Introduction
In China, gastric cancer is the second most common
type of cancer in males and the fourth most common type in females
(1). The majority of patients with
gastric cancer are diagnosed at the advanced stage, and the
prognosis for patients with advanced gastric cancer is poor
(2). Surgical resection is a
cornerstone gastric cancer treatment, particularly during the early
stage (3). Chemotherapy,
radiotherapy, targeted therapy, palliative surgery and best
supportive care have been used to treat patients with late stage
gastric cancer (4). Conversion
therapy may be feasible for prolonging overall survival. As gastric
cancer is a heterogeneous disease, therapy should be performed
based on subtype, and specific biomarkers and different treatment
targets ought to be identified for each subtype (5). Erb-b2 receptor tyrosine kinase 2 (ERBB2)
overexpression has been reported in 12–27 and 9–23% of patients
with gastric cancer according to different studies (6–12). For
patients with ERBB2+ gastric cancer, it has been advised that
trastuzumab is administered as chemotherapy. The efficacy and
safety of trastuzumab were evaluated in the Trastuzumab for Gastric
Cancer (ToGA) trial (13), and it has
been reported that trastuzumab may be used for conversion therapy
(14,15). In the present study, the treatment
regimen consisted of trastuzumab combined with oxaliplatin and
capecitabine (XELOX), and was performed following surgery. Tumors
were resected, conversion therapy was successful, and good clinical
outcomes were achieved. Written informed consent was obtained from
the patients for the present study and any accompanying images to
be published. The Ethics Committee of Sichuan Cancer Hospital
(Chengdu, China) approved the present study.
Case reports
Case 1
A 40-year-old female was admitted to Sichuan Cancer
Hospital with a complaint of lower back pain for >1 month. An
abdominal ultrasound revealed retroperitoneal lymph node
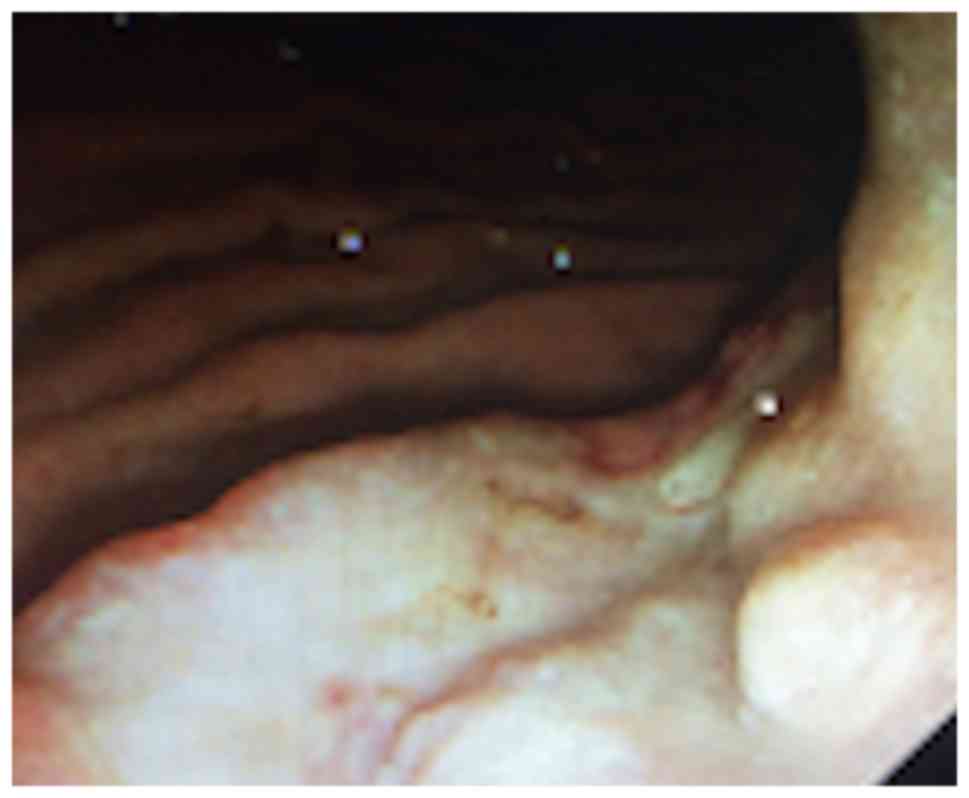
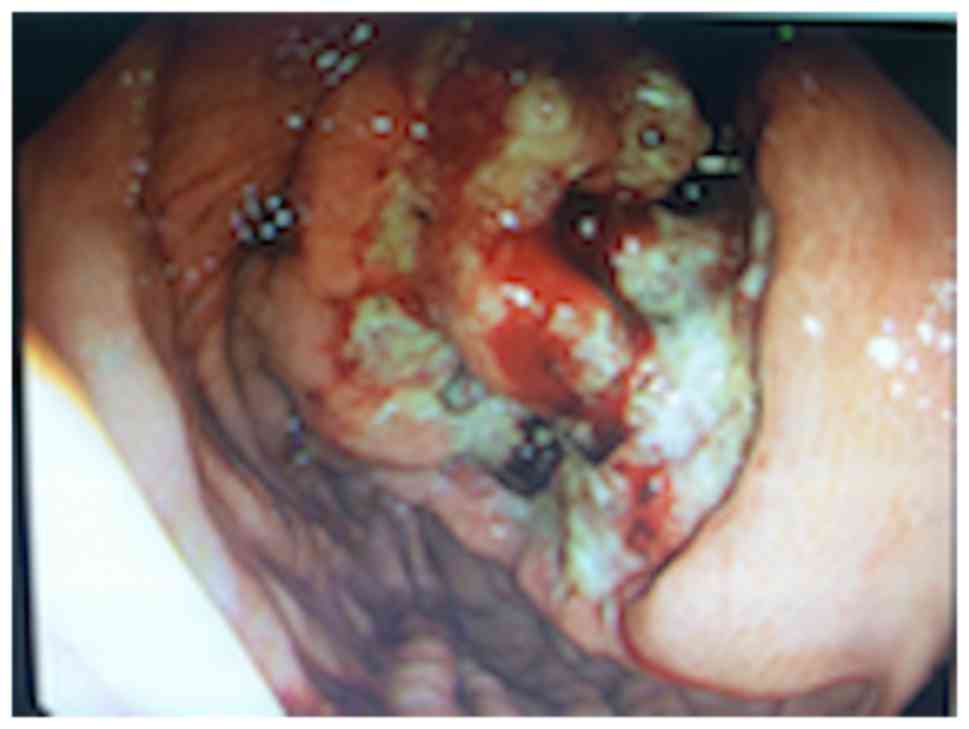
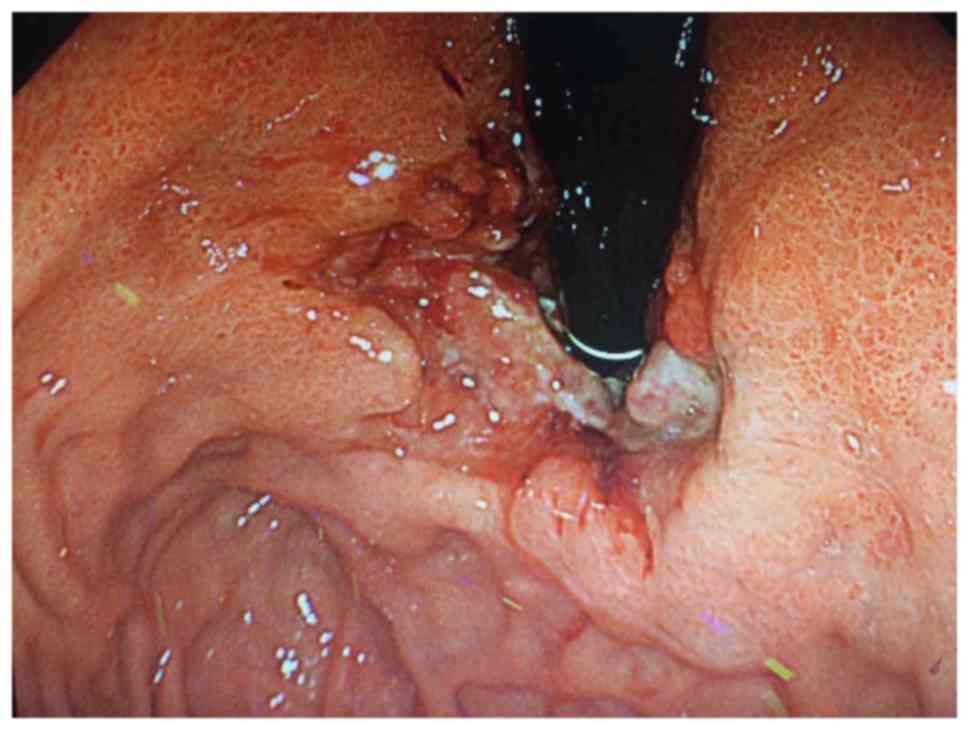
enlargement. Gastroscopy revealed a gastric tumor, rough mucosa and
a 3 cm ulcer in diameter in September 2013 (Fig. 1). Biopsy revealed a poorly
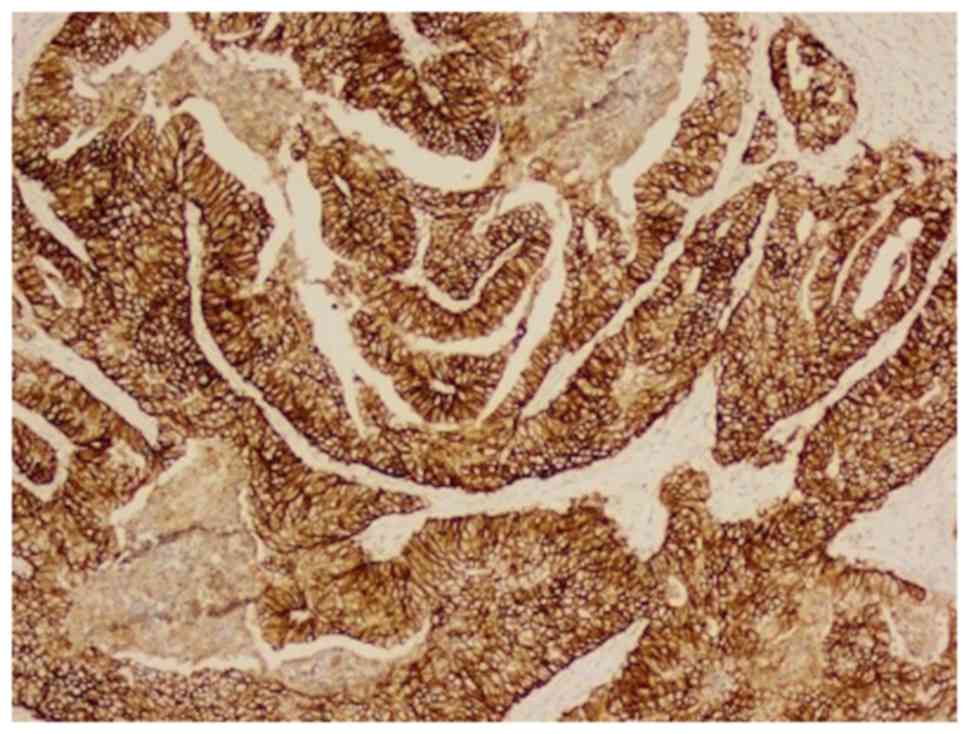
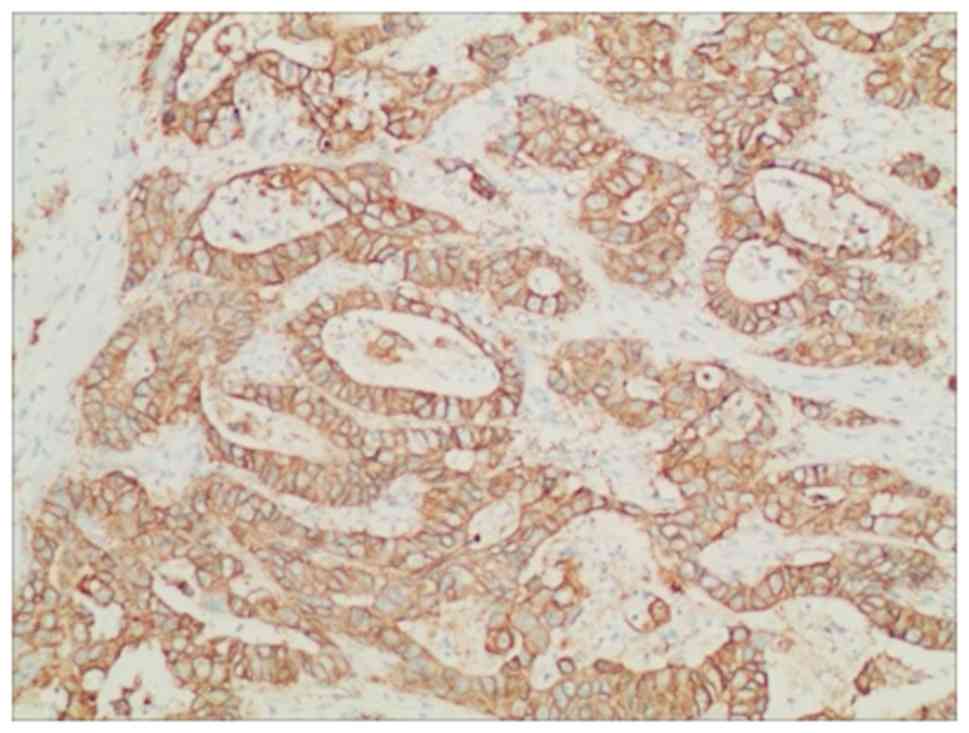
differentiated adenocarcinoma. Immunohistochemical analysis
revealed ERBB2 overexpression (Fig.
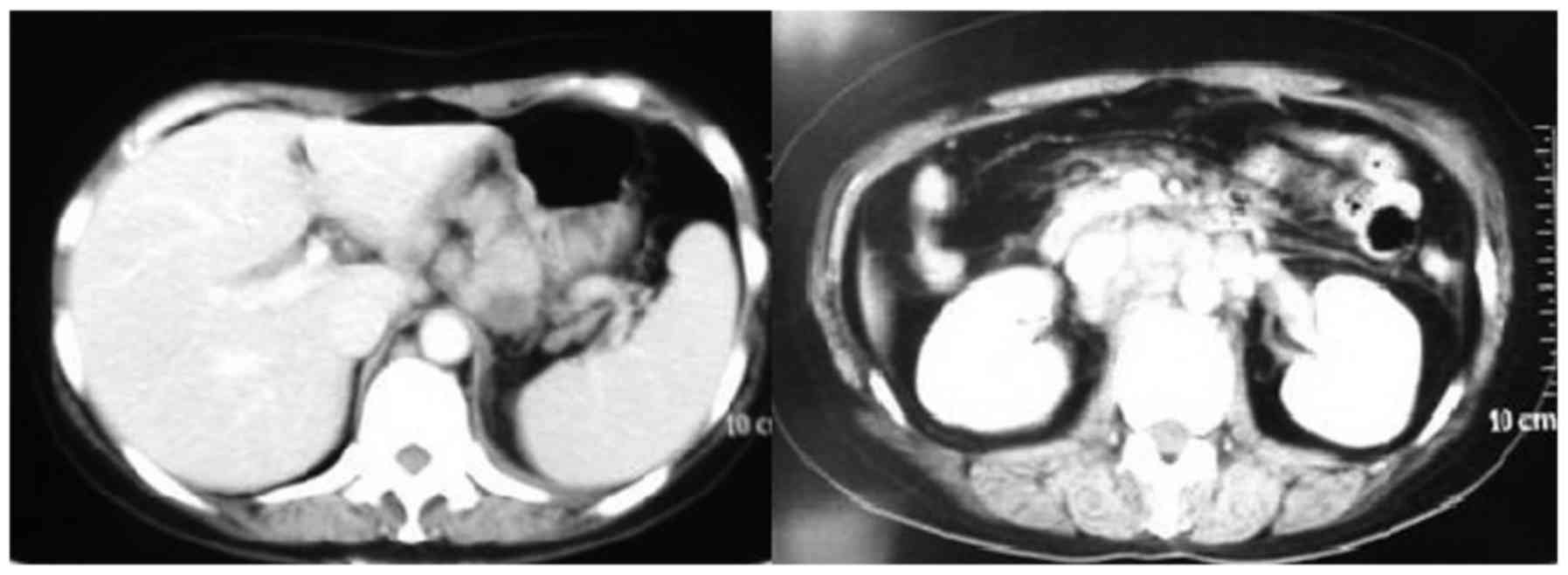
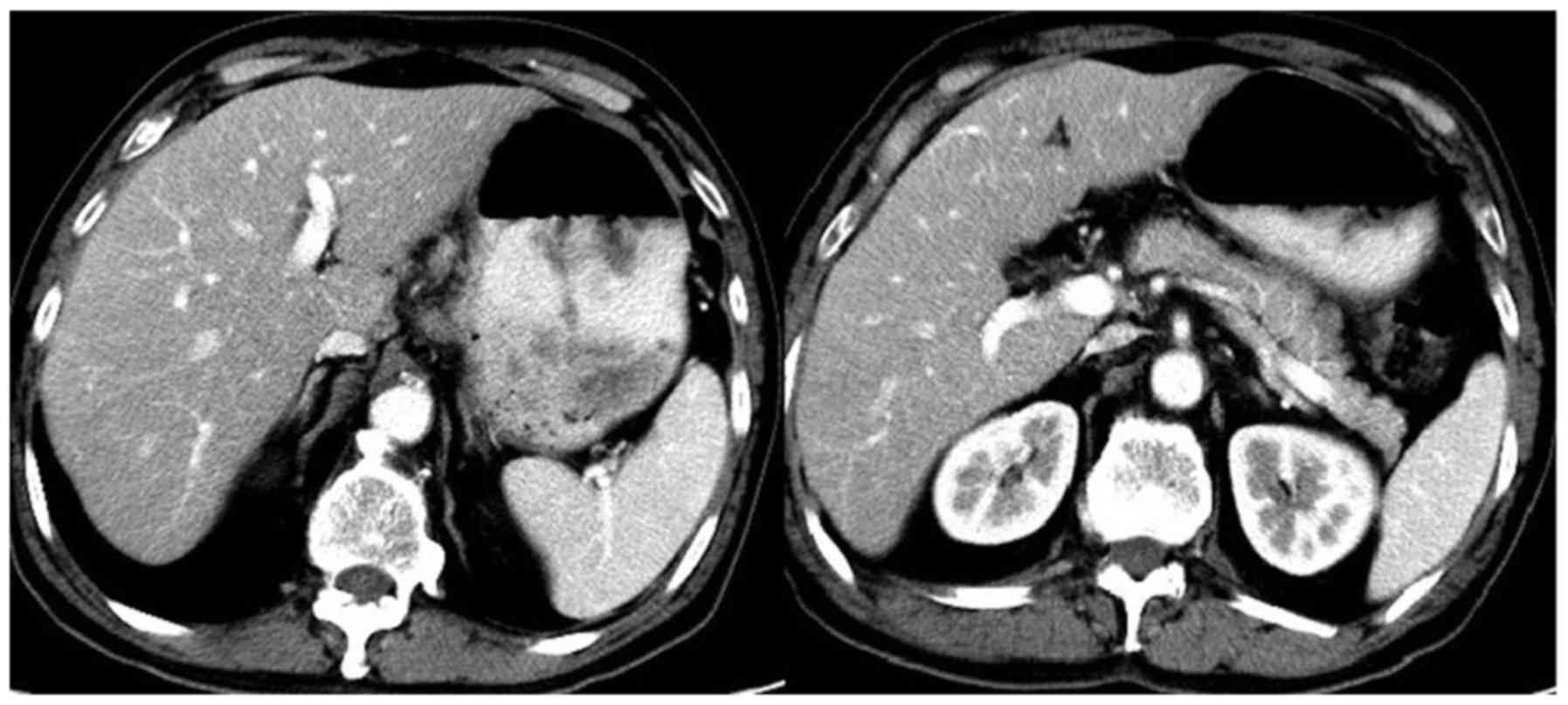
2) (16). Computed tomography
(CT) revealed that multiple lymph nodes were enlarged along the
lesser curvature of the stomach, common hepatic artery, head of the
pancreas, pancreatic vein, and the postcaval and para-aortic lymph
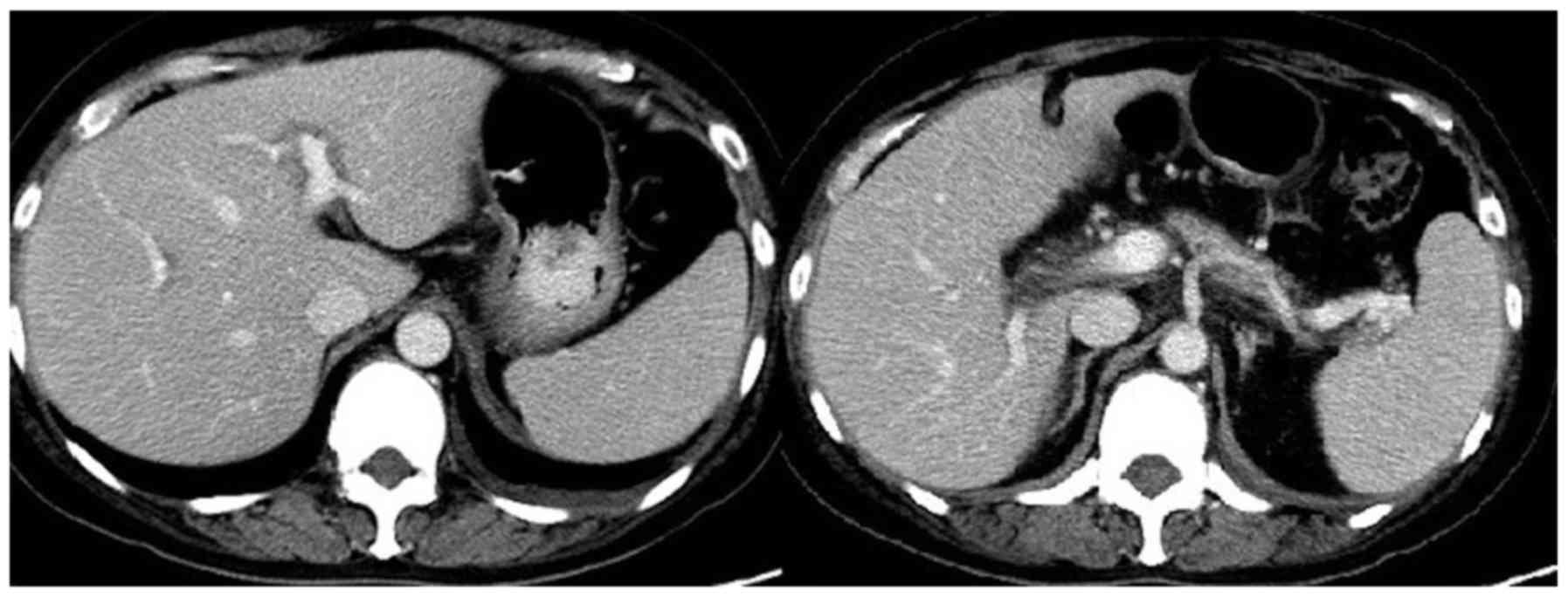
nodes (Fig. 3). The clinical
diagnosis was gastric body adenocarcinoma with abdominal cavity
lymph node metastasis; the clinical stage was cT3N2M1. The patient
received therapy that included a XELOX regimen and trastuzumab. The
XELOX regimen comprised 130 mg/m2 oxaliplatin
intravenous injection (iv) on day 1 and 1,250 mg/m2
Xeloda® orally twice a day (p.o. b.i.d.) on days 1–14,
repeated every 3 weeks. Trastuzumab was administered at 8 mg/kg iv
on day 1 of the first cycle and then 6 mg/kg iv on day 1 of future
cycles. Grade 1 neutropenia and grade 3 vomiting were observed
based on the Common Terminology Criteria for Adverse Events
(17). A CT scan was performed once
the patient finished four cycles of the combination therapy.
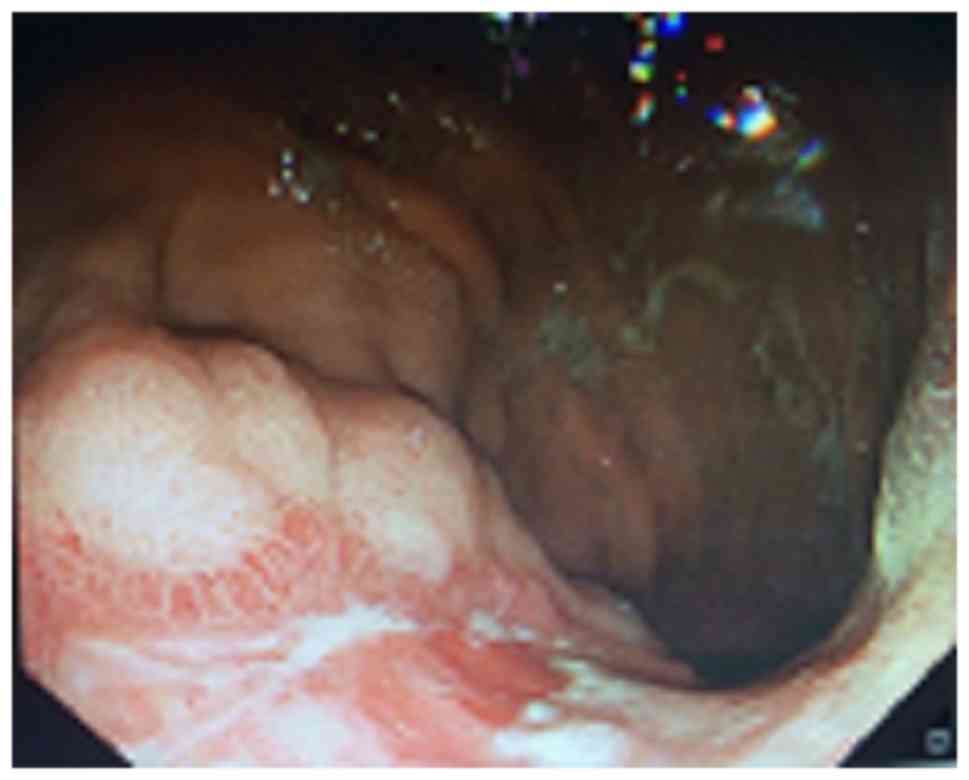
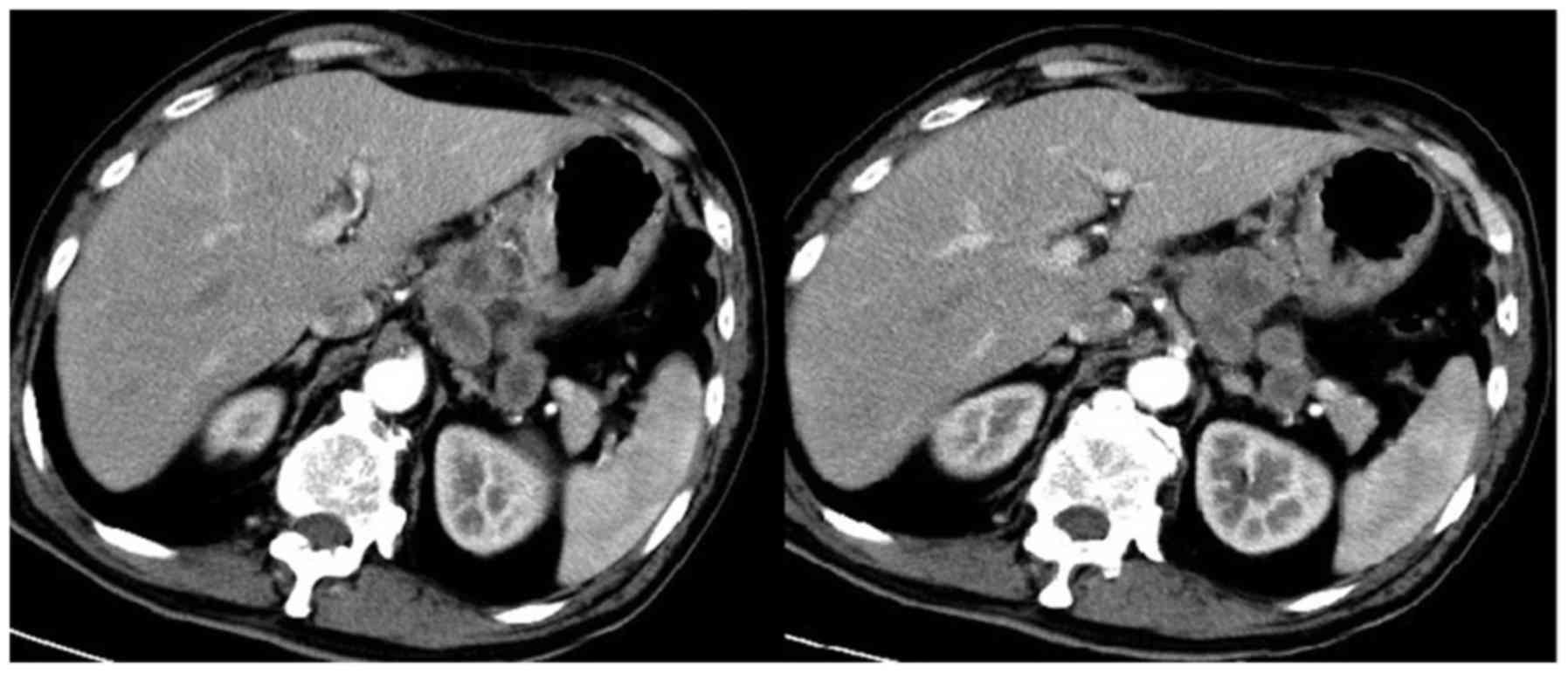
Partial response was observed using gastroscopy and CT (Figs. 4 and 5),
and the clinical stage following four cycles of trastuzumab
combined with chemotherapy was cT2N1M0 (18). Subsequently, the patient underwent
total gastrectomy, D2 lymphadenectomy and Roux-en-Y anastomosis.
Exploratory surgery revealed a 10×8 cm size tumor in the gastric
body with multiple regional lymph nodes enlarged. However, no
metastatic nodules were identified in the liver, parietal
peritoneum, mesenterium, or pelvic floor. The macroscopic type of
the tumor was classified as either the ulcerative or the
infiltrative type according to Borrmann classification (19). Erosion mucosa, a small ulcer, and a
rigid wall were detected. The pathological diagnosis was poorly
differentiated adenocarcinoma of the gastric body. The tumor had
invaded through the submucosa. Incisional margins were negative. In
addition, cancer cells were identified in a lesser curvature lymph
node (1/7 lymph nodes), whereas no cancer cells were observed in
the other lymph nodes (0/16) (18).
The pathological tumor-node-metastasis (TNM) stage was ypT1N1M0
(18). After six weeks following
surgery, the patient received four cycles of XELOX and the
trastuzumab regimen at the dose administered prior to surgery.
After the therapy ceased, the patient received a follow-up every 3
months and remained disease-free at 12 months.
Case 2
A 67-year-old male was admitted to Sichuan Cancer
Hospital with a complaint of abdominal pain for >3 weeks.
Gastroscopy revealed an ulcer of the gastric cardia (Fig. 6) in July 2013. Biopsy identified a
poorly differentiated carcinoma. Immunohistochemical analysis
revealed ERBB2 overexpression (Fig.
7) (16). CT demonstrated a
thickening of the cardia wall and that multiple lymph nodes were
enlarged in the region of the lesser curvature of the stomach
(Fig. 8). The clinical diagnosis was
cardia carcinoma with abdominal cavity lymph node metastasis. The
clinical stage was cT4aN2M0. The patient received a neoadjuvant
therapy that included a XELOX regimen and trastuzumab. The XELOX
regimen comprised 130 mg/m2 oxaliplatin iv on day 1 and
1,250 mg/m2 Xeloda p.o. b.i.d. on days 1–14, repeated
every 3 weeks. Trastuzumab was administered at a dose of 8 mg/kg iv
on day 1 of the first cycle and then 6 mg/kg iv on day 1 of future
cycles. Grade 2 neutropenia and grade 3 vomiting were observed. A
CT scan was performed after the patient finished four cycles of the
combination therapy. The lymph nodes along the lesser curvature and
the tumor decreased in size following treatment (Figs. 9 and 10). The clinical stage after four cycles of
trastuzumab combined with chemotherapy was cT2N2M0. The patient
underwent total gastrectomy, D2 lymphadenectomy and Roux-en-Y
anastomosis following the four cycles of the combination therapy.
Surgical exploration identified a 7×5 cm tumor in the cardia and
fundus of the stomach, with enlarged lymph nodes, and no evident
metastatic nodules in the liver, parietal peritoneum, mesenterium
or pelvic floor. The macroscopic type of the tumor was classified
as Borrmann III (19). The
pathological diagnosis was poorly differentiated adenocarcinoma of
the stomach. The tumor had invaded the muscular layer. Incisional
margins were negative. In addition, cancer cells were identified in
the lesser curvature lymph node (2/9), whereas no cancer cells were
identified in other lymph nodes (0/10). The pathological TNM stage
was ypT2N1M0. The patient received an additional four cycles of
XELOX and the trastuzumab regimen at a decreased dose with 100
mg/m2 for oxaliplatin and 1,000 mg/m2 for
Xeloda. After the therapy ceased, the patient received a follow-up
every 3 months and remained disease-free at 13 months.
Discussion
Gastric cancer is the fourth most common cancer
worldwide in 2008 and the high incidences of gastric cancer are
reported in China, Japan and South Korea (20). Gastric cancer is the third most
prevalent cause of cancer-associated mortality in males and the
second most prevalent in females in China in 2011 (1). Unlike in Japan, screening programs are
not performed in China, and the majority of patients with gastric
cancer present at initial diagnosis in a more advanced stage
(1).
For late stage gastric cancer, surgical resection
does not represent the optimal strategy; palliative gastrectomy,
chemotherapy, radiotherapy, gastric stent and bypass are the
current strategies for these patients (4). The Japanese Gastric Cancer Association
guidelines suggest that gastrectomy ought to be performed for
patients with a single non-curative factor (21). In contrast, the guidelines of three
European societies, the European Society for Medical Oncology, the
European Society of Surgical Oncology, and the European Society of
Radiotherapy and Oncology, suggest that gastrectomy should be
performed for patients who respond well to systemic chemotherapy
(22). However, the REGATTA trial
reported that palliative resection of the primary tumor was not
beneficial for patients (23). Other
previous studies have demonstrated that palliative resection of the
primary tumor following chemotherapy was beneficial for patients
(24,25). Conversion surgery was defined as an
initial tumor that was not suitable for surgery, therefore
chemotherapy was administered to reduce the tumor size prior to
surgical resection being performed (26). This conversion treatment may be the
optimal choice for patients with late stage gastric cancer;
chemotherapy compliance could be improved prior to surgery and
certain cytokines may promote tumor growth following surgery
(27,28).
In 1997, Nakajima et al first reported
conversion surgery for gastric cancer (29). At the same time, they also reported
that liver metastasis and peritoneal seeding were difficult to
control (29). In addition, Kanda
et al revealed that lymph node metastasis and peritoneal
dissemination were associated with an improved, and poorer
prognosis, respectively (30). To
identify patients who may benefit from conversion therapy, Yoshida
et al classified late stage gastric cancer into four
categories; patients with potentially resectable metastases without
peritoneal dissemination represented the optimal candidates for
neoadjuvant chemotherapy, while those with marginally resectable
metastases without peritoneal dissemination represented the optimal
candidates for conversion surgery (31). In a recently reported clinical trial,
40/151 patients with gastric cancer underwent conversion surgery
(32). The 5-year overall survival
rate of the patients who underwent conversion surgery was 43%,
whereas those who were treated with chemotherapy alone were
associated with an overall survival rate of 1%. The patients in the
present study presented with lymph node metastasis and responded
well to chemotherapy and targeted therapy. Therefore, conversion
surgery was performed following four cycles of chemotherapy and
targeted therapy.
The ToGA trial confirmed the efficacy and safety of
trastuzumab (13). National
Comprehensive Cancer Network guidelines suggest that all patients
with metastatic disease should be tested for ERBB2 at the time of
diagnosis (33). As a first-line
treatment, the effectiveness of trastuzumab in palliative therapy
has been supported by a previous study (13). Certain case reports have indicated
that trastuzumab was successfully applied for conversion surgery in
patients with gastric cancer (14,15).
Similarly, ERBB2 (3+) was revealed in our patients assessed in the
present study upon initial diagnosis of gastric cancer. In the
patients evaluated in the present study, targeted therapy was
combined with chemotherapy, the patients responded well, and
conversion surgeries were performed in the patients. Conversion
therapy is a novel therapy concept for which trials are
expected.
The present study provided novel insight into
conversion therapy for advanced stage gastric cancer. Further study
is required to evaluate the efficacy of combining targeted therapy
with a chemotherapy regimen in patients with gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Sichuan
Provincial Science and Technology Project (grant no.
2014FZ0089).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SX and XZ wrote the main manuscript. SX and XZ made
contribution to conception and design. SX, RX and ZD performed the
data collection. XT and JL prepared the figures and made
contribution to the pathological data collection.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patients for the present study and any accompanying images to be
published. The Ethics Committee of Sichuan Cancer Hospital
(Chengdu, China) approved the present study.
Patient consent for publication
Written informed consent was obtained from the
patients for the present study and any accompanying images to be
published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
An JY, Ha TK, Noh JH, Sohn TS and Kim S:
Proposal to subclassify stage IV gastric cancer into IVA, IVB, and
IVM. Arch Surg. 144:38–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Songun I, Putter H, Kranenbarg EM, Sasako
M and van de Velde CJ: Surgical treatment of gastric cancer:
15-year follow-up results of the randomised nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Izuishi K and Mori H: Recent strategies
for treating stage IV gastric cancer: Roles of palliative
gastrectomy, chemotherapy, and radiotherapy. J Gastrointestin Liver
Dis. 25:87–94. 2016.PubMed/NCBI
|
|
5
|
Tan P and Yeoh KG: Genetics and molecular
pathogenesis of gastric adenocarcinoma. Gastroenterology.
149:1153–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan B, Yau EX, Omar Bte SS, Ong CW, Pang
B, Yeoh KG and Salto-Tellez M: A study of HER2 gene amplification
and protein expression in gastric cancer. J Clin Pathol.
63:839–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al:
Amplification of HER-2 in gastric carcinoma: Association with
Topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hechtman JF and Polydorides AD: HER2/neu
gene amplification and protein overexpression in gastric and
gastroesophageal junction adenocarcinoma: A review of
histopathology, diagnostic testing, and clinical implications. Arch
Pathol Lab Med. 136:691–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunz PL, Mojtahed A, Fisher GA, Ford JM,
Chang DT, Balise RR, Bangs CD, Cherry AM and Pai RK: HER2
expression in gastric and gastroesophageal junction adenocarcinoma
in a US population: Clinicopathologic analysis with proposed
approach to HER2 assessment. Appl Immunohistochem Mol Morphol.
20:13–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gómez-Martin C, Garralda E, Echarri MJ,
Ballesteros A, Arcediano A, Rodriguez-Peralto JL, Hidalgo M and
López-Ríos F: HER2/neu testing for anti-HER2-based therapies in
patients with unresectable and/or metastatic gastric cancer. J Clin
Pathol. 65:751–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chua TC and Merrett ND: Clinicopathologic
factors associated with HER2-positive gastric cancer and its impact
on survival outcomes-a systematic review. Int J Cancer.
130:2845–2856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janjigian YY, Werner D, Pauligk C,
Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E,
Tafe LJ, Tang LH, et al: Prognosis of metastatic gastric and
gastroesophageal junction cancer by HER2 status: A European and USA
International collaborative analysis. Ann Oncol. 23:2656–2662.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto K, Fujitani K, Tsujinaka T, Hirao
M, Nishikawa K, Fukuda Y, Haraguchi N, Miyake M, Asaoka T, Miyamoto
A, et al: A case of HER2-positive advanced gastric cancer with
extensive lymph node metastasis treated via chemotherapy with a
trastuzumab-containing regimen followed by conversion surgery. Gan
To Kagaku Ryoho. 41:2296–2298. 2014.(In Japanese). PubMed/NCBI
|
|
15
|
Choda Y, Ninomiya M, Kanazawa T, Sato D,
Tokumoto N, Harano M, Matsukawa H, Ojima Y, Idani H, Shiozaki S, et
al: Gastric cancer with liver metastasis and peritoneal
dissemination treated with conversion surgery to achieve r0
resection after capecitabine, cisplatin, and trastuzumab
chemotherapy-a case report. Gan To Kagaku Ryoho. 41:1421–1424.
2014.(In Japanese). PubMed/NCBI
|
|
16
|
Rüschoff J, Hanna W, Bilous M, Hofmann M,
Osamura RY, Penault-Llorca F, van de Vijver M and Viale G: HER2
testing in gastric cancer: A practical approach. Mod Pathol.
25:637–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The Common Terminology Criteria for
Adverse Events Version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romero Espejo H and Siancas Navarrete J:
Classification of stomach adenocarcinomas. Rev Gastroenterol Peru.
23:199–212. 2003.(In Spanish). PubMed/NCBI
|
|
20
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; (European Society for Medical Oncology
(ESMO), : European Society of Surgical Oncology (ESSO); European
Society of Radiotherapy and Oncology (ESTRO): Gastric cancer:
ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 24 Suppl 6:vi57–vi63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujitani K, Yang HK, Mizusawa J, Kim YW,
Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, et
al: Gastrectomy plus chemotherapy versus chemotherapy alone for
advanced gastric cancer with a single non-curable factor (REGATTA):
A phase 3, randomised controlled trial. Lancet Oncol. 17:309–318.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sougioultzis S, Syrios J, Xynos ID,
Bovaretos N, Kosmas C, Sarantonis J, Dokou A, Tzivras D, Zografos
G, Felekouras E, et al: Palliative gastrectomy and other factors
affecting overall survival in stage IV gastric adenocarcinoma
patients receiving chemotherapy: A retrospective analysis. Eur J
Surg Oncol. 37:312–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ,
Park DJ, Kim JH, Kim HH and Lee JS: Survival benefit of gastrectomy
± metastasectomy in patients with metastatic gastric cancer
receiving chemotherapy. Gastric Cancer. 14:130–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terashima M: Conversion therapy for
gastric cancer: Who can make conversion as successful as Goromaru?
Gastric Cancer. 19:685–686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wada Y, Yoshida K, Hihara J, Konishi K,
Tanabe K, Ukon K, Taomoto J, Suzuki T and Mizuiri H: Sivelestat, a
specific neutrophil elastase inhibitor, suppresses the growth of
gastric carcinoma cells by preventing the release of transforming
growth factor-alpha. Cancer Sci. 97:1037–1043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada Y, Yoshida K, Tsutani Y, Shigematsu
H, Oeda M, Sanada Y, Suzuki T, Mizuiri H, Hamai Y, Tanabe K, et al:
Neutrophil elastase induces cell proliferation and migration by the
release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol
Rep. 17:161–167. 2007.PubMed/NCBI
|
|
29
|
Nakajima T, Ota K, Ishihara S, Oyama S,
Nishi M, Ohashi Y and Yanagisawa A: Combined intensive chemotherapy
and radical surgery for incurable gastric cancer. Ann Surg Oncol.
4:203–208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanda T, Yajima K, Kosugi S, Ishikawa T,
Ajioka Y and Hatakeyama K: Gastrectomy as a secondary surgery for
stage IV gastric cancer patients who underwent S-1-based
chemotherapy: A multi-institute retrospective study. Gastric
Cancer. 15:235–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshida K, Yamaguchi K, Okumura N,
Tanahashi T and Kodera Y: Is conversion therapy possible in stage
IV gastric cancer: The proposal of new biological categories of
classification. Gastric Cancer. 19:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukuchi M, Ishiguro T, Ogata K, Suzuki O,
Kumagai Y, Ishibashi K, Ishida H, Kuwano H and Mochiki E:
Prognostic role of conversion surgery for unresectable gastric
cancer. Ann Surg Oncol. 22:3618–3624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ajani JA, Bentrem DJ, Besh S, D'Amico TA,
Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et
al: Gastric cancer, version 2.2013: Featured updates to the NCCN
Guidelines. J Natl Compr Canc Netw. 11:531–546. 2013. View Article : Google Scholar : PubMed/NCBI
|