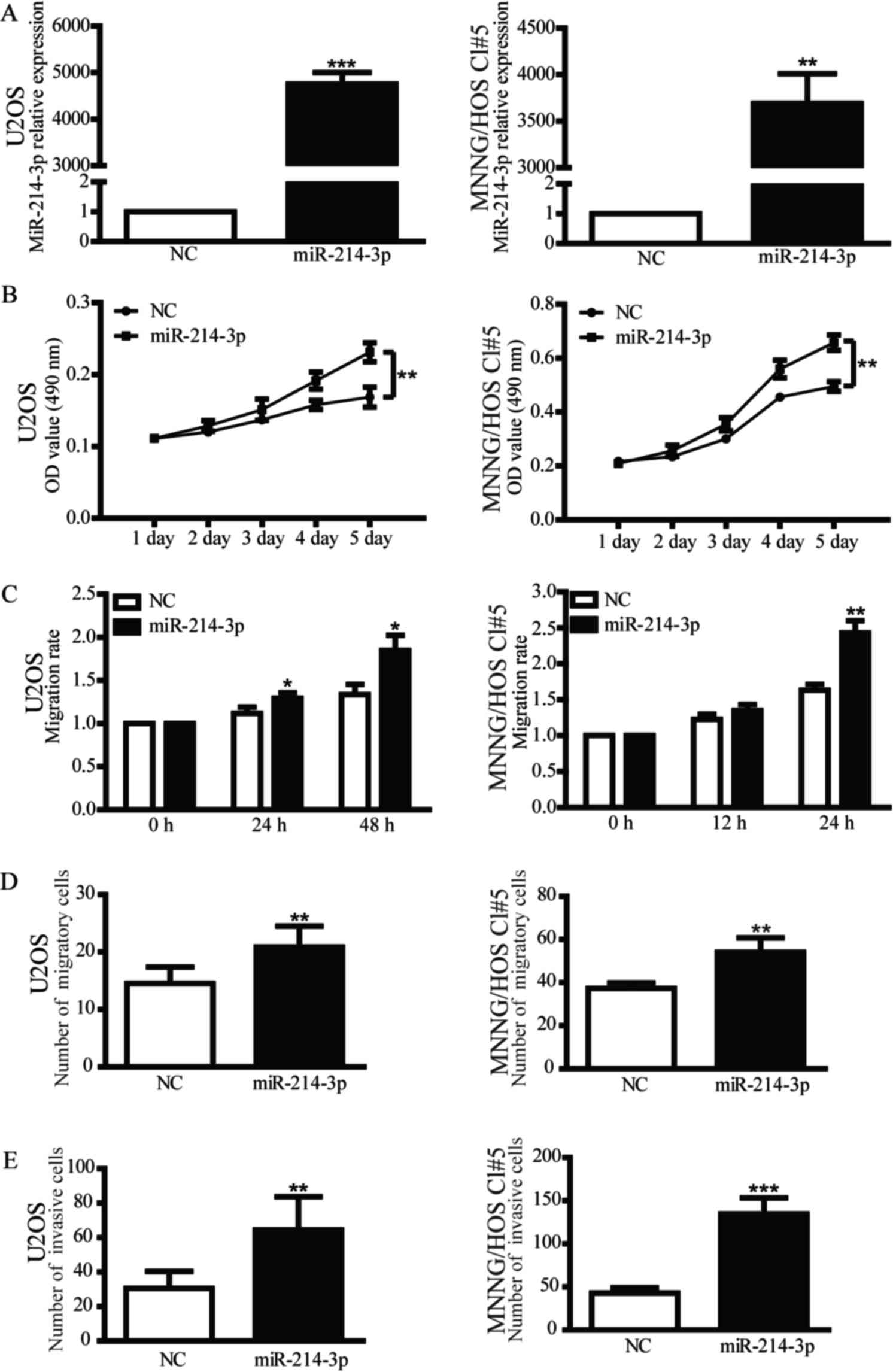
(Fig. 1A). A MTT assay was performed
to examine the proliferative ability of OS cells that overexpress
miR-214-3p. Upregulated miR-214-3p expression was able to
significantly increase the proliferation of OS cells (Fig. 1B). To evaluate the role of miR-214-3p
in migration and invasion, scratch assay and Transwell assays were
performed, revealing that miR-214-3p mimic was able to promote the
migration and invasion of OS cells (Fig.
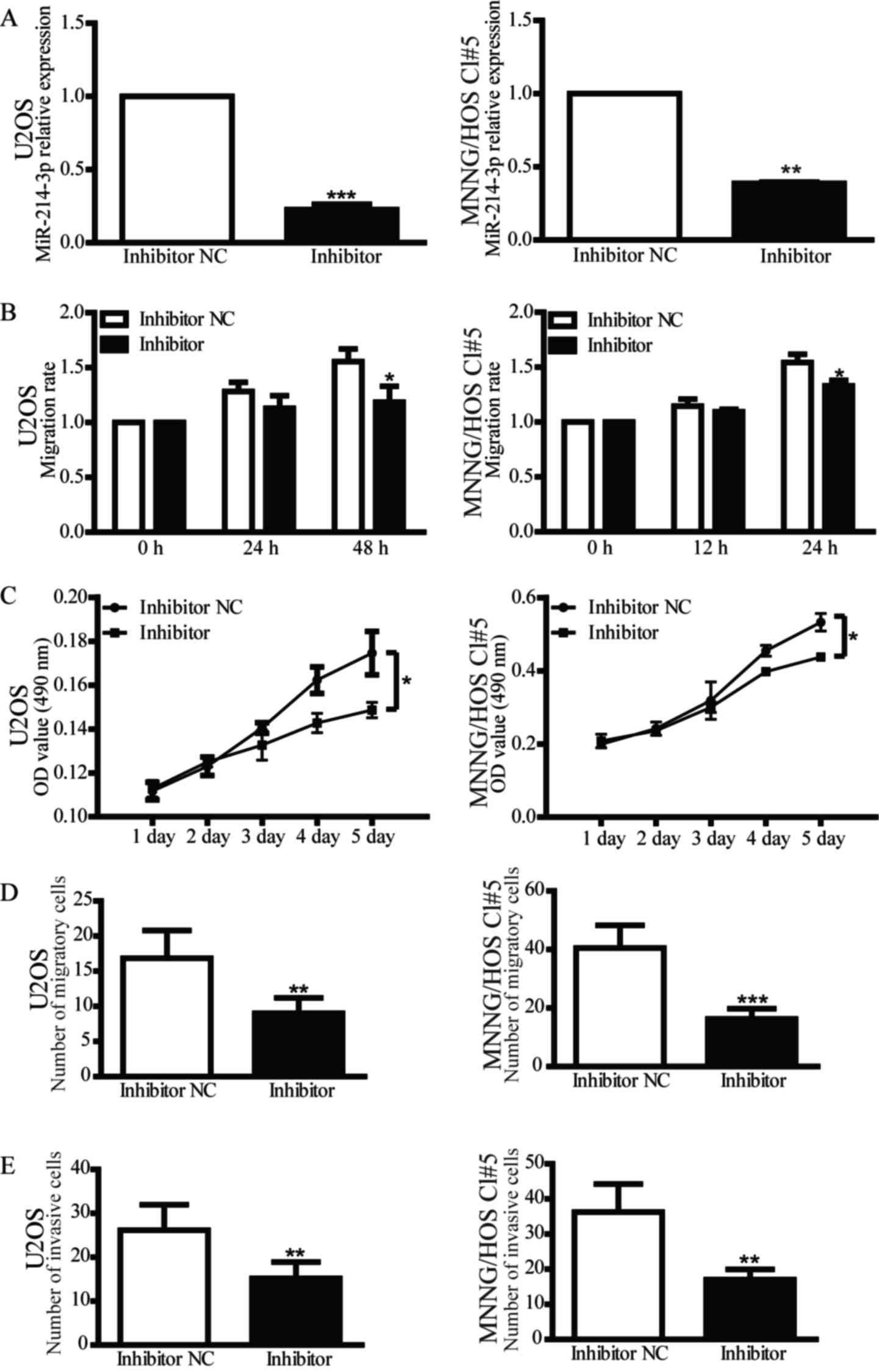
1C-E). In addition, inhibition of miR-214-3p expression in OS
cells reduced their proliferative, migratory and invasive abilities
compared with the inhibitor NC group (Fig. 2). These findings indicate that
miR-214-3p may act as an oncogene in OS cells.
CADM1 is a direct target of
miR-214-3p
To elucidate the underlying molecular mechanisms of
miR-214-3p in the proliferation, migration and invasion of OS
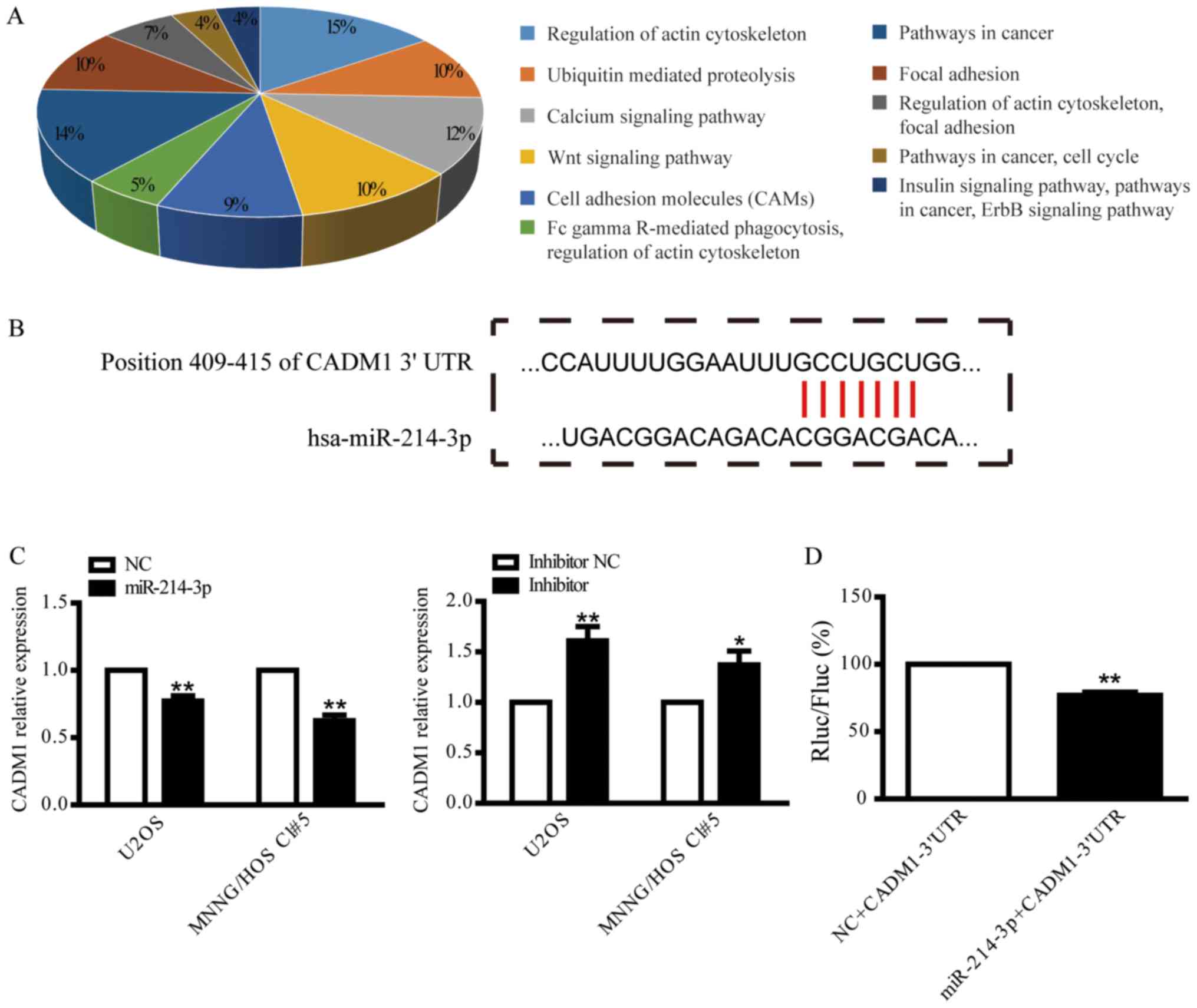
cells, the potential target genes of miR-214-3p were predicted
using TargetScan Human 7.1 and mirTarBase. The pathways of the
potential target genes were predicted using GeneCoDis (Fig. 3A). CADM1 was identified as a putative
target of miR-214-3p, and the potential binding site between
miR-214-3p and CADM1 is presented in Fig.
3B. CADM1 was selected for further validation by examining
CADM1 mRNA expression following overexpression or knockdown of
miR-214-3p in U2OS and MNNG/HOS Cl#5 cells. CADM1 expression was
suppressed by overexpression of miR-214-3p and increased by
knockdown of miR-214-3p (Fig. 3C). To
further verify whether miR-214-3p targets CADM1 directly, the CADM1
3′UTR was cloned into psiCHECK-2 prior to dual luciferase assay. As
indicated in Fig. 3D, a significant
decrease in the Renilla luciferase/Firefly luciferase ratio was
identified following co-transfection of the CADM1-3′UTR plasmid
with miR-214-3p mimic, but not with miR-NC, suggesting that
miR-214-3p may be able to repress CADM1 expression by directly
binding the 3′UTR of CADM1.
CADM1 knockdown facilitates the
proliferation, migration and invasion of OS cells by activating
P44/42 signaling
To further verify the effect of CADM1 on OS cells,
U2OS and MNNG/HOS Cl#5 cells were separately transfected with 3
CADM1 siRNAs (siCADM1s). siCADM1-3 was selected for subsequent
experiments due to its higher interference efficiency (Fig. 4A). The assays demonstrated that the
knockdown of CADM1, similarly to the overexpression of miR-214-3p,
was able to promote proliferation, migration and invasion of OS
cells (Fig. 4B-E).
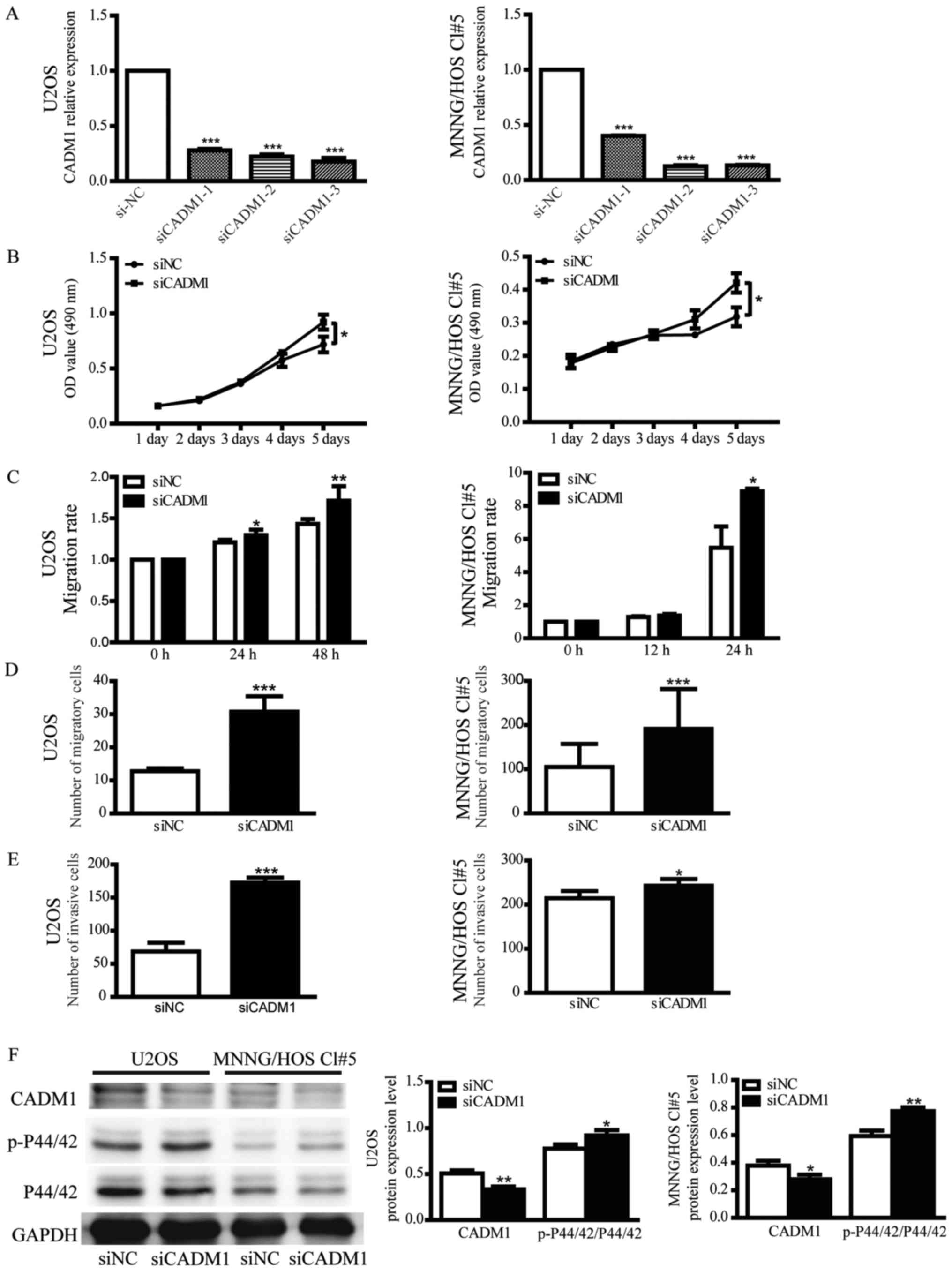 | Figure 4.Knockdown of CADM1 promotes the
proliferation, migration and invasion of OS cells by activating the
extracellular-signal-regulated kinase signalling pathway. (A)
Relative expression of CADM1 in U2OS and MNNG/HOS Cl#5 cells that
were transiently transfected with siCADM1, 2 or 3 or siNC. 18S was
used as an internal control. The value of each sample was
calculated using the 2−ΔΔCq method and analyzed by
one-way analysis of variance. Data are presented as the mean ±
standard deviation. (B) Growth curves of OS cells that were
transfected with siCADM1 or siNC represent OD values at 490 nm
measured by MTT assays. (C) A scratch assay was used to detect the
motility of U2OS and MNNG/HOS Cl#5 cells that were transfected with
siCADM1 or siNC. (D) Quantitative results of Transwell migration
assays in CADM1 knocked down-U2OS and MNNG/HOS Cl#5 cells. U2OS and
MNNG/HOS Cl#5 cells were incubated for 24 h. (E) Quantitative
results of Transwell invasion assays in CADM1 knocked down-U2OS and
MNNG/HOS Cl#5 cells. U2OS and MNNG/HOS Cl#5 cells were incubated
for 48 h. (F) The protein expression levels of CADM1, p-P44/42 MAPK
and P44/42 MAPK were measured by western blotting. GAPDH was used
as an internal control and Student's t-test was used. Data are
presented as the mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001 vs. siNC. CADM1, cell adhesion molecule
1; si, small interfering RNA; NC, negative control; OD, optical
density; OS, osteosarcoma; p-, phosphorylated; siNC, negative siRNA
control. |
In order to elucidate the mechanism of CADM1 in
regulating the growth and motility of OS cells, western blotting
was used to identify the signaling pathway implicated in the
process. The level of phosphorylated P44/42 MAPK was elevated in
CADM1-knocked down cells compared with siNC-transfected cells
(Fig. 4F), suggesting that the
knockdown of CADM1 was able to activate the P44/42 signaling
pathway, which subsequently affected the cellular functions that
are modulated by the pathway.
Discussion
Previous studies have demonstrated that miR-214
functions either as an oncogene or a tumor suppressor in a number
of human cancer types, including lung, prostate, colorectal and
esophageal cancer (15–18). Previous studies have indicated that
elevated miR-214-3p is associated with OS progression, but a
limited number of studies have focused on the function and
mechanism of miR-214-3p (10,11,19,20). In a
previous study by the authors, it was demonstrated that upregulated
miR-214 expression was associated with aggressive
clinicopathological features (tumor size, metastasis status and
response to pre-operative chemotherapy) and poor prognosis of
pediatric osteosarcoma (11). In the
present study, it was demonstrated that miR-214-3p may act as an
oncogene, where it promotes the proliferation, migration and
invasion of OS cells. The knockdown of miR-214-3p was able to
decrease the cell growth rate and mobility, which was consistent
with the results of Xu and Wang (21).
To clarify the potential mechanism of miR-214-3p in
OS, potential target genes were identified using target prediction
tools. CADM1 was identified as a candidate target of miR-214-3p,
which was verified by RT-qPCR and dual luciferase assays. 293T
cells were co-transfected with CADM1 3′UTR-containing plasmids and
scrambled miRNAs, which were used as a negative control. In the
experimental group, 293T cells were co-transfected with CADM1
3′UTR-containing plasmids and miR-214-3p mimic. However, a plasmid
containing a mutation in the seed sequence of the CADM1 3′UTR would
provide a more convincing control for indicating the direct
targeting of the CADM1 3′UTR by miR-214-3p.
CADM1 is located on chromosome 11q23.2, and is an
intercellular adhesion molecule that is part of the immunoglobulin
superfamily (22,23). Silencing of CADM1 is frequently
observed in various types of cancer, including lung, prostate,
gastric, breast, pancreatic, nasopharyngeal and cervical cancer,
and this is accompanied by increased proliferation, invasion and
metastatic potential of tumors cell (24–27).
However, to the best of our knowledge, only one previous study has
investigated CADM1 in OS, which indicated that CADM1 may be a
potential diagnostic marker (22). In
the present study, CADM1 expression was suppressed using siRNA, and
that downregulation of CADM1 resulted in increased proliferative,
migratory and invasive abilities of OS cells, which was consistent
with the effects of miR-214-3p.
Although it was demonstrated that miR-214-3p was
able to modulate the proliferative, migratory and invasive
abilities of OS cells by directly targeting the 3′UTR of CADM1, the
molecular mechanism underlying the involvement of CADM1 remains
unclear. CADM1 has been reported to be implicated in several
pathways. Vallath et al (26)
reported that CADM1 inhibited the progression of squamous cell
carcinoma by reducing signal transducer and activator of
transcription 3 activity. Zhang et al (24) demonstrated that CADM1 regulated the
G1/S phase transition and repressed tumorigenesis via the Rb-E2F
pathway in hepatocellular carcinoma (24). Murakami et al (28) demonstrated that trans-homophilic
interactions, mediated by CADM1, activated the
phosphoinositide-3-kinase pathway to reorganize the actin
cytoskeleton and form the epithelial cell structure. To the best of
our knowledge, the present study is the first to demonstrate that
the downregulation of CADM1 is able to activate the P44/42 MAPK
signaling pathway, which has been reported to be associated with
cell proliferation, migration and invasion (29,30).
In conclusion, miR-214-3p was able to activate
P44/42 MAPK signaling by downregulating CADM1 expression, thereby
promoting the proliferation, migration and invasion of OS cells.
These results indicate that miR-214-3p and CADM1 may be useful
diagnostic markers for OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Project
of Shanghai Municipal Commission of Health and Family Planning
grant no. 20134y085) and the Natural Science Foundation of Shanghai
(grant no. 14ZR1426400).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZGW made substantial contributions to conception and
design, revised the manuscript critically for important
intellectual content and gave final approval of the version to be
published. HQC and MYM performed the literature research,
experimental studies, data acquisition and data analysis. HQC wrote
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin M, Geudens I, Bruyr J, Potente M,
Bleuart A, Lebrun M, Simonis N, Deroanne C, Twizere JC, Soubeyran
P, et al: PP2A regulatory subunit Bα controls endothelial
contractility and vessel lumen integrity via regulation of HDAC7.
EMBO J. 32:2491–2503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kakimoto Y, Ito S, Abiru H, Kotani H,
Ozeki M, Tamaki K and Tsuruyama T: Sorbin and SH3 domain-containing
protein 2 is released from infarcted heart in the very early phase:
Proteomic analysis of cardiac tissues from patients. J Am Heart
Assoc. 2:e0005652013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bielack SS, Hecker-Nolting S, Blattmann C
and Kager L: Advances in the management of osteosarcoma. F1000Res.
5:27672016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhen N, Yang Q, Zheng K, Han Z, Sun F, Mei
W and Yu Y: MiroRNA-127-3p targets XRCC3 to enhance the
chemosensitivity of esophageal cancer cells to a novel
phenanthroline-dione derivative. Int J Biochem Cell Biol.
79:158–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma T, Hamilton R and Mandal CC:
miR-214: A potential biomarker and therapeutic for different
cancers. Future Oncol. 11:349–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penna E, Orso F and Taverna D: miR-214 as
a key hub that controls cancer networks: Small player, multiple
functions. J Invest Dermatol. 135:960–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allen-Rhoades W, Kurenbekova L,
Satterfield L, Parikh N, Fuja D, Shuck RL, Rainusso N, Trucco M,
Barkauskas DA, Jo E, et al: Cross-species identification of a
plasma microRNA signature for detection, therapeutic monitoring,
and prognosis in osteosarcoma. Cancer Med. 4:977–988. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z and Cai H, Lin L, Tang M and Cai H:
Upregulated expression of microRNA-214 is linked to tumor
progression and adverse prognosis in pediatric osteosarcoma.
Pediatr Blood Cancer. 61:206–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, He J, Wang Y, Zhu X, Pan Q, Xie Q
and Sun F: miR-889 promotes proliferation of esophageal squamous
cell carcinomas through DAB2IP. FEBS Lett. 589:1127–1135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liesenfeld M, Mosig S, Funke H, Jansen L,
Runnebaum IB, Dürst M and Backsch C: SORBS2 and TLR3 induce
premature senescence in primary human fibroblasts and
keratinocytes. BMC Cancer. 13:5072013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Lu C, Chu W, Zhang Y, Zhang B,
Zeng Q, Wang R, Li Z, Lv B and Liu J: microRNA-214 governs lung
cancer growth and metastasis by targeting carboxypeptidase-D. DNA
Cell Biol. 35:715–721. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava A, Goldberger H, Dimtchev A,
Ramalinga M, Chijioke J, Marian C, Oermann EK, Uhm S, Kim JS, Chen
LN, et al: MicroRNA profiling in prostate cancer-the diagnostic
potential of urinary miR-205 and miR-214. PLoS One. 8:e769942013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cristobal I, Caramés C, Madoz-Gurpide J,
Rojo F, Aguilera O and Garcia-Foncillas J: Downregulation of
miR-214 is specific of liver metastasis in colorectal cancer and
could play a role determining the metastatic niche. Int J
Colorectal Dis. 29:8852014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Q, Xu L, Li C, Yuan Y, Huang S and Chen
H: miR-214 inhibits invasion and migration via downregulating
GALNT7 in esophageal squamous cell cancer. Tumour Biol.
37:14605–14614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teicher BA: Searching for molecular
targets in sarcoma. Biochem Pharmacol. 84:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poos K, Smida J, Nathrath M, Maugg D,
Baumhoer D and Korsching E: How microRNA and transcription factor
co-regulatory networks affect osteosarcoma cell proliferation. PLoS
Comput Biol. 9:e10032102013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Z and Wang T: miR-214 promotes the
proliferation and invasion of osteosarcoma cells through direct
suppression of LZTS1. Biochem Biophys Res Commun. 449:190–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue T, Hagiyama M, Enoki E, Sakurai MA,
Tan A, Wakayama T, Iseki S, Murakami Y, Fukuda K, Hamanishi C and
Ito A: Cell adhesion molecule 1 is a new osteoblastic cell adhesion
molecule and a diagnostic marker for osteosarcoma. Life Sci.
92:91–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
You Y, Zhang J, Li Y, Li Y, Shi G, Ma L
and Wei H: CADM1/TSLC1 inhibits melanoma cell line A375 invasion
through the suppression of matrix metalloproteinases. Mol Med Rep.
10:2621–2626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Xie HY, Ding SM, Xing CY, Chen A,
Lai MC, Zhou L and Zheng SS: CADM1 regulates the G1/S transition
and represses tumorigenicity through the Rb-E2F pathway in
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
15:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Wang R, Zhang T and Dong X:
MicroRNA-126 regulates migration and invasion of gastric cancer by
targeting CADM1. Int J Clin Exp Pathol. 8:8869–8880.
2015.PubMed/NCBI
|
|
26
|
Vallath S, Sage EK, Kolluri KK, Lourenco
SN, Teixeira VS, Chimalapati S, George PJ, Janes SM and Giangreco
A: CADM1 inhibits squamous cell carcinoma progression by reducing
STAT3 activity. Sci Rep. 6:240062016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katoh M: Cardio-miRNAs and onco-miRNAs:
Circulating miRNA-based diagnostics for non-cancerous and cancerous
diseases. Front Cell Dev Biol. 2:612014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murakami S, Sakurai-Yageta M, Maruyama T
and Murakami Y: Trans-homophilic interaction of CADM1 activates
PI3K by forming a complex with MAGuK-family proteins MPP3 and Dlg.
PLoS One. 9:e1100622014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Q, Mao ZD, Shi YJ, Chen Y, Sun Y,
Zhang Q, Song L and Peng LP: MicroRNA-7 inhibits cell
proliferation, migration and invasion in human non-small cell lung
cancer cells by targeting FAK through ERK/MAPK signaling pathway.
Oncotarget. 7:77468–77481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Wang J, Lv Z, Zhao D and Luo M:
Cox-2 promotes mesenchymal stem cells differentiation into
cardiocytes by activating JNK and ERK pathway. Biochem Biophys Res
Commun. 480:101–105. 2016. View Article : Google Scholar : PubMed/NCBI
|