Introduction
Primary pulmonary lymphoepithelioma-like carcinoma
(LELC) is a rare lung malignancy (1,2) and
patients with primary pulmonary LELC have an improved prognosis
compared with patients with other types of lung cancer (2–4);
however, few prognostic factors have been described (1).
The utility of fluorine-18 fluorodeoxyglucose
positron emission tomography/computerized tomography
(18F-FDG PET/CT) for staging patients with lung cancer
has been previously validated (5)
and a number of studies have discussed the usage of
18F-FDG PET/CT in pulmonary LELC (6,7). Su
et al (8) have recently
demonstrated that 18F-FDG PET/CT can more accurately
stage cancer and better predict outcomes in patients with pulmonary
LELC. Additionally, the functional parameters of 18F-FDG
PET/CT, including maximum standardized uptake value
(SUVmax), metabolic tumor volume (MTV) and total lesion
glycolysis (TLG), which represent tumor metabolic activity, entire
tumor burden and overall metabolic activity, respectively, are
known predictors of outcomes in several malignancies, such as
breast, rectal cancer and lung cancer (9–12).
However, to the best of our knowledge, the prognostic value of
these parameters in patients with pulmonary LELC remains
unknown.
Similar to that of nasopharyngeal carcinoma (NPC),
the etiology of pulmonary LELC is substantially associated with
Epstein-Barr virus (EBV) infection in Asian individuals (13), and serum EBV titers in pulmonary LELC
are associated with tumor size and stage (14). Furthermore, circulating EBV DNA may
be positively associated with tumor burden (15).
Based on the aforementioned results, combined
evaluations of imaging parameters and blood biomarkers may lead to
the development of improved therapeutic approaches for patients
with pulmonary LELC. Therefore, the present study aimed to
investigate the prognostic value of the functional parameters of
18F-FDG PET/CT in patients with pulmonary LELC, and to
understand the association between these parameters and EBV DNA
load.
Materials and methods
Patients
Between January 2008 and December 2016, 71
individuals with pulmonary LELC were managed at Linkou Medical
Center of Chang Gung Memorial Hospital (Taoyuan, Taiwan). This
cohort has been previously described (4,8).
Pulmonary LELC was diagnosed in all patients based on pathology
findings and according to the World Health Organization criteria
(16). The present study excluded
all patients who had undifferentiated carcinoma without dense
lymphoid infiltrates. Routine nasopharyngeal evaluations were
performed in all patients to exclude metastatic NPC. Patients with
incomplete medical records and those who did not undergo
pretreatment 18F-FDG PET/CT evaluation were excluded
from the present study. A total of 32 adult patients who underwent
pretreatment 18F-FDG PET/CT for staging were included,
comprising 12 men and 20 women with a median age of 60.5 years
(range, 48–87 years). All patients were followed up until June 2017
or until death.
Staging was performed according to the 7th Edition
of the American Joint Committee on Cancer guidelines (17). Data on sex, age, smoking status,
baseline serum EBV DNA levels, stage, imaging findings and outcomes
were obtained. Restaging scans were obtained at 3-month intervals
during treatment and were evaluated by a radiologist based on the
Response Evaluation Criteria in Solid Tumors, version 1.1 (18). Follow-up information was gathered
from patient medical records, and progression-free survival (PFS;
defined as time from treatment to time of progression or death) and
overall survival (OS; defined as time from treatment to time of
death) were calculated. EBV DNA was extracted from plasma samples
and amplified using quantitative PCR as described previously
(19). Plasma EBV DNA concentration
was expressed as the number of copies of EBV genome/ml of plasma
(20).
Imaging and analysis of
18F-FDG PET/CT
All patients were asked to fast for ≥4 h prior to
the 18F-FDG PET/CT scan. Blood glucose concentrations
were measured prior to positron emission tomography (PET) studies,
and the cut-offs were <120 mg/dl for non-diabetic patients and
<200 mg/dl for diabetic patients. Subjects in the resting state
were administered intravenous injections of 18F-FDG
(5.18 MBq/kg of body weight), and images were captured at 50 min
after tracer administration. Delayed phase images were acquired 90
min after intravenous tracer injection if equivocal lesions were
suspected. The MTV and TLG were calculated only for 50-min images.
Whole-body PET/computed tomography (CT) emission scans were
obtained from the base of the skull to mid-thigh in a Discovery
ST16 scanner (GE Healthcare) or a Biograph mCT scanner (Siemens
Healthineers). Low-dose CT was used for attenuation correction of
PET data and images were reconstructed using CT-based attenuation
correction based on an ordered-subset expectation maximization
interactive reconstruction algorithm (4 iterations and 10 subsets
for the Discovery ST16; 2 iterations and 21 subsets for the
Biography mCT). Axial spatial resolution of PET at the center of
the gantry, using these reconstruction parameters, was determined
to be 4.80 and 2.16 mm for the Discovery ST16 and Biograph mCT
scanners, respectively. The SUVmax was obtained by
drawing regions of interest over the most intense slice of the
primary tumor within lesions in the lung and mediastinum. MTV was
measured by an SUV-based automated contouring program, which used
attenuation-corrected torso 18F-FDG PET images.
Boundaries were drawn to include the primary tumor and metastatic
sites in axial, coronal and sagittal 18F-FDG PET/CT
images, and voxels within the contouring margin that had an SUV
intensity >2.5 were incorporated to define the MTV. Total MTV
was calculated by adding the MTVs of all malignant lesions in each
patient. TLG was calculated by multiplying the MTV of each lesion
with the corresponding mean SUV (TLG = mean SUV × MTV) (21,22), and
total TLG was calculated by adding the TLGs of all malignant
lesions in each patient. Parametric quantification of MTV and TLG
was performed using the Syngo MI Workplace software platform,
syngoMMWP version VE40A (Siemens Healthineers).
Statistical analysis
Categorical variables were compared using Fisher's
exact test and are presented as numbers (percentages) or median
values with ranges. The correlation between PET parameters and
plasma EBV DNA load was evaluated using Pearson's correlation
analysis. Kaplan-Meier survival analysis was used to analyze PFS
and OS. Receiver operation characteristic (ROC) curves were used to
determine cut-off values for SUVmax, total MTV and TLG.
Univariate comparison of survival utilized the log-rank test. All
analyses were two-sided. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using Prism (version 5; GraphPad Software, Inc.) and SPSS
(version 20.0; IBM Corp.).
Results
Patient characteristics
Of the 71 patients with pulmonary LELC, only 32
adult patients who underwent pretreatment 18F-FDG PET/CT
for staging were included, and their data were analyzed. The
demographic characteristics of this cohort of patients, comprising
12 men and 20 women with a median age of 60.5 years (range, 48–87
years), are summarized in Table I. A
total of nine patients (28.1%) were former or current smokers.
Limited to the retrospective nature to this study, the serum EBV
DNA level was checked in only nine (28.1%) patients prior to
treatment, all of whom exhibited elevated EBV DNA levels (median,
532 copies/ml; range, 66–146,000 copies/ml).
 | Table I.Clinical characteristics of patients
with pulmonary lymphoepithelioma-like carcinoma. |
Table I.
Clinical characteristics of patients
with pulmonary lymphoepithelioma-like carcinoma.
| Characteristic | N (%) |
|---|
| Median age, years
(range) | 60.5 (48–87) |
| Sex |
|
|
Male | 12 (37.5) |
|
Female | 20 (62.5) |
| History of
smoking |
|
| Former
or current smoker | 9 (28.1) |
|
Non-smoker | 23 (71.9) |
| ECOG performance
status |
|
| 0 | 12 (37.5) |
| 1 | 20 (62.5) |
| Stage |
|
| I | 10 (31.2) |
| II | 5 (15.6) |
|
III | 11 (34.4) |
| IV | 6 (18.8) |
| EBV DNA level
(baseline), copies/ml |
|
| Median
(range) | 532
(66–146,000) |
| Primary
treatment |
|
|
Surgery | 14 (43.8) |
| Surgery
with adjuvant CT ± RT | 4 (12.5) |
|
Neoadjuvant CT ± RT with
surgery | 4 (12.5) |
|
Palliative CT ± RT | 9 (28.1) |
| RT | 1 (3.1) |
Associations among tumor stage, EBV
DNA load and 18F-FDG PET parameters
The cohort comprised 10 (31.3%) stage I, five
(15.6%) stage II, 11 (34.4%) stage III and six (18.8%) stage IV
patients with cancer. Tumor stage was significantly associated with
total MTV (R2=0.53; P<0.0001) and total TLG
(R2=0.40; P=0.0002, Table
II). Pretreatment serum EBV DNA load at baseline, evaluated in
nine patients, exhibited a significant positive correlation with
total MTV (R2=0.63; P=0.0337) and total TLG
(R2=0.77; P=0.0093, Table
II). Data on post-treatment serum EBV DNA load was available
for 8 patients, including one patient who succumbed to nosocomial
pneumonia after surgery and four who remained alive and
disease-free with no detectable serum EBV DNA after therapy.
 | Table II.Correlation analyses of
18F-FDG PET/CT functional parameters and tumor stage,
EBV DNA levels. |
Table II.
Correlation analyses of
18F-FDG PET/CT functional parameters and tumor stage,
EBV DNA levels.
|
| Total MTV | TLG |
|---|
|
|
|
|
|---|
| Parameter | R2 | P-value | R2 | P-value |
|---|
| Stage | 0.53 | <0.0001 | 0.40 | 0.0002 |
| EBV DNA,
copies/ml | 0.63 |
0.0337 | 0.77 | 0.0093 |
Survival analysis
During a median follow-up period of 34.1 months
(range, 5.1–104.8 months), there were six cases of mortality (five
patients succumbed to cancer progression; one patient succumbed to
pneumonia and severe sepsis), and tumor progression or recurrence
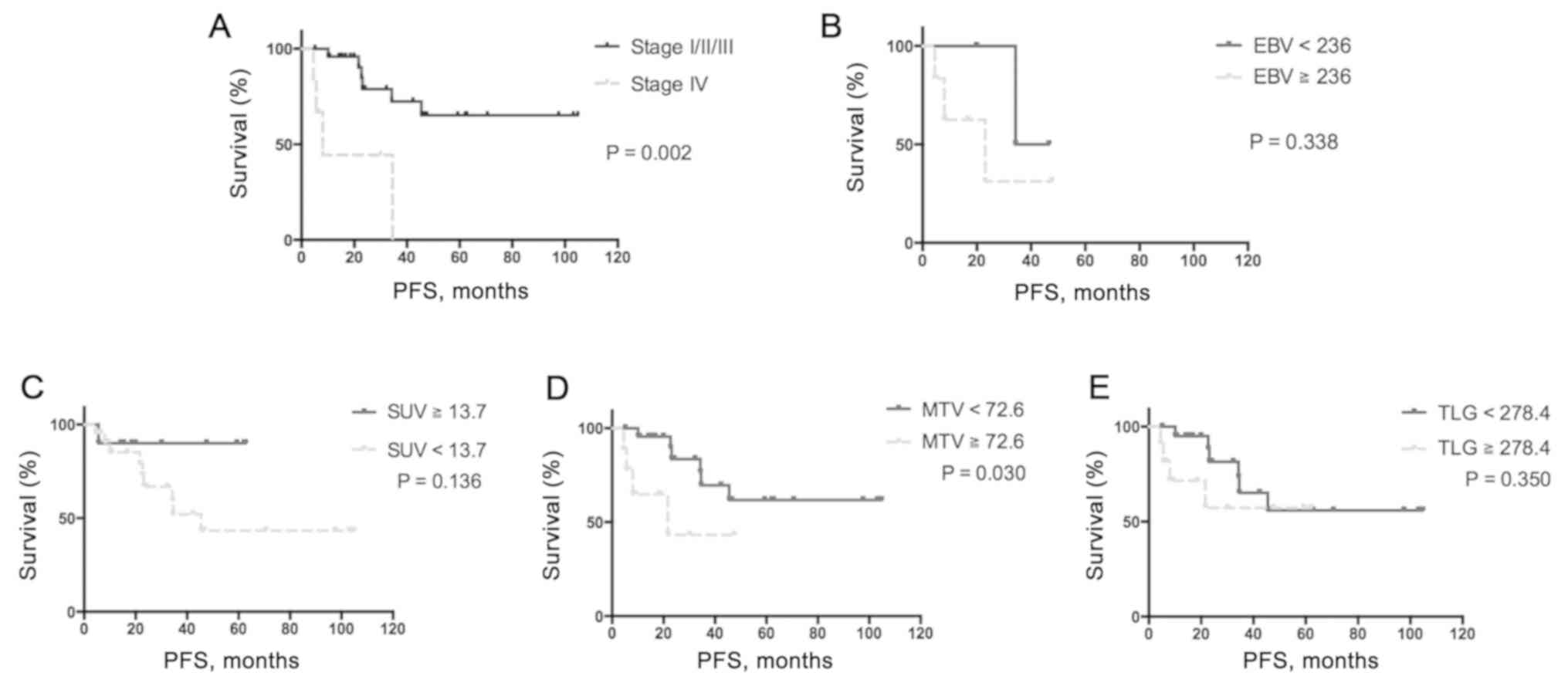
was observed in 11 patients. For survival analysis, the cut-off
values that were selected based on ROC curve analysis for
categorization of low and high tumor SUVmax, total MTV,
total TLG and serum EBV DNA, were set at 13.7 (area under the curve
(AUC), 0.65; P=0.298), 72.6 (AUC, 0.9; P=0.0056), 278.4 (AUC, 0.9;
P=0.006) and 236 (AUC, 0.75; P=0.4386) copies/ml, respectively.
Univariate analysis demonstrated that high total MTV (≥72.6 ml) was
associated with poor PFS (hazard ratio (HR), 3.60; 95% CI,
1.2–37.6; P=0.030; Table III),
whereas multivariate analysis identified only stage IV tumors as
independent predictors of worse PFS (HR, 4.85; 95% CI, 1.0–23.3;
P=0.049; Table II). Although high
EBV DNA load (≥236 copies/ml) exhibited a trend toward poorer PFS,
this was not identified to be statistically significant (Fig. 1).
 | Table III.Cox proportional hazards model of
progression free survival. |
Table III.
Cox proportional hazards model of
progression free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Stage (IV vs.
I/II/III) | 5.69
(33.6–192.0) | 0.002a | 4.85
(1.0–23.3) | 0.049a |
| EBV DNA, copies/ml
(≥236 vs. <236) | 2.79
(0.4–19.1) | 0.338 |
|
|
| SUVmax,
g/ml (≥13.7 vs. <13.7) | 4.22
(0.7–10.4) | 0.136 |
|
|
| Total MTV, ml
(≥72.6 vs. <72.6) | 3.60
(1.2–37.6) | 0.030a | 2.66
(0.6–12.8) | 0.221 |
| TLG, g (≥278.4 vs.
<278.4) | 1.81 (0.5–7.9) | 0.350 |
|
|
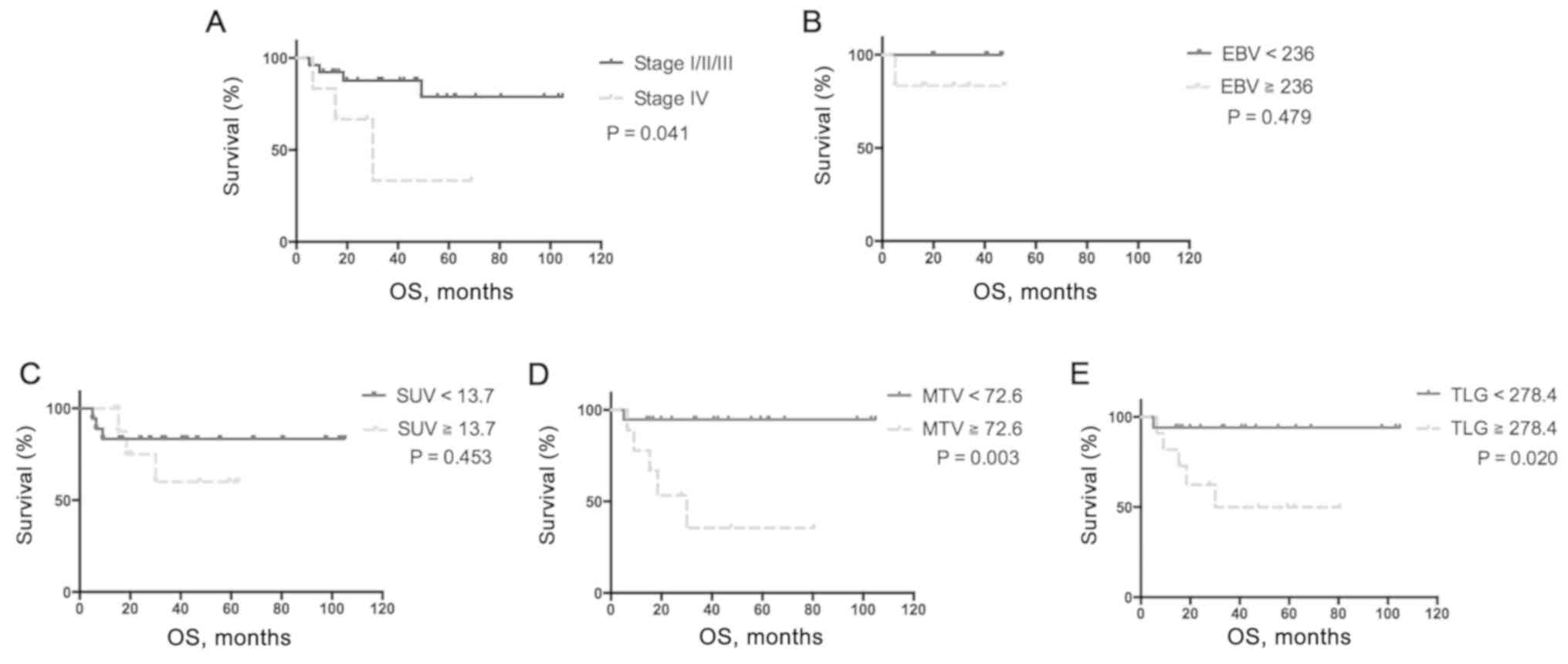
Median OS was significantly shorter in patients with
stage IV tumors, high total MTV (≥72.6 ml) and high total TLG
(≥278.4 g) compared with patients with low MTV and low TLG
(P<0.05; Fig. 2). Furthermore,
only high total MTV (≥72.6 ml) was a predictor of OS according to
multivariate analysis (HR, 12.59; 95% CI, 1.4–113.7; P=0.024;
Table IV).
 | Table IV.Cox proportional hazards model for
overall survival. |
Table IV.
Cox proportional hazards model for
overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Stage (IV vs.
I/II/III) | 4.17
(1.1–68.5) | 0.041a | 2.15 (0.2–0.9) | 0.510 |
| EBV DNA, copies/ml
(≥236 vs. <236) | 4.48
(0.1–286.5) | 0.479 |
|
|
| SUVmax,
g/ml (≥13.7 vs. <13.7) | 1.83
(0.4–10.1) | 0.453 |
|
|
| Total MTV, ml
(≥72.6 vs. <72.6) | 12.60
(2.6–90.9) | 0.003a | 12.59
(1.4–113.7) | 0.024a |
| TLG, g (≥278.4 vs.
<278.4) | 8.40
(1.4–37.8) | 0.020a | 5.97
(0.4–95.6) | 0.207 |
Two patients with stage IV pulmonary LELC who
exhibited divergent values for pretreatment total MTV and total TLG
exhibited different outcomes (Fig.
3).
Discussion
Several functional parameters of 18F-FDG
PET, including SUV, MTV and TLG, have been demonstrated to predict
outcomes in numerous solid organ tumors, such as breast, colorectal
and lung cancer (9–12). To the best of our knowledge, the
present study is the first that discusses prognostic values and the
association of EBV DNA load and pretreatment 18F-FDG PET
parameters in primary pulmonary LELC, which is probably due to the
rarity of the disease. The present study demonstrated a significant
positive association between pretreatment serum EBV DNA load and
total MTV or TLG levels. Stage IV tumors were independently
associated with poor PFS, and a high total MTV was the only
identified predictor of worse OS.
Primary pulmonary LELC is morphologically similar to
NPC (1,2,4) and is
associated with EBV infection in Asian individuals (13,14,23). Ma
et al (24) have demonstrated
a positive association between plasma EBV DNA load and MRI-based
tumor burden in NPC. Additionally, a study by Chang et al
(22) in Taiwan involving 108
patients suggested that tumor TLG, nodal TLG, total TLG and tumor
SUVmax were all significantly associated with plasma EBV
DNA load in patients with NPC. However, to the best of our
knowledge, the association between EBV viral load and PET
parameters in pulmonary LELC has not been previously explored.
Although pretreatment serum EBV DNA load was evaluated in only nine
patients in the present study, it was significantly positively
associated with total MTV and total TLG, suggesting that the plasma
EBV viral load is associated with active tumor burden.
Plasma EBV DNA load in patients with NPC has been
demonstrated to have a high prognostic value with respect to
long-term survival and distant metastasis in one meta-analysis
(25). Chang et al (14) reported that elevated EBV serology
titers represent higher tumor stage and larger tumor size in
patients with pulmonary LELC. Furthermore, Ngan et al
(15,26) demonstrated that the circulating EBV
DNA load is associated with treatment response. The results of the
present study suggested that EBV DNA load may be positively
associated with tumor burden. Data on EBV DNA load before and after
treatment was not available for eight patients, and although four
patients who had no detectable serum EBV DNA after therapy remained
disease-free, the prognostic value of serum EBV DNA could not be
evaluated due to the small sample size.
Compared with conventional CT, PET/CT provides
morphological and functional information relevant to cancer
management (21). Su et al
(8) reported that stage and
pretreatment 18F-FDG PET were independent prognostic
factors of OS in patients with pulmonary LELC; however, that study
did not evaluate the prognostic value of functional parameters of
18F-FDG PET. The most commonly used parameter is
SUVmax, which represents maximum FDG uptake in a region
of interest, and greater FDG uptake by a tumor is associated with
worse survival in patients with surgical and non-surgical lung
cancer (27,28). Previously, Shen et al
(29) have revealed that
SUVmax is a predictor of OS in patients with recurrent
NPC. However, since SUVmax largely depends on tumor
size, its prognostic value is questionable (30). Notably, Hoang et al (31) have described contradictory results
and suggest that SUVmax does not exhibit a significant
association with survival in patients with advanced non-small cell
lung cancer.
Regarding volumetric PET parameters, MTV represents
metabolically active tumor volume on PET images (32). Lee et al (33) have demonstrated that MTV has
prognostic value for PFS and OS in patients with non-small cell
lung cancer who are treated definitively. Additionally, alterations
in MTV following treatment are also significantly associated with
survival in patients with lung cancer (34,35).
Another prognostic PET parameter is TLG, which combines volumetric
and metabolic information (21).
Chung et al (36) reported
that total MTV and TLG are independent prognostic factors of PFS
and OS in patients with advanced lung adenocarcinoma in Korea.
Another retrospective study by Wang et al (37) revealed that high TLG was an
independent predictor of poor PFS in patients with epidermal growth
factor receptor (EGFR)-mutated advanced lung adenocarcinoma, and of
poor PFS and OS in patients with wild-type EGFR carrying lung
adenocarcinoma. By contrast, in the patients with pulmonary LELC
examined in the present study, neither SUVmax nor TLG
were identified to be associated with PFS or OS. Only stage IV
tumors predicted worse PFS. Additionally, the present study
demonstrated that only MTV was significantly associated with OS in
multivariate analysis. This may be due to TLG being affected by
another crucial constituent of TLG, i.e., mean SUV.
There were several limitations in the present study.
First, this was a retrospective study, which may have led to a few
biases. Pretreatment serum EBV DNA was not routinely evaluated in
all patients and posttreatment EBV DNA was not regularly followed
up. Second, for the majority of patients, 18F-FDG PET/CT
scans were only performed once, and changes of PET parameters could
not be evaluated. Additionally, all measurements were from a single
center and the sample size was small due to the rarity of the
disease. Additionally, the use of MTV and TLG in clinical practice
may be premature due to a lack of standardized estimation
methodology. A number of different methods and a wide range of
threshold levels have been proposed to calculate volume-based
PET/CT parameters (38,39). Another factor is the use of two
different PET/CT devices [Discovery ST16 scanner (GE Healthcare)
and Biograph mCT scanner (Siemens Healthineers)] in the present
study, which can affect the measurement of SUV and volumetric PET
parameters since SUV measurements can be influenced by a variety of
biological factors, including body weight and blood glucose level,
and technological factors, including inter-scanner variability and
image reconstruction parameters (40). However, to the best of our knowledge,
the present study was the first to demonstrate the predictive value
of 18F-FDG PET/CT functional parameters in pulmonary
LELC, and provide relevant and useful information to
clinicians.
Although not considered prognostic, pretreatment
serum EBV DNA levels were closely associated with MTV and TLG of
18F-FDG PET/CT in patients with pulmonary LELC. Total
MTV was found to be an independent predictor of OS and could be
valuable for guiding decision-making during pulmonary LELC
management. Further prospective, large-scale studies are warranted
to validate the findings of the present study and to evaluate the
prognostic values of changes in PET functional parameters following
treatment.
Acknowledgements
Not applicable.
Funding
YFF and CYW were funded by Chang Gung Medical
Research Program Grant (grant no. CMRPG3E1981).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYL, ITW, CYW, YCC and YFF conceived and designed
the study. ITW, YFF and CYW provided administrative support. CWW,
MHH, CYW, YCC and SML provided study materials or patients. CYL,
CWW, YFF and YCC collected and assembled data. CYW, CWW, ITW, MHH,
YFF and SML analyzed and interpreted the data. YFF and CYW had full
access to all data in the present study and take responsibility for
the integrity of the data and the accuracy of the data analysis,
and they contributed equally to this paper. All authors were
involved in writing the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Chang Gung Memorial Hospital (approval nos.
201600879B0 and 104-8614C). This was a retrospective study and no
modification in the management of patients was required, so the
need for informed consent was waived. All personal information was
encrypted in the database, and patient data accessed was
de-identified. There was no breach of privacy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EBV
|
Epstein-Barr virus
|
|
18F-FDG PET/CT
|
fluorine-18 fluorodeoxyglucose
positron emission tomography/computerized tomography
|
|
LELC
|
lymphoepithelioma-like carcinoma
|
|
MTV
|
metabolic tumor volume
|
|
NPC
|
nasopharyngeal carcinoma
|
|
SUVmax
|
maximum standardized uptake value
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
TLG
|
total lesion glycolysis
|
References
|
1
|
Liang Y, Wang L, Zhu Y, Lin Y, Liu H, Rao
H, Xu G and Rong T: Primary pulmonary lymphoepithelioma-like
carcinoma: Fifty-two patients with long-term follow-up. Cancer.
118:4748–4758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Shen J, Pan H, Huang J, Liang W and
He J: Pulmonary lymphoepithelioma-like carcinoma: A Surveillance,
Epidemiology and end results database analysis. J Thorac Dis.
7:2330–2338. 2015.PubMed/NCBI
|
|
3
|
Lin Z, Situ D, Chang X, Liang W, Zhao M,
Cai C, Liu Y and He J: Surgical treatment for primary pulmonary
lymphoepithelioma-like carcinoma. Interact Cardiovasc Thorac Surg.
23:41–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin CY, Chen YJ, Hsieh MH, Wang CW and
Fang YF: Advanced primary pulmonary lymphoepithelioma-like
carcinoma: Clinical manifestations, treatment, and outcome. J
Thorac Dis. 9:123–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lv YL, Yuan DM, Wang K, Miao XH, Qian Q,
Wei SZ, Zhu XX and Song Y: Diagnostic performance of integrated
positron emission tomography/computed tomography for mediastinal
lymph node staging in non-small cell lung cancer: A bivariate
systematic review and meta-analysis. J Thorac Oncol. 6:1350–1358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan HY, Tsoi A, Wong MP, Ho JC and Lee
EY: Utility of 18F-FDG PET/CT in the assessment of
lymphoepithelioma-like carcinoma. Nucl Med Commun. 37:437–445.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aktas GE, Can N, Demir SS and Sarikaya A:
Primary pulmonary lymphoepithelioma-like carcinoma on FDG PET/CT.
Nucl Med Mol Imaging. 51:88–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su TP, Ho KC, Wang CW, Lin CY, Liu CY,
Yang CT and Yen TC: Prognostic value and clinical impact of
pretreatment FDG PET in pulmonary lymphoepithelioma-like carcinoma.
Clin Nucl Med. 44:e68–e75. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tateishi U, Gamez C, Dawood S, Yeung HW,
Cristofanilli M and Macapinlac HA: Bone metastases in patients with
metastatic breast cancer: Morphologic and metabolic monitoring of
response to systemic therapy with integrated PET/CT. Radiology.
247:189–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu J, Khong PL, Wang S, Chan Q, Law W and
Zhang J: Quantitative assessment of diffusion-weighted MR imaging
in patients with primary rectal cancer: Correlation with
FDG-PET/CT. Mol Imaging Biol. 13:1020–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HH, Chiu NT, Su WC, Guo HR and Lee
BF: Prognostic value of whole-body total lesion glycolysis at
pretreatment FDG PET/CT in non-small cell lung cancer. Radiology.
264:559–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paesmans M, Berghmans T, Dusart M, Garcia
C, Hossein-Foucher C, Lafitte JJ, Mascaux C, Meert AP, Roelandts M,
Scherpereel A, et al: Primary tumor standardized uptake value
measured on fluorodeoxyglucose positron emission tomography is of
prognostic value for survival in non-small cell lung cancer: Update
of a systematic review and meta-analysis by the European Lung
Cancer Working Party for the International Association for the
Study of Lung Cancer Staging Project. J Thorac Oncol. 5:612–619.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayashi T, Haba R, Tanizawa J, Katsuki N,
Kadota K, Miyai Y, Bando K, Shibuya S, Nakano M and Kushida Y:
Cytopathologic features and differential diagnostic considerations
of primary lymphoepithelioma-like carcinoma of the lung. Diagn
Cytopathol. 40:820–825. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang YL, Wu CT, Shih JY and Lee YC: New
aspects in clinicopathologic and oncogene studies of 23 pulmonary
lymphoepithelioma-like carcinomas. Am J Surg Pathol. 26:715–723.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ngan RK, Yip TT, Cheng WW, Chan JK, Cho
WC, Ma VW, Wan KK, Au SK, Law CK and Lau WH: Circulating
Epstein-Barr virus DNA in serum of patients with
lymphoepithelioma-like carcinoma of the lung: A potential surrogate
marker for monitoring disease. Clin Cancer Res. 8:986–994.
2002.PubMed/NCBI
|
|
16
|
Beasley MB, Brambilla E and Travis WD: The
2004 World Health Organization classification of lung tumors. Semin
Roentgenol. 40:90–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstraw P: The 7th edition of TNM in
lung cancer: What now? J Thorac Oncol. 4:671–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hung CY, Lin TL, Kuo YC, Hsieh CH, Wang HM
and Hsu CL: Progesterone analogues reduce plasma Epstein-Barr virus
DNA load and improve pain control in recurrent/metastatic
nasopharyngeal carcinoma patients under supportive care. Biomed J.
40:212–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu CL, Chang KP, Lin CY, Chang HK, Wang
CH, Lin TL, Liao CT, Tsang NM, Lee LY, Chan SC, et al: Plasma
Epstein-Barr virus DNA concentration and clearance rate as novel
prognostic factors for metastatic nasopharyngeal carcinoma. Head
Neck. 34:1064–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khiewvan B, Ziai P, Houshmand S, Salavati
A, Ziai P and Alavi A: The role of PET/CT as a prognosticator and
outcome predictor in lung cancer. Expert Rev Respir Med.
10:317–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang KP, Tsang NM, Liao CT, Hsu CL, Chung
MJ, Lo CW, Chan SC, Ng SH, Wang HM and Yen TC: Prognostic
significance of 18F-FDG PET parameters and plasma Epstein-Barr
virus DNA load in patients with nasopharyngeal carcinoma. J Nucl
Med. 53:21–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han AJ, Xiong M and Zong YS: Association
of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the
lung in southern China. Am J Clin Pathol. 114:220–226. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma BB, King A, Lo YM, Yau YY, Zee B, Hui
EP, Leung SF, Mo F, Kam MK, Ahuja A, et al: Relationship between
pretreatment level of plasma Epstein-Barr virus DNA, tumor burden
and metabolic activity in advanced nasopharyngeal carcinoma. Int J
Radiat Oncol Biol Phys. 66:714–720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Chen Y, Chen L, Guo R, Zhou G,
Tang L, Mao Y, Li W, Liu X, Du X, et al: The clinical utility of
plasma Epstein-Barr virus DNA assays in nasopharyngeal carcinoma:
The dawn of a new era? A systematic review and meta-analysis of
7836 cases. Medicine (Baltimore). 94:e8452015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ngan RK, Yip TT, Cheng WW, Chan JK, Cho
WC, Ma VW, Wan KK, Au JS and Law CK: Clinical role of circulating
Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like
carcinoma of the lung. Ann N Y Acad Sci. 1022:263–270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davies A, Tan C, Paschalides C, Barrington
SF, O'Doherty M, Utley M and Treasure T: FDG-PET maximum
standardised uptake value is associated with variation in survival:
Analysis of 498 lung cancer patients. Lung Cancer. 55:75–78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Dong M, Sun X, Li W, Xing L and Yu
J: Prognostic Value of 18F-FDG PET/CT in surgical non-small cell
lung cancer: A meta-analysis. PLoS One. 11:e01461952016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen T, Tang LQ, Luo DH, Chen QY, Li PJ,
Mai DM, Guo SS, Liu LT, Qian CN, Guo X, et al: Different prognostic
values of plasma Epstein-Barr virus DNA and maximal standardized
uptake value of 18F-FDG PET/CT for nasopharyngeal carcinoma
patients with recurrence. PLoS One. 10:e01227562015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soret M, Bacharach SL and Buvat I:
Partial-volume effect in PET tumor imaging. J Nucl Med. 48:932–945.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoang JK, Hoagland LF, Coleman RE, Coan
AD, Herndon JE II and Patz EF Jr: Prognostic value of fluorine-18
fluorodeoxyglucose positron emission tomography imaging in patients
with advanced-stage non-small-cell lung carcinoma. J Clin Oncol.
26:1459–1464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Obara P and Pu Y: Prognostic value of
metabolic tumor burden in lung cancer. Chin J Cancer Res.
25:615–622. 2013.PubMed/NCBI
|
|
33
|
Lee P, Bazan JG, Lavori PW, Weerasuriya
DK, Quon A, Le QT, Wakelee HA, Graves EE and Loo BW: Metabolic
tumor volume is an independent prognostic factor in patients
treated definitively for non-small-cell lung cancer. Clin Lung
Cancer. 13:52–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soussan M, Chouahnia K, Maisonobe JA,
Boubaya M, Eder V, Morere JF and Buvat I: Prognostic implications
of volume-based measurements on FDG PET/CT in stage III
non-small-cell lung cancer after induction chemotherapy. Eur J Nucl
Med Mol Imaging. 40:668–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Loon J, Offermann C, Ollers M, van
Elmpt W, Vegt E, Rahmy A, Dingemans AM, Lambin P and De Ruysscher
D: Early CT and FDG-metabolic tumour volume changes show a
significant correlation with survival in stage I–III small cell
lung cancer: A hypothesis generating study. Radiother Oncol.
99:172–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung HW, Lee KY, Kim HJ, Kim WS and So Y:
FDG PET/CT metabolic tumor volume and total lesion glycolysis
predict prognosis in patients with advanced lung adenocarcinoma. J
Cancer Res Clin Oncol. 140:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang D, Zhang M, Gao X and Yu L:
Prognostic value of baseline 18F-FDG PET/CT functional parameters
in patients with advanced lung adenocarcinoma stratified by EGFR
mutation status. PLoS One. 11:e01583072016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XY, Zhao YF, Liu Y, Yang YK, Zhu Z
and Wu N: Comparison of different automated lesion delineation
methods for metabolic tumor volume of 18F-FDG PET/CT in patients
with stage I lung adenocarcinoma. Medicine (Baltimore).
96:e93652017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carlier T and Bailly C: State-Of-The-Art
and recent advances in quantification for therapeutic Follow-Up in
oncology using PET. Front Med (Lausanne). 2:182015.PubMed/NCBI
|
|
40
|
Adams MC, Turkington TG, Wilson JM and
Wong TZ: A systematic review of the factors affecting accuracy of
SUV measurements. AJR Am J Roentgenol. 195:310–320. 2010.
View Article : Google Scholar : PubMed/NCBI
|