Introduction
Head and neck cancer (HNC) is the sixth most common
cancer worldwide. At present, ~650,000 new cases of HNC are
diagnosed globally each year (1).
Cisplatin is the most widely used cytotoxic drug and, as the
epidermal growth factor receptor (EGFR) is almost invariably
expressed in HNC, drugs targeting EGFR have also been employed as
HNC therapeutics. Anti-EGFR monoclonal antibodies, cetuximab and
panitumumab, have produced some improvement in survival when used
in combination with cytotoxic drugs to treat recurrent or
metastatic disease (2,3). However, recent large clinical trials of
anti-EGFR monoclonal antibodies or tyrosine kinase inhibitors used
in addition to platinum-based chemoradiation regimens have produced
disappointing results (4,5). Moreover, adding cetuximab to
chemoradiotherapy may enhance toxicity and, thus, requires further
investigation (6). Therefore, it is
important to improve the therapeutic efficacy of current HNC
interventions. We recently described a novel method that allows
suboptimal doses of biotinylated agents to become effective
therapies in models of HNC. The treatment is based on the
intra-tumor injection of AvidinOX, an oxidized avidin derivative
that exhibits the distinctive property of forming Schiff's bases
with tissue proteins (7–11), followed by systemically administered
radioactive biotin (12) or
biotinylated cetuximab (bCet) (13).
In the present study, we show that the injection of AvidinOX into
FaDu pharynx squamous cell carcinoma cells xenografted in the mouse
tongue increases the anti-tumor activity of a suboptimal bCet dose
administered intraperitoneally after 24 h. The data also indicate
that the treatment can be repeated at least twice, 1 week apart,
and that the therapeutic efficacy could be further improved by
including a low dose of cisplatin in the protocol. AvidinOX is
currently under clinical investigation for use in targeting
177lutetium-biotinDOTA (177Lu-ST2210)
(14) to inoperable tumor lesions
(ClinicalTrials.gov NCT02053324 and
NCT03188328). The present results support that using an intra-tumor
injection of AvidinOX prior systemic administration of low dose
bCet (with or without cisplatin) may be useful in patients with
HNC.
Materials and methods
Cells and reagents
Human FaDu squamous cell carcinoma of the pharynx
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA; ATCC® HTB-43™) and maintained in a
humidified atmosphere with 5% CO2 at 37°C. Working cell
banks were established and all experiments were performed using
cells within 6–8 passages after thawing.
AvidinOX® (registered brand of Alfasigma
S.p.A., Rome, Italy) was prepared according to previously described
methods (13) and the protein
solution (3.0 mg/ml in acetate buffer, pH 5.5) was stored at −80°C
until use. Cetuximab (Erbitux®; Merck KGaA, Darmstadt,
Germany) was biotinylated and controlled as previously described
(12). Cisplatin
(cis-diamine-platinum-dichloride; cat. no. P4394; Sigma-Aldrich;
Merck KGaA) was dissolved in saline solution before
administration.
Animal model
Female athymic nude mice, 5–6 weeks old, were
purchased from Charles River Laboratories (Wilmington, MA, USA).
The present study was approved by the Ethics Committee of Alfasigma
S.p.A., (Pomezia, Rome, Italy) and authorized by the Italian
Ministry of Health (46/2014-PR). Animal studies were performed in
accordance with the ‘Directive 2010/63/UE’ on the protection of
animals used for scientific purposes, made effective in Italy by
the Legislative Decree 4 March 2014, n. 26, and ARRIVE guidelines
(15). At the end of the treatment
period, mice were euthanized by CO2 asphyxia (air
displacement rate, 20%/min) as indicated in the American Veterinary
Medical Association Panel on Euthanasia and according to the
guidelines described by the United Kingdom Co-ordinating Committee
on Cancer Research (1998). Mice were maintained in a pathogen-free
facility and the tongue was inoculated with 50 µl suspension of
FaDu cells (4×105) in Hanks' balanced salt solution with
Matrigel RGF (1:1). Tumor-bearing mice were randomized into groups
of 10 and received 75 µg/25 µl AvidinOX intratumorally or the same
volume of AvidinOX formulation buffer (vehicle) 24 h before each
drug administration. At 5 days before the first drug treatment,
mice were switched to a biotin-free diet. Treatments were first
administered at 19 days after tumor transplantation. bCet and
cisplatin were delivered intraperitoneally at the indicated doses
according to the schedule Q7dx2. The doses of bCet and cisplatin
were selected, based on preliminary studies, to induce tumor growth
inhibition of ~20%. Tumor measurements were performed using a
digital Vernier caliper twice per week and tumor volume (TV)
calculated using the following equation: Volume=length ×
(width)2/2. Efficacy of treatment was assessed as TV
inhibition percentage (TVI%) in treated vs. control mice,
calculated as: TVI%=100-(mean TV treated/mean TV control ×100).
Body weight was recorded throughout the study. Tumor doubling time
was extrapolated from semi log best-fit curves of mean tumor
volumes plotted against time. Doubling time was the ln 2/b where b
was the slope of linear regressions. Mice were euthanized 5 days
after the second treatment. At 24 h before sacrifice, mice received
a further intraperitoneal injection of bCet, cisplatin or both.
Histology and immunohistochemistry
(IHC)
Tumor masses were harvested and fixed in 10%
phosphate-buffered formalin for 12 h at 4°C. Samples were then
dehydrated in ascending concentrations of ethanol, cleared with
xylene and paraffin embedded. Tissue slices were obtained using a
rotary microtome (5 µm sections) and processed for IHC. For
histology, hematoxylin/eosin staining was performed according to
standard methods.
Briefly, after deparaffination and rehydration,
sections were treated with 0.01 mol/l citrate buffer and 0.05%
Tween 20 (pH 6.0; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
a microwave for 15 min for antigen retrieval, followed by quenching
of endogenous peroxidase activity with 3%
H2O2 in PBS (v/v) for 5 min. Sections were
then incubated with specific antibodies against phospho-γ-H2A
histone family member X (H2A.X; Ser139; cat. no. 20E3) and cleaved
caspase-3 (cat. no. 9664), from Cell Signaling Technology, Inc.,
(Danvers, MA, USA), or against platelet and endothelial cell
adhesion molecule 1 (CD31; cat. no. ab28364) or vascular
endothelial growth factor-C (VEGF-C; cat. no. ab135506) from Abcam
(Cambridge, UK), diluted in blocking buffer (5% goat serum in 0.05%
Triton X-100 and PBS for γ-H2A.X and cleaved caspase-3; 10% goat
serum in PBS for CD31; and 5% goat serum in 0.05% Triton X-100 and
TBS for VEGF-C) overnight at 4°C in a humidified chamber. Negative
controls were incubated without primary antibodies under identical
conditions. Sections were then incubated with the appropriate
biotinylated secondary antibody (1:300), followed by conjugated
horseradish peroxidase-streptavidin (ABC kit; Vector Laboratories,
Inc., Burlingame, CA, USA) and 3,3′-diaminobenzidine (ABC kit)
working solution, then counterstained with hematoxylin. Images were
captured using an optical microscope (Eclipse E800; Nikon
Corporation, Tokyo, Japan) with a JVC KY-F55B color video digital
camera.
IHC staining for γ-H2A.X and cleaved caspase-3 was
quantified as the number of positive cells (brown cells) ×
100/total number of cells, in five fields from two serial
sections/mouse. The mean number of CD31-positive vessels per
mm2 viable tumor for each xenograft was also quantified
in 24 randomly selected fields for each tumor section. The results
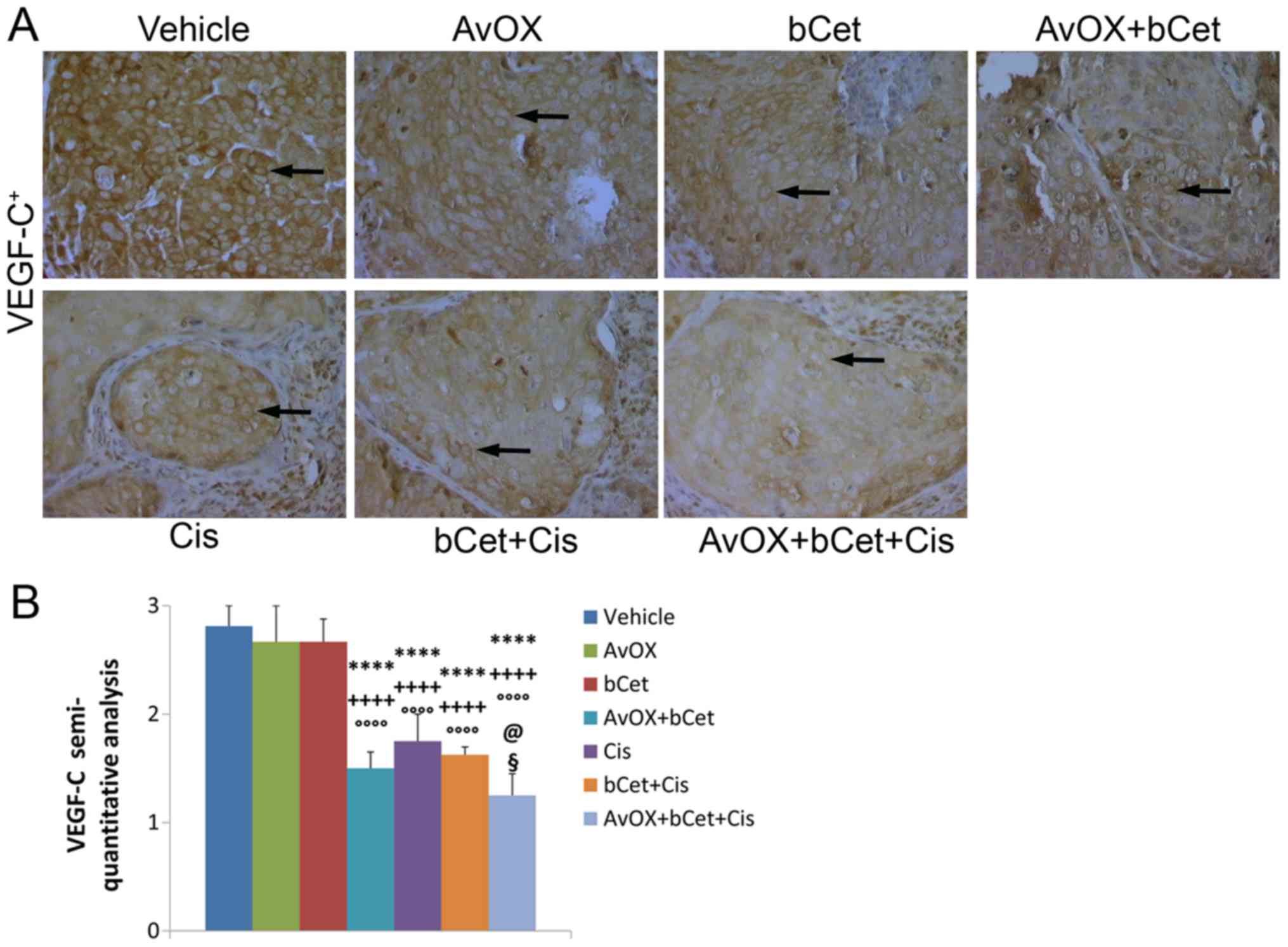
for VEGF-C were expressed according to semi-quantitative criteria:
negative staining, score 0; 1–20% positive cells, score 1+; 21–50%
positive cells, score 2+; and >50% positive cells, score 3+. The
staining intensity was scored on a scale as weak, moderate or
strong (16).
All analysis of tissue sections was performed by two
independent pathologists and data was subsequently confirmed by
computerized measurements (17).
Data are present as the mean ± standard error (SE) of 10
mice/group.
Statistical analysis
All data are presented as the mean ± standard error
or standard deviation, and statistical analyses were performed
using two-way analysis of variance followed by Bonferroni's
multiple-group comparisons (GraphPad Prism v.7.05 Software;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
The effect of the combination of drugs was evaluated
for the following groups: AvidinOX+cisplatin+bCet vs.
cisplatin+bCet; AvidinOX+bCet vs. bCet; and cisplatin+bCet vs.
single drugs; according to the method reported by Romanelli et
al (18). R was calculated as
the ratio of expected and observed T/C% values. An R index of 1
indicates an additive effect, R >1 indicates synergism.
Results
Tumor growth inhibition
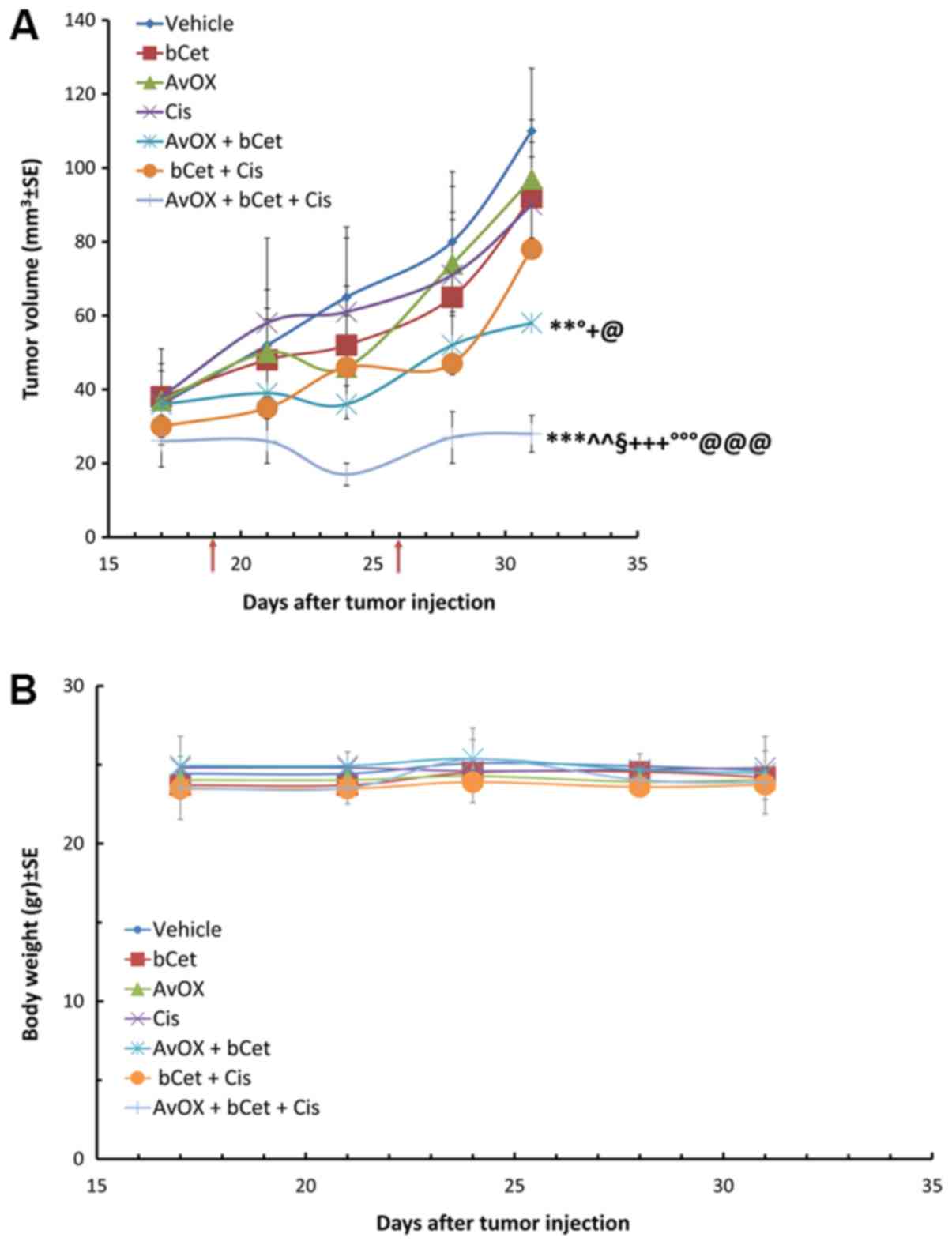
Mice with human FaDu tongue xenografts were treated
with AvidinOX intra-tumorally, followed by intraperitoneal
injection of bCet, with or without a low dose of cisplatin. Data in
Fig. 1A confirm results obtained in
a previous study using FaDu subcutaneous tumor xenografts, which
demonstrated the anti-tumor efficacy of low dose bCet in
AvidinOX-treated tumors. These results are supported by in
vitro data indicating that AvidinOX-anchored bCet causes
induction of EGFR degradation, inhibition of EGFR nuclear
translocation and downstream signaling, plus upregulation of
pro-apoptotic and cell damage markers (13). In the current study, the tumor growth
inhibition of bCet was further improved by additional
administration of low dose cisplatin; in fact, tumor masses treated
with AvidinOX in mice receiving low doses of intraperitoneal bCet
and cisplatin were significantly smaller than the tumor masses of
mice treated with AvidinOX+bCet, or bCet+cisplatin. No toxicity was
observed among all experimental groups, as indicated by body weight
measurement (Fig. 1B).
As shown in Table I,
tumor volume inhibition at the end of the study (day 31) was
significantly higher in mice treated with AvidinOX and low dose
bCet, when a low dose of cisplatin was also administered compared
to the other groups. The observed effect was higher than expected,
based on the results of the AvidinOX+bCet or bCet+cisplatin
treatment groups. The expected/observed ratio values of 1.4 and 2.5
indicate synergistic effects of AvidinOX+bCet and
AvidinOX+bCet+cisplatin, respectively. Tumor doubling time in
AvidinOX+bCet+cisplatin treated mice was also the lowest among the
experimental groups confirming that the addition of low dose
cisplatin to AvidinOX-targeted bCet can further delay tumor
growth.
 | Table I.Tumor growth inhibition of
AvOX-targeted bCet with and without cisplatin. |
Table I.
Tumor growth inhibition of
AvOX-targeted bCet with and without cisplatin.
| Groups | Dose (µg/mouse)
Q7dx2: 19, 26 | TV ± SE day +31
(mm3) | TVI% day +31 | Observed T/C% | Expected T/C% | Expected/observed
ratio | TV doubling time
(days ± SD) |
|---|
| Vehicle | 0 | 110±17 | – |
|
|
| 9.0±0.5 |
| bCet | 40 | 92±15 | 16 | 84 |
|
| 11.8±1.5 |
| AvOX | 75 | 97±16 | 12 | 88 |
|
| 10.4±1.9 |
| Cis | 5 | 90±13 | 18 | 82 |
|
| 12.8±1.9 |
| AvOX + bCet | 75+40 | 58±8a,c,e,g | 47 | 53 | 74 | 1.4k | 19.3±3.0 |
| bCet + Cis | 40+5 | 78±12 | 29 | 71 | 69 | 1.0 | 10.7±1.6 |
| AvOX + bCet +
Cis | 75+40+5 | 28±5b,d,f,h,i,j | 75 | 25 | 63 | 2.5k | 43.9±6.0 |
Immunohistochemistry analysis
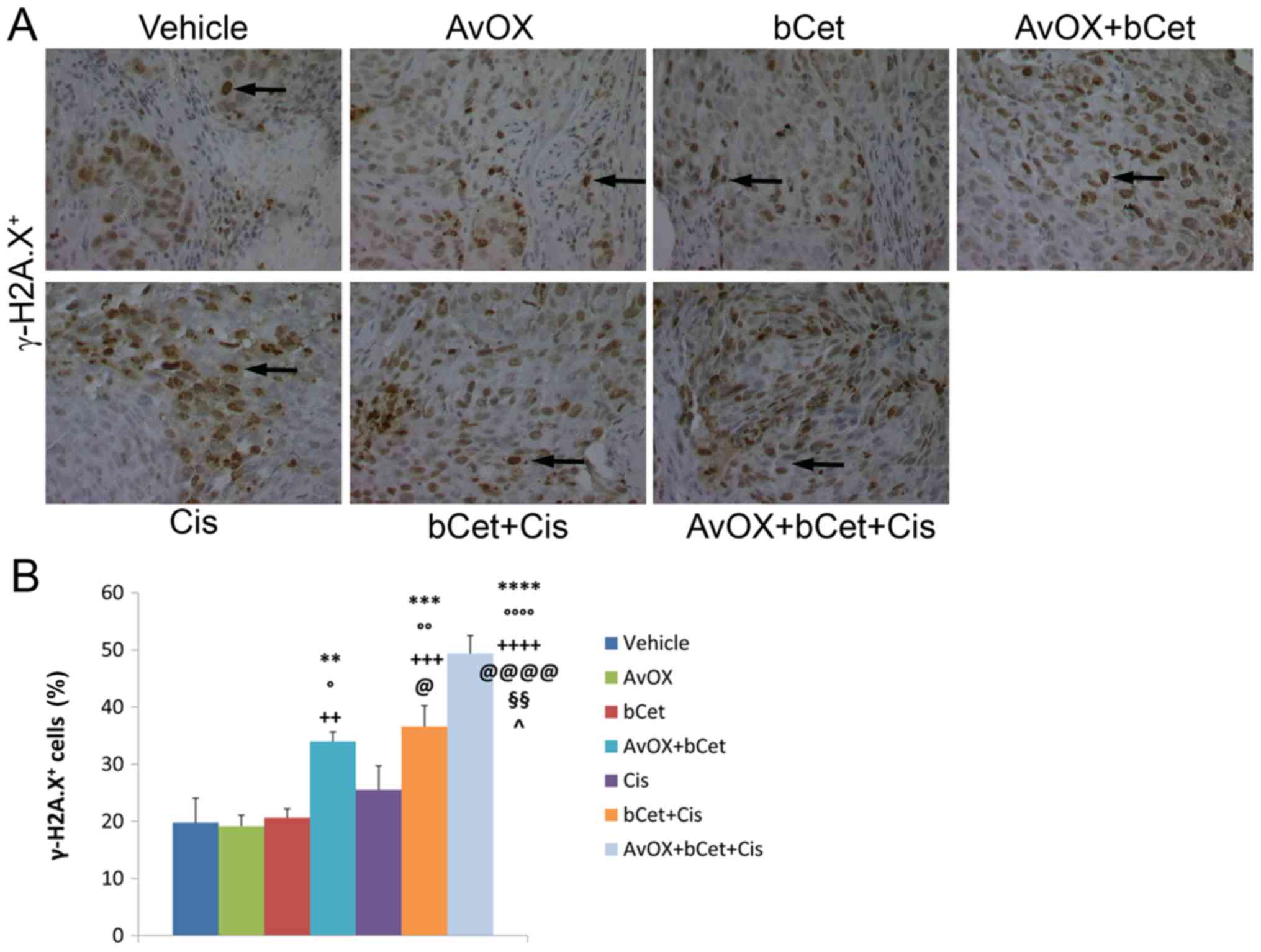
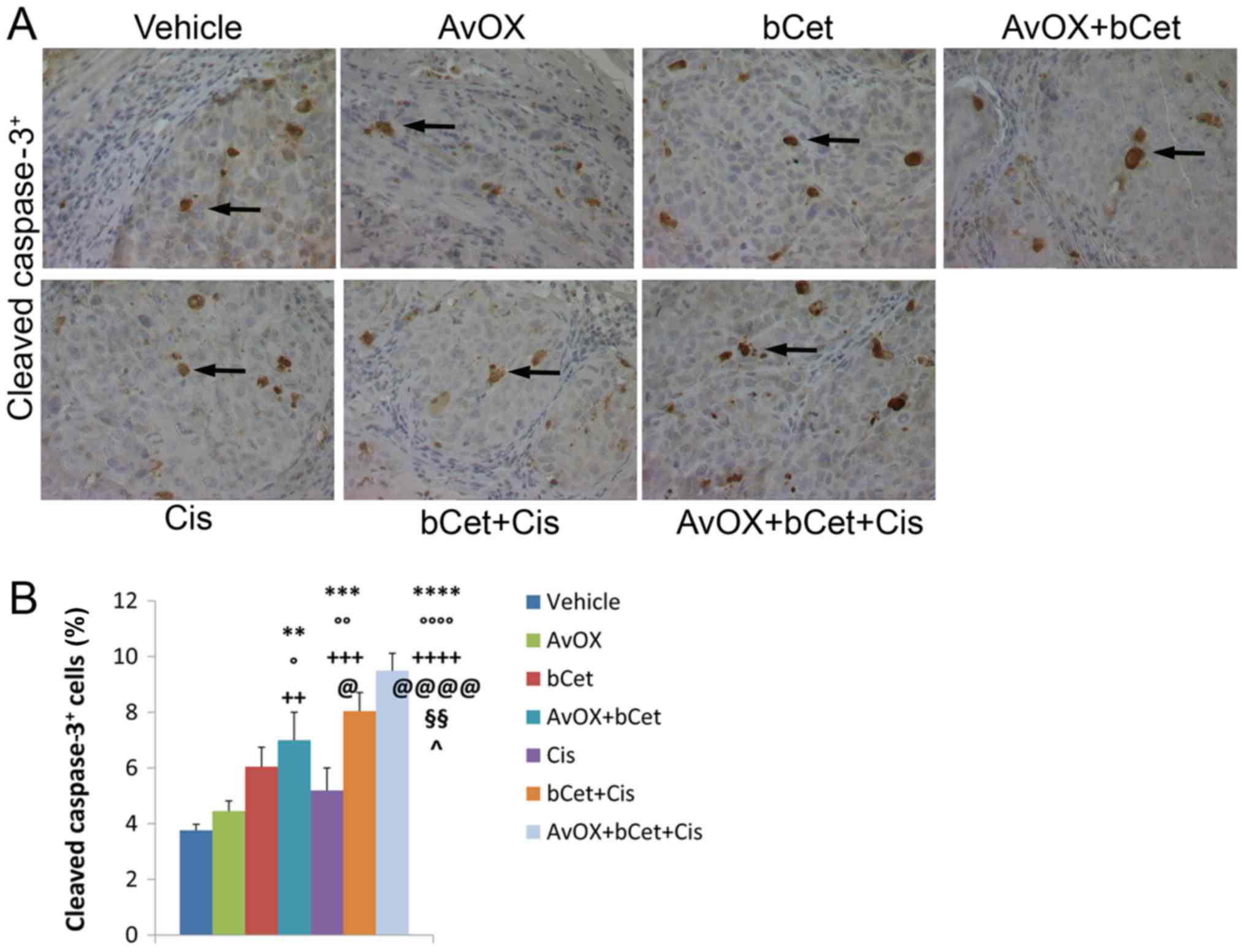
Consistent with tumor growth inhibition,
immunohistochemistry confirmed that tumor masses of mice treated
with AvidinOX, bCet and cisplatin exhibited the highest level of
tumor cell damage, as measured by the number of cells expressing
phosphorylated γ-H2A.X (Fig. 2A) and
cleaved caspase-3 (Fig. 3A). In
fact, the treatment with AvidinOX+bCet+cisplatin induced a
statistically significant increase in the number of γ-H2A.X and
cleaved caspase-3-expressing cells as compared to the
bCet+cisplatin or AvidinOX+bCet treatment groups (Figs. 2B and 3B). Additional serial sections from the
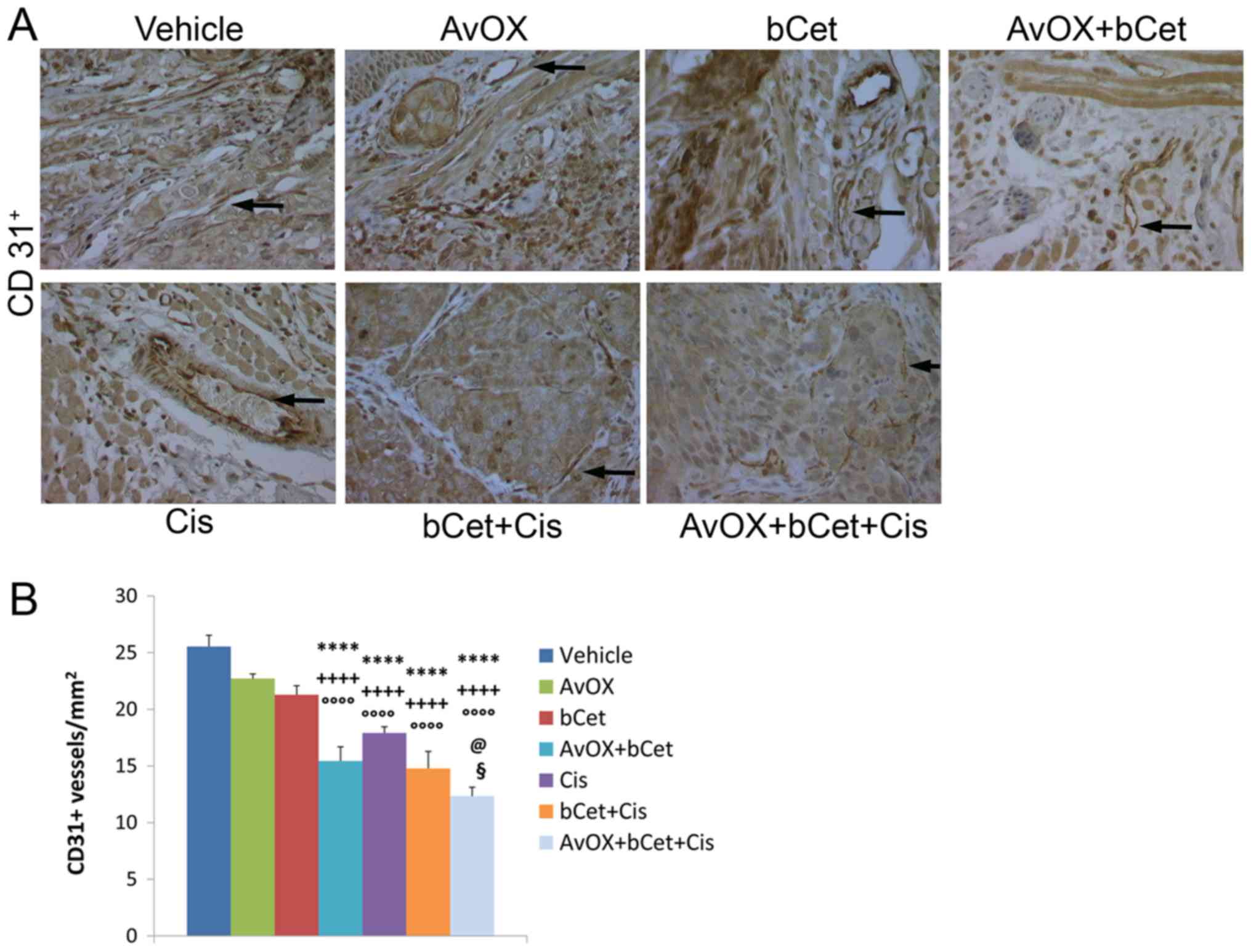
tumor masses of each experimental group were used to investigate
angiogenesis. Microvessel density and lymphangiogenic activity were
evaluated by counting the number of CD31+ cells and
VEGF-C protein expression, respectively. The results showed that
the anti-angiogenic activity of bCet was significantly enhanced by
AvidinOX, whereas AvidinOX did not significantly improve the effect
of bCet+cisplatin (Figs. 4 and
5). The combination of
bCet+cisplatin without AvidinOX was better than bCet alone, but not
better than cisplatin.
Discussion
There is a need to develop local treatments for
cancer lesions that are derived from or reside within
tissues/organs that are very different in terms of stromal
composition, vascularization, density and other features. AvidinOX
has been previously shown to form Schiff's bases with proteins in a
variety of tissues and in several animal species (7–13). Long
tissue residence of AvidinOX is also currently being confirmed in
human patients with liver metastases and other inoperable tumor
lesions as indicated by selective and consistent uptake of
intravenous 177Lu-ST2210 administered 1 and up to 15
days after intra-tumor AvidinOX (ClinicalTrials.gov NCT02053324 and NCT03188328, data
not shown). AvidinOX is a novel delivery tool for targeting a
variety of biotinylated moieties. In fact, we recently published
results that support the potential use of nebulized AvidinOX as an
anchoring agent for nebulized biotinylated monoclonal antibodies
(including cetuximab) for the topical treatment of lung cancer.
Surprisingly, the in vitro potency of bCet, panitumumab,
trastuzumab and pertuzumab is improved by the presence of AvidinOX
on the surface of tumor cells (19,20),
suggesting that AvidinOX could be used as a delivery platform for a
variety of potential therapeutic applications. Intra-tumor
administration of AvidinOX shares similarities with other
procedures common in clinical practice for diagnostic (i.e.
biopsies) and therapeutic (seed/catheter deposition,
alcoholization) purposes. Such procedures have not been linked with
an increased risk of metastasis and we consider that AvidinOX
injection would be similar.
The present work confirms and extends data from a
previous study that showed the efficacy of using AvidinOX in mice
bearing subcutaneous tumor xenografts treated with systemic low
doses of bCet (13). The current
study demonstrated that this therapeutic approach is also effective
in an orthotopic model (tongue xenograft) and that efficacy can be
further improved by adding a low dose of cisplatin to the treatment
protocol. Significant tumor reduction, together with a lack of body
weight loss, lead us to foresee that this treatment might have a
good therapeutic index. With regards to the tumor mass, the
AvidinOX+bCet+cis treatment caused the highest tumor inhibition,
which was significantly increased compared with all other treatment
groups, including bCet+cis. This result, also validated by
histological evaluation of DNA damage and apoptosis, indicated that
AvidinOX significantly improved the effects of cetuximab and
cisplatin. Overall, data highlight that induction of apoptosis of
FaDu cells can be increased by the addition of cisplatin to
AvidinOX-targeted bCet treatment. It is to note that a defect of
the apoptotic signaling in other tumor cells could make them
cisplatin resistant (21). For
example, tumor cells lacking a functional p53 that it is known to
promote cisplatin-dependent apoptosis by binding to
cisplatin-modified DNA and by counteracting the anti-apoptotic
function of Bcl-xL (22), might
resist cisplatin-induced apoptosis while being still responsive to
AvidinOX-anchored bCet.
However, AvidinOX apparently did not have a
significant effect on angiogenesis markers compared with the
bCet+cis treatment. We speculate that while AvidinOX increases the
bCet tumor toxicity, its effect, if any, on angiogenesis is not
direct. In other words, we previously described that when EGFR is
engaged by AvidinOX-anchored biotinylated anti-EGFR antibodies, the
receptor physiology is compromised as measured by inhibition of
receptor internalization and nuclear translocation and by increased
lysosomal degradation, all leading to tumor cell death (13,20). On
the other hand, we believe that the AvidinOX-anchorage of
biotinylated anti-EGFR antibodies has no direct effects on
angiogenesis thus not significantly improving the activity of
bCet+cis. Lymphangiogenesis has a central role in the formation of
lymph node metastases in HNC patients and, besides common markers
like CD31 and VEGF-C here addressed, there are several additional
markers like podoplanin and Lyve-1 that are now being correlated
with propensity to metastatization (23). Therefore, further studies will be
necessary to understand if AvidinOX+bCet+cis might offer some
advantage in controlling lymphangiogenesis, compared to standard
cetuximab+cisplatin treatment protocols.
Finally, yet importantly, the present study
demonstrated the feasibility of using AvidinOX-based approaches in
combinatorial protocols, which are becoming increasingly popular to
prevent or bypass tumor resistance (24). Additionally, we demonstrated the use
of adding cisplatin to the AvidinOX-targeted bCet treatment
combination. The next logical combination could be the addition of
radioactive biotin. In fact, irradiation, in combination with
chemotherapy or immunotherapy, is the most commonly used
therapeutic option for HNC (25),
and we previously demonstrated the therapeutic efficacy of
90Y-BiotinDOTA (90Y-ST2210) in a model of
tongue cancer (12). Considering
these results, AvidinOX-driven protocols could be used to explore
additional treatment combinations, including other biotinylated
monoclonal antibodies against EGFR family members (such as
anti-ErbB2) (20), antibodies
against check point inhibitors with or without radioactive biotin,
or other drugs/devices, as currently pursued in several clinical
protocols (26–29).
Acknowledgements
The authors would like to thank Professor Luigi
Giusto Spagnoli (Histo-cyto Service, Rome, Italy) for supervising
the immunohistochemistry procedures.
Funding
The present study was supported by Alfasigma S.p.A
(Rome, Italy).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RDS conceived the study and wrote the manuscript. LV
designed the study, analyzed the data and wrote the manuscript. AR
established the tumor model and performed the treatments. VC
conducted the immunohistochemistry analyses. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Alfasigma S.p.A. (Pomezia, Rome, Italy).
Patient consent for publication
Not applicable.
Competing interests
LV, AR and RDS are employees of Alfasigma S.p.A.
which holds the patents of AvidinOX.
References
|
1
|
De Felice F, Polimeni A, Valentini V,
Brugnoletti O, Cassoni A, Greco A, De Vincentiis M and Tombolini V:
Radiotherapy controversies and prospective in head and neck cancer:
A literature-based critical review. Neoplasia. 20:227–232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermorken JB, Stöhlmacher-Williams J,
Davidenko I, Licitra L, Winquist E, Villanueva C, Foa P, Rottey S,
Skladowski K, Tahara M, et al: Cisplatin and fluorouracil with or
without panitumumab in patients with recurrent or metastatic
squamous-cell carcinoma of the head and neck (SPECTRUM): An
open-label phase 3 randomised trial. Lancet Oncol. 14:697–710.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermorken JB, Licitra L,
Stöhlmacher-Williams J, Dietz A, Lopez-Picazo JM, Hamid O, Hamid O,
Hossain AM, Chang SC and Gauler TC: Phase II study of pemetrexed in
combination with cisplatin and cetuximab in recurrent or metastatic
squamous cell carcinoma of the head and neck. Eur J Cancer.
49:2877–2883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan
PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage III to
IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martins RG, Parvathaneni U, Bauman JE,
Sharma AK, Raez LE, Papagikos MA, Yunus F, Kurland BF, Eaton KD,
Liao JJ, et al: Cisplatin and radiotherapy with or without
erlotinib in locally advanced squamous cell carcinoma of the head
and neck: A randomized phase II trial. J Clin Oncol. 31:1415–1421.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Numico G, Franco P, Cristofano A,
Migliaccio F, Spinazzé S, Silvestris N, Cante D, Sciacero P, La
Porta MR, Girelli F and Ricardi U: Is the combination of Cetuximab
with chemo-radiotherapy regimens worthwhile in the treatment of
locally advanced head and neck cancer? A review of current
evidence. Crit Rev Oncol Hematol. 85:112–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Santis R, Albertoni C, Rosi A, Leoni B,
Petronzelli F, D'Alessio V, Nucera E, Salvatori G, Paganelli G,
Verdoliva A, et al: OXavidin for tissue targeting biotinylated
therapeutics. J Biomed Biotechnol. 2009:9214342009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Santis R, Leoni B, Rosi A, Albertoni C,
Forni G, Cojoca R, Iezzi M, Musiani P, Paganelli G, Chinol M and
Carminati P: AvidinOX for highly efficient tissue-pretargeted
radionuclide therapy. Cancer Biother Radiopharm. 25:143–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Santis R, Anastasi AM, Pelliccia A,
Rosi A, Albertoni C, Verdoliva A, Petronzelli F, D'Alessio V,
Serani S and Nuzzolo CA: Chemical linkage to injected tissues is a
distinctive property of oxidized avidin. PLoS One. 6:e210752011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nucera E, Nicoletti C, Chiapparino C,
Pacello ML, D'Alessio V, Musarò A and De Santis R: AvidinOX™ for
tissue targeted delivery of biotinylated cells. Int J Immunopathol
Pharmacol. 25:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verdoliva A, Bellofiore P, Rivieccio V,
Catello S, Colombo M, Albertoni C, Rosi A, Leoni B, Anastasi AM and
De Santis R: Biochemical and biological characterization of a new
oxidized avidin with enhanced tissue binding properties. J Biol
Chem. 285:9090–9099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albertoni C, Leoni B, Rosi A, D'Alessio V,
Carollo V, Spagnoli LG, van Echteld C and De Santis R: Radionuclide
therapy of unresectable tumors with AvidinOX and (90) Y-biotinDOTA:
Tongue cancer paradigm. Cancer Biother Radiopharm. 30:291–298.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vesci L, Milazzo FM, Anastasi AM,
Petronzelli F, Chiapparino C, Carollo V, Roscilli G, Marra E,
Luberto L, Aurisicchio L, et al: Intra-tumor AvidinOX allows
efficacy of low dose systemic biotinylated Cetuximab in a model of
head and neck cancer. Oncotarget. 7:914–928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urbano N, Papi S, Ginanneschi M, De Santis
R, Pace S, Lindstedt R, Ferrari L, Choi S, Paganelli G and Chinol
M: Evaluation of a new biotin-DOTA conjugate for pretargeted
antibody-guided radioimmunotherapy (PAGRIT). Eur J Nucl Med Mol
Imaging. 34:68–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kilkenny C, Browne WJ, Cuthill JC, Emerson
M and Altman DG: Improving bioscence research reporting: The ARRIVE
guidelines for reporting animal research. Osteoarthritis Cartilage.
20:256–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massi D, Puig S, Franchi A, Malvehy J,
Vidal-Sicart S, González-Cao M, Baroni G, Ketabchi S, Palou J and
Santucci M: Tumour lymphangiogenesis is a possible predictor of
sentinel lymph node status in cutaneous melanoma: A case-control
study. J Clin Pathol. 59:166–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to imageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romanelli S, Perego P, Pratesi G, Carenini
N, Tortoreto M and Zunino F: In vitro and in vivo interaction
between cisplatin and topotecan in ovarian carcinoma systems.
Cancer Chemother Pharmacol. 41:385–390. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Santis R, Rosi A, Anastasi AM,
Chiapparino C, Albertoni C, Leoni B, Pelliccia A, Santapaola D,
Carollo V, Marra E, et al: Efficacy of aerosol therapy of lung
cancer correlates with EGFR paralysis induced by AvidinOX-anchored
biotinylated Cetuximab. Oncotarget. 5:9239–9255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milazzo FM, Anastasi AM, Chiapparino C,
Rosi A, Leoni B, Vesci L, Petronzelli F and De Santis R:
AvidinOX-anchored biotinylated trastuzumab and pertuzumab induce
down-modulation of ErbB2 and tumor cell death at concentrations
order of magnitude lower than not-anchored antibodies. Oncotarget.
8:22590–22605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Basu A and Krishnamurthy S: Cellular
responses to cisplatin-induced DNA damage. J Nucleic Acids.
2010(pii): 2013672010.PubMed/NCBI
|
|
23
|
Arimoto S, Hasegawa T, Takeda D, Saito I,
Amano R, Akashi M and Komori T: Lymphangiogenesis and lymph node
metastasis in oral squamous cell carcinoma. Anticancer Res.
38:6157–6162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lonser RR, Sarntinoranont M, Morrison PF
and Oldfield EH: Convection-enhanced delivery to the central
nervous system. J Neurosurg. 122:697–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beitler JJ, Zhang Q, Fu KK, Trotti A,
Spencer SA, Jones CU, Garden AS, Shenouda G, Harris J and Ang KK:
Final results of local-regional control and late toxicity of RTOG
9003: A randomized trial of altered fractionation radiation for
locally advanced head and neck cancer. Int J Radiat Oncol Biol
Phys. 89:13–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kfoury M, Disdero V, Vicier C, Le Saux O,
Gougis P, Sajous C and Vignot S: Immune checkpoints inhibitors:
Recent data from ASCO's meeting 2017 and perspectives. Bull Cancer.
105:686–695. 2018.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoneda K, Imanishi N, Ichiki Y and Tanaka
F: Immune checkpoint inhibitors (ICIs) in non-small cell lung
cancer (NSCLC). J UOEH. 40:173–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Durante M and Formenti SC:
Radiation-induced chromosomal aberrations and immunotherapy:
Micronuclei, cytosolic DNA, and interferon-production pathway.
Front Oncol. 8:1922018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ni K, Lan G, Chan C, Quigley B, Lu K, Aung
T, Guo B, La Riviere P, Weichselbaum RR and Lin W: Nanoscale
metal-organic frameworks enhance radiotherapy to potentiate
checkpoint blockade immunotherapy. Nat Commun. 9:23512018.
View Article : Google Scholar : PubMed/NCBI
|