Introduction
Study of malignant tumor has always been a focus for
the concern of human health because of their high morbidity and
mortality. It was estimated that 5.3 million men and 4.7 million
women would develop a malignant tumor annually and 6.2 million
would die from the disease by the World Cancer Report 2014 of WHO.
In 2014, approximately 14.1 million people were expected to develop
cancer (1). Although malignant tumor
mainly involves in the elderly, the morbidity and mortality of
children with the disease present a rising trend. It has been the
second cause of death in children merely much less than accidental
emergency (2).
The etiology and pathogenesis of malignant tumor
remain unclear, yet most of them have been related with
accumulation of relative gene mutation or aberrant expression of
gene. Shank-associated RH domain-interacting protein (SHARPIN) was
firstly found as a scaffolding partner for Shank proteins. The
Shank family of proteins highly expresses in postsynaptic density
of excitatory synapses in brain. There are multiple domains of
Shank for protein-protein interactions including proline-rich
region, SAM domain, PDZ domain, SH3 domain and ankyrin domain. The
Shank family is composed of three members: Shank1, Shank2 and
Shank3. SHARPIN interacts with Shank through the ankyrin repeat
domain of Shank1, which plays an important role in the formation
and maintenance of excitatory synaptic structure. The succedent
studies have shown that SHARPIN expresses in various organs
relatively abundant including heart, brain and testis besides
postsynaptic density, and localizes in the membrane and nuclei of
cells (3), indicating that SHARPIN
may play some roles in physiology and pathology process except for
functioning as a scaffolding partner of Shank1. In 1993, study by
Hogenesch et al showed that the phenotype of chronic
proliferative dermatitis mutant (cpdm) presented as chronic
progressive dermatitis, absent Peyer's patches, abnormal structure
of lymph node and spleen, immune dysfunction, and eosinophilic
inflammation in multiple organs (4).
In 2007, study by Seymour et al found that the genetic
foundation of cpdm phenotype derived from spontaneous
mutation in the mouse Sharpin gene, suggesting that
Sharpin may participate in cell proliferation, apoptosis,
organ development, immune and inflammatory reaction (5). There were also evidences showed that
increased expression of SHARPIN may involve in initiation and
development of malignant tumor (6).
Our study widely explored the feature of SHARPIN
expression in multiple malignant tumors originated from different
germ layers by immunohistochemistry and immunofluorescence,
confirmed the previous findings about SHARPIN's upregulation in
entodermal and mesodermal cancers, and identified SHARPIN's
downregulation, loss of function and translocation in ectodermal
cancers, offering its complicated characters as oncogene or
anti-oncogene.
Materials and methods
Materials
The study was conducted with the approval of the
Institutional Review Board and Ethics Committee of Shenzhen
Hospital, Southern Medical University (Shenzhen, China) and in
accordance with the Declaration of Helsinki. Informed consent was
obtained from all of the patients.
Samples of malignant tumors and their corresponding
visceral organ tissues were obtained from the tissue bank of
Shenzhen Hospital, Southern Medical University. Normal skin
specimens were collected from the patients undergoing surgery at
the plastic and constructive surgery department of Shenzhen
Hospital, Southern Medical University. Malignant tumors were
recruited as follows: Six kinds of malignant entodermal tumors
including intrahepatic cholangiocellular carcinoma (ICC) (N=6),
hepatocellular carcinoma (HCC) (N=5), lung cancer (N=6), esophageal
cancer (N=6), laryngocarcinoma (N=5) and pancreatic cancer (N=7).
Three kinds of malignant mesodermal tumors including breast cancer
(N=10), endometrial cancer (N=4) and chromophobe renal cell
carcinoma (CRCC) (N=4). Five kinds of malignant ectodermal tumors
including basal cell carcinoma (BCC) (N=7), squamous cell carcinoma
(SCC) (N=5), Paget's disease (N=8), melanomas (N=7) and mycosis
fungoides (MF) (N=3).
All samples were evaluated by two pathologists with
a standard microscopic technique. Each case from the same block was
stained with hematoxylin and eosin simultaneously for confirmation
of the histologic diagnosis and tissue morphology and
integrity.
Immunohistochemistry
Immunohistochemistry was performed with
paraffin-embedded tissue sections from the above malignant tumors
and their corresponding normal tissues. The procedure of
immunohistochemistry was done according to the manufacturer's
instructions. Briefly, immunostaining were implemented with
anti-SHARPIN antibody (Santa Cruz Biotechnology Inc., Dallas, TX,
USA) at 4°C using Histostain™-SP kits (OriGene Technologies, Inc.,
Beijing, China). After deparaffinization and hydration, antigen
retrieval was carried out in a pressure cooker using 10 mM sodium
citrate buffer (pH 6.0) at full power for 5 min, and then the
tissue sections were treated with 3% hydrogen peroxide for 15 min,
followed by treating with normal goat serum for 15 min. The primary
antibody was diluted (1:400) with primary antibody dilution buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and
incubated with tissue sections for overnight at 4°C. Then the
slides were incubated with biotinylated goat anti rabbit IgG for 20
min and biotinylated horseradish peroxidase for 30 min and treated
with 3,3-diaminobenzidine for 3 min sequentially, followed by being
counterstained with Meyer's hematoxylin and mounted. Careful rinses
were performed in every step using phosphate-buffered saline buffer
(PBS) 3 times each of 5 min. Primary antibody dilution buffer
incubated sample was used to be a negative control, and normal skin
specimen incubated with anti-SHARPIN antibody (Santa Cruz
Biotechnology Inc.) was used to be a positive control.
Immunofluorescence
The process of immunofluorescence was performed in
accordance with the manufacturer's specifications.
Deparaffinization, hydration and antigen retrieval of
paraffin-embedded tissue sections were performed as
immunohistochemistry. Subsequently, the tissue sections were rinsed
for 3 times each of 5 min using PBS, then blocked by immunology
staining blocking buffer (Beyotime Institute of Biotechnology) for
60 min. The anti-SHARPIN antibody (BD Biosciences, Franklin Lakes,
NJ, USA) was diluted (1:400) with PBS (Beyotime Institute of
Biotechnology) and incubated for overnight at 4°C after decanting
immunology staining blocking buffer. Decanting the primary antibody
and rinsed for 3 times were as above described, tissue sections
were treated with Immunol Fluorence Staining kit with Alexa Fluor
488-Labeled Goat Anti-Rabbit IgG (Beyotime Institute of
Biotechnology) at room temperature for 1 h at dark, and then
stained with 300 nM 4′,6-diamidino-2-phenyindole (DAPI; Leagene,
Beijing, China) for 15 min and mounted on glass slides using Anti
fade Mounting Medium (Beyotime Institute of Biotechnology). Imaging
was processed with Olympus BX51 (Olympus, Corp., Tokyo, Japan).
Histologic scoring and statistical
analysis
Each sample was scored by two pathologists blindly.
SHARPIN protein was stained and assessed in tumors and their
corresponding normal organ tissues. Each sample of tumor and the
corresponding normal tissue was assessed using the cross-product (H
score) (7), that is counting the
percentage of sample cells staining at each of four staining
intensities: 0 means no staining, 1 represents faint yellow, 2 is
on behalf of deep yellow, 3 shows brown meaning a strong positive
stain. For instance, one tumor sample staining at 2 of 60% tumor
cells and 3 of 40% tumor cells, a combined H score is [(60×2) +
(40×3)]=240 out of maximum of 300. Scores from both pathologists
showed a good correlation in which 85% of all the samples exhibited
agreement within a range of 40 points. Samples in which a
discrepancy of >50 points in scoring were reassessed and
examined using the same standard microscope. The average of scores
from both pathologists was used as the final H scores.
Data analyses were evaluated with IBM SPSS
Statistics 23 (IBM Corp., Armonk, NY, USA), and values were
expressed as mean ± SD of 3 independent experiments. The
significant differences between two or three groups were compared
using Independent-Samples T Test or One-Way ANOVA, respectively. In
Post Hoc Multiple Comparisons of One-Way ANOVA, S-N-K analysis was
use when equal variances assumed, while Dunnett's T3 analysis was
used when equal variances not assumed. P<0.05 was considered to
indicate a statistically significant difference.
Results
In order to assess SHARPIN expression in multiple
malignant tumors and their corresponding normal tissues,
immunohistochemistry was carried out with anti-SHARPIN antibody. We
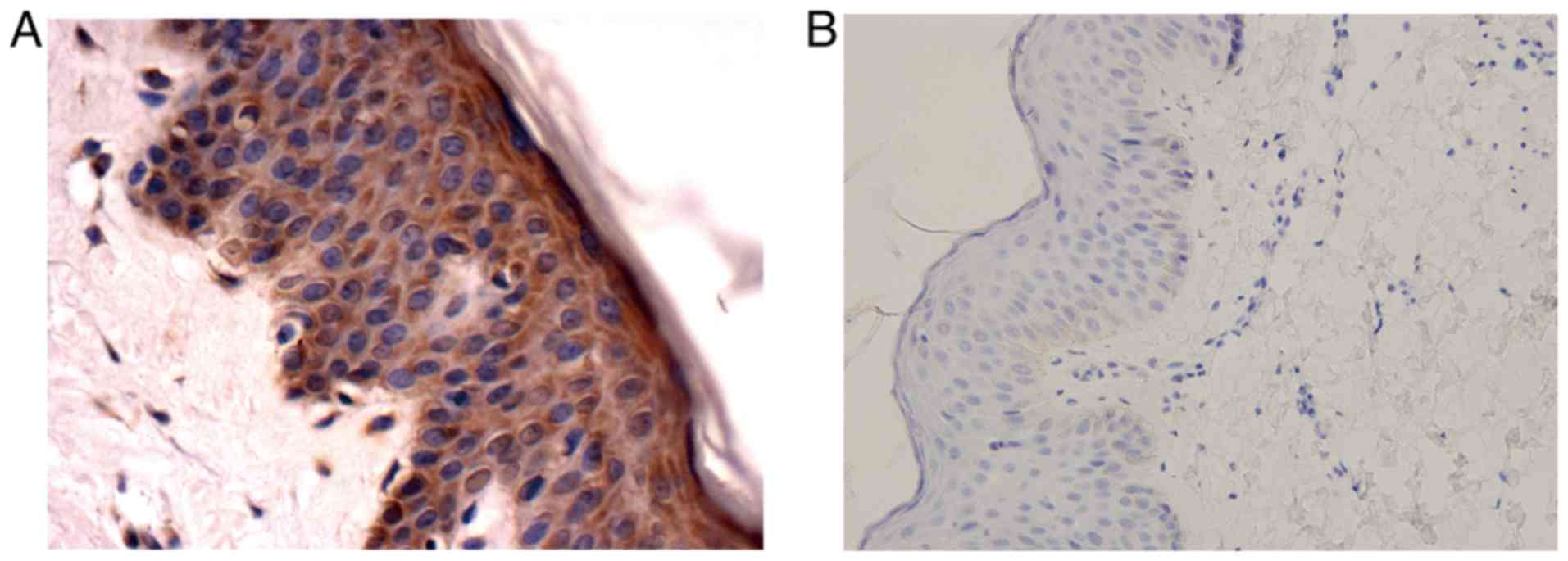
checked the specificity of the antibody firstly with normal skin
tissue. As mentioned above, sample treated with primary antibody
dilution buffer served as a negative control while sample treated
with anti-SHARPIN antibody served as a positive control. Compared
with the negative control which showed no stain, the positive
control exhibited a strong positive signal (Fig. 1). Then various malignant tumors and
their corresponding paracancers and/or normal tissues were carried
out immunohistochemistry to evaluate SHARPIN expression. H score
was used to assess SHARPIN expression in cancer, paracancer and the
corresponding normal tissue.
All the recruited normal organ tissues exhibited
positive signal in which liver, kidney and larynx showed a faint
stain. SHARPIN showed a strongest signal in both of the normal skin
tissue and breast duct, and moderate signal in other tissues
(Fig. 2).
SHARPIN expression in entodermal
cancers
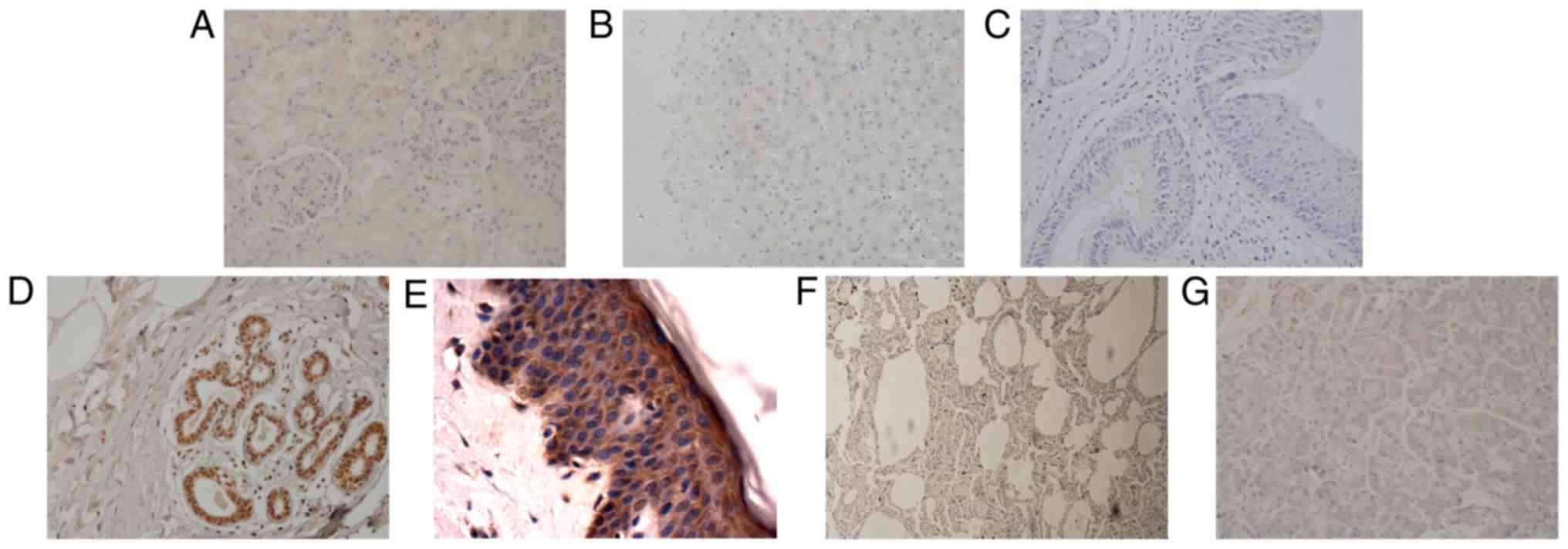
Six kinds of malignant tumors originated from
entoderm, including ICC, HCC, lung cancer, esophageal cancer,
laryngocarcinoma and pancreatic cancer, showed an elevated
expression of SHARPIN (Fig. 3). Among
those kinds of tumors, there are no reports about SHARPIN
expression in ICC and laryngocarcinoma by now, and SHARPIN
expression in HCC and pancreatic cancer is in accordance with
previous findings (6). However,
results of lung cancer and esophageal cancer do not accord with
previous study which showed no difference of SHARPIN mRNA
expression between cancer and normal tissue (6). Our explanation is that the SHARPIN mRNA
in cancer tissue may be over translated.
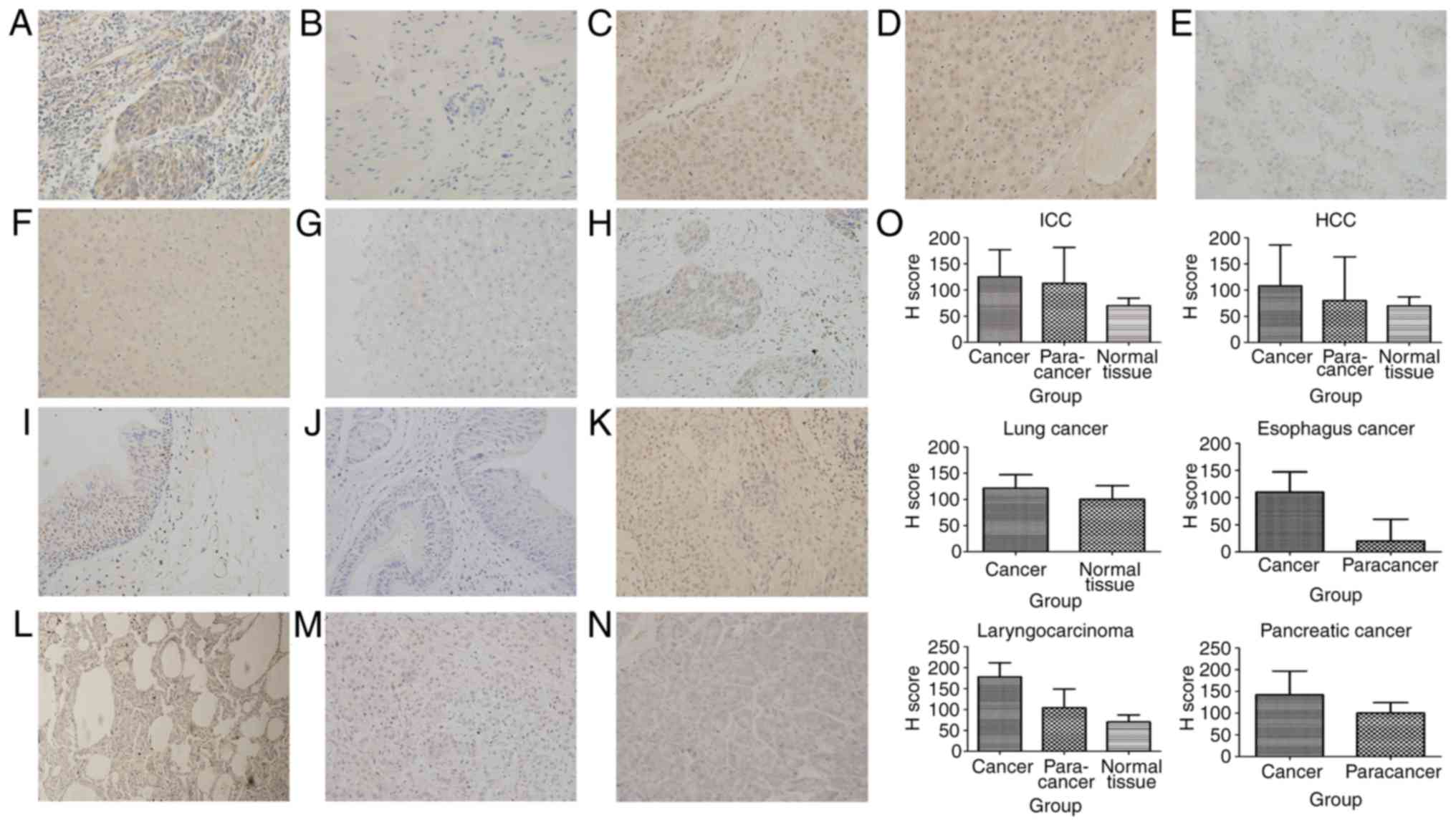 | Figure 3.Tumors derived from entoderm exhibit
upregulated expression of SHARPIN compared with their corresponding
normal organ tissues. (A) Esophageal cancer, (B) paracancer of
esophageal cancer (C) HCC, (D) paracancer of HCC, (E) ICC, (F)
paracancer of ICC, (G) normal liver, (H) laryngocarcinoma, (I)
paracancer of laryngocarcinoma, (J) normal larynx, (K) lung cancer,
(L) normal lung, (M) pancreatic cancer, (N) normal pancreas, (O) H
score of malignant tumors, paracancers and normal tissues derived
from entoderm which are expressed in a histogram (A-N, original
magnification, ×400). SHARPIN, Shank-associated RH
domain-interacting protein; HCC, hepatocellular carcinoma; ICC,
cholangiocellular carcinoma. |
SHARPIN expression in mesodermal
cancers
Three kinds of malignant tumors originated from
mesoderm, including breast cancer, endometrial cancer, CRCC,
exhibited an upregulated expression of SHARPIN (Fig. 4). Our experimental results about
breast cancer are in accordance with previous study (8,9), and
provided the first immunohistochemistrical findings in endometrial
cancer and CRCC. Former studies also described enhanced SHARPIN
expression in prostate cancer, renal clear cell adenoma and
papillary serous adenocarcinoma of ovary which also originate from
mesoderm (6), but were not included
in our sample pool.
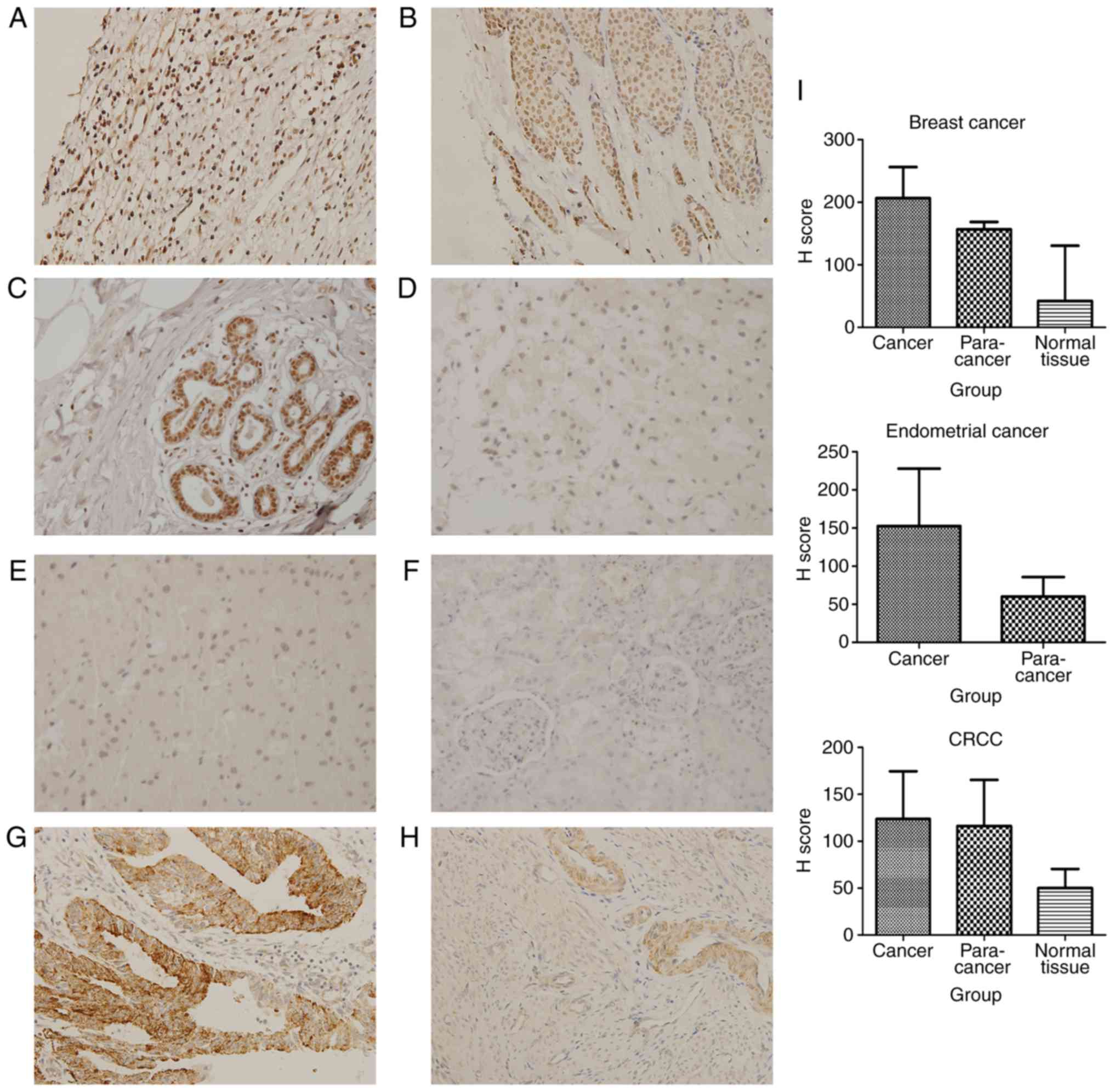 | Figure 4.Tumors derived from mesoderm exhibit
upregulated expression of SHARPIN compared with their corresponding
normal organ tissues. (A) Breast cancer, (B) paracancer of breast
cancer, (C) normal breast tissue, (D) CRCC, (E) paracancer of CRCC,
(F) normal kidney; (G) endometrial cancer, (H) paracancer of
endometrial cancer, (I) H score of malignant tumors, paracancers
and normal tissues derived from mesoderm which are expressed in a
histogram. (A-H, original magnification, ×400). SHARPIN,
Shank-associated RH domain-interacting protein; CRCC, chromophobe
renal cell carcinoma. |
SHARPIN expression in ectodermal
cancers
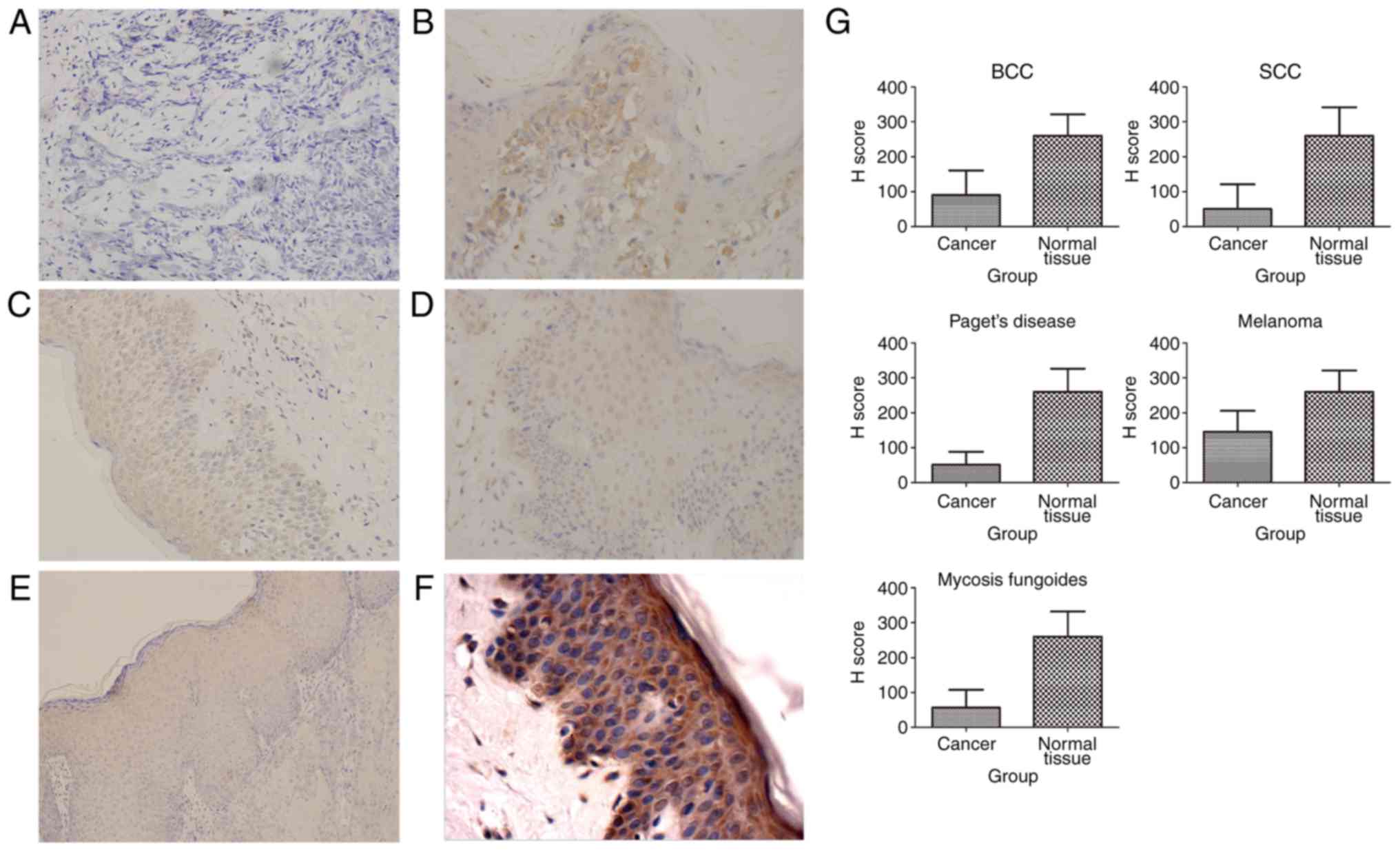
Five kinds of malignant tumors originated from
ectoderm, including BCC, SCC, Paget's disease, melanomas and MF,
showed a decreased expression of SHARPIN (Fig. 5). No investigations about SHARPIN's
expression in ectodermal cancers were published.
Independent-Samples T Test of H score showed
significant statistic difference of SHARPIN expression between some
cancer nest and their corresponding paracancer or normal tissue, in
which those tumors included MF, SCC, Paget's disease, BCC,
melanomas, esophageal cancer. One-Way ANOVA analysis of H score
showed significant statistic difference of SHARPIN expression among
breast cancer, paracancer of breast cancer and normal breast
tissue, in which SHARPIN expression in breast cancer was higher
than paracancer of breast cancer, while SHARPIN expression in
paracancer of breast cancer was higher than normal breast
tissue.
Subcellular location of SHARPIN in
cancers
Immunofluorescence was performed to confirm SHARPIN
expression and evaluate the subcellular localization of SHARPIN in
various malignant tumors and their corresponding normal tissues.
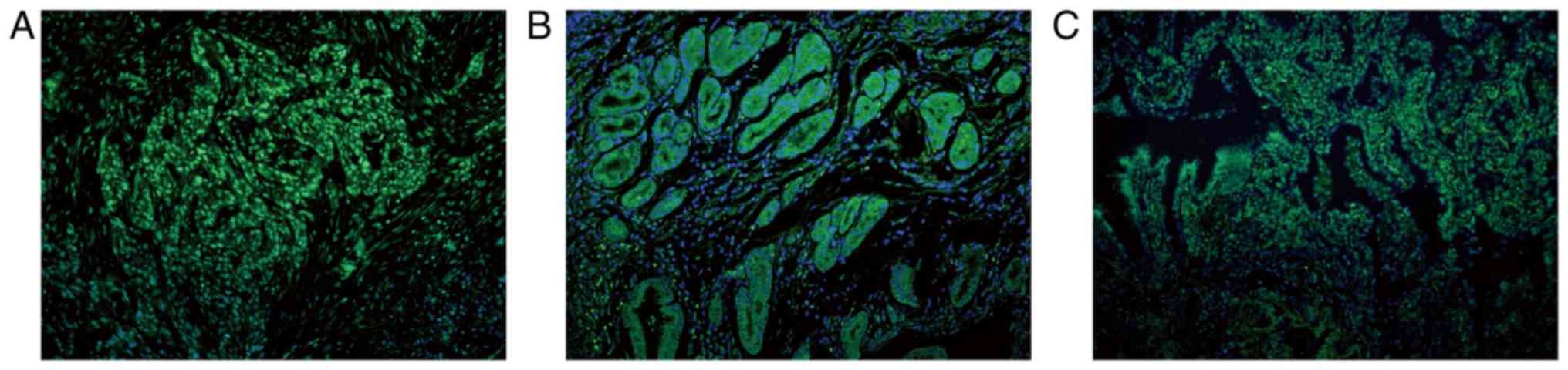
For those recruited normal tissues, SHARPIN mainly expressed in the
cytoplasm of cells and showed only a faint or no stain in the
nucleus except for the normal lung tissue which exhibited a
positive stain in the nucleus but not in the cytoplasm (Fig. 6). For those different kinds of
malignant tumors, tumors in which SHARPIN expressed mainly in the
cytoplasm but not nucleus or only a faint signal in the nucleus
included ICC, HCC, laryngocarcinoma, pancreatic cancer, endometrial
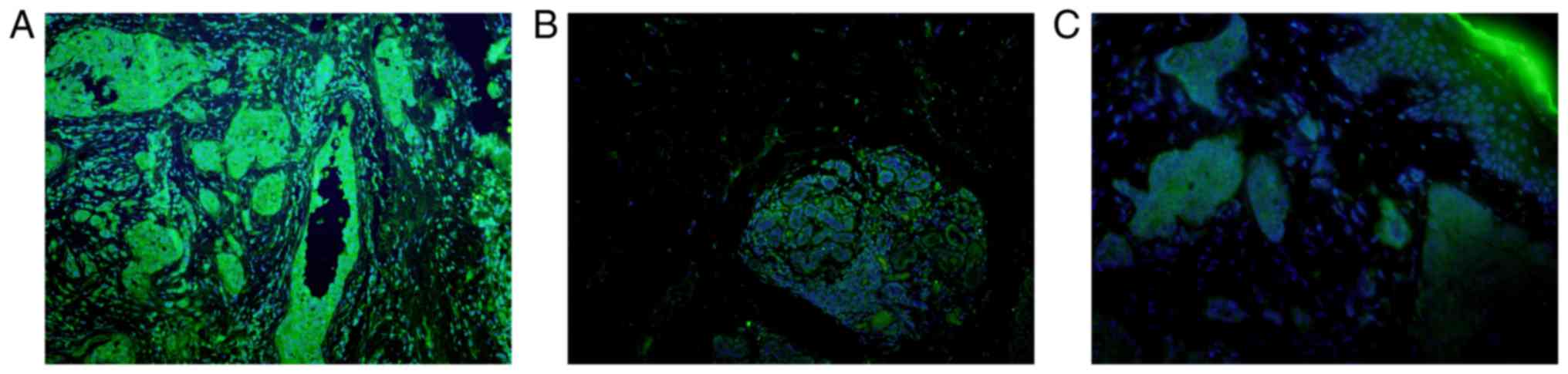
cancer, CRCC, SCC, Paget's disease, melanomas and MF (Fig. 7). However, tumors in which SHARPIN
mainly showed positive signal in the nucleus but not or only weak
signal in the cytoplasm included lung cancer, esophagus cancer
(Fig. 8). Malignant tumors in which
SHARPIN expressed in both of cytoplasm and nucleus but mainly in
the cytoplasm included breast cancer and BCC (Fig. 9).
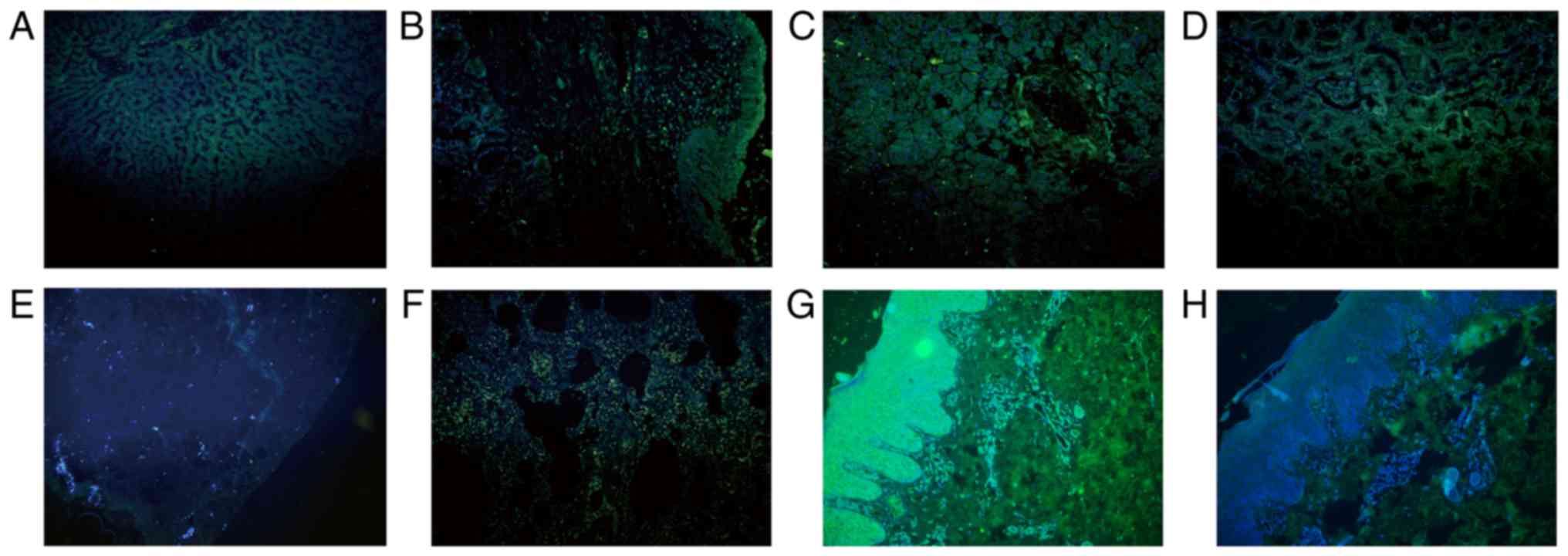 | Figure 6.SHARPIN is mainly expressed in the
cytoplasm of cells in the normal tissues recruited, with the
exception for the normal lung tissue. (A) Liver, (B) larynx, (C)
pancreas, (D) kidney, (E) breast, (F) lung samples. (G) Formalin
fixed and paraffin embedded normal skin sample incubated with
anti-SHARPIN antibody and (H) normal skin sample incubated with
PBS, which acted as the imunofluorescence negative control. Blue
and green staining in figure of immunofluorescence indicate nucleus
staining and SHARPIN positive staining of cells, respectively (A-H,
original magnification, ×200). SHARPIN, Shank-associated RH
domain-interacting protein; PBS, phosphate-buffered saline
buffer. |
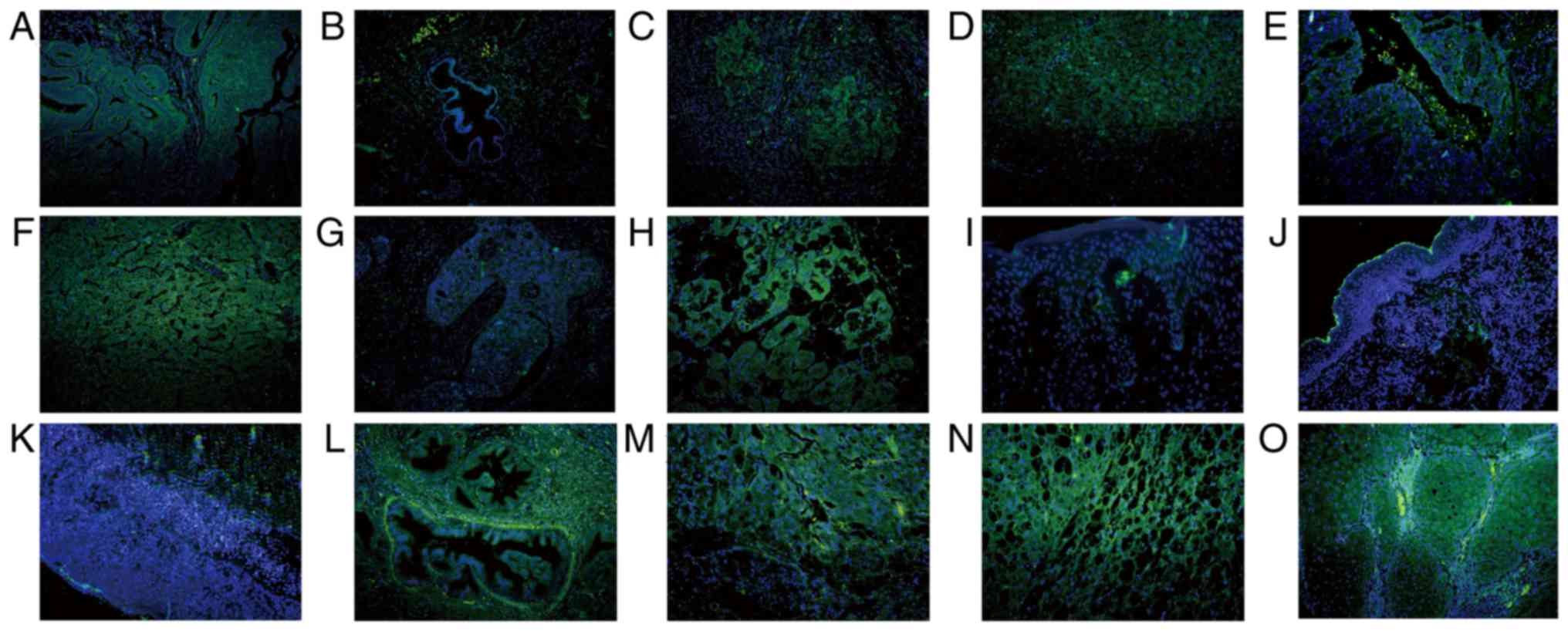 | Figure 7.Subcellular location of SHARPIN in
different cancer types in which SHARPIN mainly localizes in the
cytoplasm of malignant cells. Endometrial cancer, HCC, ICC,
laryngocarcinoma, melanomas, mycosis fungoides, Paget's disease,
pancreatic cancer, CRCC and SCC were examined. (A) Endometrial
cancer and (B) paracancer of endometrial cancer; (C) HCC and (D)
paracancer of HCC; (E) ICC and (F) paracancer of ICC; (G)
laryngocarcinoma and (H) paracancer of laryngocarcinoma; (I)
melanomas; (J) mycosis fungoides; (K) Paget's disease; (L)
pancreatic cancer; (M) CRCC and (N) paracancer of CRCC; and (O)
SCC. Blue and green staining in figure of immunofluorescence
indicate nucleus staining and SHARPIN positive staining of cells,
respectively (A-O, original magnification, ×200). SHARPIN,
Shank-associated RH domain-interacting protein; HCC, hepatocellular
carcinoma; ICC, cholangiocellular carcinoma; BCC, basal cell
carcinoma; SCC, squamous cell carcinoma. |
Discussion
All of human organs and tissues stem from embryo.
Entoderm, mesoderm and ectoderm take shape successively by the
third week of embryonic development. From the fourth week to the
eighth week, those germ layers have differentiated into their
corresponding tissues and organ anlage respectively. Among those
germ layers, ectoderm differentiates into central nervous system,
peripheral nervous system, epidermis and appendage of skin, breast,
retina, crystalline lens, inner ear and olfactory epithelium, etc.
Mesoderm differentiates into motor system including bone, cartilage
and skeletal muscle, dermis and subcutaneous connective tissue of
skin, most of urinary system and genital system, heart, blood
vessel and lymphatic, etc. Entoderm differentiates into liver,
pancreas, digestive glands of digestive tube, larynx, trachea,
bronchus and lung. On the other side, the epithelium of some organs
may originate from entoderm or ectoderm, however, the rest tissues
of those organs derive from mesoderm, e.g., the skin of breast
originates from ectoderm while the rest part derives from mesoderm.
So breast cancer is categorized into tumor from mesoderm, except
for mammary Paget's disease affecting breast duct, nipple and
mammary areola which is not included in our experiment. All of the
recruited cases of Paget's disease are extramammary Paget's disease
from ectoderm mainly affecting on scrotum, perineum, crissum or
axilla.
SHARPIN is a kind of linear ubiquitin chain related
protein which has multiple functions. Recent studies have indicated
that SHARPIN can induce cell survival via activating NF-κB
signaling pathway in hepatocytes (10), epithelial cells (11), and even osteosarcoma cells (12). In addition, SHARPIN can modulate
keratinocytes apoptosis mediated by mitochondria (13). SHARPIN also involves in tumorigenesis
and tumor progression reported by recent studies. After analysis of
expression and function between SHARPIN and PTEN in 2010, He et
al considered that SHARPIN affects tumorigenesis via inhibition
of PTEN function (14). As a tumor
suppressor, PTEN dephosphorylates
phosphotidylinositol-3,4,5-triphosphate (PIP3) at the plasma
membrane, and in the nucleus it regulates genome stability
(15). PTEN can be inactivated by
PTEN negative regulators (PTEN-NRs). As a PTEN-NRs,
shank-interacting protein-like 1 (SIPL1), namely SHARPIN, can
interact with PTEN via its UBL domain, resulting in inhibition of
the PIP3 phosphatase activity of PTEN. SIPL1 inhibits function of
PTEN in PTEN-positive human primary cervical cancer tissue.
Knockdown of SIPL1 expression by siRNA inhibits the growth of both
human prostate carcinoma cells DU145 and HeLa cells in vitro
and in vivo in axenograft tumor model, and upregulated
expression of SIPL1 protects human U87 glioma cells from growth
inhibition induced by PTEN (14). In
2015 Bii identified SHARPIN as a breast cancer metastasis gene and
prognostic biomarker by a novel gamma retroviral shuttle vector
insertional mutagenesis screen (8).
Study by De Melo and Tang described a positive correlation of
SHARPIN with breast cancer tumorigenesis (9). In 2010, Jung et al analyzed
genome-wide differences in gene expression in 11 kinds of visceral
malignant tumors originated from breast, colon, kidney, liver,
lung, esophagus, ovary, pancreas, prostate, rectum, and stomach.
Among of those malignant tumors, the expression of SHARPIN in renal
clear cell adenoma, HCC, papillary serous adenocarcinoma of ovary
and pancreas adenocarcinoma increases, experiment in vitro
identified that overexpression of SHARPIN is related with
tumorigenesis (6). Consequently, it
is postulated that SHARPIN potentially involves in the development
and proliferation of cells, overexpression of SHARPIN may closely
promote the initiation and development of malignant tumor.
Immunoblot analysis revealed that SHARPIN protein
relatively highly expresses in lung, brain and spleen, and
expresses at a lower level in testis, kidney, skeletal muscle,
liver and heart (3). In our study, 7
kinds of normal tissues including liver, lung, larynx, pancreas,
breast, kidney and skin exhibited positive signal, indicating that
SHARPIN widely expresses in human normal tissue and maybe serve as
a multiple functional protein more than an interactor of Shank
proteins. Those results and enhanced expression of SHARPIN in
breast cancer, HCC and pancreatic cancer showed in our study are in
accordance with the previous study which confirmed the
repeatability and the results' reliability of our work. Our study
also showed that all of the recruited tumors and paracancer samples
originated from entoderm and mesoderm (including prostate cancer,
renal clear cell adenoma and papillary serous adenocarcinoma of
ovary from mesoderm which previous studies reported but not
recruited in our study) showed an upregulated expression of
SHARPIN, while tumor originated from ectoderm exhibited a
downregulated expression. These wide-in-depth discoveries may
suggest that SHARPIN serves as a promoting effect in the
pathogenesis of tumors derived from entoderm and mesoderm, while
plays a suppressor role in tumors derived from ectoderm. It is
reasonable to reassess the role of SHARPIN in the initiation and
development of malignant tumors. SHARPIN could play a different or
even opposite role in malignant tumors derived from different germ
layers.
Indeed, the function of a specific gene in different
tissues may be different; it is spatial-temporal dependence even in
a kind of cell to react appropriately to various changes of
internal environment and external environment. For example,
Zinc-fingers and homeoboxes 1 (ZHX1) is a transcription repressor
which involves in pathogenesis of multiple human cancers; study by
Kwon et al showed that the expression of ZHX1 increases in
cholangiocarcinoma (CCA) tissues and ZHX1 promotes CCA cell
proliferation, migration, and invasion, functioning as an oncogene
in CCA (16); however, study by Ma
et al exhibited that ZHX1 expression is downregulated in
gastric cancer, ZHX1 could inhibit cell growth by inducing
cell-cycle arrest and apoptosis, showing a role of tumor suppressor
in gastric cancer (17).
In this study, there are five kinds of common skin
tumors recruited from ectoderm including BCC, SCC, Paget's disease,
melanomas and MF. Reduced expression of SHARPIN in those skin
cancers is contrary to previous studies which enhanced expression
of SHARPIN was observed in various of tumors such as prostate
cancer, breast cancer and HCC. SHARPIN may function as a tumor
suppressor in those skin cancers, which is a novel discovery of
SHARPIN function. Reduced SHARPIN expression in part of malignant
cells and loss expression of SHARPIN in the other part of malignant
cells were observed in these skin cancers.
Primary hepatic carcinoma can be divided into HCC,
ICC and mixed hepatocellular carcinoma by histological
differentiation. HCC occurs in hepatocytes while ICC occurs in
intrahepatic biliary epithelial cells. Previous study described
elevated expression of SHARPIN in HCC, while enhanced SHARPIN
expression was observed in both HCC and ICC of our study,
indicating a pro-oncogenic role in the tumorigenesis of primary
hepatic carcinoma. Both of renal clear cell carcinoma (RCCC) and
CRCC belong to renal cell carcinoma differing in the shape of
malignant cells. RCCC account for approximately 70–80% and CRCC
account for 5% of renal cell carcinoma. Former study exhibited
increased SHARPIN expression in RCCC (6), while enhanced SHARPIN expression was
also observed in CRCC in our study, suggesting that SHARPIN may
function as a pro-oncogenic factor in the tumorigenesis of renal
cell carcinoma.
Immunoblot analysis of adult rat brain revealed that
SHARPIN protein widely expresses among subcellular fractions, with
moderate amount in light membrane fractions and crude synaptosomal,
a large amount in cytosolic fractions (3). There were also other studies showed that
SHARPIN localizes in membranes and nuclei of cells (6,18). To
assess the subcellular localization of SHARPIN in various malignant
tumors and their corresponding normal tissues, we conducted
immunofluorescence analysis of SHARPIN expression in the above
tissues. For normal tissues recruited, SHARPIN mainly localized in
the cytoplasm of cells and showed no or only a faint signal in the
nucleus except for the normal lung tissue which exhibited an
opposite phenomenon. For these 14 kinds of malignant tumors
recruited in this study, SHARPIN also mainly localized in the
cytoplasm of cells and presented no or only a faint signal in the
nucleus except for lung cancer and esophagus cancer, in which
malignant cells have aberrantly big nucleus but basically no
cytoplasm, exhibited signal in the nucleus of cells but not in the
cytoplasm. It is postulated that SHARPIN mainly has a role in the
cytoplasm of cells. Indeed, previous studies have shown that
SHARPIN can interact with NF-κB, PTEN, integrin and MAPK in the
cytoplasm (14,19,20). Also
SHARPIN have a role in the nucleus, SHARPIN can combines with EYA1
and EYA2 (eyes absent homolog 1 and 2) directly, which enhances
relative targeted gene expression in the development of several
tissues (21). In our study, SHARPIN
localized in the nucleus of malignant cells of tumors such as lung
cancer and esophagus cancer, but its relationship with malignant
tumor still remains unclear.
We conducted a wide-range preliminary screening
research about SHARPIN expression in various cancers derived from
different germ layers, verifying the previous studies about SHARPIN
expression in 7 kinds of normal tissues and 3 kinds of tumors
including HCC, breast cancer and pancreatic cancer, and identified
that the SHARPIN expression pattern in ectodermal cancers is
different from entodermal and mesodermal malignancies, indicating a
dual role in tumorigenesis in which SHARPIN could function as a
pro-oncogenic role in entoderm and mesoderm or a tumor suppression
factor in ectoderm. However, a limitation of the study is that no
experiments were conducted to confirm the role of SHARPIN in the
tumors, and further in vitro and in vivo study is
ongoing to investigate the role of SHARPIN in skin
malignancies.
Acknowledgements
Not applicable.
Funding
The present research was supported by a grant from
National Natural Science Foundation of China (grant no.
81371724).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed and guided this study, and outlined and
revised the manuscript. BC performed the majority of the
experiments and drafted the manuscript. FL, YY and YZ collected
samples and assisted in the study design. JW conducted data
analysis, data interpretation and generated the figures. ST
performed data collection and the literature search. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted with the approval of the
Institutional Review Board and Ethics Committee of Shenzhen
Hospital, Southern Medical University and in accordance with the
Declaration of Helsinki. Informed consent was obtained from all of
the patients.
Patient consent for publication
The patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaatsch P: Epidemiology of childhood
cancer. Cancer Treat Rev. 36:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim S, Sala C, Yoon J, Park S, Kuroda S,
Sheng M and Kim E: Sharpin, a novel postsynaptic density protein
that directly interacts with the shank family of proteins. Mol Cell
Neurosci. 17:385–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hogenesch H, Gijbels MJ, Offerman E, van
Hooft J, van Bekkum DW and Zurcher C: A spontaneous mutation
characterized by chronic proliferative dermatitis in C57BL mice. Am
J Pathol. 143:972–982. 1993.PubMed/NCBI
|
|
5
|
Seymour RE, Hasham MG, Cox GA, Shultz LD,
Hogenesch H, Roopenian DC and Sundberg JP: Spontaneous mutations in
the mouse Sharpin gene result in multiorgan inflammation, immune
system dysregulation and dermatitis. Genes Immun. 8:416–421. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung J, Kim JM, Park B, Cheon Y, Lee B,
Choo SH, Koh SS and Lee S: Newly identified tumor-associated role
of human Sharpin. Mol Cell Biochem. 340:161–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bollag G, Hirth P, Tsai J, Zhang J,
Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al:
Clinical efficacy of a RAF inhibitor needs broad target blockade in
BRAF-mutant melanoma. Nature. 467:596–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bii VM, Rae DT and Trobridge GD: A novel
gammaretroviral shuttle vector insertional mutagenesis screen
identifies SHARPIN as a breast cancer metastasis gene and
prognostic biomarker. Oncotarget. 6:39507–39520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Melo J and Tang D: Elevation of SIPL1
(SHARPIN) increases breast cancer risk. PLoS One. 10:e01275462015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sieber S, Lange N, Kollmorgen G, Erhardt
A, Quaas A, Gontarewicz A, Sass G, Tiegs G and Kreienkamp HJ:
Sharpin contributes to TNFα dependent NFκB activation and
anti-apoptotic signalling in hepatocytes. PLoS One. 7:e299932012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerlach B, Cordier SM, Schmukle AC,
Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H,
Wong WW, et al: Linear ubiquitination prevents inflammation and
regulates immune signalling. Nature. 471:591–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomonaga M, Hashimoto N, Tokunaga F,
Onishi M, Myoui A, Yoshikawa H and Iwai K: Activation of nuclear
factor-kappa B by linear ubiquitin chain assembly complex
contributes to lung metastasis of osteosarcoma cells. Int J Oncol.
40:409–417. 2012.PubMed/NCBI
|
|
13
|
Liang Y and Sundberg JP: SHARPIN regulates
mitochondria-dependent apoptosis in keratinocytes. J Dermatol Sci.
63:148–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He L, Ingram A, Rybak AP and Tang D:
Shank-interacting protein-like 1 promotes tumorigenesis via PTEN
inhibition in human tumor cells. J Clin Invest. 120:2094–2108.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Y, Paluch BE, Wang X and Jiang X: PTEN
at a glance. J Cell Sci. 125:4687–4692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwon RJ, Han ME, Kim JY, Liu L, Kim YH,
Jung JS and Oh SO: ZHX1 promotes the proliferation, migration and
invasion of cholangiocarcinoma cells. PLoS One. 11:e01655162016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Huang M, Wang Z, Liu B, Zhu Z and Li
C: ZHX1 inhibits gastric cancer cell growth through inducing
cell-cycle arrest and apoptosis. J Cancer. 7:60–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Potter CS, Sundberg JP and
Hogenesch H: SHARPIN is a key regulator of immune and inflammatory
responses. J Cell Mol Med. 16:2271–2279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tokunaga F, Nakagawa T, Nakahara M, Saeki
Y, Taniguchi M, Sakata S, Tanaka K, Nakano H and Iwai K: SHARPIN is
a component of the NF-κB-activating linear ubiquitin chain assembly
complex. Nature. 471:633–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rantala JK, Pouwels J, Pellinen T, Veltel
S, Laasola P, Mattila E, Potter CS, Duffy T, Sundberg JP,
Kallioniemi O, et al: SHARPIN is an endogenous inhibitor of
β1-integrin activation. Nat Cell Biol. 13:1315–1324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Landgraf K, Bollig F, Trowe MO, Besenbeck
B, Ebert C, Kruspe D, Kispert A, Hänel F and Englert C: Sipl1 and
Rbck1 are novel Eya1-binding proteins with a role in craniofacial
development. Mol Cell Biol. 30:5764–5775. 2010. View Article : Google Scholar : PubMed/NCBI
|