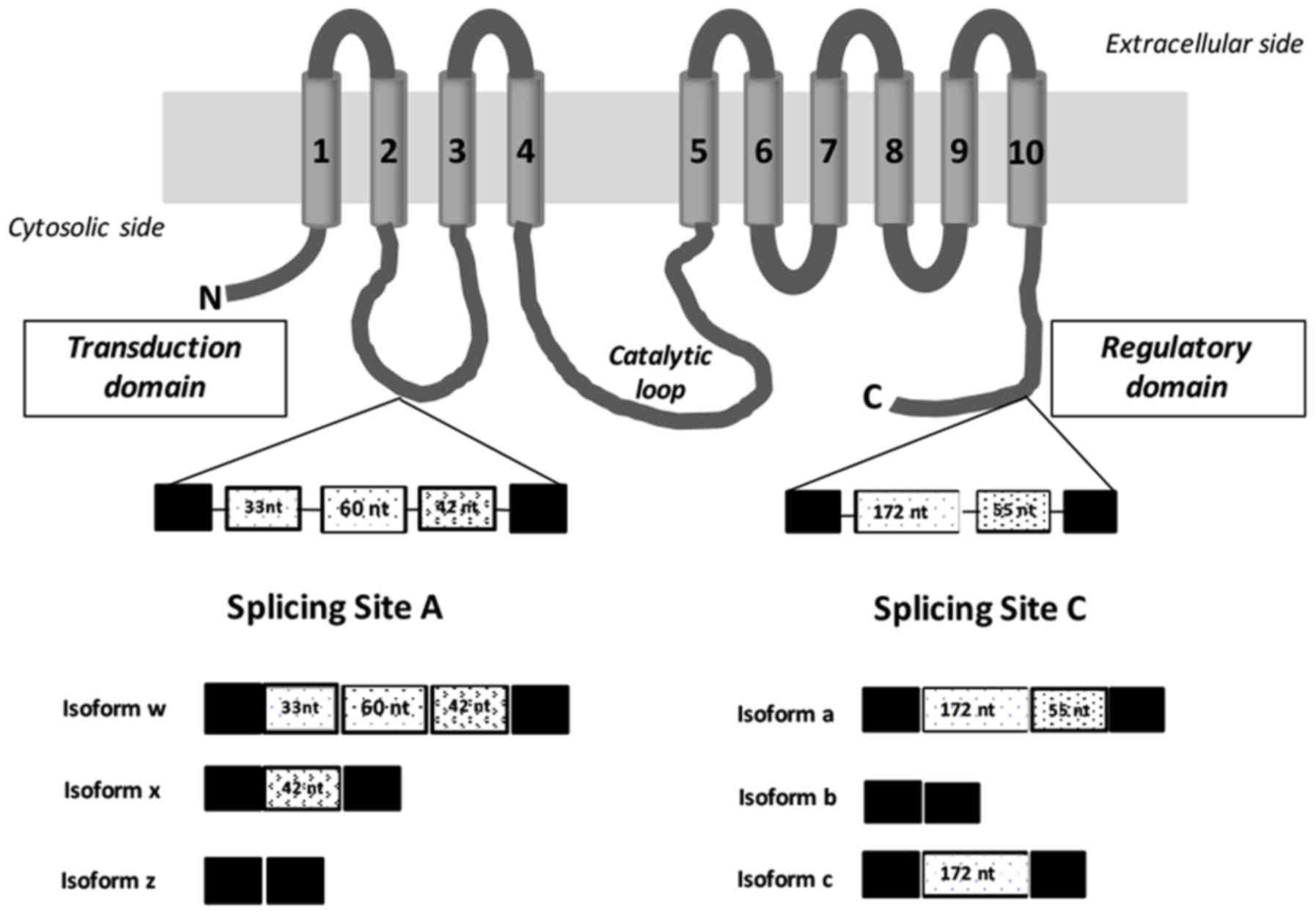
Introduction
The human plasma membrane calcium ATPase (PMCA2)
cooperates with other calcium transport systems and with soluble
Ca2+ binding proteins to control calcium homeostasis in
eukaryotic cells (1–3). The precise control of intracellular
calcium levels is required due to the critical role of
Ca2+ in the modulation of vital physiological processes,
including lactation, proliferation and apoptosis (3–5). In
humans, PMCA2 is expressed in a limited range of tissues, with high
expression levels observed in the nervous system, including
cerebellar Purkinje cells and cochlear hair cells, and the
lactating epithelial mammary gland, and reduced expression levels
of the enzyme are observed in pancreatic beta, corneal epithelium
and muscle cells (6–10). Recent studies have identified elevated
PMCA2 mRNA levels in human breast cancer (11–15).
Furthermore, it has been established that two alternative splicing
domains at the N-terminal first intracellular loop (site A; 2w, 2×,
2z) and at the C-terminal region (site C; 2a, 2b, 2c) produce >9
different variants through mechanisms in which exons are included
or excluded (Fig. 1). Site A includes
exons (33, 60 and 42 bp) that encode residues in the vicinity of a
phospholipid-responsive domain located in the cytoplasmic loop
between the second and third transmembrane domain, which may be
incorporated into the mature mRNA in diverse combinations (16–19),
affecting protein targeting. Site C comprises of exons (172 and 55
bp) that may be included or excluded in the mature mRNA, inducing
changes in the length of the C-terminal tail, which provide
different regulatory and trafficking characteristics to the splice
isoforms (17–20). All of these alternative-splicing
variants have been detected in a tissue-specific gene expression
manner, indicating that diversity in PMCA2 expression variants
serve an important role in Ca2+ signaling in the
physiological and disease conditions; however, splice sites have
only been studied independently and for a number of isoforms, these
sites have not yet been confirmed at the protein level (7,20).
Although the expression of PMCA2 site A or C splice variants has
been described in different tissues (18) and breast cancer cell lines (11), there is limited knowledge regarding
their expression in different types of human breast tumor tissues.
Previous studies regarding tumor cells have identified 2w/b as the
most important isoform expressed in breast cancer; however, no
comprehensive investigation regarding PMCA2 splice variants has
been conducted in human breast and breast cancer tissues. In the
present study, the identification of three PMCA2 splice variants
(2w, 2× and 2z) produced by alternative exon choice in the
N-terminal region of the protein, and one PMCA2 splice variant (2b)
in the C-terminal domain in breast cancer tumor and adjacent
non-tumor tissues of Spanish patients was reported. Relative
expression levels of the different splice isoforms of PMCA2 were
also associated with estrogen receptor (ER), progesterone receptor
(PR), human epidermal growth factor receptor 2 (HER2) and lymph
node status, and tumor stage and histological classification. The
data presented in the present study indicate variable expression of
the different PMCA2 isoform mRNAs in breast tumor and normal
tissues and highlight the relevance of this p-type ATPase in the
context of breast cancer and the physiology of the breast.
Materials and methods
Tissue collection and tumor
specimens
In total, 85 tumor and 69 adjacent non-tumor tissue
specimens, obtained from 85 Spanish patients with primary breast
cancer, were examined in the present study. Paired tumor and
non-tumor tissues were available for 69 of the patients with breast
cancer. RNA samples were obtained from Biobanc de l'Hospital Clínic
de Barcelona, Institut d'Investigacions Biomèdiques August Pi i
Sunyer (Barcelona, Spain); however, they were unable to provide
information regarding age and sex distribution, and the data range
of recruitment/sample collection. Informed consent was obtained
from all patients who donated samples to this tumor bank. The study
protocol was approved by the National Research Ethics Committee of
the Hospital Clinic and the Regional Research Ethics committee of
the Madrid Community. All tumor tissues were histologically
classified as ductal or lobular carcinomas by the Biobanc de
l'Hospital Clínic de Barcelona, Institut d'Investigacions
Biomèdiques August Pi i Sunyer (Table
I). Data on ER, PR and HER2 receptor status, determined through
immunohistochemistry conducted by the Biobanc de l'Hospital Clínic
de Barcelona, Institut d'Investigacions Biomèdiques August Pi i
Sunyer, were available for 78, 79 and 54 tumor tissues,
respectively, and were positive in 55, 52 and 36 cases,
respectively (Table I). For 7, 6 and
31 tumor tissues, no data were available regarding ER, PR and HER2
status, respectively. A total of 38 primary breast tumor tissues
were lymph-node positive and 42 were lymph-node negative, with no
information available for the 5 remaining tumor tissues. A total of
24 of the tumor tissues were <2 cm, 45 were 2–5 cm and 13 were
>5 cm; the size data were not available for 3 tumors (Table I). Disease was staged as recommended
by the American Joint Committee on Cancer based on tumor extension
(T), spread to the lymph nodes (N) and the presence of metastasis
(M) [TNM system, (21)]. A total of
18 tumor tissues were classified as stage I, 35 as stage II and 25
as stage III, and 7 were not staged.
 | Table I.Relative mRNA levels (mean and
standard deviation) for the 2w, 2z, 2× and 2b splice isoforms of
PMCA2 in breast tumor tissue vs. adjacent non-tumor tissue by
histological and clinical features. |
Table I.
Relative mRNA levels (mean and
standard deviation) for the 2w, 2z, 2× and 2b splice isoforms of
PMCA2 in breast tumor tissue vs. adjacent non-tumor tissue by
histological and clinical features.
|
| PMCA2 splice
isoform |
|---|
|
|
|
|---|
| Clinicopathological
data | 2w | 2z | 2× | 2b |
|---|
| ER
statusa |
|
|
|
|
|
Positive (n=55), mean ±
SD | 4.14±10.96 | 2.60±5.01 | 2.22±3.09 | 3.25±5.52 |
|
Negative (n=23), mean ±
SD | 3.64±8.08 | 3.36±4.70 | 2.42±3.69 | 2.11±2.50 |
|
P-value | 0.048 | 0.588 | 0.917 | 0.977 |
| PR
statusa |
|
|
|
|
|
Positive (n=52), mean ±
SD | 2.69±4.27 | 1.77±2.64 | 1.95±2.98 | 3.45±5.68 |
|
Negative (n=27), mean ±
SD | 6.38±16.12 | 4.77±7.19 | 2.84±3.68 | 1.81±2.19 |
|
P-value | 0.352 | 0.024 | 0.215 | 0.717 |
| HER2
statusa |
|
|
|
|
|
Positive (n=36), mean ±
SD | 2.62±3.47 | 1.98±3.0 | 2.47±3.44 | 3.90±4.60 |
|
Negative (n=18), mean ±
SD | 3.11±5.45 | 2.67±3.52 | 1.88±2.52 | 0.175±2.66 |
|
P-value | 0.248 | 0.811 | 0.378 | 0.014 |
| Nodal
statusa |
|
|
|
|
|
Positive (n=38), mean ±
SD | 4.18±13.65 | 2.27±3.82 | 1.65±2.70 | 1.75±1.51 |
|
Negative (n=42), mean ±
SD | 3.47±4.94 | 3.18±5.65 | 2.74±3.57 | 3.74±6.29 |
|
P-value | 0.165 | 0.112 | 0.054 | 0.881 |
| Tumor size
(cm)b |
|
|
|
|
| ≤2
(n=24), mean ± SD | 3.16±5.39 | 3.28±6.97 | 1.97±2.20 | 4.36±7.78 |
| 2–5
(n=45), mean ± SD | 5.09 ± 12.69 | 2.99 ± 3.96 | 2.76±3.91 | 2.46±2.71 |
| ≥5
(n=13), mean ± SD | 0.78±0.66 | 0.89±0.54 | 0.89±0.64 | 1.40±1.50 |
|
P-value | 0.100 | 0.07 | 0.168 | 0.476 |
| Histological
classificationa |
|
|
|
|
|
Ductal/CDI (n=59), mean ±
SD | 4.26±11.48 | 2.58±3.93 | 1.93±2.89 | 2.63±5.27 |
|
Lobulillar/CLI (n=26), mean ±
SD | 2.59±3.35 | 4.33±9.26 | 3.44±4.70 | 4.27±5.76 |
|
P-value | 0.285 | 0.689 | 0.028 | 0.006 |
| Tumor
stageb |
|
|
|
|
| I
(n=18), mean ± SD | 3.40±5.81 | 3.32±7.81 | 1.79±2.10 | 4.09±7.88 |
| II
(n=35), mean ± SD | 6.34±14.85 | 3.14±4.22 | 3.30±4.47 | 2.61±3.03 |
| III
(n=5), mean ± SD | 1.19±1.35 | 1.72±2.61 | 1.13±0.83 | 1.74±1.48 |
|
P-value | 0.515 | 0.097 | 0.250 | 0.796 |
RNA isolation and cDNA synthesis
RNA samples were directly obtained from Biobanc de
l'Hospital Clínic de Barcelona, Institut d'Investigacions
Biomèdiques August Pi i Sunyer. RNA was extracted using an RNeasy
plus Universal Mini kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocols. RNA integrity and
concentration was determined using an Agilent Bioanalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA). cDNA was synthesized at
the Universidad Europea de Madrid using Superscript III
First-Strand Synthesis SuperMix (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocols. For cDNA synthesis, 250 ng RNA from each
sample was used as the starting material. Negative RT was performed
according to the manufacturer's protocols without adding
SuperScript™ III enzyme for a set of representative samples (3
tumor and 3 adjacent non-tumor tissues).
Design of isoform specific
primers
The primers used for reverse
transcription-polymerase chain reaction (RT-PCR) and
RT-quantitative PCR (RT-qPCR) were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Primer sets for RT-PCR were based
on published studies of PMCA2 mRNA expression in different human
tissues and breast cancer cell lines (6,16,18), and are included in Table II. In the present study, pair 5AF-5AR
was used for splicing site A and pair 8BF-8BR was used for splicing
site C (Table II). These primers
were gene-specific and selected to flank the alternative splicing
sites A (2w, 363 bp; 2×, 270 bp; 2y, 320 bp; and 2z, 228 bp) and C
(2a, 584 bp; 2b, 357 bp; and 2c, 529 bp). Primers for RT-qPCR
quantification of human PMCA2 mRNA were designated using Primer3
software v3.0.1 (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Amplification primer sets for qPCR were based on published
human PMCA2 sequences available at www.ensembl.org, and amplicons were intron-spanning
and 50–152 bp in length. These primers were isoform-specific and
selected to flank alternative splice sites A and/or C (Table II). Designs were optimized to ensure
high efficiencies, and verified by generating standard curves using
serial dilutions of either cDNA or a purified polymerase PCR
template.
 | Table II.Primers for PMCA isoforms expression
analysis using quantitative polymerase chain reaction analysis and
for normal reverse transcription-polymerase chain reaction (RT-PCR)
(5AF-5AR; 8BF-8BR). |
Table II.
Primers for PMCA isoforms expression
analysis using quantitative polymerase chain reaction analysis and
for normal reverse transcription-polymerase chain reaction (RT-PCR)
(5AF-5AR; 8BF-8BR).
| Splicing isoform
site | Strand | Sequence
(5′→3′) | Amplicon size
(bp) | Isoform |
|---|
| Oligo name for
PMCA-2 Site A |
|
|
|
|
|
1AF | Forward |
GTGTGAAGAAGGGGGATGGC | 104 | 2w |
|
1AR | Reverse |
CTGCATTTTACCATTGACTAGGCTGG |
|
|
|
2AF | Forward |
TCAGACTGGCATCATCTTTACCCTCC | 80 | 2× |
|
2AR | Reverse |
CCTGCATTTTACCTTTTTTGTCTTTCTTCTC |
|
|
|
3AF | Forward |
GTGTGAAGAAGGGGGATGGC | 109 | 2y |
|
3AR | Reverse |
CCGTCCTGTTGTTTGGCATTGAC |
|
|
|
4AF | Forward |
GACAAAAAAGCCAAACAACAGGACGG | 73 | 2z |
|
4AR | Reverse |
CTCGGCACTCTTGAGGGGCTG |
|
|
|
5AF | Forward |
TGACTGCTGTGGGTGTG | 363 | 2w |
|
5AR | Reverse |
CAGCTTGGTGAGCTTGC | 270 | 2× |
|
|
|
| 320 | 2y |
|
|
|
| 228 | 2z |
| Oligo
name for PMCA-2 Site C |
|
|
|
|
|
6BF | Forward |
GCCCTAGTCGCGTGTCGTTGTCC | 77 | 2a |
|
6BR | Reverse |
CTAGCCCTGCCCGGCTGACG |
|
|
|
7BF | Forward |
GGCAGGCAGGCTCACACAGAAG | 52 | 2b |
|
7BR | Reverse |
CACGACGCGGATCTGTGTCTGG |
|
|
|
8BF | Forward |
CACCATCCCTACCAGCAGGCT | 584 | 2a |
|
|
|
| 357 | 2b |
|
|
|
| 529 | 2c |
|
8BR | Reverse |
GCTCGAGTTCTGCTTGAGCGC |
|
|
| Housekeeping oligo
name |
|
|
|
|
|
9HF | Forward |
TACTTGGATAACTGTGGTAATTCTAGAG | 110 | 18S |
|
9HR | Reverse |
AGGGGCTGACCGGGTTGG |
| rRNA |
Nomenclature
A naming system was used for full-length PMCA
molecules described by Chicka and Strehler (19) and also was used to label splice
variants with the isoform number and letter. Accordingly, 2w refers
to the ‘w’ splice variant of PMCA2, and PMCA2w/b refers to PMCA2
with variant 2w at splice-site A and variant 2b at splice-site C.
Furthermore, PMCA2w refers to PMCA2 with 2w at splice-site A and
either variant at splice-site C.
RT-PCR analysis for alternative
splicing of PMCA2
RNA aliquots of 250 ng were subjected to RT as
aforementioned. The obtained cDNA (25 µl) was used to assess the
PMCA2 alternative splicing at sites A and C. PCR was performed
using recombinant Taq DNA polymerase (Invitrogen; Thermo Fisher
Scientific, Inc.) and 2 mM primers, as previously described
(6,16,18). For
amplification of isoforms PMCA2 cDNA of site A, following an
initial 5 min at 95°C, amplification of 38 cycles consisted of 95°C
for 1 min, 52°C for 1 min and 72°C for 1 min, and then a final step
of 72°C for 10 min. For amplification of isoforms PMCA2 cDNA of
site C, following an initial 5 min at 95°C, amplification of 38
cycles consisted of 95°C for 1 min, 59°C for 1 min and 72°C for 1
min, and then a final step of 72°C for 10 min. The PCR product
bands were visualized by electrophoresis on 2% agarose gels for
bands of site A, and 1.5% agarose for bands of site C using 0.5
mg/ml ethidium bromide for UV transillumination. Analysis of bands
was performed with Image Lab 6.0 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). In order to confirm the specificity of
the PCR products obtained for sites A and C, nested PCR was
performed. For each PMCA2 isoform, an aliquot of the product of the
first round of PCR was used as template. In the second round of
PCR, specific oligos for each PMCA2 isoform (Table II) and identical conditions to
RT-qPCR quantification were used. The nested PCR product bands were
visualized by electrophoresis on 3% agarose gels using RedSafe for
UV transillumination. Analysis of bands was performed with Image
Lab 6.0 software.
RT-qPCR for alternative splicing of
PMCA2
RT-qPCR was performed in triplicate using a Bio-Rad
CFX96 Real-Time PCR Thermocycler (Bio-Rad Laboratories, Inc.).
Using Bio-Rad CFX Manager 3.0 software (Bio-Rad Laboratories,
Inc.), cycle quantifications (Cq) were calculated for each
reaction. Cycle-fluorescence growth curves for tumor and non-tumor
samples were measured using Bio-Rad CFX Manager 3.0 software
(Bio-Rad Laboratories, Inc.). By plotting fluorescence against the
cycle number, the real-time PCR instrument generates an
amplification plot that represents the accumulation of product over
the duration of the entire PCR reaction. All samples were assayed
in 96-well plates. The results provided are based on the means of
three experiments. All expression data were normalized to 18S
ribosomal RNA (rRNA). To determine mRNA expression for PMCA2 and
18S rRNA, SYBR® Green assays in a final volume of 10
µl/well were conducted. The thermocycling conditions for each
isoform were 95°C for 30 sec, followed by 38 cycles at 95°C for 5
sec and 65°C (isoforms: 2b and 2w) or 63°C (isoforms: 2y, 2× and
2z) for 10 sec for all assays. For all reactions quantifying 18S
rRNA, primers were included at 50 nM and were cycled in separate
plates at a 1:1,000 dilution of total cDNA using the same method
for each specific target. For melting curve analysis, a
dissociation step (50°C for 5 sec, and then increased by 0.5°C
every 5 sec until 95°C) was added. The melting peak curve was
obtained using Bio-Rad CFX Manager 3.0 software. The change in
fluorescence/change in temperature (−ΔF/ΔT) is plotted against
temperature to obtain a clear view of the melting dynamics. Agarose
gel electrophoresis of the amplified products for all primer sets
revealed single bands of the expected size (Table II). The bands were extracted using
the MinElute Gel Extraction kit (Qiagen GmbH, Hilden, Germany; 10
µl final volume). An aliquot (3 µl) of purified product was
sequenced (NZYTech, Lda., Lisbon, Portugal) using the RT-qPCR
specific primers for each isoform (Table
II). Sequences were analyzed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome)
and human PMCA2 gene sequence information from Ensembl [ATP2B2
ENSG00000157087; www.ensembl.org, Ensembl Release 92 (22)].
Data and statistical analysis
PMCA2 mRNA RT-qPCR data were analyzed using the
comparative Cq method. Normalized mRNA expression was calculated
using the ΔCq method (23) by
subtracting the mean Cq-value of triplicates of selected isoforms
from the mean Cq-value of triplicates of the housekeeping gene.
Additionally, the expression levels of each isoform of PMCA2 mRNA
relative to their expression levels in adjacent non-tumor tissues
was estimated using the following steps and equations: i)
Normalized level of mRNA expression of each PMCA isoform in all
tumor and adjacent non-tumor samples: ΔCq=Cq of PMCA2 isoform-Cq
housekeeping gene (18S rRNA). ii) Relative mRNA expression of each
isoform in tumor tissue: ΔΔCq=ΔCq tumor sample-ΔCq adjacent
non-tumor tissue. When no corresponding non-tumor tissue was
available, relative mRNA expression was calculated vs. a non-tumor
tissue sample from another patient with the identical tumor type.
The relative expression (E) of each isoform (mean ± standard
deviation) in a tumor sample=2(−ΔΔCq), where E>1 indicates
over-expression and E<1 indicates under-expression.
The Kolmogorov-Smirnov test was used to confirm the
normality of the data. In order to compare variables across
different categorical clinicopathological characteristics including
ER, PR and HER2 status, lymph node involvement and explanatory
variables, including tumor size, histological classification and
stage, the non-parametric Kruskal-Wallis test was used and the
Mann-Whitney U test was used for pairwise comparison. All
statistical tests were performed using the software package SPSS
(version 21; IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Characterizing alternative splice
variant PMCA2 mRNA expression by qualitative RT-PCR
RT-PCR for PMCA2 using splice variant-sensitive
primers for sites A and C determined PMCA2 products in tumor and
adjacent non-tumor samples. A total of 20 samples were analyzed by
RT-PCR and representative examples of samples for sites A and C are
depicted in Fig. 2A and B. According
to the oligos used for amplification, for site A, four RT-PCR
product bands (363, 320, 270 and 228 bp) should be observed;
however, the present tumor and non-tumor samples were determined to
express three mRNA isoforms [PMCA2w (363 bp), PMCA2× (270 bp) and
PMCA2z (228 bp)], and it was not possible to detect PMCA2y (320 bp)
using this technique (Fig. 2A). For
site C, three RT-PCR product bands (584, 357 and 529 bp) should
have been detected; however, tumor and non-tumor samples primarily
expressed PMCA2b (357 bp) mRNA, with PMCA2a (584 bp) and PMCA2c
(520 bp) not detected (Fig. 2B).
Bands corresponding to particular PMCA2 splice variants were then
identified by comparing bp sizes of the RT-PCR products to those of
published expected bp sizes (6,16,18).
 | Figure 2.RT-PCR analysis of PMCA2 performed
with oligos, as previously described, in order to amplify sites A
and C isoforms. Total RNA was isolated from representative human
breast tumor samples and adjacent tissue samples. The RT-PCR
products for sites A and C were run on 2 and 1.6% electrophoresis
agarose gels, respectively. RT-PCR amplifications were performed
without or with reverse transcriptase. (A) For site A, three PCR
amplification products were detected in the tumor and non-tumor
tissue samples: PMCA2w (363 bp), PMCA2× (270 bp) and PMCA2z (228
bp). (B) For site C, one band corresponding to PMCA2b mRNA (357 bp)
was observed in all the breast tissue samples and no other products
were detected. The sizes of a number of DNA molecular weight
markers are depicted. bp, base pairs; n, negative PCR control
without complementary DNA; N, adjacent tissue samples; T, human
breast tumor samples; -, without reverse transcriptase; +, with
reverse transcriptase; RT-PCR, reverse transcription, polymerase
chain reaction; PMCA2, plasma membrane calcium ATPase 2. |
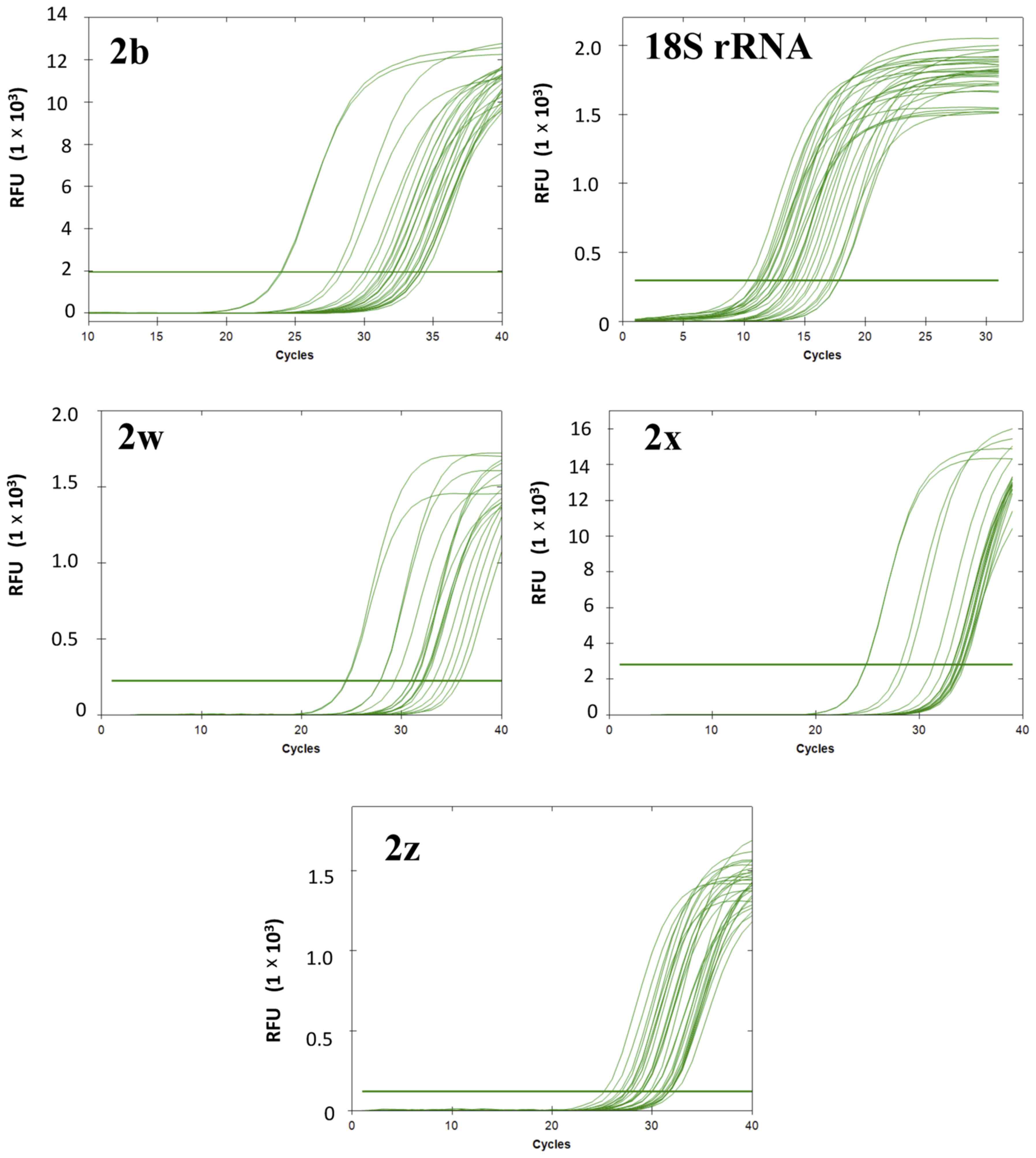
Product specificity of RT-qPCR
analysis
The expression of PMCA2 splice isoforms and of the
reference gene (18S rRNA) was determined in tumor and non-tumor
samples. As an example of the RT-qPCR results, cycle-fluorescence
growth curves for each of the splicing isoforms (PMCA2b, PMCA2w,
PMCA2z and PMCA2×) and 18S rRNA in a number of representative
samples are depicted in Fig. 3. The
PMCA2 splicing isoforms exhibited Cq values ranging from
11.05–17.61 for the reference gene (18S rRNA), 24.28–34.63 for
PMCA2b, 25.36–33.34 for PMCA2z, 25.58–34.63 for PMCA2× and
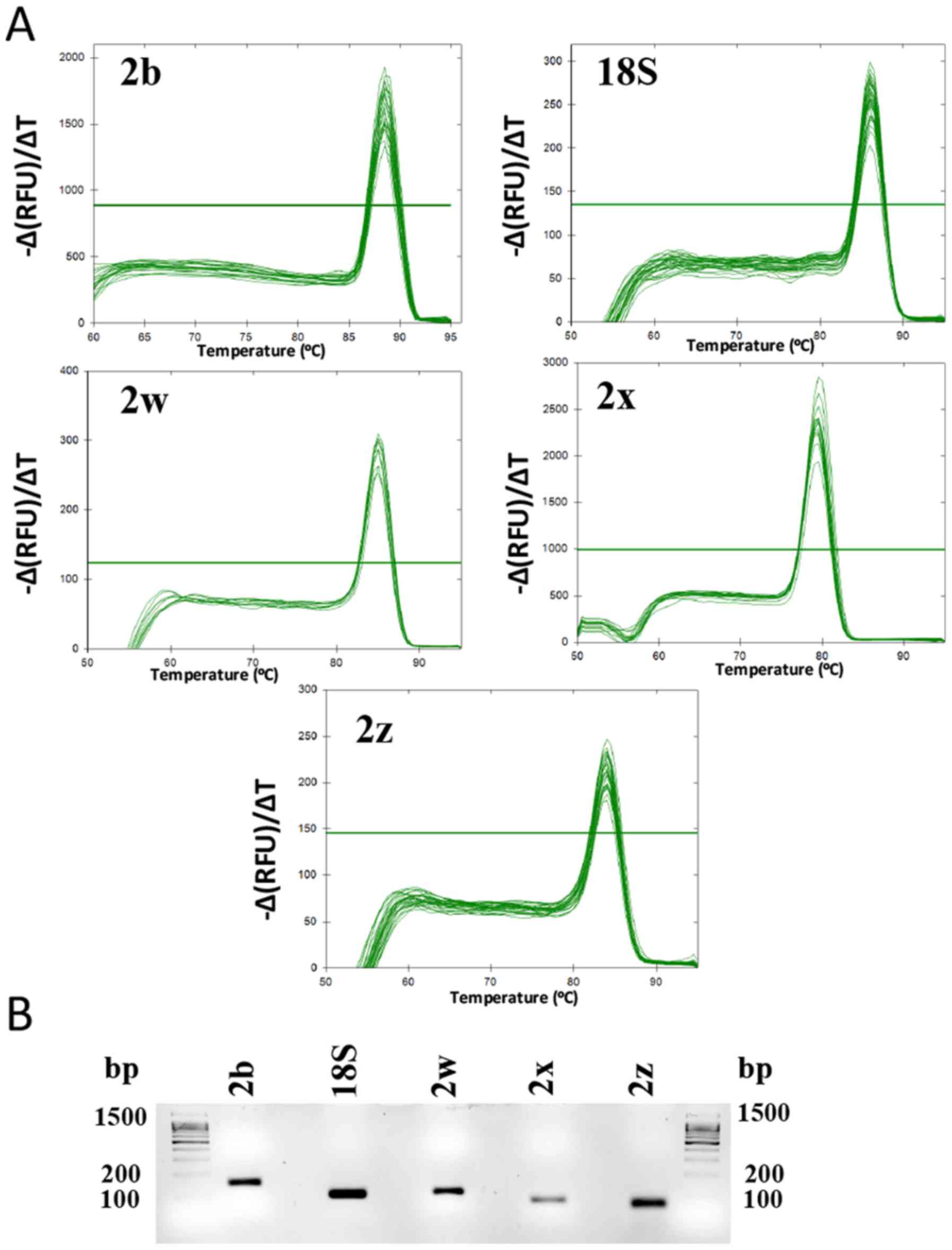
23.87–34.72 for PMCA2w. In the melt peak analysis, a single
homogeneous peak for all primer sets was detected (Fig. 4A). Agarose gel electrophoresis
analysis of the amplified products for all primer sets revealed
single bands of the expected size (Table
II; Fig. 4B). Furthermore, the
specificity of the PCR products was confirmed by a nested PCR for
each PMCA2 isoform, where single bands of the expected size were
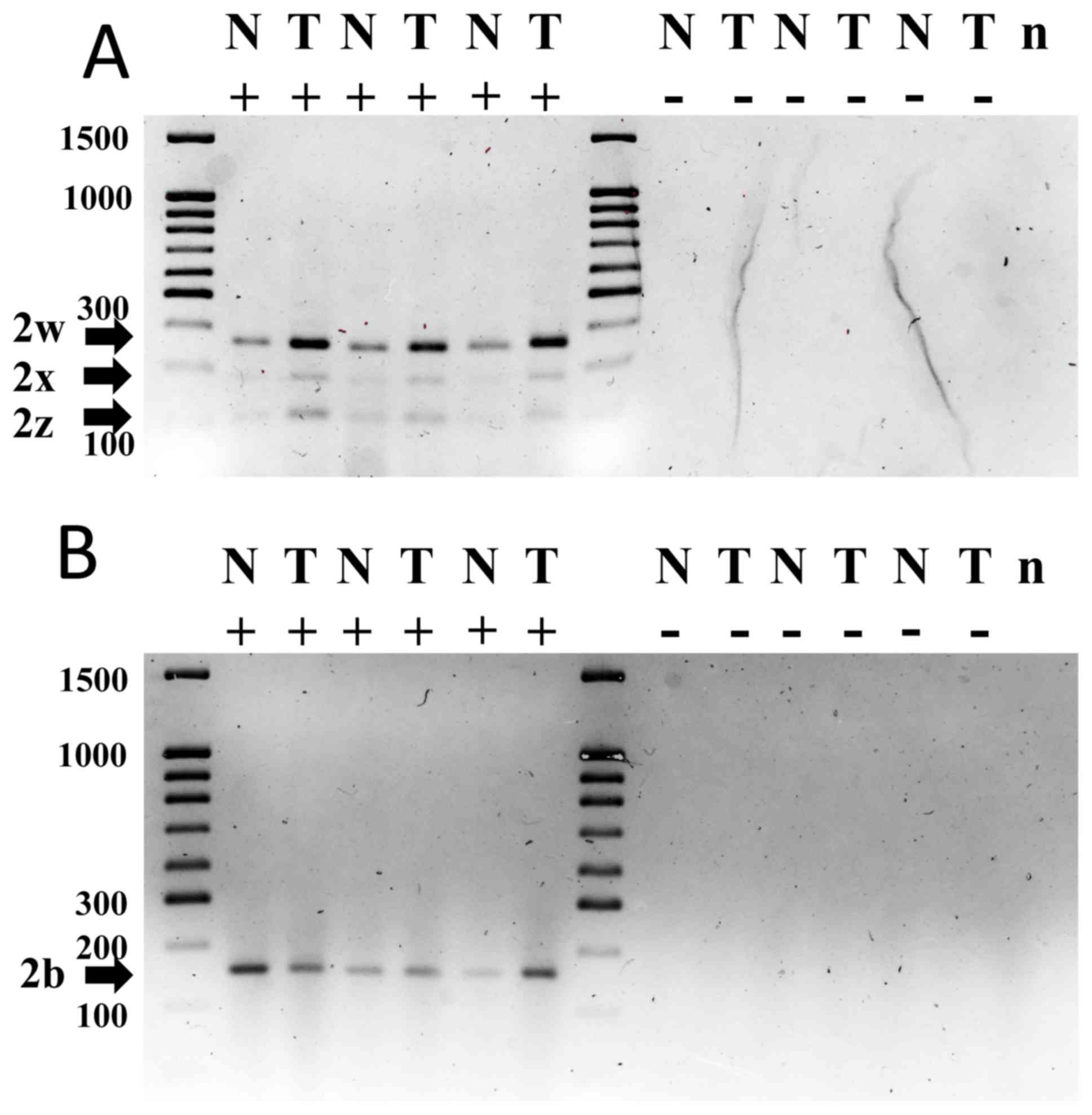
observed for each of the PMCA2 isoforms (Fig. 5). Sequence data analysis of the qPCR
products (Fig. 4B) using BLAST
verified the identity of each of the amplified products as Human
ATP2B2 using [Ensemble version: ENSG00000157087.18; Ensembl Release
92 (22)]. PMCA2b: ATP2B2-202
ENST00000360273.6, ATP2B2-205 ENST00000452124.2 and ATP2B2-204
ENST00000397077.6; PMCA2w: ATP2B2-202 ENST00000360273.6 and
ATP2B2-214 ENST00000645850.1; PMCA2×: ATP2B2-205 ENST00000452124.2
and ATP2B2-215 ENST00000643662.1; PMCA2z: ATP2B2-204
ENST00000397077.6; and Human 18S rRNA: GenBank: KY962518.1 and
Chromosome 21: 8,437,037-8,437,147 [www.ensembl.org, Ensembl Release 92 (22)].
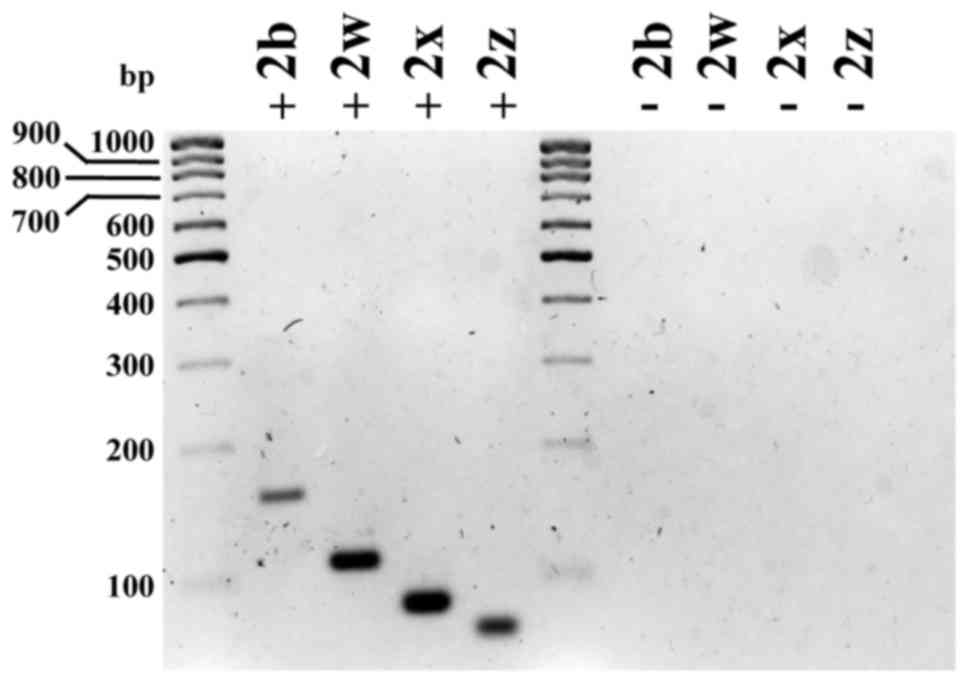 | Figure 5.Nested PCR for plasma membrane
calcium ATPase 2 using specific RT-qPCR oligos revealed single
bands of the expected size, confirming the specificity of the PCR
products: 2w, 152 bp; 2w, 104 bp; 2×, 80 bp; 2z, 73 bp. RT-PCR was
performed using total RNA isolated from tissue adjacent to the
tumor (N). For electrophoresis, PCR products were run on 3% agarose
gel. The sizes of a number of DNA molecular weight markers are
depicted. bp, base pairs; +, with cDNA; -, without cDNA; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
Differential expression of alternative
splicing PMCA2 mRNA isoforms in breast tumor tissues, compared with
in adjacent tissues, by qPCR
mRNA expression levels of PMCA2 splice isoforms in
the 85 mammary carcinoma tissues and 69 adjacent non-tumor tissues
were determined by qPCR. These experiments demonstrated that, for
site A, the isoforms expressed in tumor and adjacent tissues were
PMCA2w, PMCA2× and PMCA2z. It was not possible to quantify the
expression of isoform PMCA2y. Table I
lists the relative mRNA expression levels of the different splice
isoforms (PMCA2w, PMCA2z, PMCA2× and PMCA2b) recorded in tumor
tissues, compared with in non-tumor tissues. These experiments
confirmed that, for site C, the isoform PMCA2b was expressed in
tumor and adjacent tissues, and it was not possible to quantify the
expression of the PMCA2a isoform. mRNA levels for the different
isoforms demonstrated high biological variability and increased
expression was observed in the breast tumor tissues, compared with
in adjacent tissues (Table I).
Associating PMCA2 mRNA levels with the
clinical and histological characteristics of human breast tumor
tissues
The expression of PMCA2 splice isoform mRNA was also
assessed in association with the clinical characteristics of the
patients, including tumor histological classification and size,
disease stage, and lymph node involvement (Table I). Significant associations were
determined between PMCA2 expression and tumor histology, with
PMCA2b expression levels being significantly reduced in ductal-type
tumor tissues, compared with in lobulillar-type tumor tissues
(P<0.006; Table I), and PMCA2×
expression was significantly increased in lobulillar-type tumor
tissues, compared with in ductal-type tumor tissues (P<0.028).
Additionally, non-significant associations were demonstrated
between PMCA2z expression and tumor size (P<0.07). Furthermore,
PMCA2× mRNA expression was increased in lymph node-negative tumor
tissues, compared with lymph node-positive tumor tissues, but this
difference was not significant (P=0.054; Table I).
Differential expression of alternative
splicing PMCA2 mRNA isoforms, according to the hormone receptor
status
The splice PMCA2 isoform mRNA expression was
investigated in association with the molecular markers of breast
cancer ER, PR and HER2 status (Table
I). Significant associations were determined between PMCA2z
expression and PR status (P<0.024), with significantly increased
expression in PR-negative tumor tissues, compared with in
PR-positive tumor tissues. Furthermore, when comparing expression
levels of mRNA of the PMCA2 isoforms according to the ER status,
significantly increased PMCA2w expression determined in the
ER-positive tumor tissues, compared with in the ER-negative tumor
tissues (P<0.048; Table I) and
PMCA2b overexpression observed in the HER2-positive tumor tissues,
compared with in HER2-negative tumor tissues.
Discussion
In humans, the expression of PMCA2 mRNA is limited
to a reduced number of tissues, including the brain and mammary
gland (20). Besides a role in
mammary gland physiology, PMCA2 mRNA levels are elevated in breast
cancer cell lines (11,12,24) and a
number of studies have detected a strong association between high
PMCA2 mRNA expression and reduced survival time (3,14). The
present data indicated that PMCA2 mRNA is expressed in different
types of human breast tumor and adjacent non-tumor tissues. Despite
high variability in expression levels, these were increased in the
tumor specimens, compared with in adjacent healthy tissue (Table I). The differential expression of
PMCA2 could induce changes in Ca2+ efflux and this
capacity has been associated with the diminished sensitivity of
cells to apoptosis and an enhanced response of cancer cells to
proliferative stimuli (25–28). Following thorough consideration, a
cohort of Spanish patients with breast tumor types were selected to
compare the expression levels of PMCA2 splice variants in breast
tumor and adjacent non-tumor tissues. These were then used to
examine the relevance of PMCA2 in a context of human breast cancer
and the physiology of the human breast. Currently, the expression
of PMCA2 splice isoforms has been described in specialized tissues
(20,29), including inner ear hair cells, the
nervous system and the lactating gland. PMCA2 exhibits the highest
binding affinity for calmodulin, but simultaneously, in the absence
of calmodulin, it exhibits high basal activity indicating its
importance in specialized cells, where there is a requirement to
pump Ca2+ at a high rate (30,31).
Splicing at Site A inserts up to 3 exons and at site C one or two
exons (Fig. 1), indicating that up to
nine different variants may be produced by alternative splicing.
The present data indicated that at least three different PMCA2 mRNA
splice variants (PMCA2w/b PMCA2z/b and PMCA2×/b) are expressed at
different levels in a number of subtypes of breast tumor and normal
breast tissue. This observation is consistent with data from rodent
and pre-lactation human breast models, indicating that PMCA2 is a
key pump responsible for Ca2+ efflux from the maternal
compartment into milk (32–34), but prompts further questions due to
>1 splice isoform were detected. Previous studies regarding the
mammary gland in pregnant and lactating rats demonstrated that
following parturition and during lactation, total PMCA2 protein
expression is upregulated in the rat mammary gland due to a
predominant increase in the PMCA2w/b splice variant (32,34–37), in
order to match the demands of Ca2+ homeostasis in the
mammary gland. This data is consistent with functional studies
indicating that the 2b splice variant has an increased affinity for
calmodulin, compared with the splice variant 2a (31,32), and
the 2w splice option at splice site A results in the localization
of PMCA2 at apical membranes (10,19,38–40).
Consistently, high expression levels of the PMCA2w/b isoform have
been observed on the epithelial cell luminal membrane in tissue
specimens from a patient with breast cancer in the third trimester
of pregnancy (41); however, we are
unaware of the significance and localization of the other two
isoforms 2z/b and 2×/b detected in this study in the normal
physiology of the human mammary gland and in breast cancer.
Previous studies regarding recombinant protein expression have
indicated that alternative splicing at site A influences the apical
or basolateral localization of PMCA2, including PMCA2w, which is
located at the apical membrane, and PMCA2× and PMCA2z, which are
located at the basolateral membrane, and this occurs regardless of
whether the COOH terminal splices correspond to the 2b or 2a
isoforms (19); therefore, it would
be notable to examine whether the isoforms 2z/b and 2×/b are
translated into functional proteins. Furthermore, it would also be
notable to examine the 5′untranslated region (UTR) of PMCA2 in
humans, due to the four different mice transcriptional start
regions being described in this UTR region (36), but only two are specifically used to
control PMCA2 expression in the mammary gland during lactation.
PMCA2w/a expression was not detected in the present human mammary
tissue samples. This variant results in a truncated form of the
pump due to the frame shift, which has been detected exclusively in
the outer hair cells of the inner ear (29).
In the present study, there was a significantly
increased expression of PMCA2×/b in lobulillar-type tumor tissues,
compared with in ductal-type tumor tissues (P<0.028). This data
is similar to recent evidence indicating that lobulillar and ductal
are two separate subtypes of breast cancer at the clinical and
molecular level, as demonstrated by different gene profiles
(42). The detection of high levels
of PMCA2 mRNA is consistent with the data of previous studies
demonstrating that PMCA2 overexpression results in the reduced
transcription activity of nuclear factor of activated T cells
(NFAT) in breast cancer (2,43). Furthermore, previous studies conducted
in PC12 cells indicated that NFAT inhibition is involved in the
regulation of the PMCA2× splice variant (44). These results highlighted the complex
association that exists between PMCA2 and NFAT signaling in breast
cancer.
The present results indicated high PMCA2z expression
in PR-negative tumor tissues, while PMCA2w was significantly
overexpressed in ER-positive tumor tissues, indicating differences
in tumor characteristics that may be associated with hormone
levels, impacting the mRNA levels of these two variants. In
agreement with previous data, the present data also demonstrated
that PMCA2b was significantly overexpressed in HER2-positive tumor
tissues (15); therefore, PMCA2b
could be used as a marker for HER2-positive tumor tissues, which
have been associated with a poor prognosis (45). A limitation of the present study is
the lack of availability of clinical data on patients with breast
cancer. Furthermore, PMCA2× mRNA expression was increased in lymph
node-negative tumor tissues, compared with in lymph node-positive
tumor tissues, but this difference was not significant (P=0.054);
therefore, increased PMCA2× expression may be associated with less
aggressive tumor behavior.
In conclusion, the present data determined the
expression of a number of splice variants of PMCA2 in breast tumor
and adjacent tissues, including PMCA2w, PMCA2z and PMCA2× for site
A, and PMCA2b for site C. Notably, the expression in of the PMCA2z
and PMCA2× variants, which are primarily expressed in brain tissue,
was also determined in the breast tumor types. The differential
distribution and expression of PMCA2 splice variants was dependent
on hormone receptor status and histological classification of the
tumor. PMCA2b was significantly overexpressed in HER2-positive
tumor tissues, indicating that high mRNA levels of PMCA2b could be
a marker of poor prognosis. The present data indicated PMCA2
isoform-specific differences in human breast tissues and provides
direction for future studies designed to produce further insight
into the role of the alternative splicing isoforms PMCA2w/b,
PMCA2z/b and PMCA2×/b in breast cancer.
Acknowledgements
The authors would like to thank the Biobanc Core
Facility of the Institut d'Investigacions Biomèdiques August Pi i
Sunyer (Barcelona, Spain) for technical help. The authors would
also like to thank Professor Margarita Rubio (Department of Basic
Biomedical Sciences, Faculty of Biomedical and Health Sciences,
Universidad Europea de Madrid, Madrid, Spain) for help with the
statistical analysis, Mrs Maria Gregoria Montalvo-Lominchar
(Doctoral Studies and Research School, Universidad Europea de
Madrid, Villaviciosa de Odón, Madrid, Spain) and Mrs Carolina
Sánchez (Doctoral Studies and Research School, Universidad Europea
de Madrid) for technical help with the RT-qPCR assays, and
Professor Mr Pablo Gómez del Arco, (Molecular Biology Department,
Universidad Autónoma de Madrid, Madrid, Spain) for technical help
with the sequence analysis of splicing isoforms of PMCA2 and 18S
rRNA reference gene.
Funding
The present study was funded by the Universidad
Europea de Madrid (project 2014/UEM005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ARL performed the primer design, cDNA synthesis and
qPCR assays, contributed to the study design and contributed to the
writing of the paper. MG performed qualitative PCR assays and
contributed to the writing of the paper. ALA contributed to the
study design and provided input on drafting the work and revising
it critically for intellectual content. AFS contributed to the
study design, statistical analysis and contributed to the writing
of the paper. AN performed qPCR assays, contributed to the study
design, statistical analysis and drafted the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all patients who
donated samples to this tumor bank. The study protocol was approved
by the National Research Ethics Committee of the Hospital Clinic
and the Regional Research Ethics committee of the Madrid
Community.
Patient consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
bp
|
base pairs
|
|
Cq
|
cycle quantification
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
PMCA2
|
plasma membrane calcium ATPase 2
|
|
PR
|
progesterone receptor
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
site A
|
first intracellular loop of ATPase
2
|
|
site C
|
C-terminal region of ATPase 2
|
|
TNM system
|
Tumor-Node-Metastasis system
|
|
18S rRNA
|
18S ribosomal RNA
|
|
nt
|
nucleotides
|
|
UTR
|
untranslated region
|
References
|
1
|
Strehler EE, Filoteo AG, Penniston JT and
Caride AJ: Plasma-membrane Ca(2+) pumps: Structural diversity as
the basis for functional versatility. Biochem Soc Trans.
35:919–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Padányi R, Pászty K, Hegedűs L, Varga K,
Papp B, Penniston JT and Enyedi Á: Multifaceted plasma membrane
Ca(2+) pumps: From structure to intracellular Ca(2+) handling and
cancer. Biochim Biophys Acta. 1863:1351–1363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stafford N, Wilson C, Oceandy D, Neyses L
and Cartwright EJ: The plasma membrane calcium ATPases and their
role as Major new players in human disease. Physiol Rev.
97:1089–1125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brini M, Calì T, Ottolini D and Carafoli
E: The plasma membrane calcium pump in health and disease. FEBS J.
280:5385–5397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruce JIE: Metabolic regulation of the
PMCA: Role in cell death and survival. Cell Calcium. 69:28–36.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Váradi A, Molnár E and Ashcroft SJ: A
unique combination of plasma membrane Ca2+-ATPase isoforms is
expressed in islets of Langerhans and pancreatic beta-cell lines.
Biochem J. 314:663–669. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strehler EE: Plasma membrane calcium
ATPases: From generic Ca(2+) sump pumps to versatile systems for
fine-tuning cellular Ca(2.). Biochem Biophys Res Commun. 460:26–33.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talarico EF Jr, Kennedy BG, Marfurt CF,
Loeffler KU and Mangini NJ: Expression and immunolocalization of
plasma membrane calcium ATPase isoforms in human corneal
epithelium. Mol Vis. 11:169–178. 2005.PubMed/NCBI
|
|
9
|
Talarico EF Jr and Mangini NJ: Alternative
splice variants of plasma membrane calcium-ATPases in human corneal
epithelium. Exp Eye Res. 85:869–879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calì T, Brini M and Carafoli E: Regulation
of cell calcium and role of plasma membrane calcium ATPases. Int
Rev Cell Mol Biol. 332:259–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee WJ, Roberts-Thomson SJ, Holman NA, May
FJ, Lehrbach GM and Monteith GR: Expression of plasma membrane
calcium pump isoform mRNAs in breast cancer cell lines. Cell
Signal. 14:1015–1022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee WJ, Roberts-Thomson SJ and Monteith
GR: Plasma membrane calcium-ATPase 2 and 4 in human breast cancer
cell lines. Biochem Biophys Res Commun. 337:779–783. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farmer P, Bonnefoi H, Becette V,
Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J,
Cameron D, Goldstein D, et al: Identification of molecular apocrine
breast tumours by microarray analysis. Oncogene. 24:4660–4671.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
VanHouten J, Sullivan C, Bazinet C, Ryoo
T, Camp R, Rimm DL, Chung G and Wysolmerski J: PMCA2 regulates
apoptosis during mammary gland involution and predicts outcome in
breast cancer. Proc Natl Acad Sci USA. 107:11405–11410. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong J, VanHouten JN, Dann P, Kim W,
Sullivan C, Yu H, Liotta L, Espina V, Stern DF, Friedman PA and
Wysolmerski JJ: PMCA2 regulates HER2 protein kinase localization
and signaling and promotes HER2-mediated breast cancer. Proc Natl
Acad Sci USA. 113:E282–E290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heim R, Hug M, Iwata T, Strehler EE and
Carafoli E: Microdiversity of human-plasma-membrane calcium-pump
isoform 2 generated by alternative RNA splicing in the N-terminal
coding region. Eur J Biochem. 205:333–340. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hilfiker H, Guerini D and Carafoli E:
Cloning and expression of isoform 2 of the human plasma membrane
Ca2+ ATPase. Functional properties of the enzyme and its splicing
products. J Biol Chem. 269:26178–26183. 1994.PubMed/NCBI
|
|
18
|
Stauffer TP, Hilfiker H, Carafoli E and
Strehler EE: Quantitative analysis of alternative splicing options
of human plasma membrane calcium pump genes. J Biol Chem.
268:25993–26003. 1993.PubMed/NCBI
|
|
19
|
Chicka MC and Strehler EE: Alternative
splicing of the first intracellular loop of plasma membrane
Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem.
278:18464–18470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strehler EE and Zacharias DA: Role of
alternative splicing in generating isoform diversity among plasma
membrane calcium pumps. Physiol Rev. 81:21–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC (American Joint Committee on Cancer)
Cancer Staging Manual. Springer (eds); 7th. New York: 2010
|
|
22
|
Zerbino DR, Achuthan P, Akanni W, Amode
MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, et
al: Ensembl 2018. Nucleic Acids Res. 46:D754–D761. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monteith GR, Davis FM and Roberts-Thomson
SJ: Calcium channels and pumps in cancer: Changes and consequences.
J Biol Chem. 287:31666–31673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts-Thomson SJ, Curry MC and Monteith
GR: Plasma membrane calcium pumps and their emerging roles in
cancer. World J Biol Chem. 1:248–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mu L, Tuck D, Katsaros D, Lu L, Schulz V,
Perincheri S, Menato G, Scarampi L, Harris L and Yu H: Favorable
outcome associated with an IGF-1 ligand signature in breast cancer.
Breast Cancer Res Treat. 133:321–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Curry MC, Luk NA, Kenny PA,
Roberts-Thomson SJ and Monteith GR: Distinct regulation of
cytoplasmic calcium signals and cell death pathways by different
plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer
cells. J Biol Chem. 287:28598–28608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Curry M, Roberts-Thomson SJ and Monteith
GR: PMCA2 silencing potentiates MDA-MB-231 breast cancer cell death
initiated with the Bcl-2 inhibitor ABT-263. Biochem Biophys Res
Commun. 478:1792–1797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krebs J: The plethora of PMCA isoforms:
Alternative splicing and differential expression. Biochim Biophys
Acta. 1853:2018–2024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lopreiato R, Giacomello M and Carafoli E:
The plasma membrane calcium pump: New ways to look at an old
enzyme. J Biol Chem. 289:10261–10268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brini M, Di Leva F, Ortega CK, Domi T,
Ottolini D, Leonardi E, Tosatto SC and Carafoli E: Deletions and
mutations in the acidic lipid-binding region of the plasma membrane
Ca2+ pump: A study on different splicing variants of isoform 2. J
Biol Chem. 285:30779–30791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reinhardt TA and Horst RL: Ca2+-ATPases
and their expression in the mammary gland of pregnant and lactating
rats. Am J Physiol. 276:C796–C802. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brini M and Carafoli E: Calcium pumps in
Health and disease. Physiol Rev. 89:1341–1378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reinhardt TA, Lippolis JD, Shull GE and
Horst RL: Null mutation in the gene encoding plasma membrane
Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol
Chem. 279:42369–42373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reinhardt TA and Lippolis JD: Mammary
gland involution is associated with rapid down regulation of major
mammary Ca2+-ATPases. Biochem Biophys Res Commun. 378:99–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Silverstein RS and Tempel BL: Atp2b2,
encoding plasma membrane Ca2+-ATPase type 2, (PMCA2) exhibits
tissue-specific first exon usage in hair cells, neurons, and
mammary glands of mice. Neuroscience. 141:245–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Faddy HM, Smart CE, Xu R, Lee GY, Kenny
PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ and
Monteith GR: Localization of plasma membrane and secretory calcium
pumps in the mammary gland. Biochem Biophys Res Commun.
369:977–981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hill JK, Williams DE, LeMasurier M, Dumont
RA, Strehler EE and Gillespie PG: Splice-site A choice targets
plasma-membrane Ca2+-ATPase isoform 2 to hair bundles. J Neurosci.
26:6172–6180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Antalffy G, Caride AJ, Pászty K, Hegedus
L, Padanyi R, Strehler EE and Enyedi A: Apical localization of
PMCA2w/b is enhanced in terminally polarized MDCK cells. Biochem
Biophys Res Commun. 410:322–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Padányi R, Xiong Y, Antalffy G, Lór K,
Pászty K, Strehler EE and Enyedi A: Apical scaffolding protein
NHERF2 modulates the localization of alternatively spliced plasma
membrane Ca2+ pump 2B variants in polarized epithelial cells. J
Biol Chem. 285:31704–31712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peters AA, Milevskiy MJ, Lee WC, Curry MC,
Smart CE, Saunus JM, Reid L, da Silva L, Marcial DL, Dray E, et al:
The calcium pump plasma membrane Ca(2+)-ATPase 2 (PMCA2) regulates
breast cancer cell proliferation and sensitivity to doxorubicin.
Sci Rep. 6:255052016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Desmedt C, Zoppoli G, Sotiriou C and
Salgado R: Transcriptomic and genomic features of invasive lobular
breast cancer. Semin Cancer Biol. 44:98–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holton M, Yang D, Wang W, Mohamed TM,
Neyses L and Armesilla AL: The interaction between endogenous
calcineurin and the plasma membrane calcium-dependent ATPase is
isoform specific in breast cancer cells. FEBS Lett. 581:4115–4119.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kosiorek M, Podszywalow-Bartnicka P,
Zylinska L and Pikula S: NFAT1 and NFAT3 cooperate with HDAC4
during regulation of alternative splicing of PMCA isoforms in PC12
cells. PLoS One. 9:e991182014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eroles P, Bosch A, Pérez-Fidalgo JA and
Lluch A: Molecular biology in breast cancer: Intrinsic subtypes and
signaling pathways. Cancer Treat Rev. 38:698–707. 2012. View Article : Google Scholar : PubMed/NCBI
|