Introduction
Kidney cancer is one of the top 10 types of cancer
in terms of incidence and mortality rate in men and women,
worldwide. Renal cell carcinoma (RCC) accounts for ~90% of all
kidney cancer cases (1,2). RCC mainly includes the papillary
subtype, the chromophobe subtype and clear cell RCC (ccRCC)
(3); ccRCC is the most common
subtype. Furthermore, 30% of patients with kidney cancer present
with metastatic disease (4).
Surgical resection remains the most effective therapeutic strategy
against clinically localized ccRCC. In addition, current treatments
are focused on vascular endothelial growth factor receptor
(VEGFR)-targeted therapy and mammalian target of rapamycin (mTOR)
inhibition (5,6). However, 30% patients with ccRCC are
already at an advanced stage at the time of diagnosis (7), and the current therapeutic strategies
offer limited efficacy. The development of novel therapeutic drugs
for ccRCC is therefore challenging and it is crucial to determine
efficient prognostic biomarkers of ccRCC in order to develop an
effective treatment.
As the best-known inhibitory neurotransmitter in the
brain, γ-aminobutyric acid (GABA) activates three pharmacologically
and structurally distinct classes of receptor: GABAA,
GABAB and GABAC (8). Among them, GABAA is the
major inhibitory receptor in the central nervous system (9,10).
Notably, GABAA receptor subunit θ (GABRQ) can bind with
other receptors to form a functional chloride channel that mediates
inhibitory synaptic transmission in the mature central nervous
system (9). GABA and receptor
GABAA are also present in peripheral tissues, including
cancerous cells, but their precise function is poorly understood
(10). A previous study revealed
that GABRQ is overexpressed in hepatocellular carcinoma and that
GABA promotes the proliferation of cancer cells through GABRQ
(11). However, the prognostic
significance of GABRQ in ccRCC remains unknown. To the best of our
knowledge, the present study was the first to report on the mRNA
expression levels of GABRQ in samples obtained from the
International Cancer Genome Consortium (ICGC) (12) and The Cancer Genome Atlas (TCGA)
(13,14) primary-ccRCC cohorts. The results
suggested that the mRNA expression levels of GABRQ may be
considered an effective prognostic marker of ccRCC.
Materials and methods
Patient data and characteristics
The clinical and transcriptomic data from patients
with ccRCC were downloaded from TCGA (13,14) and
ICGC (12) databases in March 2018.
To identify the prognostic significance of GABRQ (Table I). TCGA and ICGC databases are
approved for the quality of patient data and are widely used in
numerous studies. No additional quality assessment was performed,
since data that were not produced by a reputable institution were
excluded. Samples with insufficient survival information were
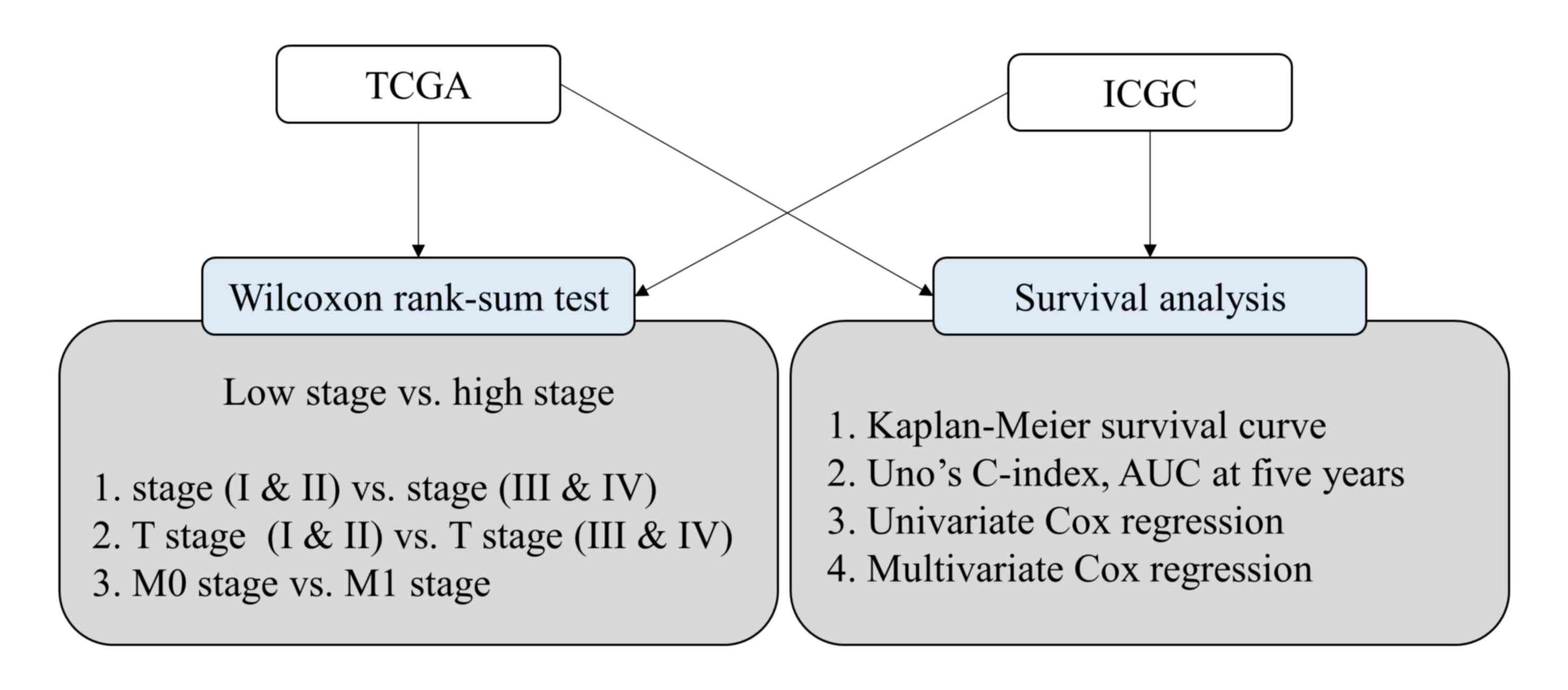
excluded (15,16). The overall study workflow is
presented in Fig. 1. Comparative
analyses between normal and tumor cells was conducted with the use
of publicly available microarray data from the Oncomine database.
To relate GABRQ copy number (17), the GSE20306 dataset (n=449) was
used.
 | Table I.Characteristics of patients from TCGA
and ICGC databases. |
Table I.
Characteristics of patients from TCGA
and ICGC databases.
| Characteristic | TCGA (%) | ICGC (%) |
|---|
| ATCC stage |
|
|
| I | 216 (48.4) | 48 (52.7) |
| II | 46 (10.3) | 12 (13.2) |
|
III | 111 (24.9) | 13 (14.3) |
| IV | 71 (15.9) | 9 (9.9) |
| Not
available | 2 (0.4) | 9 (9.9) |
| Grade |
|
|
| I | 9 (2.0) | – |
| II | 189 (42.4) | – |
|
III | 175 (39.2) | – |
| IV | 68 (15.2) | – |
| Not
available | 5 (1.1) | – |
| Sex |
|
|
|
Male | 290 (65.0) | 52 (57.1) |
|
Female | 156 (35.0) | 39 (42.9) |
| Age (mean ±
standard deviation) | 60.62±12.80 | 60.47±10.03 |
| Total number of
patients | 446 | 91 |
Statistical analyses
Wilcoxon's rank-sum test was performed to identify
the differences in GABRQ expression between early and late
stages of ccRCC in TCGA and ICGC cohorts. Survival analyses to
predict overall survival of patients with ccRCC and the associated
statistical analyses were conducted using R software (version
3.5.0; The R Foundation for Statistical Computing; 2018; http://www.R-project.org). Furthermore, to validate
the prognostic value of GABRQ, the following statistical
methods were carried out: i) Uno's C-index; ii) area under the
curve (AUC) in receiver operating characteristics (ROC) at 5 years;
and iii) P-value from log-rank test of Kaplan-Meier survival curve
to evaluate the accuracy of the discrimination, as described
previously using the ‘survival’ and ‘survAUC’ R packages (16,18). The
C-index is a well-known parameter of the fit of a survival model
within a continuous time period during a clinical study (19,20).
Regarding the survival curve analyses, the optimal cutoff value
that had the maximal Uno's C-index by 5-fold cross-validation was
determined as previously described (15,16,18).
Since the RNA sequencing data from TCGA and ICGC had been obtained
using different sequencing and normalization methods, the absolute
value of gene expression varied widely among datasets. For these
reasons, the optimal cutoff values were different for each cohort.
T and M stage information in both cohorts was sufficient to perform
subgroup analysis; however, as there was no information for N
stage, subgroup analysis was not performed (12–14).
Univariate and multivariate Cox regression analyses were performed
to compare the effects of GABRQ expression (as a categorical
value) on prognosis and other clinical variables.
Results
Patient characteristics
A total of 446 and 91 patients from the TCGA and
ICGC databases, respectively, were analyzed in the present study
(12–14,21,22). The
446 patients from TCGA comprised 290 men and 156 women. The 91
patients from the ICGC comprised 52 men and 39 women. The patient
characteristics investigated in the present study are listed in
Table I.
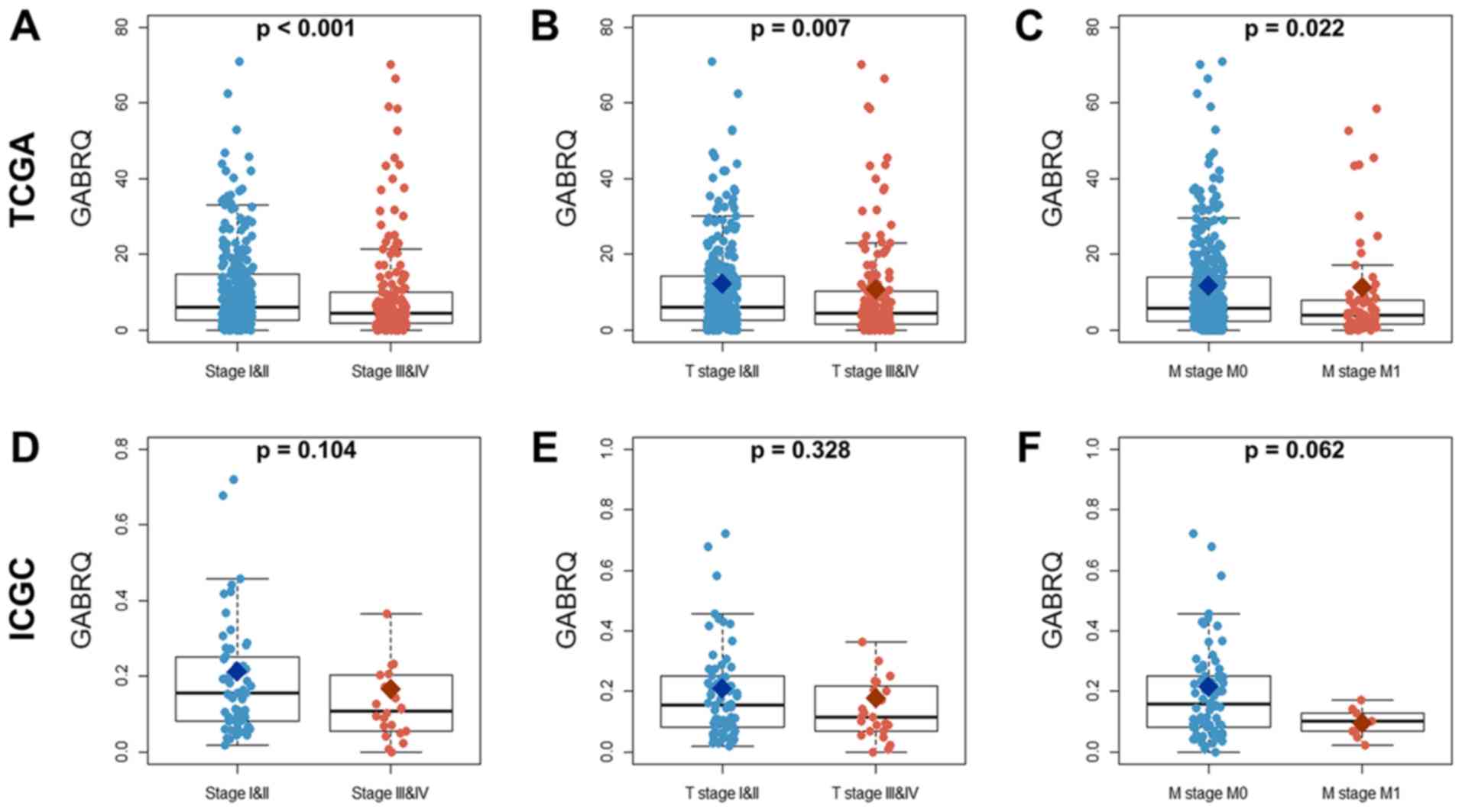
Downregulation of GABRQ at late stages
of ccRCC
The mRNA expression levels of GABRQ were
compared among samples from early (TI and II), late (TIII and IV),
nonmetastatic (M0) and metastatic (M1, primary tumor) stages of
ccRCC (Table II) from TCGA and ICGC
cohorts. The mRNA expression levels of GABRQ were much
higher in early (TI and II) and nonmetastatic (M0) ccRCC samples
compared with in late (TIII and IV) and metastatic (M1, primary
tumor) ccRCC samples in TCGA (Fig.
2). The similar trend was seen in the ICGC but this was not
statistically significant. In addition, GABRQ copy numbers
are decreased in several types of cancers, including kidney
cancers, leukemia, multiple myeloma and prostate cancers (Fig. 3).
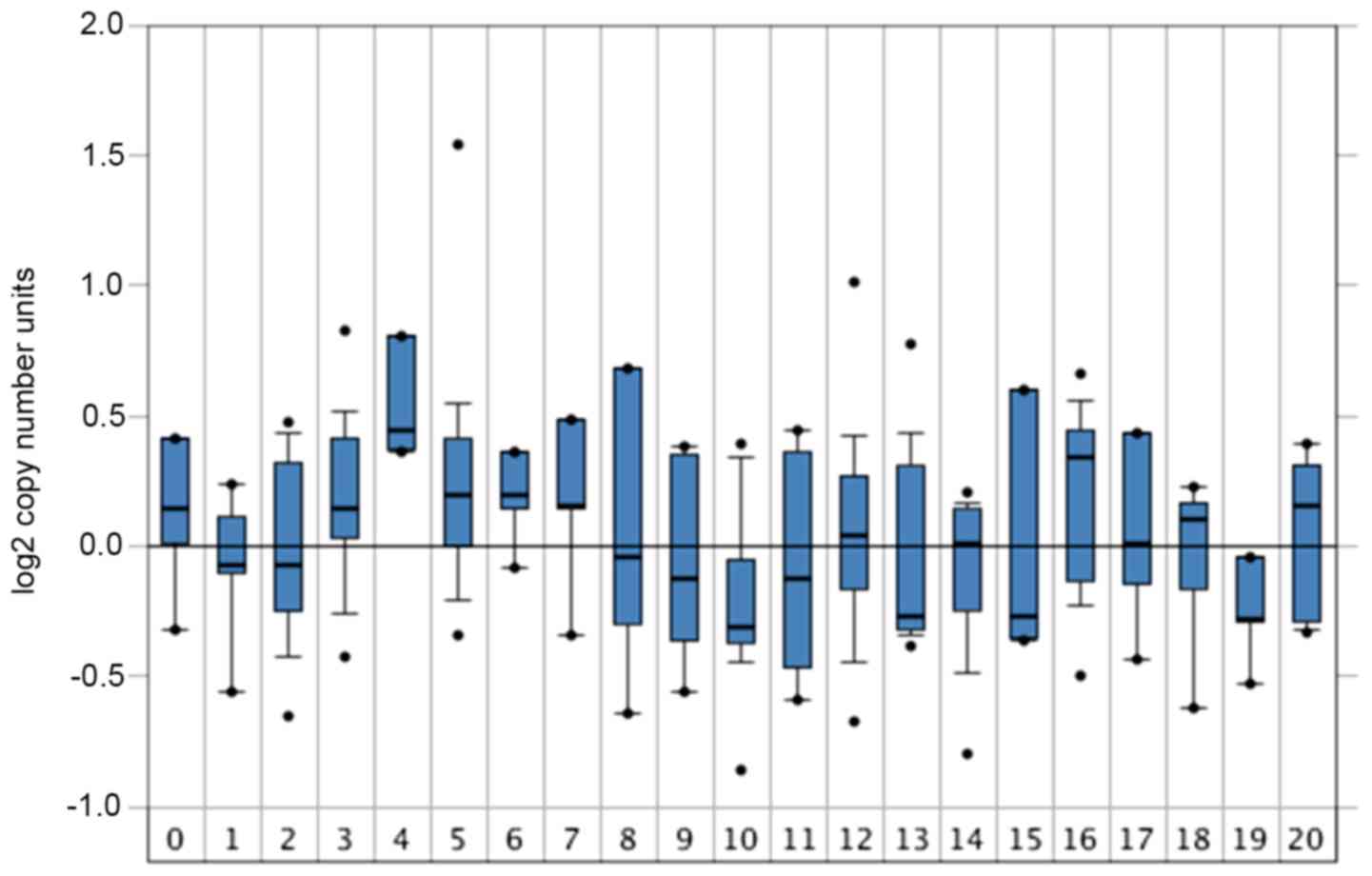 | Figure 3.GABRQ gene expression in
tumors. The copy number of GABRQ in tumors in the Oncomine
database corresponds to the GSE20306 dataset (n=449) (36). The x-axis represents the number of
patients with different types of cancer: 0, Normal (n=4); 1,
Bladder (n=9); 2, Brain and central nervous system (n=17); 3,
Breast (n=21); 4, Cervical (n=7); 5, Colorectal (n=21); 6,
Esophageal (n=4); 7, Gastric (n=5); 8, Head and neck (n=6); 9,
Kidney (n=8); 10, Leukemia (n=33); 11, Liver (n=9); 12, Lung
(n=78); 13, Lymphoma (n=41); 14, Melanoma (n=12); 15, Myeloma
(n=5); 16, Other (n=7); 18, Pancreatic (n=9); 19, Prostate (n=5)
and 20, Sarcoma (n=20). GABRQ, γ-aminobutyric acid receptor
A subunit θ. |
 | Table II.Optimal cutoff values for
γ-aminobutyric acid receptor A subunit θ expression in TCGA and
ICGC cohorts. |
Table II.
Optimal cutoff values for
γ-aminobutyric acid receptor A subunit θ expression in TCGA and
ICGC cohorts.
| Dataset | Cutoff value |
|---|
| TCGA | 4.9689 |
| ICGC | 0.1440 |
Prognostic value of GABRQ mRNA
expression in patients with ccRCC
To identify the prognostic significance of
GABRQ in ccRCC, survival curves for GABRQ mRNA
expression (Table II) and survival
within TCGA (Fig. 4) and ICGC
(Fig. 5) cohorts were analyzed.
Patients with low GABRQ mRNA expression in the primary tumor
in the two cohorts had significantly shorter overall survival time
compared with patients with higher GABRQ mRNA expression
(Figs. 4 and 5). Prognostic value was then examined using
multivariate Cox regression analysis (Table III). The multivariate analysis
conformed that GABRQ mRNA expression was an independent prognostic
factor for ccRCC.
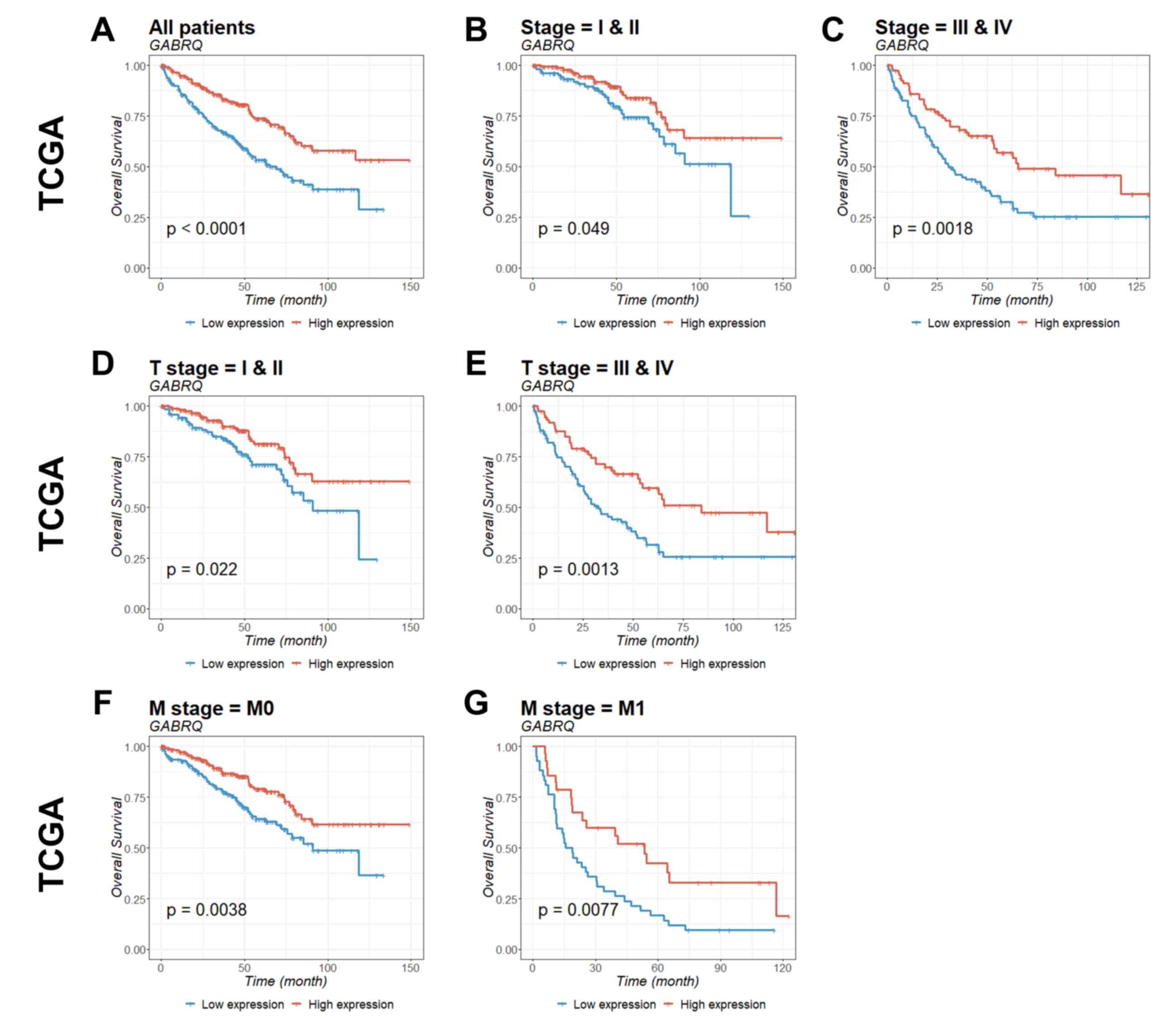 | Figure 4.Kaplan-Meier estimation of
GABRQ mRNA expression as a prognostic biomarker in patients
with ccRCC. The association of GABRQ gene expression with
overall survival among patients from TCGA was examined. Gene
expression was analyzed for (A) all patients, (B) stage I and II,
(C) stage III and IV, (D) T stage I and II, (E) T stage III and IV,
(F) M stage 0, and (G) M stage 1. P-values were calculated by the
log-rank test and are provided in the bottom left of each plot.
ccRCC, clear cell renal cell carcinoma; GABRQ,
γ-aminobutyric acid receptor A subunit θ; TCGA, The Cancer Genome
Atlas; M0, non-metastatic; M1, primary tumor. |
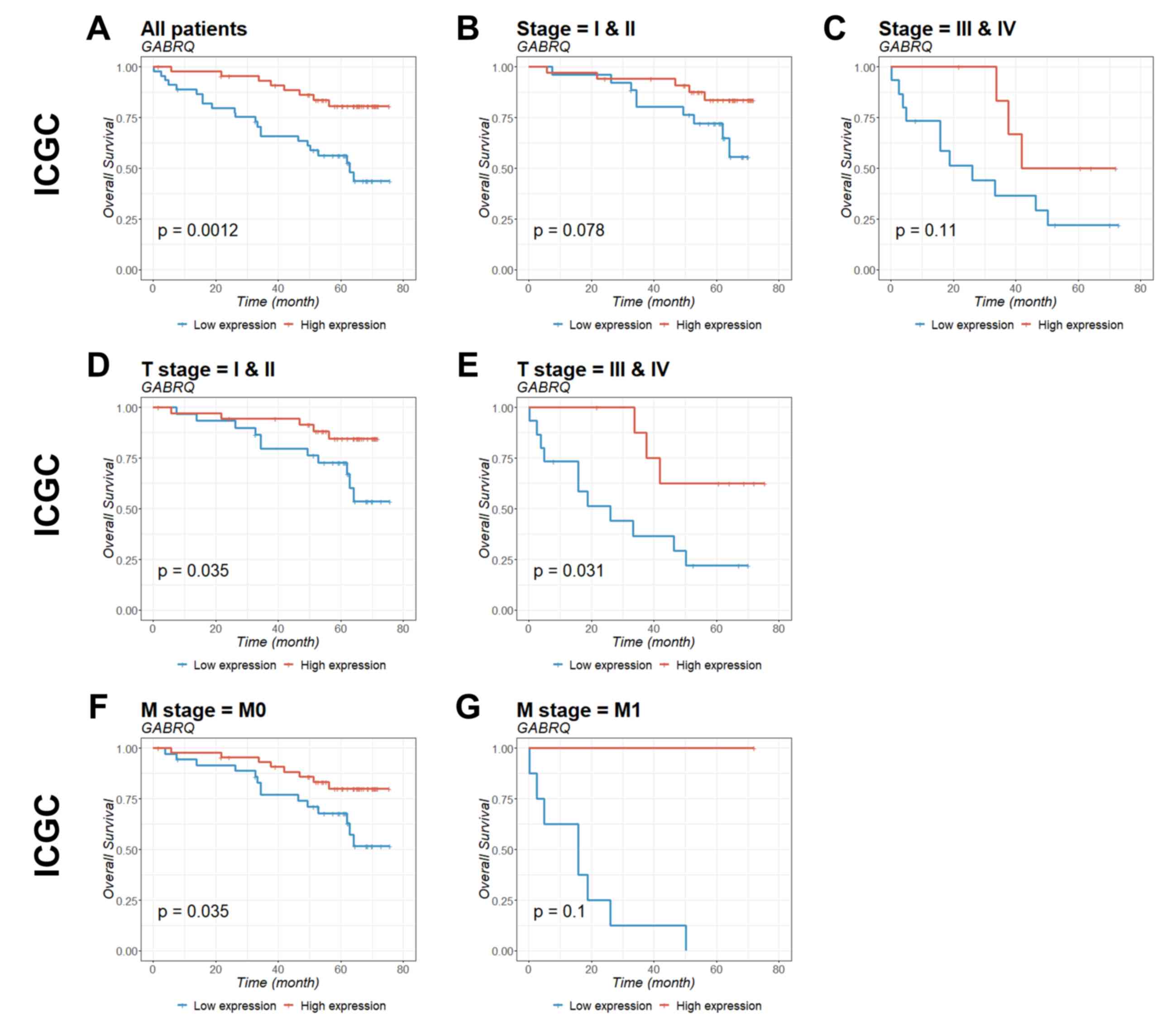 | Figure 5.Kaplan-Meier estimation of
GABRQ mRNA expression as a prognostic biomarker in patients
with ccRCC. The association of GABRQ gene expression with
overall survival among patients from the ICGC was examined. Gene
expression was analyzed for (A) all patients, (B) stage I and II,
(C) stage III and IV, (D) T stage I and II, (E) T stage III and IV,
(F) M stage 0, and (G) M stage 1. P-values were calculated by the
log-rank test and are provided in the bottom left of each plot.
ccRCC, clear cell renal cell carcinoma; GABRQ,
γ-aminobutyric acid receptor A subunit θ; ICGC, International
Cancer Genome Consortium; M0, non-metastatic; M1, primary
tumor. |
 | Table III.Univariate and multivariate analyses
of overall survival in each cohort. |
Table III.
Univariate and multivariate analyses
of overall survival in each cohort.
| A, TCGA |
|---|
|
|---|
|
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|---|
| Variable | P-value | Hazard ratio | 95% confidence
interval | P-value | Hazard ratio | 95% confidence
interval |
|---|
| GABRQ
(categorical) |
<0.001c | 0.483 | 0.348 | 0.672 |
<0.001c | 0.562 | 0.401 | 0.788 |
| Age | <0.001
c | 1.033 | 1.018 | 1.047 |
<0.001c | 1.030 | 1.015 | 1.046 |
| Stage (I, II vs.
III, IV) |
<0.001c | 3.478 | 2.474 | 4.888 |
<0.001c | 2.883 | 2.012 | 4.132 |
| Sex (female vs.
male) | 0.333 | 0.850 | 0.612 | 1.181 | 0.859 | 0.969 | 0.688 | 1.366 |
| Grade (I, II vs.
III, IV) |
<0.001c | 2.247 | 1.572 | 3.212 | 0.135 | 1.340 | 0.913 | 1.968 |
|
| B, ICGC |
|
|
| Univariate Cox
regression | Multivariate Cox
regression |
|
|
|
|
|
Variable | P-value | Hazard
ratio | 95% confidence
interval | P-value | Hazard
ratio | 95% confidence
interval |
|
| GABRQ
(categorical) | 0.002b | 0.283 | 0.126 | 0.638 | 0.014a | 0.343 | 0.146 | 0.804 |
| Age | 0.109 | 1.031 | 0.993 | 1.071 | 0.448 | 1.015 | 0.976 | 1.056 |
| Stage |
<0.001c | 4.796 | 2.264 | 10.16 |
<0.001c | 4.628 | 2.094 | 10.232 |
| (I, II vs. III,
IV) |
| Sex (female vs.
male) | 0.863 | 1.066 | 0.517 | 2.194 | 0.307 | 0.657 | 0.294 | 1.470 |
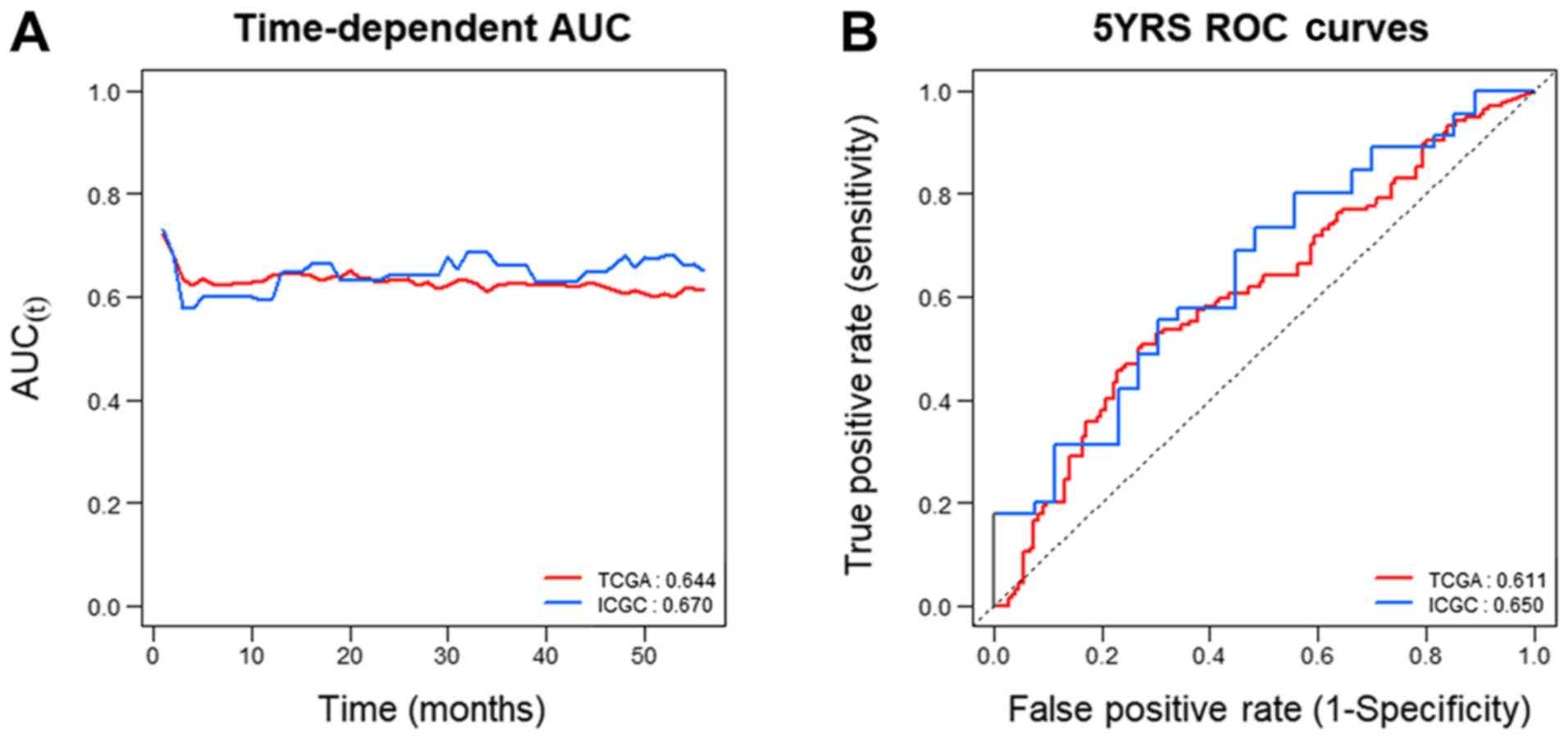
C-index and AUC of GABRQ
To assess whether GABRQ mRNA expression could
be considered a prognostic biomarker of ccRCC, Uno's C-index based
on the time-dependent AUC analysis and the AUC of the receiver
operating characteristic curve at 5 years were examined. The
results demonstrated that GABRQ mRNA had high C-index values
in the two independent cohorts (TCGA: 0.644 and ICGC: 0.670;
Fig. 6A and Table IV). The 5-year ROC curves yielded
high AUC values for both TCGA and ICGC (0.611 and 0.650,
respectively; Fig. 6B). These
results suggest that GABRQ mRNA expression is useful to predict
prognosis of patients with ccRCC.
 | Table IV.C-index values of GABRQ in the
specified categories of TCGA and ICGC cohorts. |
Table IV.
C-index values of GABRQ in the
specified categories of TCGA and ICGC cohorts.
|
| C-index |
|---|
|
|
|
|---|
| Categories | TCGA | ICGC |
|---|
| All patients | 0.644 | 0.670 |
| Stage I &
II | 0.597 | 0.639 |
| Stage III &
IV | 0.634 | 0.585 |
| T (I & II) | 0.600 | 0.621 |
| T (III &
IV) | 0.645 | 0.671 |
| M0 | 0.623 | 0.652 |
| M1 | 0.644 | 0.655 |
Discussion
Classification of ccRCC includes localized and
advanced ccRCC. Numerous therapeutic options are currently
available for localized ccRCC; however the most effective therapy
remains surgical resection. Furthermore, there are no suitable
drugs for the adjuvant treatment of local kidney cancer (23). Current treatments of advanced ccRCC
target VEGFR and mTOR (24). Due to
recent advances in biotechnology, including next-generation
sequencing, bioinformatics has rapidly developed and highlighted a
great number of potential biomarkers (25). Numerous patient databases are freely
available to the public, including the Gene Expression Omnibus and
TCGA, which contain extensive gene expression data that can be used
to determine novel biomarkers (26).
Notably, these databases can be used to identify essential
biomarkers for effective prognosis of ccRCC. In addition, molecular
markers that can be used in combination with the current cancer
staging systems need to be identified.
The present study demonstrated that GABRQ
mRNA expression could be a prognostic marker of ccRCC. In
particular, low GABRQ mRNA expression was associated with a
poor prognosis among patients with ccRCC. A previous study
indicated that GABRQ is overexpressed in hepatocellular
carcinoma and that GABA promotes the proliferation of cancer cells
through GABRQ (11). In addition,
GABA can inhibit colon cancer cell migration associated with the
norepinephrine-induced pathway (27), and via the GABAB pathway,
which is involved in prostate cancer metastasis and invasion
(28). Although GABRQ may contribute
to cancer progression, it has been reported that it can serve
additional roles in other types of disease, including essential
tremor (8) and migraines (29).
This study presented some methodological
limitations. Since the expression data of GABRQ in the two
independent cohorts were obtained through different methods, the
absolute expression values differed for each cohort. The cutoff
values of GABRQ were therefore different for each cohort.
Most cohort studies present the same technical limitations, unless
data processing was performed by the same hospital and at the same
time. To identify the involvement of GABRQ in ccRCC,
experiments need to be conducted at the protein level. However,
there are numerous studies that have determined prognostic
biomarkers based on mRNA expression (for example oncotype DX
(30), MammaPrint (31) and gene signatures tests (19,32–34)).
Although the present study did not confirm GABRQ function at the
protein level, mRNA-based studies are emerging in this field, and
represent time- and cost-effective methods.
The main conclusions from the present study may
strengthen the foundation of precision medicine via analysis of
transcriptomic data. mRNA-based prognostic markers have been
identified in numerous diseases, including cancer, and certain
markers are so accurate that they can be included in clinical
guidelines (19,32,35–37). The
results from both cohorts analyzed in this study demonstrated that
lower GABRQ mRNA expression was associated with a worse
ccRCC prognosis. In addition, GABRQ copy numbers were much
lower in numerous types of cancer, including kidney cancer,
leukemia, multiple myeloma and prostate cancer, according to
genomic analyses from Oncomine (17)
(Fig. 6). Although there are
limitations to transcriptome-based studies of GABRQ, the
results from the present study suggested that GABRQ mRNA
expression may be considered a novel prognostic biomarker of
ccRCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the MRC program
(grant no. NRF-2015R1A5A2009656), the National Research Foundation
of Korea (NRF) funded by the Korean government (MSIT) (grant no.
NRF-2018R1C1B6001290) and the Convergence Medical Institute of
Technology R&D project (grant no. CMIT2019-03) of Pusan
National University Hospital. In addition, this work was supported
by the Collaborative Genome Program for Fostering New Post-Genome
Industry of the NRF funded by the Korean government (MSIT; grant
no. NRF-2017M3C9A6047610).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, MH, SOO and YHK contributed to the design of the
study. CMH, JYK, SMP, DSP, DHS, HJS, HSY, CDKi, CDKa and MEH
acquired the data. MH, YH, DSP, DHS, HJS, HSY, CDKi, CDKa and MEH
acquired and analyzed the data. DL, SOO and YHK drafted the
manuscript. DL, DSP, DHS, HJS, HSY, CDKi, CDKa, MEH, SOO and YHK
revised and edited the manuscript. DL, SOOO and YHL acquired
funding, resources and supervised the project.
Ethics and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Srigley JR, Delahunt B, Eble JN, Egevad L,
Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et
al: The international society of urological pathology (ISUP)
Vancouver classification of renal Neoplasia. Am J Surg Pathol.
37:1469–1489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nickerson ML, Jaeger E, Shi Y, Durocher
JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko
V, et al: Improved identification of von Hippel-Lindau gene
alterations in clear cell renal tumors. Clin Cancer Res.
14:4726–4734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powles T, Albiges L, Staehler M, Bensalah
K, Dabestani S, Giles RH, Hofmann F, Hora M, Kuczyk MA, Lam TB, et
al: Updated European association of urology guidelines
recommendations for the treatment of first-line metastatic clear
cell renal cancer. Eur Urol. Dec 7–2017.doi:
10.1016/j.eururo.2017.11.016 (Epub ahead of print).
|
|
6
|
Powles T, Staehler M, Ljungberg B,
Bensalah K, Canfield SE, Dabestani S, Giles R, Hofmann F, Hora M,
Kuczyk MA, et al: Updated EAU Guidelines for clear cell renal
cancer patients who fail vegf targeted therapy. Eur Urol. 69:4–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karakiewicz PI, Briganti A, Chun FK, Trinh
QD, Perrotte P, Ficarra V, Cindolo L, De la Taille A, Tostain J,
Mulders PF, et al: Multi-institutional validation of a new renal
cancer-specific survival nomogram. J Clin Oncol. 25:1316–1322.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia-Martin E, Martinez C,
Alonso-Navarro H, Benito-León J, Lorenzo-Betancor O, Pastor P,
Puertas I, Rubio L, López-Alburquerque T, Agúndez JA and
Jiménez-Jiménez FJ: Gamma-aminobutyric acid GABRA4, GABRE, and
GABRQ receptor polymorphisms and risk for essential tremor.
Pharmacogenet Genomics. 21:436–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neelands TR, Zhang J and Macdonald RL:
GABA(A) receptors expressed in undifferentiated human
teratocarcinoma NT2 cells differ from those expressed by
differentiated NT2-N cells. J Neurosci. 19:7057–7065. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haydar TF, Wang F, Schwartz ML and Rakic
P: Differential modulation of proliferation in the neocortical
ventricular and subventricular zones. J Neurosci. 20:5764–5774.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YH, Liu Y, Li YD, Liu YH, Li F, Ju Q,
Xie PL and Li GC: GABA stimulates human hepatocellular carcinoma
growth through overexpressed GABAA receptor theta subunit. World J
Gastroenterol. 18:2704–2711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
International Cancer Genome Consortium, ;
Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabé RR, Bhan
MK, Calvo F, Eerola I, et al: International network of cancer
genome projects. Nature. 464:993–998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cancer Genome Atlas Research Network, ;
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ha M, Han ME, Kim JY, Jeong DC, Oh SO and
Kim YH: Prognostic role of TPD52 in acute myeloid leukemia: A
retrospective multicohort analysis. J Cell Biochem. 120:3672–3678.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han ME, Kim JY, Kim GH, Park SY, Kim YH
and Oh SO: SAC3D1: A novel prognostic marker in hepatocellular
carcinoma. Sci Rep. 8:156082018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho SH, Pak K, Jeong DC, Han ME, Oh SO and
Kim YH: The AP2M1 gene expression is a promising biomarker for
predicting survival of patients with hepatocellular carcinoma. J
Cell Biochem. 120:4140–4146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YH, Jeong DC, Pak K, Goh TS, Lee CS,
Han ME, Kim JY, Liangwen L, Kim CD, Jang JY, et al: Gene network
inherent in genomic big data improves the accuracy of prognostic
prediction for cancer patients. Oncotarget. 8:77515–77526.
2017.PubMed/NCBI
|
|
20
|
Uno H, Cai T, Pencina MJ, D'Agostino RB
and Wei LJ: On the C-statistics for evaluating overall adequacy of
risk prediction procedures with censored survival data. Stat Med.
30:1105–1117. 2011.PubMed/NCBI
|
|
21
|
Hoshida Y, Nijman SM, Kobayashi M, Chan
JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K,
Hashimoto M, et al: Integrative transcriptome analysis reveals
common molecular subclasses of human hepatocellular carcinoma.
Cancer Res. 69:7385–7392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shtraizent N, DeRossi C, Nayar S,
Sachidanandam R, Katz LS, Prince A, Koh AP, Vincek A, Hadas Y,
Hoshida Y, et al: MPI depletion enhances O-GlcNAcylation of p53 and
suppresses the Warburg effect. Elife. 6(pii): e224772017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramanian P and Haas NB: Recent advances
in localized RCC: A focus on VEGF and immuno-oncology therapies.
Urol Oncol. 36:23–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Chen L, Wang G, Cheng S, Qian K,
Liu X, Wu CL, Xiao Y and Wang X: Fifteen hub genes associated with
progression and prognosis of clear cell renal cell carcinoma
identified by coexpression analysis. J Cell Physiol.
234:10225–10237. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan L, Tan J, Li H and Jin X: Biomarker
identification in clear cell renal cell carcinoma based on
miRNA-seq and digital gene expression-seq data. Gene. 647:205–212.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao C, Zhou C, Zhuang J, Liu L, Liu C, Li
H, Liu G, Wei J and Sun C: MicroRNA expression in cervical cancer:
Novel diagnostic and prognostic biomarkers. J Cell Biochem.
119:7080–7090. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joseph J, Niggemann B, Zaenker KS and
Entschladen F: The neurotransmitter gamma-aminobutyric acid is an
inhibitory regulator for the migration of SW 480 colon carcinoma
cells. Cancer Res. 62:6467–6469. 2002.PubMed/NCBI
|
|
28
|
Azuma H, Inamoto T, Sakamoto T, Kiyama S,
Ubai T, Shinohara Y, Maemura K, Tsuji M, Segawa N, Masuda H, et al:
Gamma-aminobutyric acid as a promoting factor of cancer metastasis;
induction of matrix metalloproteinase production is potentially its
underlying mechanism. Cancer Res. 63:8090–8096. 2003.PubMed/NCBI
|
|
29
|
Plummer PN, Colson NJ, Lewohl JM, MacKay
RK, Fernandez F, Haupt LM and Griffiths LR: Significant differences
in gene expression of GABA receptors in peripheral blood leukocytes
of migraineurs. Gene. 490:32–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wittner BS, Sgroi DC, Ryan PD, Bruinsma
TJ, Glas AM, Male A, Dahiya S, Habin K, Bernards R, Haber DA, et
al: Analysis of the MammaPrint breast cancer assay in a
predominantly postmenopausal cohort. Clin Cancer Res. 14:2988–2993.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HY, Yu SL, Chen CH, Chang GC, Chen
CY, Yuan A, Cheng CL, Wang CH, Terng HJ, Kao SF, et al: A five-gene
signature and clinical outcome in non-small-cell lung cancer. N
Engl J Med. 356:11–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goh TS, Lee JS, II Kim J, Park YG, Pak K,
Jeong DC, Oh SO and Kim YH: Prognostic scoring system for
osteosarcoma using network-regularized high-dimensional
Cox-regression analysis and potential therapeutic targets. J Cell
Physiol. 234:13851–13857. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pak K, Kim YH, Suh S, Goh TS, Jeong DC,
Kim SJ, Kim IJ, Han ME and Oh SO: Development of a risk scoring
system for patients with papillary thyroid cancer. J Cell Mol Med.
23:3010–3015. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim YH, Jeong DC, Pak K, Han ME, Kim JY,
Liangwen L, Kim HJ, Kim TW, Kim TH, Hyun DW and Oh SO: SLC2A2
(GLUT2) as a novel prognostic factor for hepatocellular carcinoma.
Oncotarget. 8:68381–68392. 2017.PubMed/NCBI
|
|
36
|
Nault JC, De Reynies A, Villanueva A,
Calderaro J, Rebouissou S, Couchy G, Decaens T, Franco D, Imbeaud
S, Rousseau F, et al: A hepatocellular carcinoma 5-gene score
associated with survival of patients after liver resection.
Gastroenterology. 145:176–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van't Veer LJ, Dai H, van de Vijver MJ, He
YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ,
Witteveen AT, et al: Gene expression profiling predicts clinical
outcome of breast cancer. Nature. 415:530–536. 2002. View Article : Google Scholar : PubMed/NCBI
|