Introduction
PM2.5 refers to the suspended particles in the air
with a diameter ≤2.5 µm. PM2.5 can stay in the air for a
long time, and high concentration of PM2.5 can lead to serious air
pollution. With special morphological and physical properties,
PM2.5 can enter the bloodstream through the respiratory tract to
cause reproductive toxicity, and increase the incidence of cancer
(1–4).
PM2.5 was first recognized as a type of carcinogen by the World
Health Organization in October 2013. PM2.5 can induce a variety of
malignancies including brain tumors, leukemia and cervical cancer.
Studies have shown that long-term exposure to PM2.5 can reduce life
expectancy, and can also increase the incidence of a variety of
chronic diseases, such as asthma, bronchitis and cardiovascular
disease (5–8).
IL-1 is an important immunomodulatory factor that
plays important roles in regulating the immune function of cells,
and participates in the endocrine system, nervous system and the
inflammatory reaction in the human body (9). IL-6 is a type of inflammatory factor
that plays a pivotal role in female reproductive tract infections
and is expressed in cervical epithelial cells, renal cell carcinoma
epithelial cells, and multiple myeloma tissues (10). It has been reported that (11) PM2.5 inhalation can cause lymphocyte
aggregation, neutrophil infiltration and other inflammatory
reactions. Inflammatory cytokines IL-1 and IL-6 promote cell
aggregation, and the levels of IL-1 and IL-6 reflect early
inflammatory reaction in the human body (12). In this study, HeLa cells were
stimulated by PM2.5 to observe the effects of PM2.5 on
proliferation and invasion of HeLa cells and on the expression
levels of IL-1 and IL-6.
Materials and methods
Main reagents and equipment
HeLa cells (The Institute of Basic Medical Sciences
of the Chinese Academy of Medical Sciences, Beijing, China);
RPMI-1640 medium (R8758; Sigma-Aldrich; Merck KGaA, St. Louis, MO,
USA); trypsin (15400054; Gibco; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA); fetal bovine serum (04-001-1ACS; Shanghai
Enzyme-linked Biotechnology Co., Ltd., Shanghai, China); rabbit
anti-human IL-1 and IL-6 monoclonal antibodies, horseradish
peroxidase, goat anti-rabbit IgG secondary antibody, and IL-1 and
IL-6 enzyme-linked immunosorbent assay (ELISA) kit (701304, 701028,
MA110371, A-11034, KHC0011, BMS213-2; Thermo Fisher Scientific,
Inc.). TRIzol reagent and RT-PCR kit (15596018; Invitrogen; Thermo
Fisher Scientific, Inc.). Reverse transcription kit and TaqMan
miRNA kit (4374966; Applied Biosystems; Thermo Fisher Scientific,
Inc.). High-speed refrigerated centrifuge (AVANTIJ-15R; Beckman
Coulter, Inc., Brea, CA, USA). Microplate reader (51119700DP;
Gibco; Thermo Fisher Scientific, Inc.). Constant temperature
CO2 incubator and ABI 7900 real-time PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.).
The study was approved by the Ethics Committee of
the Third Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China).
Preparation of PM2.5 turbid liquid and
particle treatment
A piece of glass fiber filter paper (200 × 250 mm)
was placed in a thermostat at 100°C for 1–2 h. During sampling,
filter paper was placed on the filter paper holder with tweezers
and fixed. The flow rate of the sampler was controlled between 1.1
and 1.7 m3/min. After sampling, the filter paper was
collected and placed in a desiccator for 6 h. The filter paper
containing the particles was cut into 1-cm2 pieces and
immersed in three kinds of steam water. Ultrasonic oscillation was
performed 4 times, 30 min per time. Then fluid was filtered through
6 layers of gauze, followed by centrifugation at 10,000 × g, 4°C
for 20 min. Suspension was collected in a glass plate, vacuum
frozen, vacuum dried, and stored in a low temperature freezer. A
certain amount of sample was mixed with DMEM cell culture, followed
by ultrasonic oscillation for 15 min. Stock solution was sterilized
and stored at 4°C. Ultrasonic oscillation for another 5 min was
performed before use (13).
Cell culture and treatment
HeLa cells were collected during logarithmic growth
phase, and were digested with 0.25% trypsin. Cells were resuspended
in cell culture medium containing 10% fetal bovine serum to a final
density of 2.5×105/ml. After incubation for 24 h, the
cells were washed 3 times with PBS. Finally, 20 µl of
particle suspension were added for the final concentrations of 0,
10, 25, 50, 100 and 200 µg/ml, respectively. Concentration
of 0 µg/ml was used as negative control. Each treatment was
repeated 3 times, and the cells were cultured in an incubator
(37°C, 5% CO2).
MTT assay to detect cell
proliferation
HeLa cells at a density of 4.5×103
cells/ml were transferred to a 96-well plate with 200 ml in each
well. Three replicate wells were set for each experiment. The cells
were cultured in an incubator (37°C, 5% CO2) for 4 h,
followed by the addition of 20 µl (5 mg/ml) of MTT reagent
into each well. After cell culture for another 5 h, supernatant was
removed and 200 ml DMSO were added. After shaking for 15 min, a
microplate reader (490 nm) was used to read the OD values and the
cell growth curve was plotted.
Transwell assay
Cell invasion ability was measured by Transwell
assay. Briefly, 80 µl of diluted Matrigel solution were used
to cover the entire upper membrane. After keeping at room
temperature for 1.5 h, 500 µl cell culture medium containing
20% FBS were added to the lower chamber, while HeLa cells
(4×103) in serum-free medium were added into the upper
chamber. After incubation for 24 h, the cells that failed to invade
the membranes were removed and H&E staining was performed.
Three visual fields were selected under an optical microscope to
count the cells 3 times.
Western blotting
Total protein was extracted from the collected cells
using RIPA pyrolysis (P0013B; Beyotime Institute of Biotechnology,
Shanghai, China). BCA method (P0009; Beyotime Institute of
Biotechnology) was used to detect the protein concentration, and
the calculated protein was added to 5X SDS sample buffer at 1/4
volume. For SDS-PAGE electrophoresis separation, the constant
voltage was 80 V at 8% stacking gel, and it was changed to 120 V at
5% separate gel; in the ice bath at 80 V for 100 min. The membrane
was transferred to difluoroethylene film, the protein bands were
dyed with Ponceau S., washed after soak in PBST for 5 min and
stored in 5% skimmed milk powder at 4°C for the night. After the
antibodies were diluted with PBST containing 1% skimmed milk
powder, IL-1, IL-6 monoclonal antibodies (1:1,000) were added and
sealed at 4°C for the night. After the primary antibody was
removed, the membrane was washed with TBST, and horseradish
peroxidase labeled goat anti-rabbit secondary antibody (1:5,000)
was added. It was incubated at 37°C for 1–2 h, and rinsed 5 times
with TBST, 5 min each time. Developed in the darkroom, the liquid
was blotted on the film with filter paper, ECL gloss agent
(Beyotime Institute of Biotechnology) was applied, and exposed
after 5 min. The gray value was analyzed using Quantity One
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) by
scanning protein bands, where the relative expression level of
protein was equal to the gray value of target protein bands/GAPDH
protein bands.
RT-qPCR
The collected cells were extracted with TRIzol for
total RNA extraction, and the extracted total RNA was tested for
purity, concentration and integrity using ultraviolet
spectrophotometry and agarose gel electrophoresis. Total RNA was
reverse transcribed using a reverse transcription kit, and the
operation steps were strictly followed according to the
manufacturer's instructions. cDNA preservation of reverse
transcription was collected and part was taken for subsequent
experiments. PCR kits were used for PCR amplification experiments,
and the PCR reaction system was 2X TransStart® Top Green
qPCR SuperMix (TransGen Biotech, Beijing, China) 10 µl, upstream
and downstream primers (each 0.4 µl), cDNA 2 µl, 50X ROX Reference
Dye II (Thermo Fisher Scientific, Inc.) 0.4 µl. nuclease-free water
was added to complete to 20 µl. The PCR reaction conditions were:
95°C predegeneration for 30 sec, 95°C for 5 sec, 60°C for 30 sec
and 40 cycles. Three duplicate wells were set for each sample, and
the experiment was conducted three times. In this study, GAPDH was
used as internal reference, and 2−ΔCq was used to
analyze the data (14) (Table I).
 | Table I.Primer sequences |
Table I.
Primer sequences
| Gene | Upstream primers | Downstream
primers |
|---|
| IL-1 |
5′-GCACAGTTCCCCAACTGGTA-3′ |
5′-AAGACACGGGTTCCATGGTG-3′ |
| IL-6 |
5′-TTCGGCAAATGTAGCATC-3′ |
5′-AATAGTGTCCTAACGCTCATAC-3′ |
| GAPDH |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
ELISA
According to the manufacturer's instructions, the
test was performed as follows: the collected cells were added to 50
µl of standard substance solution at different concentrations in
blank micropores; 50 µl of distilled water were added to the blank
control well and 50 µl of antibody were added. In the rest of the
micropores, 40 µl of the sample were firstly added and then 10 µl
of the biotin-labeled antibody were added. The plate was sealed and
incubated at 37°C for 30 min. When the plate was washed in each
well, it was ensured that it was full and not overflowing; it was
left to stand for 30 sec, discarded and patted dry, 5 times in
total. After 50 µl enzyme-labeled solution was added to each hole,
the plate was sealed again and incubated at 37°C for 60 min. The
plate was washed again for 5 times, and patted dry with absorbent
paper at the last time. A total of 100 µl/well horseradish
peroxidase marker was added before sealing the plate and incubated
in the dark under 37°C for 15 min. Another 100 µl/well chromogenic
substrate TMB was added, and incubated in the dark at room
temperature for 20 min. Finally, 50 µl/well stop buffer was added,
and enzyme standard instrument was used for testing within 15 min.
The maximum absorption wavelength of 450 nm was measured. Three
sets of repeat wells were set, and the experiment was repeated for
3 times.
Statistical analysis
SPSS 20.0 software package (IBM Corp., Armonk, NY,
USA) was used for the statistical analysis of the collected data
and GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla,
CA, USA) for the figures. Measurement data were expressed as mean ±
standard deviation (mean ± SD), and were compared by t-test
(between two groups) and one-way analysis of variance (among
multiple groups). LSD test was used as a post hoc test. Repeated
analysis of variance was used for comparisons among multiple
time-points. P<0.05 was considered to indicate a statistically
significant difference.
Results
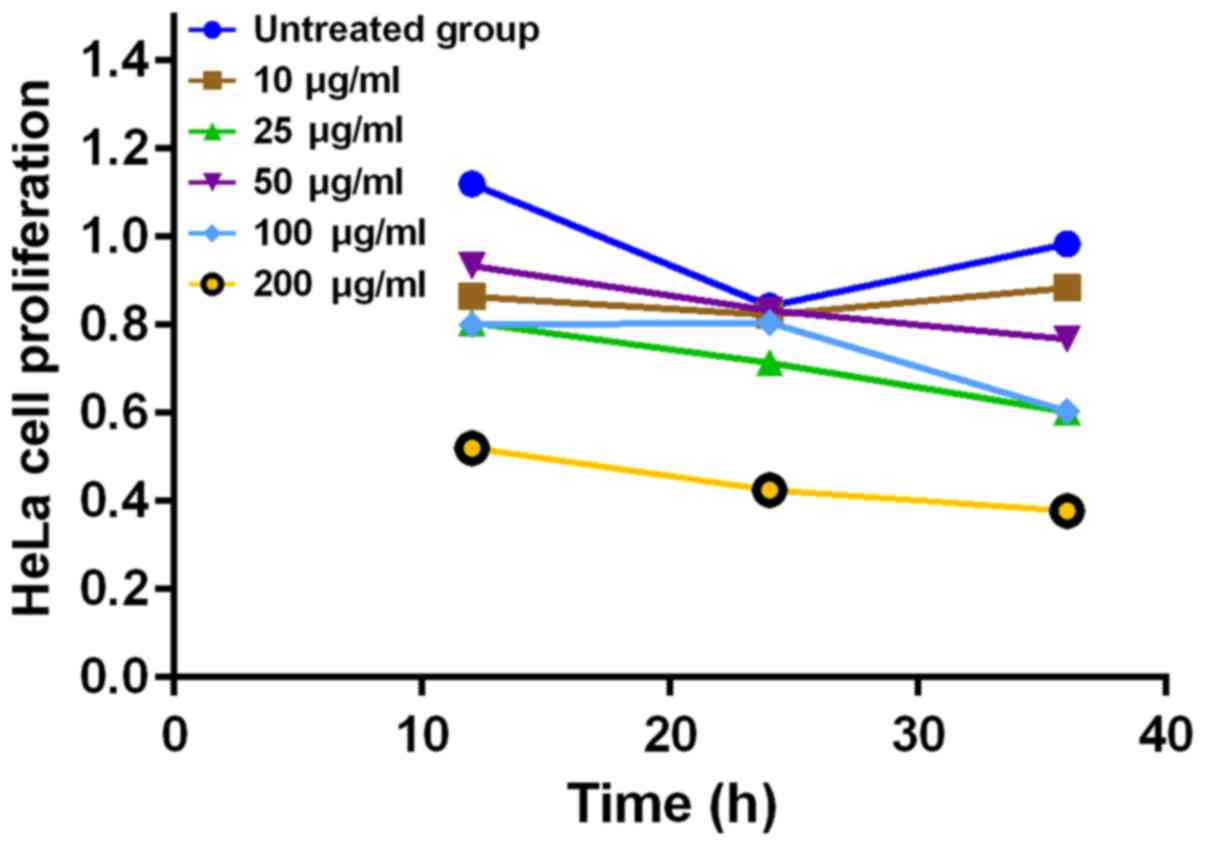
Effects of different concentrations of
PM2.5 on HeLa cell proliferation
The results showed that PM2.5 at 10 µg/ml had
no significant effect on cell proliferation, while PM2.5 inhibited
the proliferation of HeLa cells at the concentrations of 50–120
µg/ml, in a concentration-dependent manner (P<0.05)
(Fig. 1).
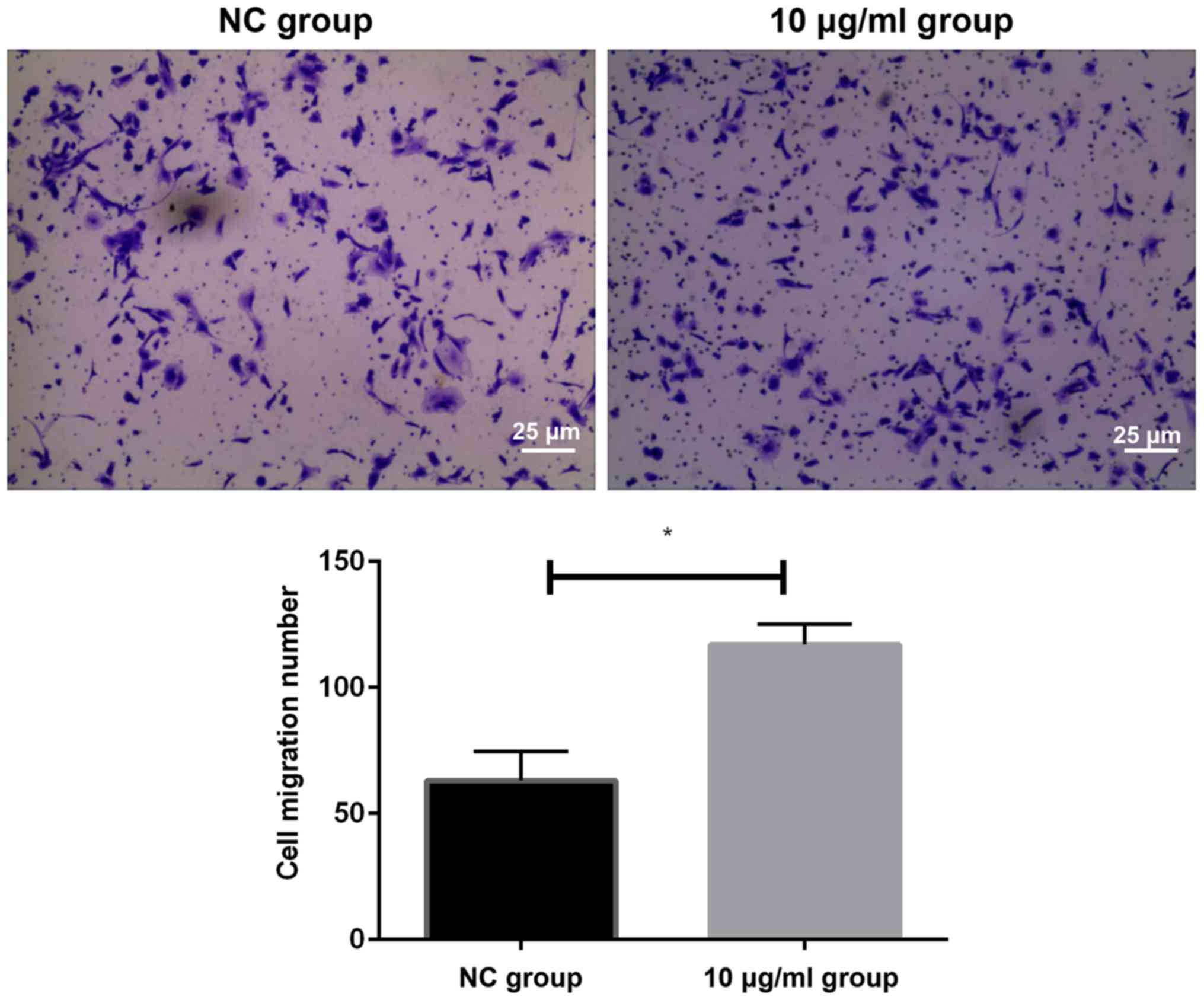
Effects of PM2.5 on the invasion of
HeLa cells as detected by Transwell assay
We detected the migration of cells in two groups by
Transwell invasion assay. The results showed that the number of
migrating cells in HeLa cells after 10 µg/ml PM2.5
stimulation was significantly higher than that in NC group
(P<0.05). It could be concluded that an appropriate
concentration of PM2.5 could increase the invasion ability of HeLa
cells (Fig. 2).
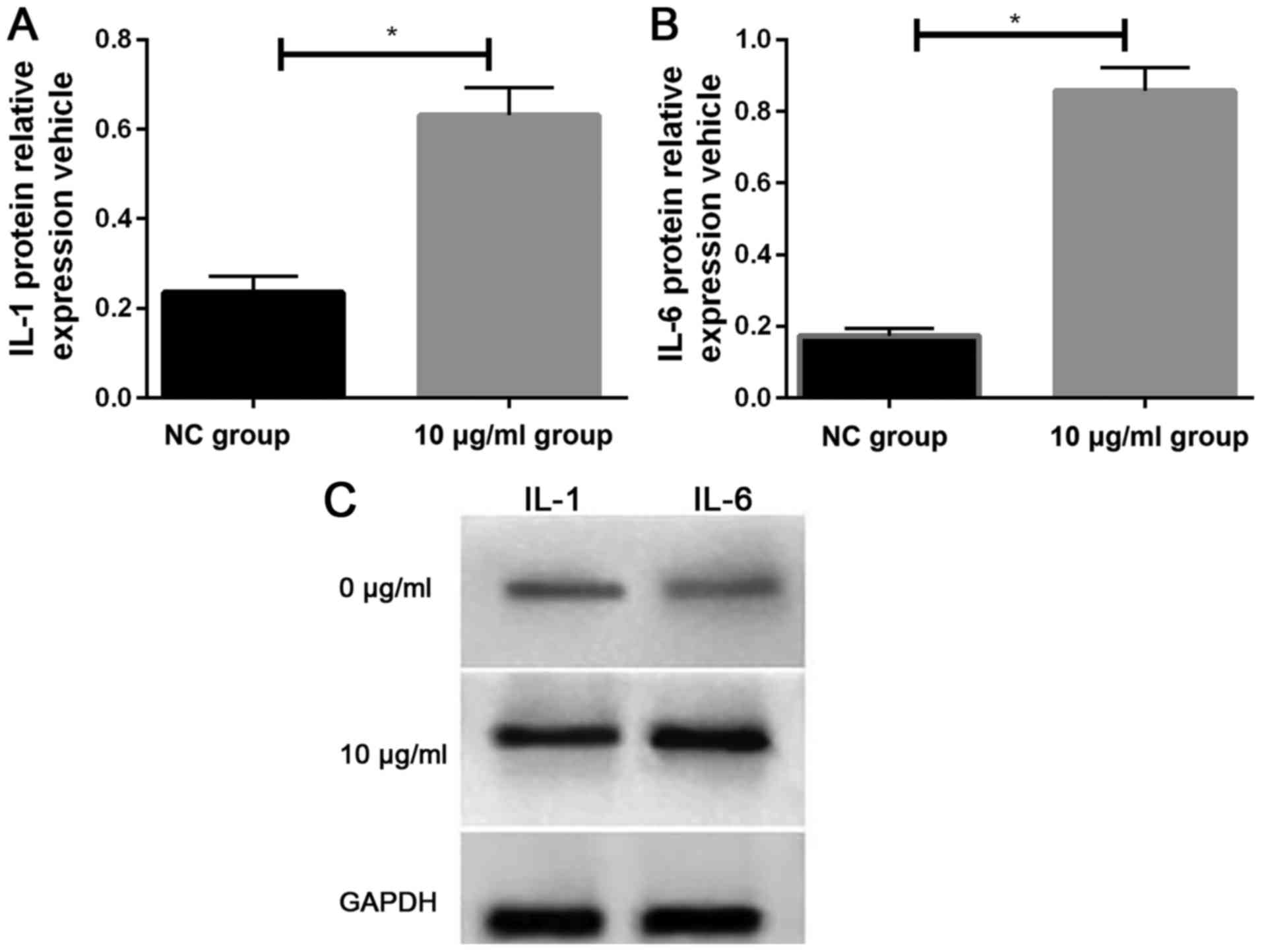
Effects of PM2.5 on the expression
levels of IL-1 and IL-6 as detected by western blotting
We detected the expression of IL-1 and IL-6 protein
in two groups of cells by western blotting. It was found that the
relative expression levels of IL-1 and IL-6 proteins in HeLa cells
stimulated by 10 µg/ml PM2.5 for 24 h were significantly
higher than those in HeLa cells of NC group (Fig. 3).
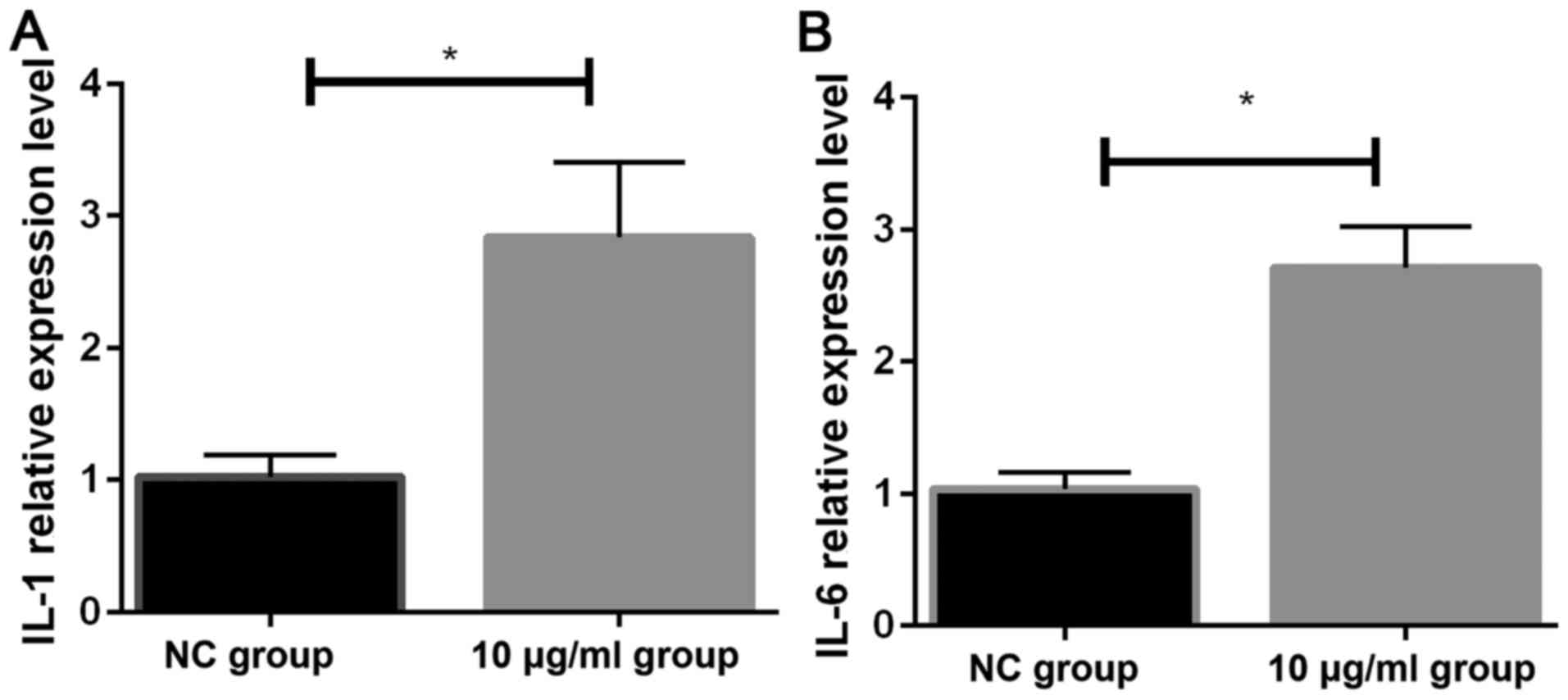
Effects of PM2.5 on the expression
levels of IL-1 and IL-6 as detected by RT-qPCR
The expression of IL-1 mRNA and IL-6 mRNA in two
groups of cells was detected by RT-qPCR. The relative expression
levels of IL-1 mRNA (2.84±0.57) and IL-6 mRNA (2.71±0.31) in HeLa
cells stimulated by 10 µg/ml PM2.5 were significantly higher
that the expression levels of IL-1 mRNA (1.021±0.171) and IL-6 mRNA
(1.035±0.023) in NC group (both P<0.05) (Fig. 4).
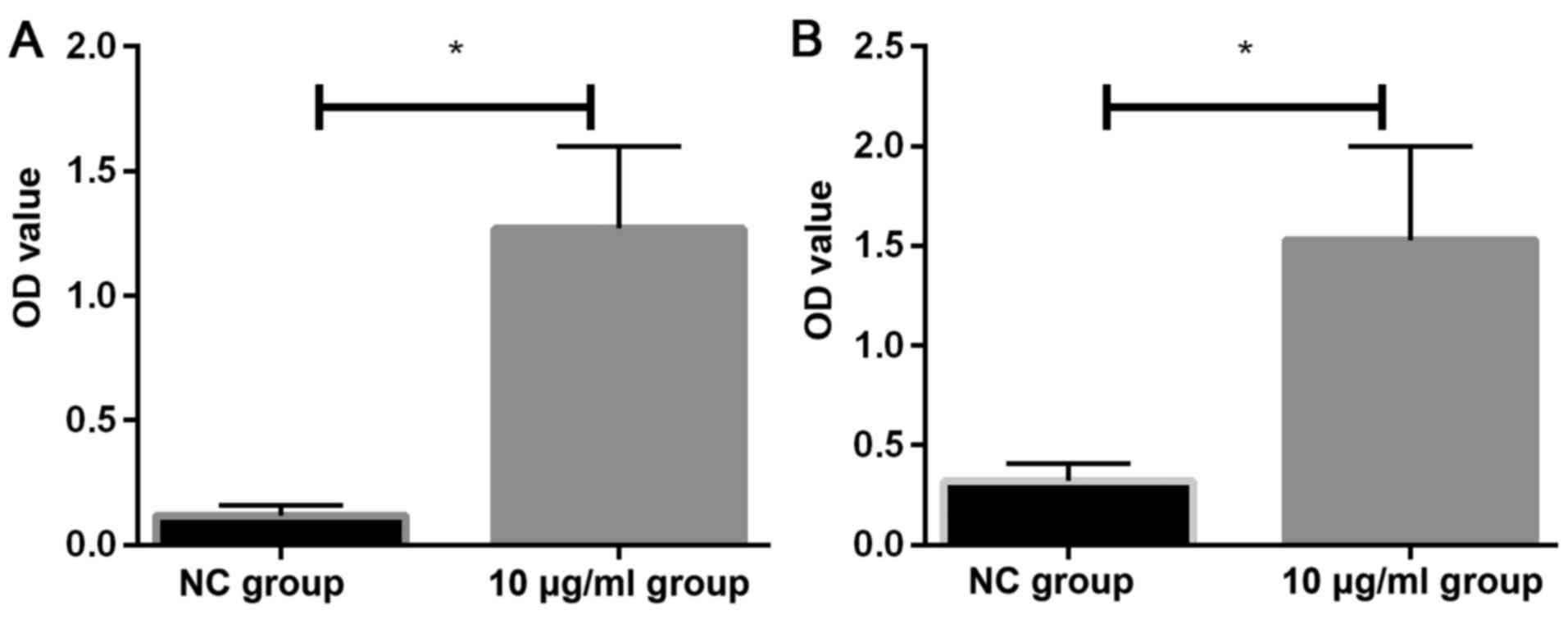
Effects of PM2.5 on the levels of IL-1
and IL-6 proteins as detected by ELISA
The expression levels of IL-1 and IL-6 in two groups
of cells were detected by ELISA. The expression levels of IL-1
(1.27±0.33) and IL-6 (1.53±0.47) in HeLa cells after 24 h
stimulation with 10 µg/ml PM2.5 were significantly higher
than the levels of IL-1 (0.12±0.04) and IL-6 (0.32±0.09) in NC
group (P<0.05) (Fig. 5).
Discussion
As the most common carcinogenic substance, PM2.5
exists in the air in the form of solid or liquid. PM2.5 contains
harmful and toxic substances that can exist in air for a long time,
bringing adverse effects on human health, air quality and
environment (15). PM2.5 inhalation
affects lung gas exchange, and promotes the occurrence of
respiratory diseases, cardiovascular and cerebrovascular diseases,
and other diseases such as asthma, arrhythmia and myocardial
infarction (16).
IL-1 is an inflammatory mediator released by
activated giant cells and related inflammatory cells. IL-1 can
cause infiltration of granulocytes and macrophages, which in turn
lead to the occurrence of pulmonary inflammation. IL-1 can be
combined with gp130/IL6R to form heterosextrains to stimulate
protein tyrosine kinase, leading to the expression of oncogenes and
the occurrence of tumorigenesis (17). It has been reported that IL-1
expression levels are significantly lower in normal individuals
than those in cervical cancer patients, and studies have shown that
IL-1 is a risk factor for cervical cancer (18,19). IL-6
is a multifunctional cytokine, and its receptors belong to the
erythroid receptor superfamily which are overexpressed on the
surface of non-lymphoid and lymphoid cells. It has been
demonstrated (20) that IL-6 can be
secreted by both epithelial cells and fibroblasts in the cervical
mucosa. IL-6 not only promotes the proliferation of cervical
epithelial cells but also promotes the proliferation of
non-tumor-derived HPV-transfected immortalized cells, eventually
leading to the transition from cervical lesions to malignant
tumors.
HeLa cells are human cervical adenocarcinoma cells.
Compared with other cancer cells, HeLa cells have high
proliferation ability but the price is low. PM2.5 levels in cities
are generally between 8 and 14 µg/m3, and high
PM2.5 concentrations limit individuals' outdoor activities.
Therefore, we selected 10 µg/ml PM2.5 to treat HeLa cells.
This study found that the proliferation rate of HeLa cell is
negatively associated with PM2.5 concentration. However, PM2.5 at
10 µg/ml showed no significant effect on proliferation of
HeLa cells (P>0.05), while higher concentrations of PM2.5
inhibited cell proliferation in a concentration-dependent manner.
Our finding is consistent with previous studies which have reported
that high concentration of PM2.5 can inhibit the proliferation of
cells (21). However, Yang et
al (22) have reported that high
concentration of PM2.5 promotes the proliferation of H1299 cell
lines, suggesting that effects of PM2.5 on cell proliferation may
be different for different types of cells under the same
conditions. Transwell assay showed that, compared with NC group,
HeLa cells treated with 10 μg/ml PM2.5 showed significantly
increased invasion ability (P<0.05), indicating that PM2.5 at 10
μg/ml can promote the invasion of HeLa cells. Invasion
ability of HeLa cells is relatively weak, and the increased
invasion ability may increase the invasion of tumor cells to
surrounding tissues, which in turn increase the degree of
malignancy of cervical cancer. RT-qPCR, western blotting and ELISA
were performed to detect the expression of IL-1 and IL-6 in HeLa
cells treated with PM2.5 at 10 µg/ml. The results of RT-qPCR
and western blotting showed that the expression levels of IL-1 and
IL-6 in HeLa cells were significantly increased after treatment
with PM2.5 at 10 µg/ml for 24 h. However, IL-1 and IL-6 are
secretion inflammatory factors and their protein levels usually do
not change significantly. Therefore, supernatant of cell culture
was collected, and ELISA was performed to detect the levels of IL-1
and IL-6 proteins. Results showed that treatment with PM2.5 at 10
µg/ml significantly increased the secretion levels of IL-1
and IL-6 proteins. We are unable to perform flow cytometry and
Ki-67 because of the limitations of the present funds and
conditions. We will try to improve our study in the following
research.
In conclusion, this study has preliminarily proven
that PM2.5 plays a certain role in the occurrence and development
of cervical cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the project of Guangdong
Provincial Science and Technology Department ‘The effect of PM2.5
exposure on the development and metastasis of cervical cancer and
its possible mechanism’ (2014A020212687).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH contributed to the cell culture and treatment. WL
performed RT-qPCR. YC was responsible for western blotting. KH and
JZ assisted with MTT assay. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Third Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel MM, Chillrud SN, Correa JC, Hazi Y,
Feinberg M, Kc D, Prakash S, Ross JM, Levy D and Kinney PL:
Traffic-related particulate matter and acute respiratory symptoms
among New York City area adolescents. Environ Health Perspect.
118:1338–1343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner MC, Krewski D, Pope CA III, Chen Y,
Gapstur SM and Thun MJ: Long-term ambient fine particulate matter
air pollution and lung cancer in a large cohort of never-smokers.
Am J Respir Crit Care Med. 184:1374–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng X, Zhang F, Rui W, Long F, Wang L,
Feng Z, Chen D and Ding W: PM2.5-induced oxidative stress triggers
autophagy in human lung epithelial A549 cells. Toxicol In Vitro.
27:1762–1770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee IM, Bauman AE, Blair SN, Heath GW,
Kohl HW III, Pratt M and Hallal PC: Annual deaths attributable to
physical inactivity: Whither the missing 2 million? Lancet.
381:992–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamra GB, Guha N, Cohen A, Laden F,
Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P,
Yorifuji T, et al: Outdoor particulate matter exposure and lung
cancer: A systematic review and meta-analysis. Environ Health
Perspect. 122:906–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahalingaiah S, Hart JE, Laden F, Terry
KL, Boynton-Jarrett R, Aschengrau A and Missmer SA: Air pollution
and risk of uterine leiomyomata. Epidemiology. 25:682–688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nursan C, Müge AT, Cemile D, Pinar T and
Sevin A: Parent's knowledge and perceptions of the health effects
of environmental hazards in Sakarya, Turkey. J Pak Med Assoc.
64:38–41. 2014.PubMed/NCBI
|
|
8
|
Kampa M and Castanas E: Human health
effects of air pollution. Environ Pollut. 151:362–367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Tahhan MA, Etewa RL and El Behery MM:
Association between circulating interleukin-1 beta (IL-1β) levels
and IL-1β C-511T polymorphism with cervical cancer risk in Egyptian
women. Mol Cell Biochem. 353:159–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka T, Narazaki M and Kishimoto T:
Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol.
10:pii a0284562018. View Article : Google Scholar
|
|
11
|
Wagner JG, Kamal AS, Morishita M, Dvonch
JT, Harkema JR and Rohr AC: PM2.5-induced cardiovascular
dysregulation in rats is associated with elemental carbon and
temperature-resolved carbon subfractions. Part Fibre Toxicol.
11:252014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christiansen JG, Jensen HE, Jensen LK,
Koch J, Aalbaek B, Nielsen OL and Leifsson PS: Systemic
inflammatory response and local cytokine expression in porcine
models of endocarditis. APMIS. 122:292–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong C, Song WM and Shi YW: Study on the
oxidative injury of the vascular endothelial cell affected by
PM2.5. Wei Sheng Yan Jiu. 34:169–171. 2005.PubMed/NCBI
|
|
14
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buczyńska AJ, Krata A, Van Grieken R,
Brown A, Polezer G, De Wael K and Potgieter-Vermaak S: Composition
of PM2.5 and PM1 on high and low pollution event days and its
relation to indoor air quality in a home for the elderly. Sci Total
Environ. 490:134–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Lin Z, Jia R, Li G, Xi Z and Wang
D: Transgenerational effects of traffic-related fine particulate
matter (PM2.5) on nematode Caenorhabditis elegans. J Hazard Mater.
274:106–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sparmann A and Bar-Sagi D: Ras-induced
interleukin-8 expression plays a critical role in tumor growth and
angiogenesis. Cancer Cell. 6:447–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian N, Chen X, Han S, Qiang F, Jin G,
Zhou X, Dong J, Wang X, Shen H and Hu Z: Circulating IL-1beta
levels, polymorphisms of IL-1B, and risk of cervical cancer in
Chinese women. J Cancer Res Clin Oncol. 136:709–716. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song Z, Lin Y, Ye X, Feng C, Lu Y, Yang G
and Dong C: Expression of IL-1α and IL-6 is associated with
progression and prognosis of human cervical cancer. Med Sci Monit.
22:4475–4481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naik Dayalu SL, Kumar V and Joshi R: HPV
Inflammation mediate IL-6 through STAT3 signaling pathway in
different grades of cervical cancer. J Cancer Res Molecul Med.
3:1–5. 2016.
|
|
21
|
Alfaro-Moreno E, Torres V, Miranda J,
Martínez L, García-Cuellar C, Nawrot TS, Vanaudenaerde B, Hoet P,
Ramírez-López P, Rosas I, et al: Induction of IL-6 and inhibition
of IL-8 secretion in the human airway cell line Calu-3 by urban
particulate matter collected with a modified method of PM sampling.
Environ Res. 109:528–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang B, Chen D, Zhao H and Xiao C: The
effects for PM2.5 exposure on non-small-cell lung cancer induced
motility and proliferation. Springerplus. 5:20592016. View Article : Google Scholar : PubMed/NCBI
|