With the sixth highest incidence and fourth highest
mortality rates, liver cancer is one of the most important causes
of cancer-associated mortality worldwide. In 2018, ~840,000 cases
of liver cancer were diagnosed in addition to 782,000 mortalities,
of which ≥85% were associated with hepatocellular carcinoma (HCC)
(1,2). Although the development of predictive
and treatment methods has increased the survival rates of patients
with HCC, the early diagnosis and 5-year survival rates remain poor
(2). Clinically, HCC can be
diagnosed or prognosed using ultrasound, computerized tomography
and the detection of serum α-fetoprotein (AFP) levels (3). However, the sensitivity and specificity
of these methods is unsatisfactory. The complexity of the molecular
mechanisms of HCC has prevented the elucidation of its development
and therefore, the means with which to successfully treat the
disease. Thus, it is necessary to identify novel therapeutics and
drug targets by screening HCC-associated gene networks.
Branched chain amino acid transaminase 1 (BCAT1) is
able to catalyze the synthesis of α-ketoglutarates from branched
chain amino acids (BCAAs) and the subsequent production of
glutamates and branched-chain α-ketoacids. BCAT1 is reportedly
involved in the progression of various malignancies, including
breast (4), ovarian (5), gastric (6) and pancreatic cancer (7), as well as HCC (8,9). The
overexpression of BCAT1 promoted cellular proliferation,
migration and invasion in various types of tumor via multiple
signaling pathways (10,11). In addition, previous studies have
revealed that BCAT1 is involved in DNA methylation and
associated with inflammatory diseases (12,13).
However, one study has suggested that BCAT1 expression is
not associated with the overall survival of patients with HCC
(14), thus its exact role and
mechanism in hepatocarcinogenesis remain controversial.
In tumors, the abnormal expression of specific genes
has long been associated with overall patient survival. In the
present study, two datasets from HCCDB were used to analyze the
overall survival of HCC patients with high and low BCAT1
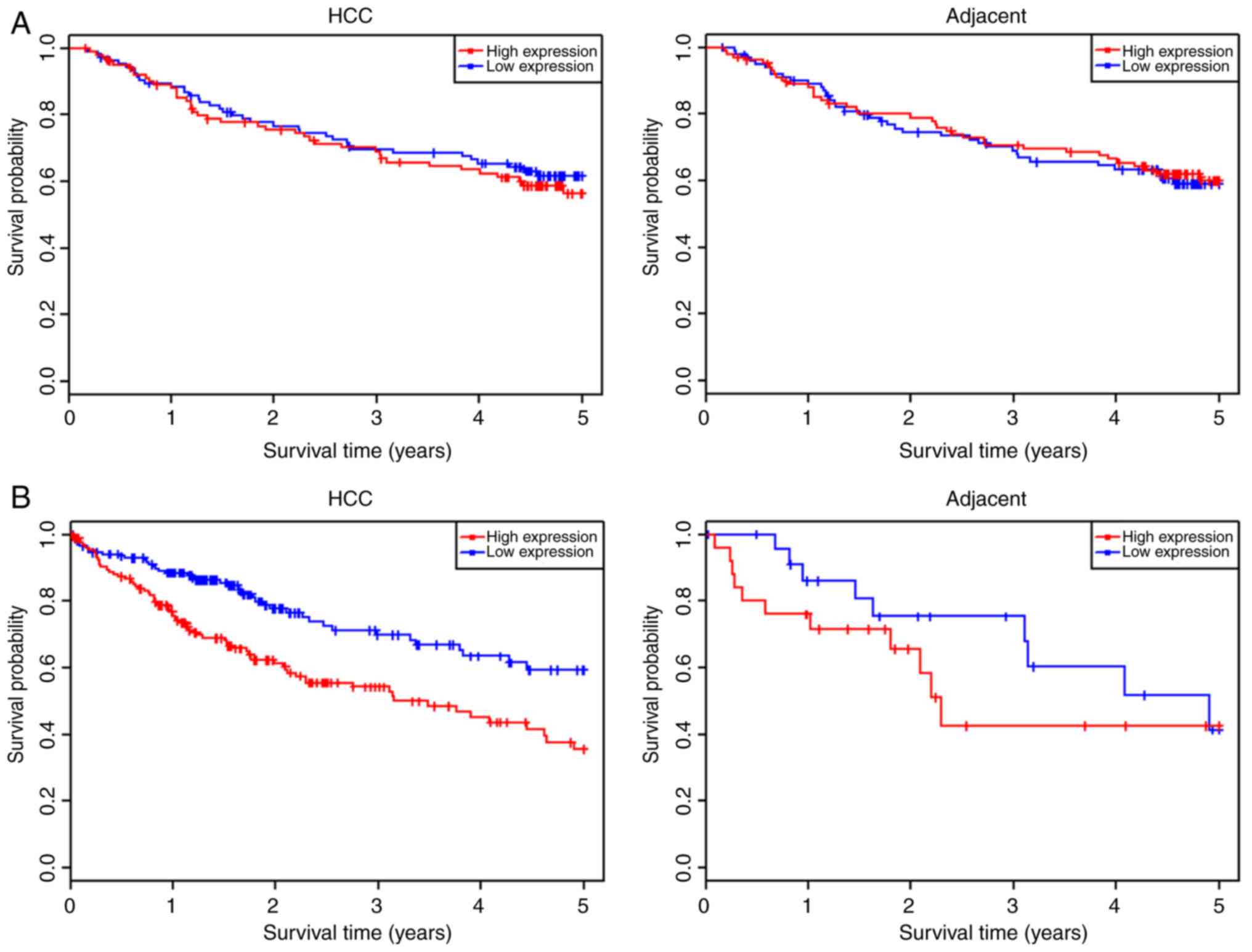
expression levels. This revealed that where the expression level of
BCAT1 was not significantly different between the HCC and
adjacent tissues, the overall survival of patients in the high- and
low-BCAT1 expression cohorts did not differ significantly
(Fig. 4A). By contrast, if the
expression level of BCAT1 in HCC tissues was significantly
increased compared within the adjacent tissues, patients with high
BCAT1 expression levels possessed a lower overall survival
probability than those with low expression levels (Fig. 4B).
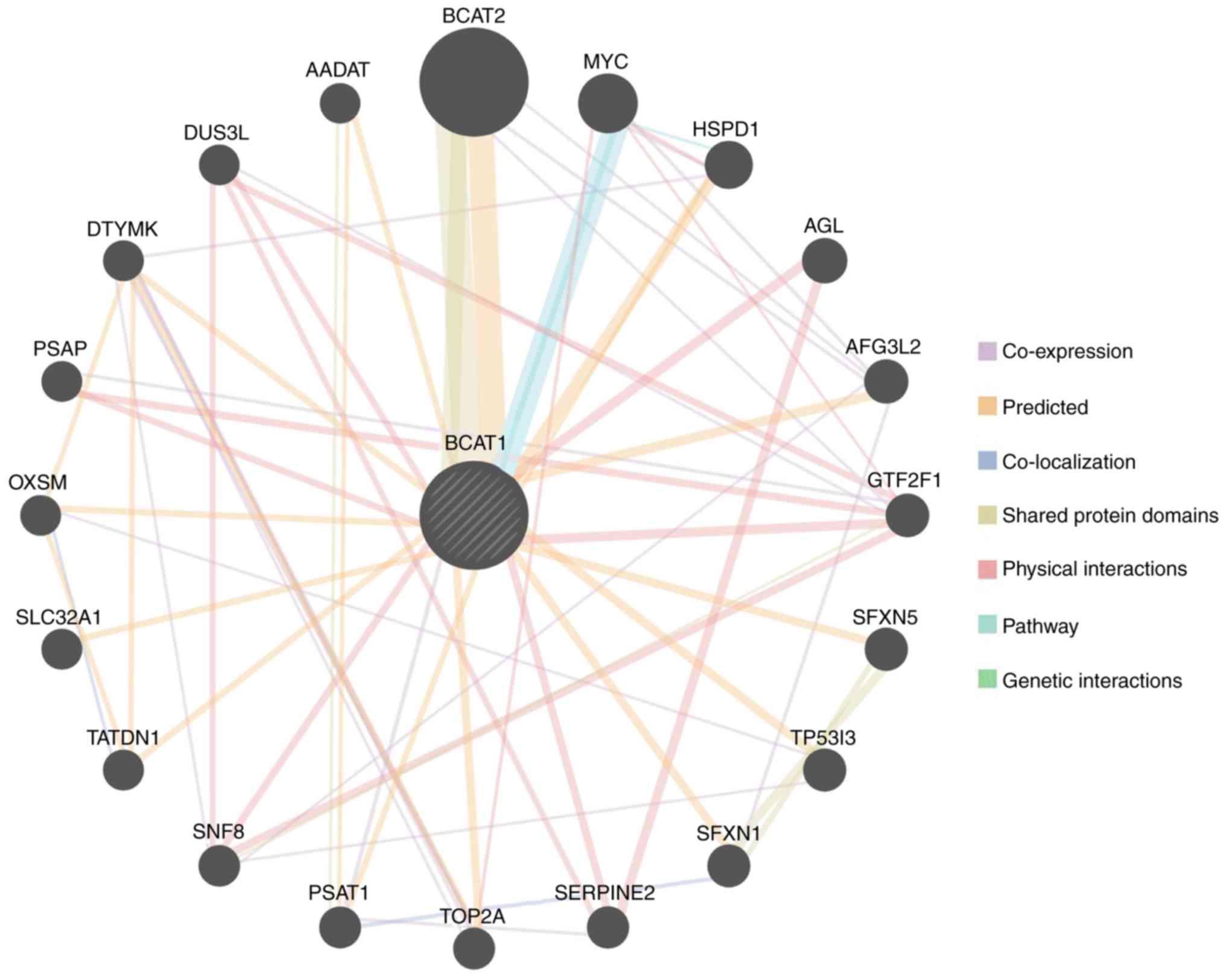
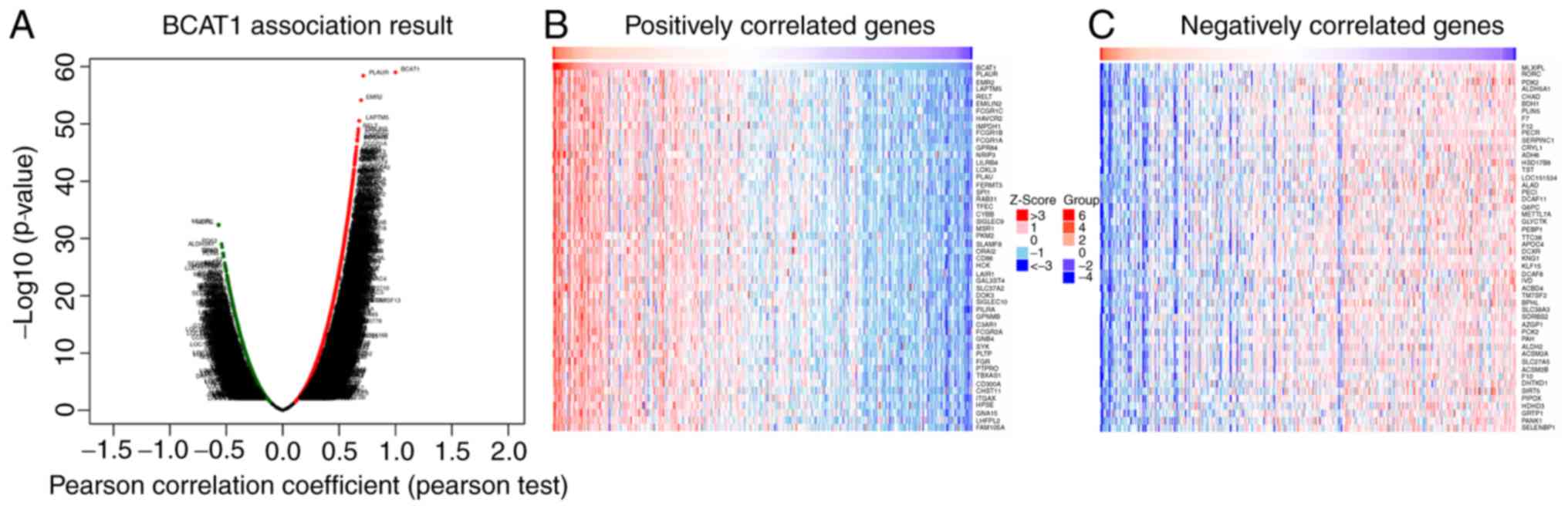
LinkedOmics online tools were then used to
investigate the genes that were co-expressed with BCAT1 in
HCC. A volcano plot revealed that the expression of 2,970 genes
negatively correlated with that of BCAT1 [green spot; false
discovery rate (FDR) <0.05], while the expression of 9,077 genes
positively correlated with BCTA1 (red spot; FDR <0.05;
Fig. 6A). The top 50 negatively and
positively correlated genes are displayed in Fig. 6B and C. These results indicate that
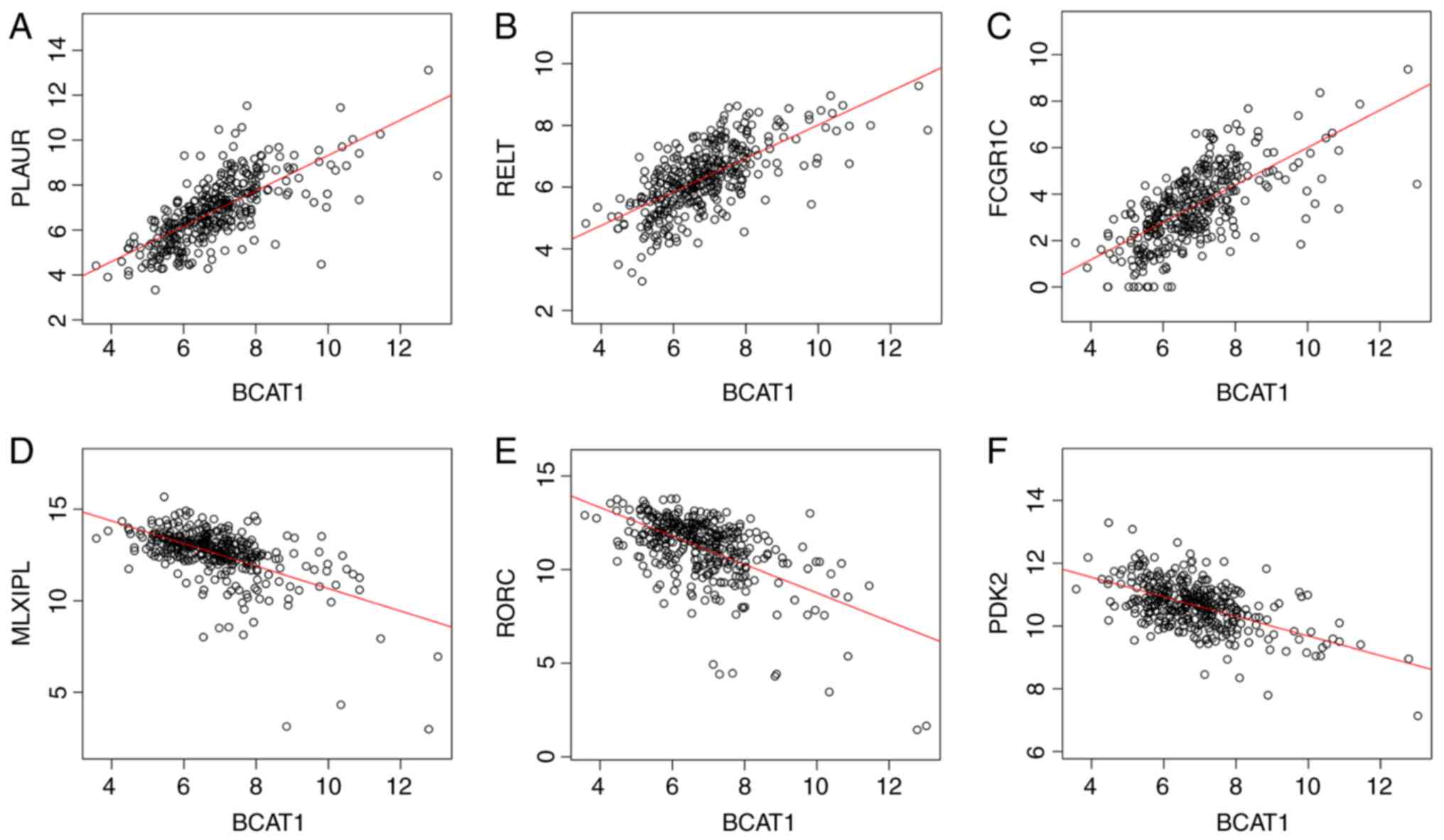
BCAT1 serves a critical role in HCC development. Pearson's
correlation coefficient analysis revealed a strong positive
association between the expression levels of BCAT1 and
plasminogen activator, urokinase receptor (Pearson's
correlation coefficient=0.7142; P=3.89×10−59; Fig. 7A), RELT (Pearson's correlation
coefficient=0.6708; P=7.628×10−50; Fig. 7B), Fc fragment of IgG receptor
Ic (Pearson's correlation coefficient=0.6651;
P=9.94×10−49; Fig. 7C),
and a negative association with MLX interacting protein like
(MLXIPL; Pearson's correlation coefficient=−0.5685;
P=3.787×10−33; Fig. 7D),
RAR related orphan receptor C (RORC; Pearson's
correlation coefficient=−0.5677; P=4.868×10−33; Fig. 7E) and pyruvate dehydrogenase
kinase 2 (PDK2; Pearson's correlation coefficient=−0.5421;
P=1.001×10−29; Fig. 7F),
which are involved in ‘extracellular matrix degradation’, ‘NF-κB
pathway and immune response’, ‘IgG component’, ‘triglyceride
synthesis’, ‘lymphoid organogenesis and thymopoiesis’ and ‘pyruvate
dehydrogenation’, respectively.
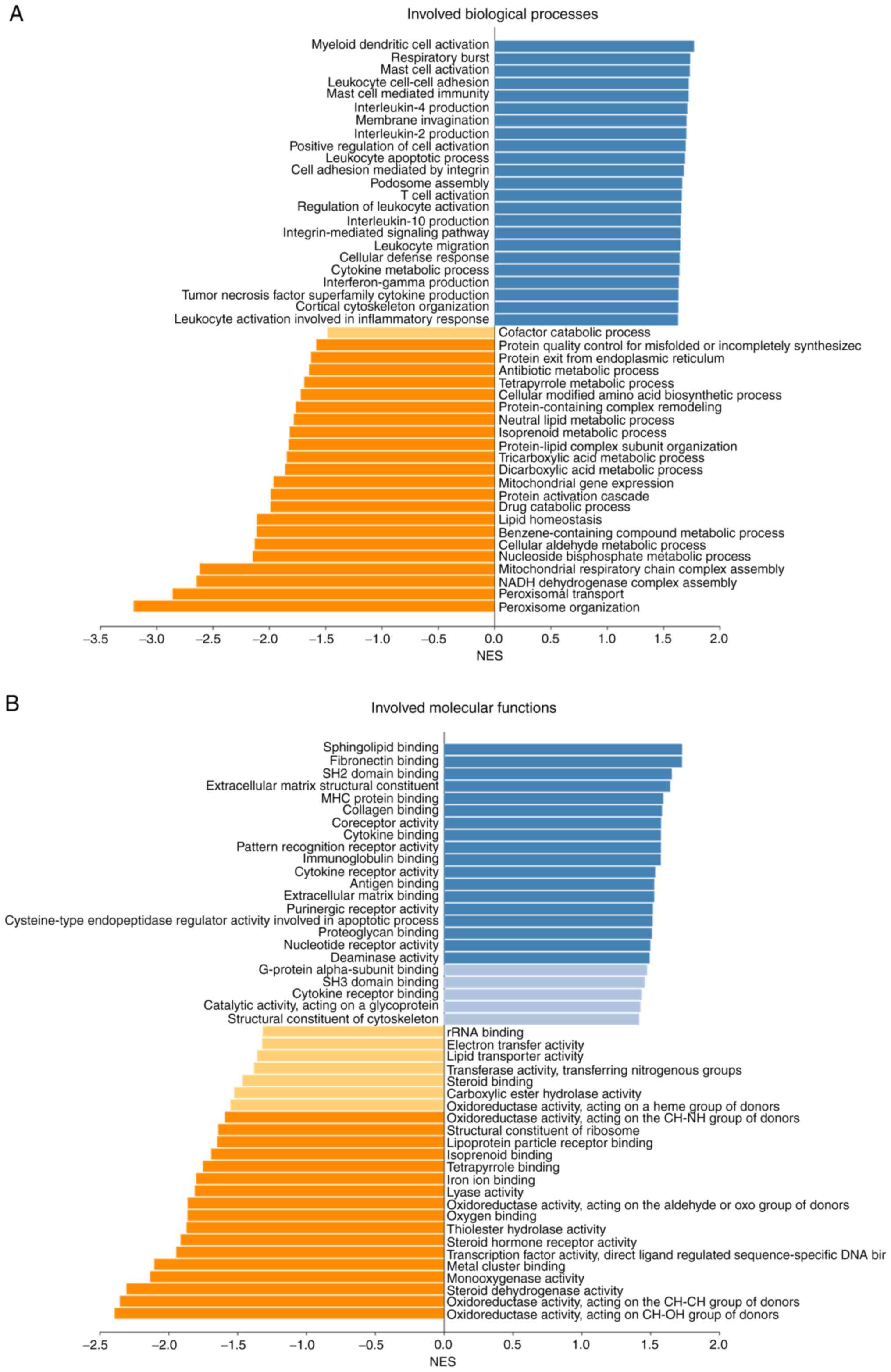
Biological process and molecular function analyses
were conducted using gene set enrichment analysis, which showed
that BCAT1-associated DEGs were involved in a number of
biological processes and molecular functions, including ‘protein
activation cascade’, ‘respiratory burst’, ‘T cell activation’,
‘sphingolipid binding’, ‘MHC protein binding’ and ‘cytokine
receptor activity’ (Fig. 8). These
data indicate that BCAT1 serves an important role in immune
system activation, cellular responses to stimulation, metabolism
and a number of other processes.
The proliferation of cancer cells depends on extra
nutrients acquired from the tumor microenvironment (44,45).
BCAAs are essential for tumor growth and the progression of
multiple biological pathways (46).
A number of studies have revealed that the enzymes catalyzing BCAAs
into their corresponding nutrients are predominantly overexpressed
in cancer (7,13,47) and
that BCAT1 is one of the most important enzymes involved in BCAA
catabolism. The authors' previous study indicated that BCAT1
was significantly upregulated in HCC and that this was associated
with poor patient prognosis (43).
To further investigate the function networks of BCAT1 in
HCC, bioinformatics analyses were performed using a selection of
public databases to provide insights for future research into the
metabolic regulation of HCC.
With a lack of appropriate markers of early stage
HCC, 70–80% of patients are diagnosed at the advanced stage of
disease and lose the opportunity to undergo surgery. Clinically,
AFP has long been regarded as the standard for liver cancer
diagnosis; however, a large number of HCC patients are AFP-negative
(2), thus additional biomarkers for
the early detection of HCC are required. A previous study explored
the use of serum methylated BCAT1 and IKAROS family zinc
finger 1 (IKZF1) for the detection of colorectal neoplasia;
this revealed that for the diagnosis of colorectal cancer, the
detection of BCAT1/IKZF1 DNA in the serum had a
comparable sensitivity, but a higher specificity than the
traditional fecal immunochemical test (48). The present study demonstrated that
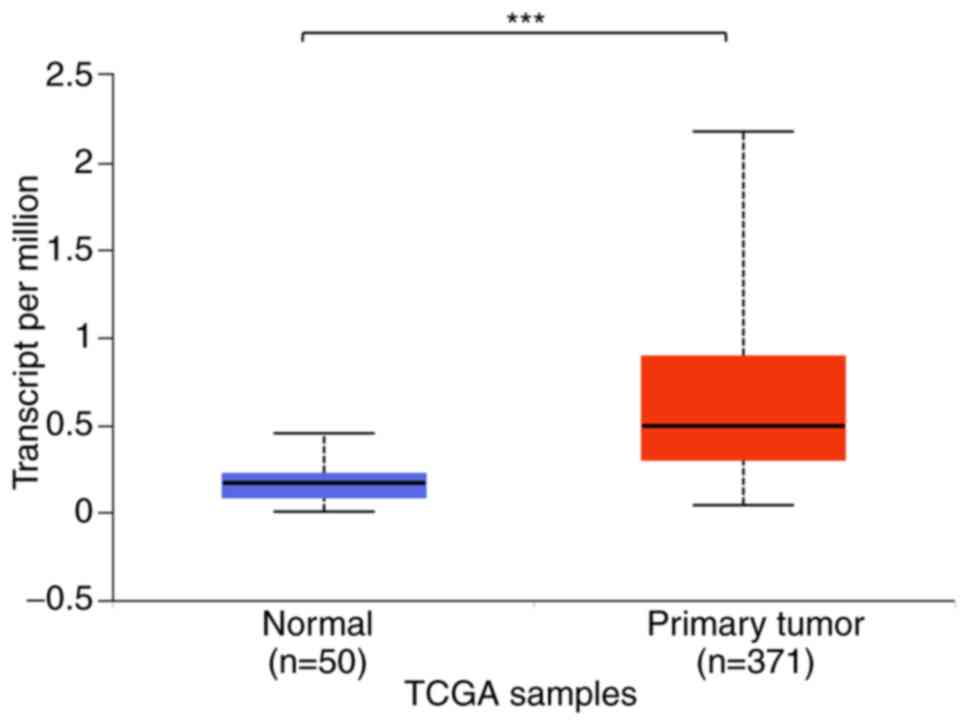
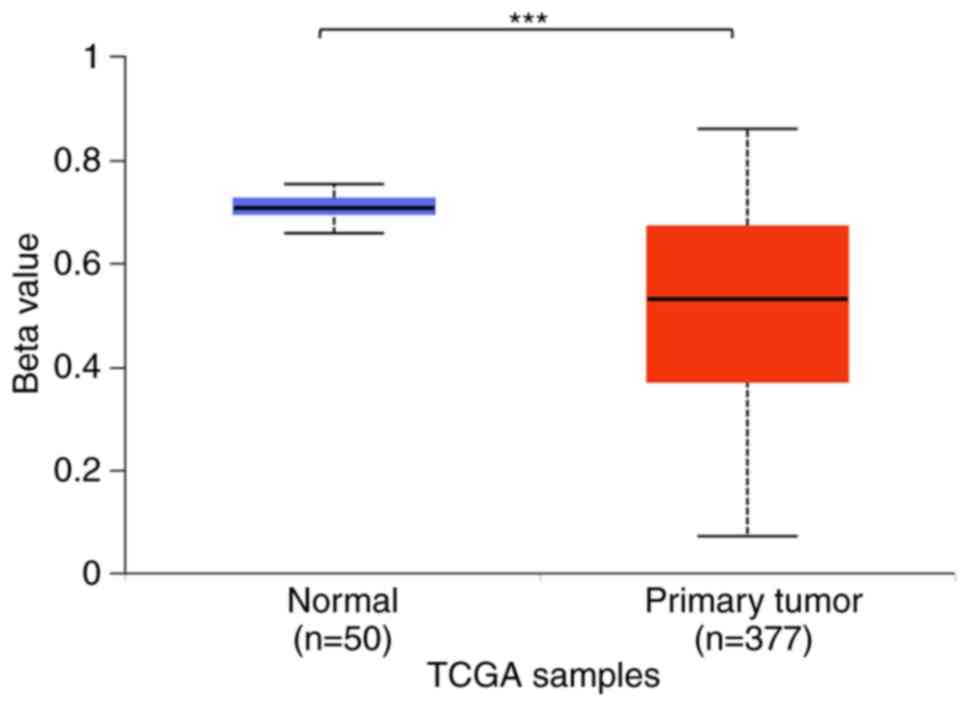
BCAT1 mRNA expression levels in HCC tissues were increased
compared with those in normal tissues. In addition, the level of
BCAT1 promoter methylation was decreased in HCC tissues
compared within normal tissues as that indicated for mRNA
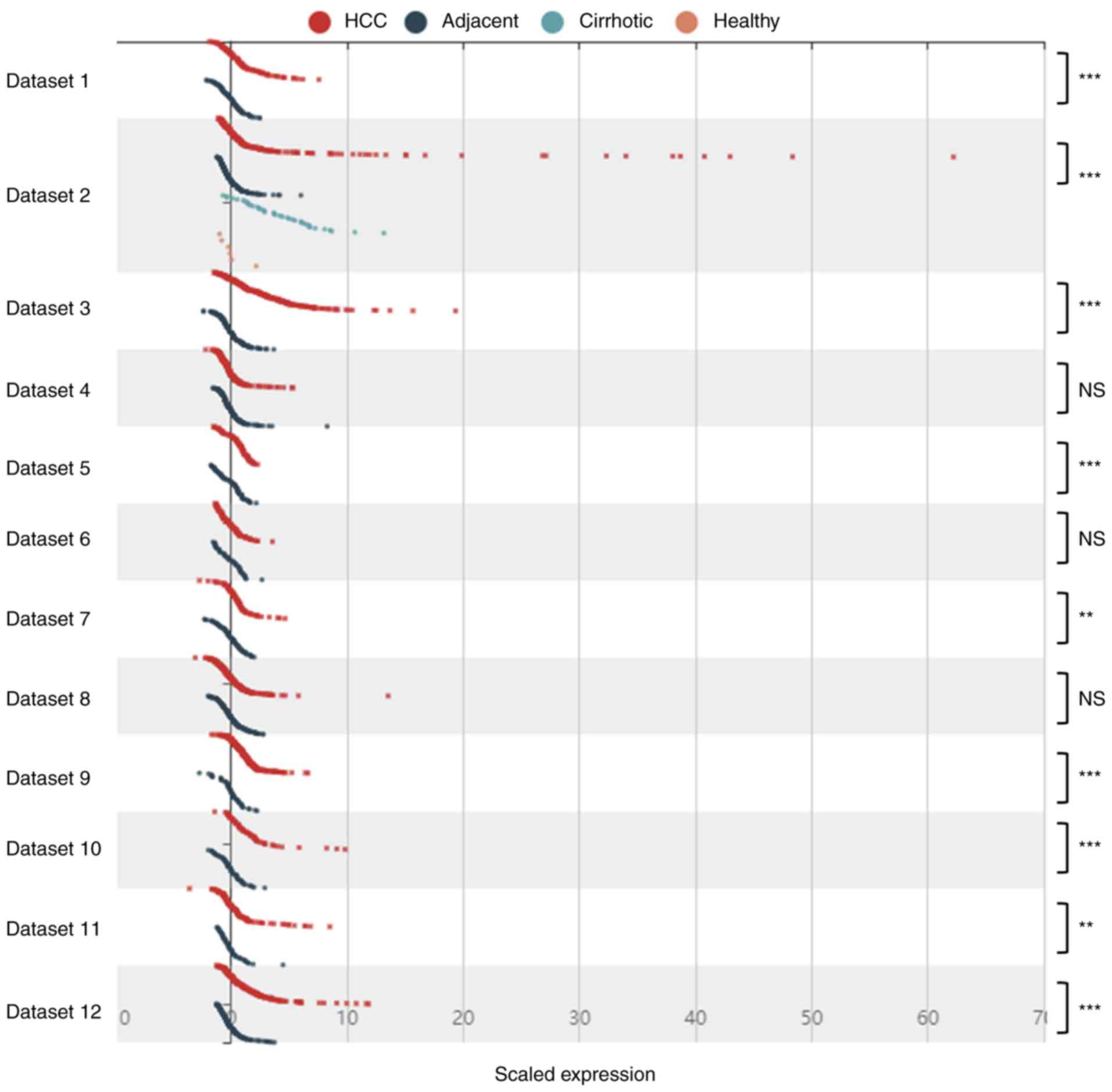
expression levels. However, analysis of 12 datasets from different
regions indicated that in some cases, the expression level of
BCAT1 was not significantly different between the HCC and
adjacent normal tissues. These results suggested that while
BCAT1 may serve as a marker for the detection of HCC,
regional variations may apply. For this reason, further
large-scale, multicenter studies are required to confirm the role
of BCAT1 in the prediction and diagnosis of HCC.
Nonetheless, the analysis of BCAT1 expression and promoter
methylation levels inpatients demonstrated its potential use as a
reliable biomarker of HCC.
The abnormal expression of oncogenes is closely
associated with patient survival (49,50).
Overexpression of MTHFD1 predicted poorer overall survival in
patients with HCC (51). Also, the
expression levels of SIRT1weresignificantlyincreasedin HCC and
associated with poor patient survival (52). In this study, although the majority
of studies illustrated an upregulation in BCAT1 expression
levels in HCC tissues (compared with adjacent tissues), selected
cases presented an opposing result. Notably, further analyses
within the present study indicated that patients with no
significant differences in the level of BCAT1 expression
between tumor and paracancerous tissues possessed a BCAT1
expression-independent survival rate. Meanwhile, patients with
higher BCAT1 expression levels in HCC tissues exhibited a
BCAT1-dependent survival probability. These results suggest
that additional methods may be required to demonstrate the role of
BCAT1 in HCC; this may include not only focusing on mRNA and
protein expression, but also assessing the products of BCAT1
enzymatic reactions. BCAT1 is one of the key enzymes involved in
amino acid metabolism, such that a repeatable method such as
metabolomics analysis may further clarify the role of BCAT1.
However, the results ultimately revealed that BCAT1 serves
an important role in the survival of patients with HCC.
Tumorigenesis is a complex process with abnormal
changes in protein-protein interactions and signaling pathways
(53). BCAT1 was identified
to be involved in the proliferation (4,54),
apoptosis (55), invasion and
metastasis (56) of cancer cells,
and to be associated with poor prognosis inpatients with various
types of cancer (57,58). In addition, BCAT1 was reported
to regulate mTOR signaling (59), as
well as the Wnt (60) and FoxO
signaling pathways (61). However,
the effects of BCAT1 on other pathways and cellular
functions remain unclear.
However, there were limitations to the present
study. First, only 371 tumor cases and 50 normal controls were
employed for analysis, and a larger number of patients is required
to verify the present findings. In addition, few patients at cancer
stage 4, between 81 and 100 years of age and with extreme obesity
were included, which may suggest that a large number of HCC
patients die prior to diagnosis. Secondly, only bioinformatics
analysis results were displayed in this study. Further verification
experiments, such as PCR experiments, western blot assays and
co-immunoprecipitation, are necessary to verify the present
results. Another limitation is that transcriptome sequencing cannot
directly provide information on protein activity, which can only be
verified by follow-up studies.
In conclusion, the present study provides
bioinformatics evidence of the significance of BCAT1 in
hepatocarcinogenesis and its potential as an early detection
marker. The results indicated that the function and effects of
BCAT1 in HCC are multifaceted, and that BCAT1 may be
associated with immune activation, metabolic pathways and the cell
cycle. Furthermore, a BCAT1 miRNA-target network was
analyzed using TargetScan, demonstrating that thousands of miRNAs
were predicted to interact with BCAT1 (data not shown) and
providing a clear direction for future studies. More importantly,
analysis results of the present study of this well-known gene
indicated that the interactions between BCAT1 and numerous
other critical proteins were uncharted. This will help us to
determine the future research direction and draw a complete
molecular network of BCAT1 participating in HCC. Also, the
present study provided researchers, especially young researchers
who lack experimental and bioinformatics analysis platforms, with
guidance on finding directions for functional genome research.
Not applicable.
The present study was supported in part by the
National Natural Science Foundation of China (grant no. 81773148),
the National Key Sci-Tech Special Project of China (grant no.
2018ZX10302207), the Natural Science Foundation of Guangxi (grant
no. 2018GXNSFDA138001), the Basic Ability Enhancement Program for
Young and Middle-aged Teachers of Guilin Medical University (grant
no. 2018glmcy073) and the Basic Ability Enhancement Program for
Young and Middle-aged Teachers of Guangxi (grant no.
2019KY0553).
RZY and WJL conceived, designed the research and
drafted the manuscript; HFZ and WTX were responsible for the
acquisition and analysis of data; HFZ and MJL analyzed and
interpreted the data; RZY revised the manuscript for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Eng J Med. 380:1450–1462. 2019. View Article : Google Scholar
|
|
3
|
Dhanasekaran R, Limaye A and Cabrera R:
Hepatocellular carcinoma: Current trends in worldwide epidemiology,
risk factors, diagnosis, and therapeutics. Hepat Med. 4:19–37.
2012.PubMed/NCBI
|
|
4
|
Thewes V, Simon R, Hlevnjak M, Schlotter
M, Schroeter P, Schmidt K, Wu Y, Anzeneder T, Wang W, Windisch P,
et al: The branched-chain amino acid transaminase 1 sustains growth
of antiestrogen-resistant and ERalpha-negative breast cancer.
Oncogene. 36:4124–4134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ZQ, Faddaoui A, Bachvarova M, Plante
M, Gregoire J, Renaud MC, Sebastianelli A, Guillemette C, Gobeil S,
Macdonald E, et al: BCAT1 expression associates with ovarian cancer
progression: Possible implications in altered disease metabolism.
Oncotarget. 6:31522–31543. 2015.PubMed/NCBI
|
|
6
|
Xu Y, Yu W, Yang T, Zhang M, Liang C, Cai
X and Shao Q: Overexpression of BCAT1 is a prognostic marker in
gastric cancer. Hum Pathol. 75:41–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mayers JR, Torrence ME, Danai LV,
Papagiannakopoulos T, Davidson SM, Bauer MR, Lau AN, Ji BW, Dixit
PD, Hosios AM, et al: Tissue of origin dictates branched-chain
amino acid metabolism in mutant Kras-driven cancers. Science.
353:1161–1165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng YH, Hu WJ, Chen BC, Grahn TH, Zhao
YR, Bao HL, Zhu YF and Zhang QY: BCAT1, a key prognostic predictor
of hepatocellular carcinoma, promotes cell proliferation and
induces chemoresistance to cisplatin. Liver Int. 36:1836–1847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu M, Liu Q, Jia Y, Tu K, Yao Y, Liu Q and
Guo C: BCAT1 promotes tumor cell migration and invasion in
hepatocellular carcinoma. Oncol Lett. 12:2648–2656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tonjes M, Barbus S, Park YJ, Wang W,
Schlotter M, Lindroth AM, Pleier SV, Bai AHC, Karra D, Piro RM, et
al: BCAT1 promotes cell proliferation through amino acid catabolism
in gliomas carrying wild-type IDH1. Nat Med. 19:901–908. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou W, Feng X, Ren C, Jiang X, Liu W,
Huang W, Liu Z, Li Z, Zeng L, Wang L, et al: Over-expression of
BCAT1, a c-Myc target gene, induces cell proliferation, migration
and invasion in nasopharyngeal carcinoma. Mol Cancer. 12:532013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raffel S, Falcone M, Kneisel N, Hansson J,
Wang W, Lutz C, Bullinger L, Poschet G, Nonnenmacher Y, Barnert A,
et al: BCAT1 restricts αKG levels in AML stem cells leading to
IDHmut-like DNA hypermethylation. Nature. 551:384–388. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papathanassiu AE, Ko JH, Imprialou M,
Bagnati M, Srivastava PK, Vu HA, Cucchi D, McAdoo SP, Ananieva EA,
Mauro C and Behmoaras J: BCAT1 controls metabolic reprogramming in
activated human macrophages and is associated with inflammatory
diseases. Nat Commun. 8:160402017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HG, Xie R, Shen P, Huang XD, Ji GZ
and Yang XZ: BCAT1 expression in hepatocellular carcinoma. Clin Res
Hepatol Gastroenterol. 40:e55–e56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38:W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46:D956–D963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Vasaikar S, Shi Z, Zhang B and
Greer M: WebGestalt 2017: A more comprehensive, powerful, flexible
and interactive gene set enrichment analysis toolkit. Nucleic Acids
Res. 45:W130–W137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lian Q, Wang S, Zhang G, Wang D, Luo G,
Tang J, Chen L and Gu J: HCCDB: A database of hepatocellular
carcinoma expression atlas. Genomics Proteomics Bioinformatics.
16:269–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tung EK, Mak CK, Fatima S, Lo RC, Zhang C,
Dai H, Poon RT, Yuen MF, Lai CL, Li JJ, et al: Clinicopathological
and prognostic significance of serum and tissue Dickkopf-1 levels
in human hepatocellular carcinoma. Liver Int. 31:1494–1504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamb JR, Zhang C, Xie T, Wang K, Zhang B,
Hao K, Chudin E, Fraser HB, Millstein J, Ferguson M, et al:
Predictive genes in adjacent normal tissue are preferentially
altered by sCNV during tumorigenesis in liver cancer and may rate
limiting. PloS One. 6:e200902011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sung WK, Zheng H, Li S, Chen R, Liu X, Li
Y, Lee NP, Ariyaratne PN, Tennakoon C, Mulawadi FH, et al:
Genome-wide survey of recurrent HBV integration in hepatocellular
carcinoma. Nat Genet. 44:765–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong KF, Liu AM, Hong W, Xu Z and Luk JM:
Integrin α2β1 inhibits MST1 kinase phosphorylation and activates
Yes-associated protein oncogenic signaling in hepatocellular
carcinoma. Oncotarget. 7:77683–77695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu AM, Yao TJ, Wang W, Wong KF, Lee NP,
Fan ST, Poon RT, Gao C and Luk JM: Circulating miR-15b and miR-130b
in serum as potential markers for detecting hepatocellular
carcinoma: a retrospective cohort study. BMJ Open. 2:e0008252012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burchard J, Zhang C, Liu AM, Poon RT, Lee
NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G, et al:
microRNA-122 as a regulator of mitochondrial metabolic gene network
in hepatocellular carcinoma. Mol Sys Biol. 6:4022010. View Article : Google Scholar
|
|
26
|
Lim HY, Sohn I, Deng S, Lee J, Jung SH,
Mao M, Wang K, Shi S, Joh JW, et al: Prediction of disease-free
survival in hepatocellular carcinoma by gene expression profiling.
Ann Surg Oncol. 20:3747–3753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX and Wang XW: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roessler S, Long EL, Budhu A, Chen Y, Zhao
X, Ji J, Walker R, Jia HL, Ye QH, Qin LX, et al: Integrative
genomic identification of genes on 8p associated with
hepatocellular carcinoma progression and patient survival.
Gastroenterology. 142:957–966.e912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao X, Parpart S, Takai A, Roessler S,
Budhu A, Yu Z, Blank M, Zhang YE, Jia HL, Ye QH, et al: Integrative
genomics identifies YY1AP1 as an oncogenic driver in EpCAM(+)
AFP(+) hepatocellular carcinoma. Oncogene. 34:5095–5104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Gao B, Tan PY, Handoko YA, Sekar
K, Deovasogamani A, Seshachalam VP, OuYang HY, Shi M, Xie C, et al:
Genome-wide CRISPR knockout screens identify NCAPG as an essential
oncogene for hepatocellular carcinoma tumor growth. FASEB J.
33:8759–8770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoshida Y, Villanueva A, Kobayashi M, Peix
J, Chaing DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et
al: Gene expression in fixed tissues and outcome in hepatocellular
carcinoma. N Engl J Med. 359:1995–2004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiang DY, Villanueva A, Hoshida Y, Peix
J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M,
et al: Focal gains of VEGFA and molecular classification of
hepatocellular carcinoma. Cancer Res. 68:6779–6788. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villanueva A, Hoshida Y, Battiston C,
Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashu M,
Kumada H, et al: Combining clinical, pathology, and gene expression
data to predict recurrence of hepatocellular carcinoma.
Gastroenterology. 140:1501–1512.e1502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toffanin S, Hoshida Y, Lachenmayer A,
Villanueva A, Cabellos L, Minguez B, Savic R, Ward SC, Thung S,
Chiang DY, et al: MicroRNA-based classification of hepatocellular
carcinoma and oncogenic role of miR-517a. Gastroenterology.
140:1618–1628.e1616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kishikawa T, Otsuka M, Tan PS, Ohno M, Sun
X, Yoshikawa T, Shibata C, Takata A, Kojima K, Takehana K, et al:
Decreased miR122 in hepatocellular carcinoma leads to
chemoresistance with increased arginine. Oncotarget. 6:8339–8352.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kojima K, April C, Canasto-Chibuque C,
Chen X, Deshmukh M, Venkatesh A, Tan PS, Kobayashi M, Kumada H, Fan
JB and Hoshida Y: Transcriptome profiling of archived sectioned
formalin-fixed paraffin-embedded (AS-FFPE) tissue for disease
classification. PloS One. 9:e869612014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Villa E, Critelli R, Lei B, Marzocchi G,
Cammà C, Giannelli G, Cabibbo G, Enea M, Colopi S, Caporali C, et
al: Neoangiogenesis-related genes are hallmarks of fast-growing
hepatocellular carcinomas and worst survival. Results from a
prospective study. Gut. 65:861–869. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zubiete-Franco I, Garcia-Rodriguez JL,
Lopitz-Otsoa F, Serrano Macia M, Simon J, Fernàndez-Tussy P,
Barbier-Torres L, Fernàndez-Ramos D, Gutiérrez-de-Juan V, López de
Davalillo S, et al: SUMOylation regulates LKB1 localization and its
oncogenic activity in liver cancer. EBioMedicine. 40:406–421. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villanueva A, Portela A, Sayols S,
Battiston C, Hoshida Y, Méndez-González J, Imbaud S, Letouzé E,
Hernandez-Gea V, Corenlla H, et al HEPTROMIC Consortium, : DNA
methylation-based prognosis and epidrivers in hepatocellular
carcinoma. Hepatology. 61:1945–1956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dong B, Lee JS, Park YY, Yang F, Xu G,
Huang W, Finegold J and Moore DD: Activating CAR and β-catenin
induces uncontrolled liver growth and tumorigenesis. Nat Commun.
6:59442015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Makowska Z, Boldanova T, Adametz D,
Quagliata L, Vogt JE, Dill MT, Matter MS, Roth V, Terracciano L and
Heim MH: Gene expression analysis of biopsy samples reveals
critical limitations of transcriptome-based molecular
classifications of hepatocellular carcinoma. J Pathol Clin Res.
2:80–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grinchuk OV, Yenamandra SP, Iyer R, Singh
M, Lee HK, Lim KH, Chow PK and Kuznetsov VA: Tumor-adjacent tissue
co-expression profile analysis reveals pro-oncogenic ribosomal gene
signature for prognosis of resectable hepatocellular carcinoma. Mol
Oncol. 12:89–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liao MJ, Yang XM, Yao RZ and Shi N: BCAT1
overexpression associates with clinical progression and poor
prognosis in patients with hepatocellular carcinoma. Int J Clin Exp
Med. 12:4202–4209. 2019.
|
|
44
|
Lyssiotis CA and Kimmelman AC: Metabolic
interactions in the tumor microenvironment. Trends Cell Biol.
27:863–875. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reina-Campos M, Moscat J and Diaz-Meco M:
Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol.
48:47–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ananieva EA and Wilkinson AC:
Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr
Metab Care. 21:64–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dey P, Baddour J, Muller F, Wu CC, Wang H,
Liao WT, Lan Z, Chen A, Gutschner T, Kang Y, et al: Genomic
deletion of malic enzyme 2 confers collateral lethality in
pancreatic cancer. Nature. 542:119–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Symonds EL, Pedersen SK, Baker RT, Murray
DH, Gaur S, Cole SR, Gopalsamy G, Mangira D, LaPointe LC and Young
GP: A blood test for methylated BCAT1 and IKZF1 vs. a fecal
immunochemical test for detection of colorectal neoplasia. Clin
Transl Gastroenterol. 7:e1372016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Q, Pan X, Zhu D, Deng Z, Jiang R and
Wang X: Circular RNA MAT2B promotes glycolysis and malignancy of
hepatocellular carcinoma via the miR-338-3p/PKM2 axis under hypoxic
stress. Hepatology. Apr 20–2019.(Epub ahead of print).
|
|
50
|
Itzel T, Spang R, Maass T, Munker S,
Roessler S, Ebert MP, Schlitt HJ, Herr W, Evert M and Teufel A:
Random gene sets in predicting survival of patients with
hepatocellular carcinoma. J Mol Med (Berl). 97:2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu H, Wang H, Xu HR, Zhang YC, Yu XB, Wu
MC, Jin GZ and Cong WM: Overexpression of MTHFD1 in hepatocellular
carcinoma predicts poorer survival and recurrence. Future Oncol.
15:1771–1780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang H, Zhang X, Tao Y, Shan L, Jiang Q,
Yu Y, Cai F and Ma L: Prognostic and clinicopathologic significance
of SIRT1 expression in hepatocellular carcinoma. Oncotarget.
8:52357–52365. 2016.PubMed/NCBI
|
|
53
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang L and Han J: Branched-chain amino
acid transaminase 1 (BCAT1) promotes the growth of breast cancer
cells through improving mTOR-mediated mitochondrial biogenesis and
function. Biochem Biophys Res Commun. 486:224–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Eden A and Benvenisty N: Involvement of
branched-chain amino acid aminotransferase (Bcat1/Eca39) in
apoptosis. FEBS Lett. 457:255–261. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ji D, Jiang C, Zhang L, Liang N, Jiang T,
Yang B and Liang H: LncRNA CRNDE promotes hepatocellular carcinoma
cell proliferation, invasion, and migration through regulating
miR-203/BCAT1 axis. J Cell Physiol. 234:6548–6560. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qi LN, Xiang BD, Wu FX, Ye JZ, Zhong JH,
Wang YY, Chen YY, Chen ZS, Ma L, Chen J, et al: Circulating tumor
cells undergoing EMT provide a metric for diagnosis and prognosis
of patients with hepatocellular carcinoma. Cancer Res.
78:4731–4744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cho HR, Jeon H, Park CK, Park SH, Kang KM
and Choi SH: BCAT1 is a new MR imaging-related biomarker for
prognosis prediction in IDH1-wildtype glioblastoma patients. Sci
Rep. 7:177402017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li H, Ye D, Xie W, Hua F, Yang Y, Wu J, Gu
A, Ren Y and Mao K: Defect of branched-chain amino acid metabolism
promotes the development of Alzheimer's disease by targeting the
mTOR signaling. Biosci Rep. 38(pii): BSR201801272018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jour G, Vasudevaraja V, Prieto VG, Snuderl
M, Torres-Cabala CA, Al-Rohil R, Sulman EP, Ballester LY and Aung
PP: BCAT1 and miR-2504: Novel methylome signature distinguishes
spindle/desmoplastic melanoma from superficial malignant peripheral
nerve sheath tumor. Mod Pathol. 32:338–345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Honda M, Takehana K, Sakai A, Tagata Y,
Shirasaki T, Nishitani S, Muramatsu T, Yamashita T, Nakamoto Y,
Mizukoshi E, et al: Malnutrition impairs interferon signaling
through mTOR and FoxO pathways in patients with chronic hepatitis
C. Gastroenterology. 141:128–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chu Y, Li D and Zhang H, Ding J, Xu P, Qiu
X and Zhang H: PIG3 suppresses gastric cancer proliferation by
regulating p53-mediated apoptosis. J Biol Regul Homeost Agents.
32:1185–1189. 2018.PubMed/NCBI
|
|
63
|
Moua P, Checketts M, Xu LG, Shu HB,
Reyland ME and Cusick JK: RELT family members activate p38 and
induce apoptosis by a mechanism distinct from TNFR1. Biochem
Biophys Res Commun. 491:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choi BK, Kim SH, Kim YH, Lee DG, Oh HS,
Han C, Kim YI, Jeon Y, Lee H and Kwon BS: RELT negatively regulates
the early phase of the T-cell response in mice. Eur J Immunol.
48:1739–1749. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Go Y, Jeong JY, Jeoung NH, Jeon JH, Park
BY, Kang HJ, Ha CM, Choi YK, Lee SJ, Ham HJ, et al: Inhibition of
pyruvate dehydrogenase kinase 2 protects against hepatic steatosis
through modulation of tricarboxylic acid cycle anaplerosis and
ketogenesis. Diabetes. 65:2876–2887. 2016. View Article : Google Scholar : PubMed/NCBI
|