Introduction
Cervical cancer is one of the most common cancer
types in women living with human immunodeficiency virus (HIV)
(1). Most patients present with
locally advanced disease (2),
defined as stages IB2-IVA by the International Federation of
Gynaecology and Obstetrics (FIGO), and concurrent chemoradiation
remains the standard of treatment for these patients. However, the
majority of recurrences occur within two years after treatment
(3,4). A defective immune surveillance might
contribute to poor outcomes. Theoretically, tumours can evade
immune surveillance by upregulating programmed cell death-ligand 1
(PD-L1) expression. PD-L1 is known to play a key role in the
inhibition of T cell-mediated immune responses, leading to the
progression of tumours. PD-L1 on malignant cells is often
upregulated within the cancer microenvironment (5). Several mechanisms contributing to the
upregulation of PD-L1 on malignant cells, including epigenetic
factors, oncogenic signalling and acquired immune responses, have
been identified. Constitutive oncogenic signalling has been
discovered to induce PD-L1 expression on malignant cells either
through the phosphatidylinositol-3-kinase-protein kinase B
(PI3K-AKT) pathway or signal transducer and activator of
transcription (STAT) 3 signalling (6,7). In
addition, the acquired immune response is considered to manifest
through PD-L1 upregulation on malignant cells by endogenous
antitumour immunity-related factors in the cancer microenvironment,
such as interferon-γ (IFN-γ) produced by tumour-infiltrating
lymphocytes (8).
PD-L1 overexpression has been identified in many
solid cancer types (9), such as
malignant melanoma (10), pulmonary
cancer (11) and colorectal cancer
(12). Wu et al (9) demonstrated that PD-L1 overexpression is
related to worse overall survival in gastric carcinoma,
hepatocellular carcinoma, oesophageal carcinoma, and transitional
cell carcinoma, whereas this relationship is not present in
pulmonary cancer and malignant melanoma.
Interestingly, amplification of chromosome 9p24.1
has recently been demonstrated as an essential mechanism for
increased PD-L1 protein expression in nodular sclerosing classical
Hodgkin lymphoma and primary mediastinal large B-cell lymphoma
(13). Consequently, 9p24.1 gene
locus amplification has been discovered in subsets of colorectal
carcinoma, triple-negative breast cancer, glioblastoma and gastric
adenocarcinoma (14,15).
More recently, the genetic basis of increased PD-L1
expression was identified in cervical and vulvar squamous cell
carcinoma. The genes encoding PD-L1 and PD-L2, CD274 and
PDCD1LG2, respectively, were coamplified or overexpressed
due to chromosomal gains in 67% of cervical and 43% of vulvar
squamous cell carcinoma cases assessed by fluorescence in situ
hybridization (FISH) (16). The data
show that 9p24.1 gene copy number alterations are an important
mechanism of increased PD-L1 expression in cervical squamous cell
carcinoma. However, this study did not investigate the correlation
of genetic changes with clinical outcomes.
In the highly active antiretroviral therapy era,
several studies have demonstrated that the incidence of
AIDS-defining cancer among HIV-positive patients has significantly
decreased over the past few decades (17–19).
Regarding cervical cancer, a recent meta-analysis showed a
reduction in the incidence and progression of cervical
intraepithelial neoplasia and the incidence of invasive cervical
cancer after antiretroviral therapy (ART) (20). However, the interactions between ART
and high-risk human papillomavirus (HPV) and invasive cervical
cancer in HIV-positive patients are poorly understood. Several
previous studies have shown that ART possesses anti-HPV and
anticancer properties in addition to improving functional immunity
(21).
Several antitumour mechanisms have been discovered,
including inhibition of angiogenesis, invasion of cancer cells and
induction of apoptosis (21).
Consequently, ART might hold promise for treating cancer.
Furthermore, it is possible that ART might participate in a variety
of anticancer mechanisms and associate with PD-L1 expression via
the downregulation of common signalling pathways or cytokines.
Hence, we aimed to explore this relationship using PD-L1, a
prognostic and predictive biomarker in various solid tumours. The
associations of ART, PD-L1 protein expression, and PD-L1 gene copy
number status with clinical outcomes were studied by comparing
ART-exposed subjects with ART-unexposed controls.
Materials and methods
Patients
The retrospective cohort consisted of 48
HIV-infected patients and 123 uninfected controls with
International Federation of Gynaecology and Obstetrics stage (FIGO)
stage IB2-IVA cervical cancer who underwent tissue biopsies of
squamous cell carcinoma and adenocarcinoma of the cervix between
December 2008 and December 2016 at the Faculty of Medicine,
Navamindradhiraj University, the National Cancer Institute, and
Rajavithi Hospital. The present study was approved by the
Institutional Review Boards of Navamindradhiraj University, the
National Cancer Institute, and Rajavithi Hospital. All patients
provided informed consent. H&E-stained sections were reviewed
by two pathologists (KL and NP). Complete clinicopathologic data
were available for all patients. The inclusion and exclusion
criteria are described below.
Inclusion criteria: Subjects were eligible if they
i) had stage IB2-IVA cervical cancer; ii) were HIV-positive and had
previously been exposed to ART more than one year before the
diagnosis of cervical cancer (classified as the ART-exposed group);
and iii) were HIV-positive and had never been exposed to ART before
cervical cancer diagnosis (classified as the ART-untreated
group).
Exclusion criteria: Subjects were excluded from the
study for the following reasons: i) Previous exposure to
chemoradiation therapy before cervical cancer diagnosis; ii) known
history of the following underlying illnesses (autoimmune diseases,
diabetes mellitus, and hepatitis B or C virus coinfection); iii)
taking immunosuppressive or antituberculous drugs within one year
before the diagnosis of cervical cancer; and iv) presence of
synchronous or metachronous malignancy.
Immunohistochemistry
Immunohistochemistry (IHC) was performed in all
cases with a monoclonal antibody recognizing PD-L1. Whole tissue
sections (4 µm) were cut and stained for PD-L1 (clone SP263;
Ventana Medical Systems, Inc.) on an automated staining platform
(Benchmark ULTRA; Ventana Medical Systems, Inc.). An OptiView DAB
IHC Detection Kit (Ventana Medical Systems, Inc.) was used
according to the manufacturer's instructions for the visualization
of the primary anti-PD-L1 antibody. Human placental tissue was used
as a positive control in all immunohistochemical reactions.
Immunohistochemical expression of PD-L1 in malignant cells was
evaluated by counting the proportion of positive malignant cells
and quantifying IHC staining intensity in a 4-tiered scoring system
according to Hofmann's criteria (22) as follows: Score 0 indicated no
appreciable staining or staining in less than 10% of malignant
cells; score 1+ indicated weak appreciable partial membranous
staining in >10% of malignant cells; score 2+ indicated moderate
complete membrane staining in >10% of malignant cells; and score
3+ indicated intense complete membrane staining in >10% of
malignant cells. Based on a previous study involving other
malignancies (23), tumours with 5%
or more cells showing positive PD-L1 staining, regardless of the
intensity, were considered ‘positive’.
For p16 IHC, 4-µm tissue microarray (TMA) sections
were cut from formalin-fixed paraffin-embedded tissue, and IHC was
performed on a Leica Bondmax platform (Leica Micro-systems)
according to the manufacturer's instructions. Mouse monoclonal
anti-p16 (clone JC8, 1:600 dilution; Santa Cruz Biotechnology) was
used as a primary antibody. p16 IHC was scored as positive if there
was strong and diffuse nuclear and cytoplasmic staining present in
greater than 70% of malignant cells.
PD-L1 fluorescence in situ
hybridization
Tissue microarrays (TMAs) with 3 mm core diameter
were obtained from representative cervical cancer tissues.
Dual-colour FISH analysis was performed on 4 µm FFPE TMA sections.
The SPEC CD274, PDCD1LG2/CEN9 Dual Color Probe (Zytovision) was
used according to the manufacturers guidelines.
At least 50 malignant cells were detected based on
DAPI-stained nuclei. PD-L1 amplification was defined as a
PD-L1/CEP9 ratio ≥2.0. Polysomy was defined as a mean copy number
of PD-L1 ≥3.0, with a PD-L1/CEP9 ratio <2.0. All other instances
were considered disomy as previously reported (24).
Statistical analysis
Statistical analysis was performed using Stata
Statistical Software (College Station, TX: StataCorp LP; http://www.stata.com). The distribution of qualitative
data was compared between groups using χ2 tests or
Fisher's exact tests, depending on the cell counts of corresponding
contingency tables. For survival analysis, the Kaplan-Meier method
was used to compute recurrence-free survival (RFS), cancer-specific
survival (CSS), and locoregional recurrence-free survival (LRR).
Univariate and multivariate analyses were performed using Cox
proportional hazards models, and the differences between groups
were analysed using the log-rank test. For all statistical
analyses, P<0.05 was considered statistically significant.
Results
Patient Characteristics
The clinicopathological characteristics of the
cervical cancer patients in the HIV-positive or HIV-negative
cohorts are shown in Table I. The
median follow-up time was 40 (range: 1–120) months for the
HIV-positive cohort and 28 (range: 2–82) months for the
HIV-negative cohort. The median CD4 count was 312 (interquartile
range (IQR): 158.5–439.0). Among the HIV-positive patients (n=48),
there was no significant difference in the mean age of ART-exposed
patients (n=23) and ART-untreated patients (n=25) [43.70 (9.07) vs.
40.68 (9.83) years; P=0.276]. The median time on ART was 21 (range:
12.5–91) months. Compared to ART-untreated patients, ART-exposed
patients (n=23) usually had FIGO stage IB2-IIB disease (82.6 vs.
48.0%; P=0.012), increased CD4 counts (74.0 vs. 40.0%; P=0.022),
undetectable viral loads (82.6 vs. 40.0%; P=0.003), reduced tumour
sizes (34.8 vs. 4.0%; P=0.009) and reduced likelihood of having
parametrial invasion (52.2 vs. 92.0%; P=0.002). There were no
differences in the histologic subtypes of squamous cell carcinoma
(73.9 vs. 92.0%; P=0.130), presence of metastatic lymph nodes (30.4
vs. 36.0%; P=0.683) or use of radio (chemo) therapy (100.0 vs.
96.0%; P=1.000) between the two groups. No significant correlation
was observed between the NRTI+NNRTI group and the NRTI+PI group
with regard to patient age [40.69 (10.14) vs. 41.43 (5.97) years;
P=0.441], higher CD4 counts (75.0 vs. 71.4%; P=1.000), undetectable
viral loads (81.2 vs. 85.7%; P=1.000), FIGO stage 1B2-IIB disease
(81.3 vs. 85.7%; P=1.000), tumour size ≥4 cm (75.0 vs. 42.9%;
P=0.182), the presence of parametrial invasion (75.0 vs. 71.4%;
P=1.000), histologic subtype of squamous cell carcinoma (68.8 vs.
85.7%; P=0.621), or the presence of metastatic nodes (37.3%;
P=0.366). Additionally, ART-exposed patients had younger age
[median age, 43.70 (9.07) vs. 55.15 (12.67) years; P<0.001],
more likelihood of FIGO stage IB2-IIB disease (82.6 vs. 62.6%;
P=0.063), and lower prevalence of parametrial invasion (52.2 vs.
79.7%; P=0.005) than HIV-negative patients (n=123). No other
correlations were observed between the two groups.
 | Table I.Clinicopathological characteristics
of patients with locally advanced cervical cancer (n=171). |
Table I.
Clinicopathological characteristics
of patients with locally advanced cervical cancer (n=171).
|
| HIV |
|
|
| ART regimen |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | ART-treated
(n=23) | ART-untreated
(n=25) | Non-HIV
(n=123) |
P-valuea |
P-valueb | NRTI+NNRTI
(n=16) | NRTI+PI (n=7) |
P-valuec |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
<60 | 22 (95.7) | 23 (92.0) | 81 (65.9) | >0.05 | 0.004 | 15 (93.8) | 7 (100.0) | >0.05 |
|
≥60 | 1 (4.3) | 2 (8.0) | 42 (34.1) |
|
| 1 (6.3) | 0 (0.0) |
|
| Histology |
|
|
|
|
|
|
|
|
|
SCC | 17 (73.9) | 23 (92.0) | 110 (89.4) | 0.130 | 0.083 | 11 (68.8) | 6 (85.7) | 0.621 |
|
Adeno | 6 (26.1) | 2 (8.0) | 13 (10.6) |
|
| 5 (31.3) | 1 (14.3) |
|
| Tumor size
(cm) |
|
|
|
|
|
|
|
|
|
<4 | 8 (34.8) | 1 (4.0) | 30 (24.4) | 0.009 | 0.297 | 4 (25.0) | 4 (57.1) | 0.182 |
| ≥4 | 15 (65.2) | 24 (96.0) | 93 (75.6) |
|
| 12 (75.0) | 3 (42.9) |
|
| FIGO stage |
|
|
|
|
|
|
|
|
| Stage
IB2-IIB | 19 (82.6) | 12 (48.0) | 77 (62.6) | 0.012 | 0.063 | 13 (81.3) | 6 (85.7) | >0.05 |
| Stage
IIIA-IVA | 4 (17.4) | 13 (52.0) | 46 (37.4) |
|
| 3 (18.8) | 1 (14.3) |
|
| CD4 count
(cells/µl) |
|
|
|
|
|
|
|
|
|
≤350 | 6 (26.0) | 15 (60.0) |
| 0.022 |
| 4 (25.0) | 2 (28.6) | >0.05 |
|
>350 | 17 (74.0) | 10 (40.0) |
|
|
| 12 (75.0) | 5 (71.4) |
|
| HIV viral load
(copies/µl) |
|
|
|
|
|
|
|
|
|
Undetectable | 19 (82.6) | 10 (40.0) |
| 0.003 |
| 13 (81.2) | 6 (85.7) | >0.05 |
|
≥50 | 4 (17.4) | 15 (60.0) |
|
|
| 3 (18.8) | 1 (14.3) |
|
| Parametrial
invasion |
|
|
|
|
|
|
|
|
|
Absent | 11 (47.8) | 2 (8.0) | 25 (20.3) | 0.002 | 0.005 | 8 (50.0) | 3 (42.9) | >0.05 |
|
Present | 12 (52.2) | 23 (92.0) | 98 (79.7) |
|
| 8 (50.0) | 4 (57.1) |
|
| Metastatic lymph
node |
|
|
|
|
|
|
|
|
|
Absent | 16 (69.6) | 16 (64.0) | 93 (75.6) | 0.683 | 0.541 | 10 (62.5) | 6 (85.7) | 0.366 |
|
Present | 7 (30.4) | 9 (36.0) | 30 (24.4) |
|
| 6 (37.5) | 1 (14.3) |
|
| Treatment |
|
|
|
|
|
|
|
|
|
CTRT | 23 (100.0) | 24 (96.0) | 119 (96.7) | >0.05 | >0.05 | 16 (100.0) | 7 (100.0) |
|
| Radical
RT | 0 (0.0) | 1 (4.0) | 4 (3.3) |
|
| 0 (0.0) | 0 (0.0) |
|
Status of PD-L1 expression
For the entire cohort, PD-L1 expression in at least
5% of tumour cells was identified in 130/171 (76%) of cervical
carcinoma cases. The mean percentage of positive tumour cells (any
intensity of staining) was 60% (range: 15–90%). Strong membranous
staining (3+) was identified in 24/171 (14%) cases, moderate
staining (2+) in 46/171 (27%) cases, and weak staining (+1) in
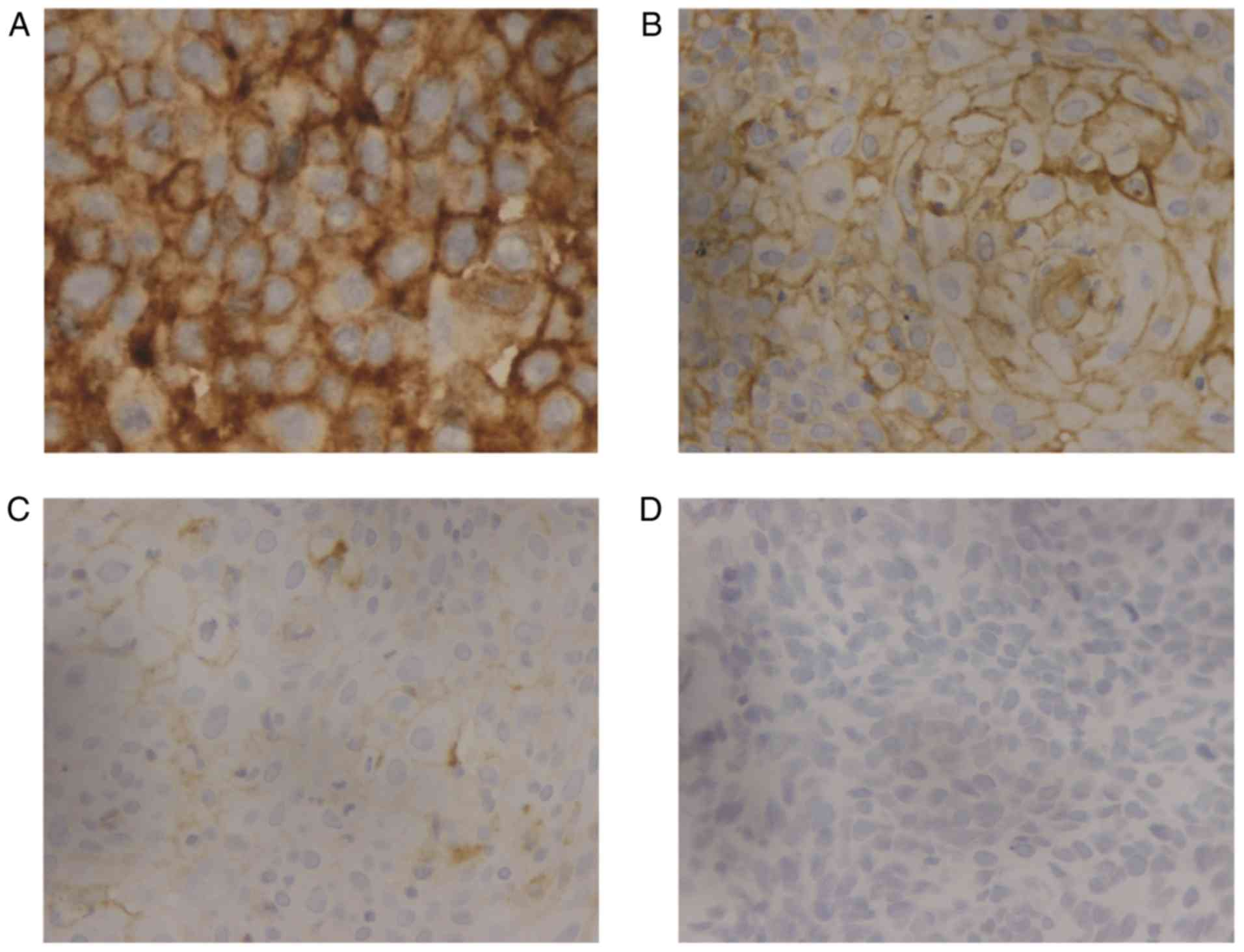
39/171 (23%) cases (Fig. 1).
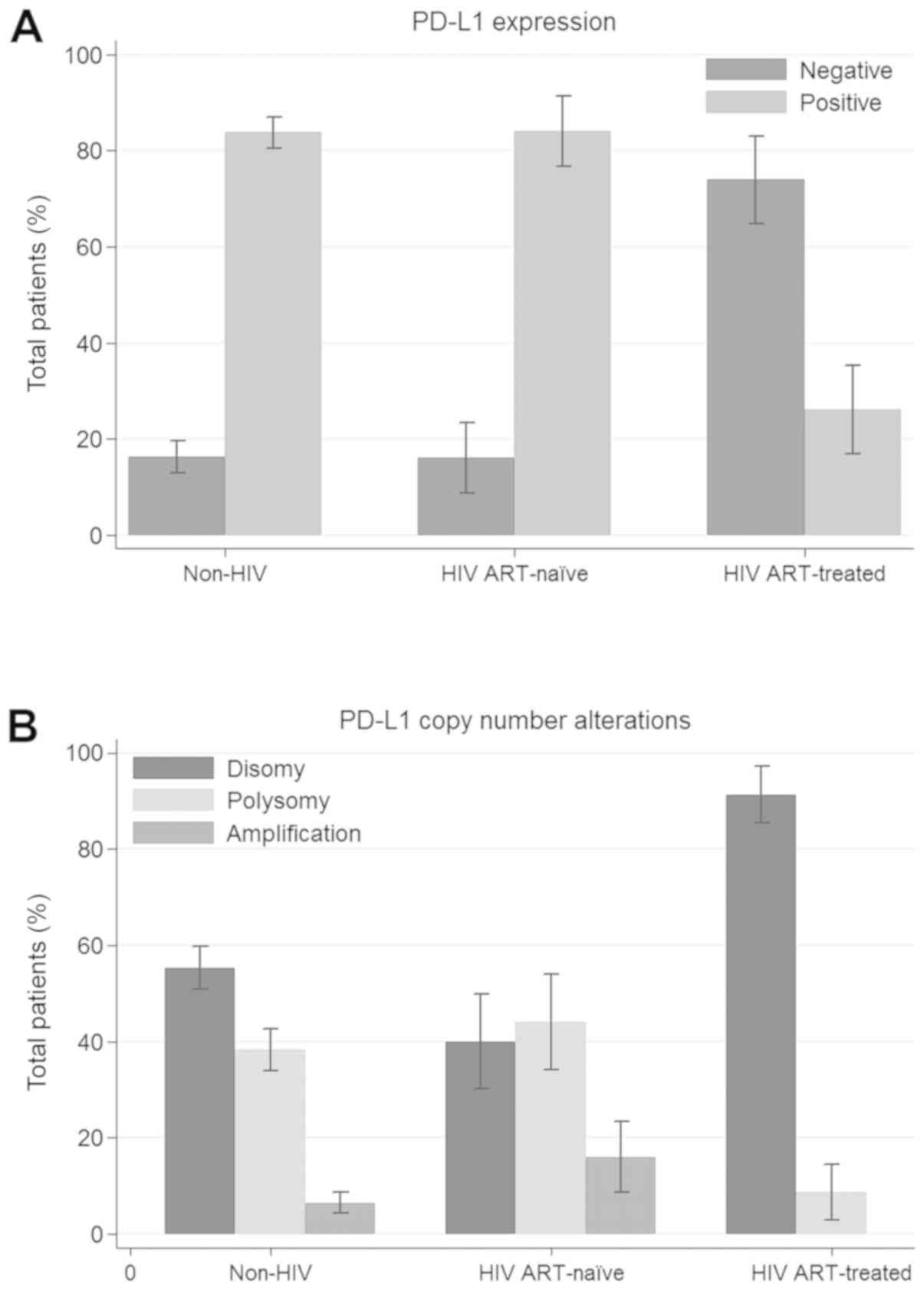
Fig. 3 shows the proportion of PD-L1
immunoreactivity in each patient group. There was a significant
difference in PD-L1 overexpression between the HIV-positive cohort
and HIV-negative cohort (56.3 vs. 83.7%; P<0.001). Among the
HIV-positive patients, compared to ART-untreated patients,
ART-exposed patients showed a significant decrease in PD-L1 protein
expression (26.1 vs. 84%; P<0.001). Additionally, ART-exposed
patients had a lower prevalence of PD-L1 immunopositivity than
HIV-negative patients (26.1 vs. 83.7%; P<0.001).
Status of p16 expression
P16 positivity was not altered by the HIV status,
ART use, or antiretroviral drug regimen. All cases in the HIV
cohort displayed p16 immunopositivity, whereas nearly all cases in
the HIV-negative cohort were p16-positive except for two cases of
adenocarcinoma. No significant correlation was observed in any of
the groups with regard to any of the relevant parameters mentioned
above (data not shown).
Status of PD-L1 gene copy number
alterations
Overall, 12/171 (7%) tumours were positive for
amplification. Gene copy number gain was restricted to tumour cells
and was not present in the inflammatory cell component. Polysomy
was observed in 60/171 (35%) cases. A total of 99/171 (58%) cases
were disomic for the PD-L1 gene locus at 9p24.1 (Fig. 2). Fig.
3 shows the proportion of PD-L1 copy number alterations in each
patient group. There was no significant difference in PD-L1
amplification, polysomy, or disomy (64.6 vs. 55.3%, 27.1 vs. 38.2%
and 8.3 vs. 6.5%, respectively; P=0.387) between the HIV-positive
cohort and the HIV-negative cohort. Among the HIV-positive cohort,
ART-exposed patients had a lower prevalence of amplification (0 vs.
28.6%; P=0.019) and polysomy (8.7 vs. 52.4%; P=0.002) and a higher
prevalence of disomy (91.3 vs. 40%; P<0.001) than their
ART-untreated counterparts. Additionally, ART-exposed patients had
a lower prevalence of polysomy (8.7 vs. 40.9%; P=0.003) and a
higher prevalence of disomy (91.3 vs. 55.3%; P=0.001) than
HIV-negative patients. There were no differences in amplification
between the two groups (0 vs. 10.5%; P=0.195).
Correlation between PD-L1 copy number
gain and PD-L1 protein expression
Results for both PD-L1 immunohistochemistry and
PD-L1 FISH are shown in Table II.
Tumours with PD-L1 gene amplification and polysomy displayed
membranous PD-L1 immunostaining (scores 1+ to 3+) by
immunohistochemistry in 11/12 (92%) and 46/60 (76%) cases,
respectively. A significantly higher frequency of cases with PD-L1
amplification were PD-L1 immunopositive than cases without
amplification (92 vs. 61.6%; P=0.03). Likewise, the proportion of
PD-L1 immunopositive tumours with PD-L1 polysomy were significantly
higher than those of tumours with disomy (76.7 vs. 52.5%; P=0.002).
Furthermore, 7/12 carcinoma cases with strong membranous PD-L1
immunostaining (score 3+) showed PD-L1 amplification, 11/60 showed
a polysomy, and 6/99 cases displayed a disomy.
 | Table II.PD-L1 FISH and PD-L1 IHC. |
Table II.
PD-L1 FISH and PD-L1 IHC.
| PD-L1 FISH | Cases (n=171) | PD-L1 IHC Score
3+ | PD-L1 IHC Score
2+ | PD-L1 IHC Score
1+ | PD-L1 IHC Score
0 |
|---|
| Amplification | 12 (7%) | 7/12 | 2/12 | 2/12 | 1/12 |
| Polysomy | 60 (35%) | 11/60 | 18/60 | 17/60 | 14/60 |
| Disomy | 99 (58%) | 6/99 | 26/99 | 20/99 | 47/99 |
Survival outcomes
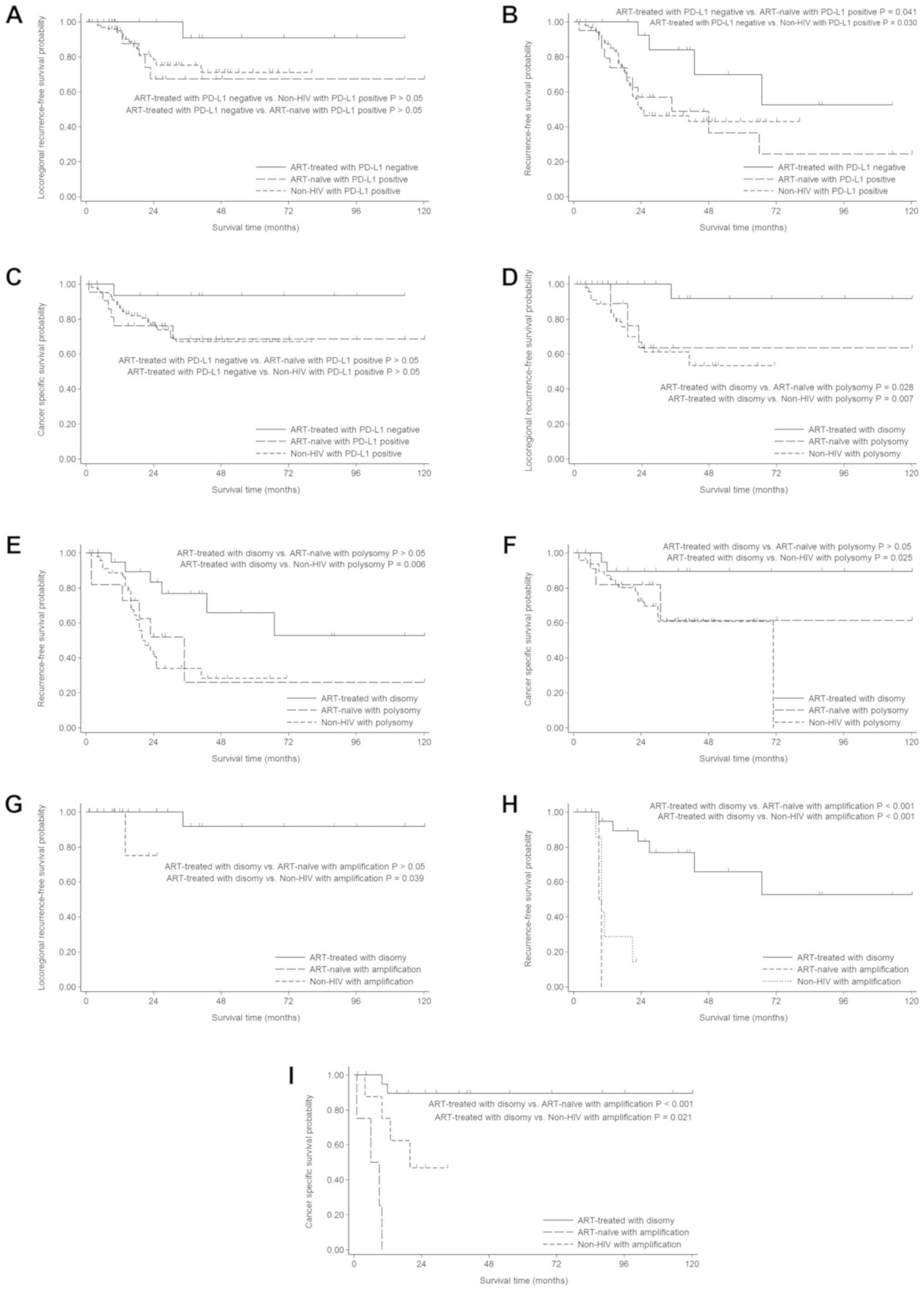
Fig. 4 shows the
Kaplan-Meier survival curves for exposed (ART-exposed) vs.
unexposed ART (ART-untreated and HIV-negative) patients, according
to the IHC-based and FISH-based expression status of PD-L1 in
tumours. Overall, ART-exposed patients had longer survival with
regard to LRR, RFS, and CSS than ART-unexposed patients. The
results of univariate and multivariate analyses evaluating the
impact of various known prognostic factors on LRR, RFS and CSS are
summarized in Table III (Tables SI–III).
 | Table III.Univariate and multivariate survival
analysis (n=171). |
Table III.
Univariate and multivariate survival
analysis (n=171).
|
|
| Univariate analysis
(HR, 95% CI, P-value) | Multivariate
analysis (HRadj, 95%CI, P-value) |
|---|
|
|
|
|
|
|---|
| Variables | Number (n) | LRR | RFS | CSS | LRR | RFS | CSS |
|---|
| Patient's
subgroups |
|
|
|
|
|
|
|
| Non-HIV
vs. ART-naïve | 123 vs. 25 | 1.11, 0.42–2.90,
>0.05 | 1.11, 0.59–2.08,
>0.05 | 1.39, 0.66–2.93,
>0.05 | 1.06, 0.40–2.78,
>0.05 | 0.93, 0.48–1.81,
>0.05 | 0.91, 0.41–2.03,
>0.05 |
|
ART-treated vs. ART-naïve | 23 vs. 25 | 0.15,
0.02–1.32, | 0.44, 0.17–1.11,
>0.05 | 0.21, 0.04–0.96,
0.044a | 0.24, 0.03–2.13,
>0.05 | 0.64, 0.22–1.85,
>0.05 | 0.55, 0.12–2.63,
>0.05 |
|
ART-treated vs. non-HIV | 23 vs. 123 | 0.17, 0.02–1.27,
>0.05 | 0.48, 0.22–1.07,
>0.05 | 0.29, 0.07–1.20,
>0.05 | 0.26, 0.03–1.94,
>0.05 | 0.60, 0.24–1.47,
>0.05 | 0.50, 0.12–2.20,
>0.05 |
| Age (years) |
|
|
|
|
|
|
|
| <60
vs. ≥60 | 126 vs. 45 | 0.81, 0.33–1.97,
>0.05 | 0.95, 0.54–1.67,
>0.05 | 1.45, 0.77–2.76,
>0.05 |
|
|
|
| Histology |
|
|
|
|
|
|
|
| SCC vs.
adeno | 150 vs. 21 | 1.75, 0.71–4.29,
>0.05 | 0.92, 0.44–1.92,
>0.05 | 0.49, 0.15–1.59,
>0.05 |
|
|
|
| Tumor size
(cm) |
|
|
|
|
|
|
|
| <4
vs. ≥4 | 39 vs. 132 | 0.90,
0.4–02.02, | 2.3,
1.17–4.52, | 6.79,
1.64–28.08, |
| 1.42,
0.69–2.95, | 2.39,
0.55–10.42, |
|
|
| >0.05 | 0.016a | 0.008a | >0.05 | >0.05 | >0.05 |
| FIGO stage |
|
|
|
|
|
|
|
| Stage
IB2-IIB vs. Stage IIIA-IVA | 108 vs. 63 | 2.55, 1.23–5.28,
0.012a | 2.87, 1.76–4.69,
<0.001a | 14.73, 6.18–35.09,
<0.001a | 1.74, 0.80–3.78,
>0.05 | 2.43, 1.37–4.30,
0.002a | 11.47, 4.70–27.99,
<0.001a |
| Parametrial
invasion |
|
|
|
|
|
|
|
| Absent
vs. present | 38 vs. 133 | 1.21, 0.52–2.84,
>0.05 | 1.97, 1.05–3.71,
0.035a |
|
| 0.81, 0.37–1.77,
>0.05 |
|
| Metastatic lymph
node |
|
|
|
|
|
|
|
| Absent
vs. present | 125 vs. 46 | 1.63, 0.77–3.45,
>0.05 | 1.98, 1.20–3.27,
0.007a | 2.19, 1.19–4.03,
0.012a |
| 1.35, 0.79–2.32,
>0.05 | 1.46, 0.77–2.77,
>0.05 |
| Treatment |
|
|
|
|
|
|
|
| CTRT
vs. radical RT | 166 vs. 5 |
|
|
|
|
|
|
| PDL1
expression |
|
|
|
|
|
|
|
|
Negative vs. positive | 41 vs. 130 | 1.66, 0.63–4.34,
>0.05 | 1.78, 0.95–3.34,
>0.05 | 1.25, 0.60–2.61,
>0.05 |
| 0.85, 0.40–1.83,
>0.05 |
|
| PDL1 copy number
alterations |
|
|
|
|
|
|
|
|
Polysomy vs. disomy | 60 vs. 99 | 3.46, 1.61–7.45,
0.002a | 2.39, 1.44–3.99,
0.001a | 2.08, 1.07–4.05,
0.031a | 2.5, 1.11–5.63,
0.027a | 1.73, 0.96–3.12,
>0.05 | 0.92, 0.45–1.87,
>0.05 |
| Amplification vs.
disomy | 12 vs. 99 | 1.73, 0.22–13.67,
>0.05 | 8.37, 3.67–19.12,
<0.001a | 7.46, 3.15–17.69,
< 0.001a | 1.44, 0.18–11.41,
>0.05 | 7.03, 2.79–17.74,
<0.001a | 4.05, 1.64–9.98,
0.002a |
For the entire cohort, FIGO stage (HR, 2.87; 95% CI,
1.76–4.69; P<0.001 vs. HR, 14.73; 95% CI, 6.18–35.09;
P<0.001), tumour size (HR, 2.30; 95% CI, 1.17–4.52; P=0.016 vs.
HR, 6.79; 95% CI, 1.64–28.08; P=0.008), nodal status (HR, 1.98; 95%
CI, 1.20–3.27; P=0.007 vs. HR, 2.19; 95% CI, 1.19–4.03; P=0.012),
PD-L1 amplification (HR, 8.37; 95% CI, 3.67–19.12; P<0.001 vs.
HR, 7.46; 95% CI, 3.15–17.69; P<0.001) and polysomy (HR, 2.39;
95% CI, 1.44–3.99; P=0.001 vs. HR, 2.08; 95% CI, 1.07–4.05;
P=0.031) were univariately associated with RFS and CSS.
Nevertheless, on multivariate analysis, FIGO stage and PD-L1
amplification continued to show a significant impact on RFS (HR,
2.43; 95% CI, 1.37–4.30; P=0.002 vs. HR, 7.03; 95% CI, 2.79–17.74;
P<0.001) and CSS (HR, 11.47; 95% CI, 4.70–27.99; P<0.001 vs.
HR, 4.05; 95% CI, 1.64–9.98; P=0.002). FIGO stage and PD-L1
polysomy showed a significant impact on LRR in univariate analysis
(HR, 2.55; 95% CI, 1.23–5.28; P=0.012 vs. HR, 3.46; 95% CI,
1.61–7.45; P=0.002); however, only PD-L1 polysomy remained an
independent predictor of LRR in the multivariate analysis (HR,
2.50; 95% CI, 1.11–5.63; P=0.027). In subgroup analyses, ART
exposure was univariately correlated with CSS (HR, 0.21; 95% CI,
0.04–0.96; P=0.044) in the HIV-positive cohort; however, no
significant difference was observed in the multivariate analysis
(HR, 0.55; 95% CI, 0.12–2.63; P=0.455).
There was no significant difference in LRR, RFS, or
CSS between the ART-exposed group and the HIV-negative group (HR,
0.26; 95% CI, 0.03–1.94; P=0.187, HR, 0.60; 95% CI, 0.24–1.47;
P=0.265, and HR, 0.50; 95% CI, 0.12–2.20; P=0.362, respectively) or
between the ART-untreated group and the HIV-negative group (HR,
0.95; 95% CI, 0.36–2.5; P=0.913, HR, 1.07; 95% CI, 0.55–2.09;
P=0.833, and HR, 1.09; 95% CI, 0.49–2.42; P=0.826, respectively) on
multivariate analysis. The data demonstrated that HIV status was
not associated with worse outcomes in Cox models.
Discussion
In the present study, our results showed an
association between ART and PD-L1 expression. We found that
ART-exposed patients had a significantly lower prevalence of PD-L1
protein expression, PD-L1 amplification and polysomy, and better
clinical outcomes than ART-untreated HIV-positive and HIV-negative
patients. As reported in earlier studies (25), HIV-positive women tended to have
aggressive cervical cancer with a poor prognosis. Nevertheless, a
recent analysis of surveillance data pertaining to the
post-antiretroviral era showed a comparable prognosis for
HIV-positive and HIV-negative populations (18). Although several factors that
contribute to poor prognosis of cervical cancer in HIV-positive
patients have been proposed, the definite aetiology in this respect
has not been clearly identified. From the standpoint of anticancer
immunity, suppression of the T cell-mediated anticancer immune
response is likely to underlie the association between HIV
infection and poor prognosis for cervical cancer.
In addition to improving functional immunity, ART
could exert a combined effect on oncogenic HPV infection and
cervical cancer. Different antiretroviral drug combinations may
show a wide spectrum of activity and improved potency (i.e.,
synergistic or additive effects) against HPV infection or cancer
cells. In a recent meta-analysis, Kelly et al demonstrated
that ART may reduce the risk for cervical cancer and its precursor
lesions in women living with HIV (20). Interestingly, these effects remained
after adjusting for immune restoration indicators, such as CD4 cell
count and duration of ART use.
In vitro studies have shown that lopinavir in
some ART regimens may have activity against oncogenic HPV through
the inhibition of the viral oncogene E6 (26). Several studies have shown that
protease inhibitors (PIs) and other anti-HIV drugs possess several
pleiotropic anticancer properties, including inhibition of cancer
cell invasion, angiogenesis, inflammatory cytokine production, and
proliferation and induction of apoptosis (21,27).
Several intracellular signalling pathways have been identified, and
some of these pathways might be linked to PD-L1 expression.
An in vitro study in cultured squamous cell
carcinoma of the head and neck (SCCHN) cell lines demonstrated that
PD-L1 expression is significantly upregulated in response to
interferon-γ (IFN-γ), a key cytokine triggering de novo
PD-L1 induction in tumour cells and normal tissues (28).
PD-L1 expression can be stimulated by
autocrine/paracrine mediators within the cancer microenvironment,
especially IFN-γ. Interactions between extrinsic stimuli and the
IFN-γ receptor could lead to the expression and activation of
various downstream signalling pathways, including nuclear factor-κ
light chain enhancer of activated B cells (NF-κB),
mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase
(PI3K), mammalian target of rapamycin (mTOR) and Janus
kinase/signal transducer and activator of transcription (JAK/STAT),
which promote cell cycle progression and the activation of
transcription factors. Such signalling pathways further regulate
the nuclear translocation of transcription factors to the PD-L1
promoter (29).
In the setting of HIV infection, several cytokines
are produced by infected cells and cells of the immune system. Both
innate and adaptive immune responses are activated during the
disease course. CD4+ T helper cells play a crucial role
in the immune system by secreting cytokines that regulate the
immune response. Th1 CD4+ subsets produce IL-2 and
IFN-γ. IFN-γ acts by stimulating macrophages and is important for
eliminating intracellular pathogens (30). Previous studies conducted by De Luca
et al documented that the production of various inflammatory
cytokines [macrophage inflammatory protein-1α (MIP-1α), macrophage
inflammatory protein-1β (MIP-1β), regulated on activation, normal T
cell expressed and secreted (RANTES), and IFN-γ] can be
significantly inhibited at 8 weeks and partially recovered at 24
weeks after the commencement of protease inhibitor therapy in
patients with advanced HIV infection (31).
In cross-sectional and longitudinal clinical
studies, comparison of ART-untreated to ART-exposed subjects
demonstrated high serum levels of many cytokines, which were
significantly reduced when ART was initiated. A cross-sectional
study of pre- and post-ART showed lower serum levels of IFN-γ with
the initiation of ART (32). A
longitudinal study displayed a statistically significant reduction
in IFN-γ after ART for 60 days or longer. In addition, a study
conducted by Piconi et al found that different NRTI
combinations (AZT+ddI and AZT+3TC) could exert different effects on
IFN-γ and interleukin-2 (IL-2) production (33).
Nevertheless, IFN-γ-induced PD-L1 expression can
fluctuate at different time points during the disease course. In
contrast, PD-L1 expression can be continuously activated via gene
amplification events involving a gene locus on chromosome 9p24.1.
The additional somatic copy number alterations resulting in an
increase in the fraction of DNA regions could be associated with
carcinogenesis and cancer progression. Several important genes are
known to be amplified and have been identified as prognostic
markers, factors associated with drug resistance, or treatment
targets in some cancer types, such as non-small cell lung cancer
(34).
The 9p24.1 chromosomal region contains the genes
encoding PD-L1, PD-L2 and JAK2.
Amplification of the chromosomal region 9p24.1 has
recently been demonstrated as an essential mechanism for increased
PD-L1 protein expression in nodular sclerosing classical Hodgkin
lymphoma and primary mediastinal large B-cell lymphoma (13) and has also been identified in
colorectal carcinoma, triple-negative breast cancer, glioblastoma
and gastric adenocarcinoma (14,15), and
cervical and vulvar carcinoma (16).
In the present study, our results demonstrated that
PD-L1 copy number gains (amplification and polysomy) can be
observed in a subset of cervical cancer patients using FISH
analysis (35.4% for HIV-positive patients and 44.7% for
HIV-negative patients).
In contrast to the results of a previous study
(16), our results showed that PD-L1
amplification can be identified in only a minority of cases (8.3%
for HIV-positive patients and 6.5% for HIV-negative patients).
These conflicting results can be explained by differences in sample
size, disease stage, or underlying diseases in the studied
population.
More importantly, we found that ART was correlated
with PD-L1 copy number status in addition to protein expression. In
the present study, we demonstrated a novel genetic association
between PD-L1 copy number gain and ART in cervical carcinoma.
However, the exact molecular mechanism for this phenomenon is still
unknown.
Based on previous genetic studies, several
signalling pathways might be downregulated after treatment with
ART, which may lead to a decrease in PD-L1 expression (35,36). The
results showed that a variety of genes are downregulated following
ART and that these genes might share common pathways with PD-L1,
such as the NF-κB, MAPK, and JAK/STAT pathways (35,36).
Moreover, several ART-responsive genes have been identified, and
the biological processes and functions of a large number of these
genes are still unknown (35). The
expression of some of these genes could be changed as a direct
consequence of exposure of human cells to ART, rather than as a
consequence of ART-mediated viral suppression (35). Further studies are needed to clarify
the relationship between ART and PD-L1 gene expression.
Considering survival outcomes, we found that both
copy number gains in the PD-L1 gene and PD-L1 overexpression
indicated poor prognosis in univariate analysis. However, PD-L1
copy number gains were superior to PD-L1 overexpression and could
act as independent and strong predictors of survival outcomes in
cervical carcinoma.
Our results may identify a new subgroup of cervical
cancer with a disease-specific genetic alteration. Further studies
are required to evaluate the impact of PD-L1 copy number gain on
pathogenesis, disease progression and prognosis in this newly
identified subgroup of cervical cancer patients. In addition, the
identification of PD-L1 gene copy number gain as a powerful
mechanism for PD-L1 expression in the present study may provide a
rationale for the treatment of cervical cancer patients in this
subgroup.
In addition to the abovementioned mechanisms, ART
could affect the response to radiation, as some PIs potently
sensitize cancer cells to radiation (37). In vitro results have
demonstrated that the commonly used combination of tenofovir,
emtricitabine, and efavirenz sensitizes tumours to external beam
radiation therapy (EBRT) (<4 Gy per fraction) but protects
tumours from brachytherapy (≥4 Gy per fraction) (38).
In conclusion, although the immunotherapeutic drug
pembrolizumab has been approved for locally advanced cervical
cancer, the cost of cancer treatment is relatively high, and the
response to pembrolizumab alone remains low. In addition to
immune-checkpoint inhibitors, various therapeutic options, such as
HPV vaccines and adoptive T-cell therapy, are currently being
developed for the treatment of cervical cancer (39). However, the development of new drug
treatments is both time-consuming and expensive. Subsequently, the
repositioning of previously approved drugs for alternative
purposes, such as cancer treatment, is reasonable. Hopefully, our
preliminary data will be useful and lead to new treatment options
for these patients in the future.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Sujitra
Tanvanich, Mrs. Unaporn Sitthivilai and Mrs. Pornpimon Kongjan
(Department of Anatomical Pathology, Navamindradhiraj University)
for theoretical guidance and writing assistance during the present
study. The authors would also like to thank Dr Totsapon Jiamton
(Department of Obstetrics and Gynecology, Navamindradhiraj
University) and Dr Wanniga Saengsuri (Division of Gynecologic
Oncology, Taksin Hospital) for clinical advice during the present
study. Finally, the authors would like to thank Ms. Oraphan
Wanacharoen (BCC MDx Co., Ltd.) for her technical
assistance.
Funding
The current study was supported by a grant from the
Faculty of Medicine of Navamindradhiraj University (Bangkok,
Thailand; grant no. 12461).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KL and CS designed the study and wrote the
manuscript. SC and NP performed the statistical analysis and
revised the manuscript. SV and JT performed the retrospective
analyses and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Medical Ethics Committee of Navamindradhiraj University (Bangkok,
Thailand). Informed consent was obtained from all patients prior to
enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
1993 revised classification system for HIV
infection and expanded surveillance case definition for AIDS among
adolescents and adults. MMWR Recomm Rep. 41:1–19. 1992.
|
|
2
|
Indian Council of Medical Research, .
National Cancer Registry ProgrammeNational Printing Press; India:
Bangalore: pp. 60–61. 2001
|
|
3
|
Hong JH, Tsai CS, Lai CH, Chang TC, Wang
CC, Chou HH, Lee SP and Hsueh S: Recurrent squamous cell carcinoma
of cervix after definitive radiotherapy. Int J Radiat Oncol Biol
Phys. 60:249–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez CA, Grigsby PW, Camel HM, Galakatos
AE, Mutch D and Lockett MA: Irradiation alone or combined with
surgery in stage IB, IIA, and IIB carcinoma of the uterine cervix:
Update of a nonrandomized comparison. Int J Radiat Oncol Biol Phys.
31:703–716. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marzec M, Zhang Q, Goradia A, Raghunath
PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA and
Wasik MA: Oncogenic kinase NPM/ALK induces through STAT3 expression
of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad
Sci USA. 105:20852–20857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu P, Wu D, Li L, Chai Y and Huang J:
PD-L1 and survival in solid tumors: A Meta-analysis. PLoS One.
10:e01314032015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Velcheti V, Schalper KA, Carvajal DE,
Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L
and Rimm DL: Programmed death ligand-1 expression in non-small cell
lung cancer. Lab Invest. 94:107–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M,
Meng YL, Yang AG and Wen WH: B7-H1 expression is associated with
poor prognosis in colorectal carcinoma and regulates the
proliferation and invasion of HCT116 colorectal cancer cells. PLoS
One. 8:e760122013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Green MR, Monti S, Rodig SJ, Juszczynski
P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub
TR, et al: Integrative analysis reveals selective 9p24.1
amplification, increased PD-1 ligand expression, and further
induction via JAK2 in nodular sclerosing Hodgkin lymphoma and
primary mediastinal large B-cell lymphoma. Blood. 116:3268–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barrett MT, Anderson KS, Lenkiewicz E,
Andreozzi M, Cunliffe HE, Klassen CL, Dueck AC, McCullough AE,
Reddy SK, Ramanathan RK, et al: Genomic amplification of 9p24.1
targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple
negative breast cancer. Oncotarget. 6:26483–26493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howitt BE, Sun HH, Roemer MG, Kelley A,
Chapuy B, Aviki E, Pak C, Connelly C, Gjini E, Shi Y, et al:
Genetic basis for PD-L1 expression in squamous cell carcinomas of
the cervix and vulva. JAMA Oncol. 2:518–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eltom MA, Jemal A, Mbulaiteye SM, Devesa
SS and Biggar RJ: Trends in Kaposi's sarcoma and non-Hodgkin's
lymphoma incidence in the United States from 1973 through 1998. J
Natl Cancer Inst. 94:1204–1210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schneider E, Whitmore S, Glynn KM,
Dominguez K, Mitsch A and McKenna TM; Centers for Disease Control
Prevention (CDC), : Revised surveillance case definitions for HIV
infection among adults, adolescents, and children aged <18
months and for HIV infection and AIDS among children aged 18 months
to <13 years-United States, 2008. MMWR Recomm Rep. 57:1–12.
2008.PubMed/NCBI
|
|
19
|
Shiels MS, Pfeiffer RM, Gail MH, Hall HI,
Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ
and Engels EA: Cancer burden in the HIV-infected population in the
United States. J Natl Cancer Inst. 103:753–762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly H, Weiss HA, Benavente Y, de Sanjose
S and Mayaud P: Association of antiretroviral therapy with
high-risk human papillomavirus, cervical intraepithelial neoplasia,
and invasive cervical cancer in women living with HIV: A systematic
review and meta-analysis. Lancet HIV. 5:e45–e58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chow WA, Jiang CL and Guan M: Anti-HIV
drugs for cancer therapeutics: Back to the future? Lancet Oncol.
10:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hofmann M, Stoss O, Shi D, Buttner R, van
de Vijver M, Kim W, Ochiai A, Ruschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue Y, Yoshimura K, Mori K, Kurabe N,
Kahyo T, Mori H, Kawase A, Tanahashi M, Ogawa H, Inui N, et al:
Clinical significance of PD-L1 and PD-L2 copy number gains in
non-small-cell lung cancer. Oncotarget. 7:32113–32128. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dryden-Peterson S, Bvochora-Nsingo M,
Medhin H, Suneja G, Asmelash A, Pusoentsi M, Russell A, Efstathiou
J, Chabner B, Lockman S, et al: HIV infection and survival among
women with cervical cancer. J Clin Oncol. 34:3749–3757. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Batman G, Oliver AW, Zehbe I, Richard C,
Hampson L and Hampson IN: Lopinavir up-regulates expression of the
antiviral protein ribonuclease L in human papillomavirus-positive
cervical carcinoma cells. Antivir Ther. 16:515–525. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xulu KR and Hosie MJ: HAART induces cell
death in a cervical cancer cell line, HCS-2: A scanning electron
microscopy study. J Microsc Ultrastruct. 5:39–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsushima F, Tanaka K, Otsuki N, Youngnak
P, Iwai H, Omura K and Azuma M: Predominant expression of B7-H1 and
its immunoregulatory roles in oral squamous cell carcinoma. Oral
Oncol. 42:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
pd-l1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tudela EV, Singh MK, Lagman M, Ly J, Patel
N and Venketaraman V: Cytokine levels in plasma samples of
individual with HIV infection. Austin J Clin Immunol.
1:10032014.
|
|
31
|
De Luca A, Giancola ML, Cingolani A,
Ammassari A, Murri R and Antinori A: Circulating levels and ex vivo
production of beta-chemokines, interferon gamma, and interleukin 2
in advanced human immunodeficiency virus type 1 infection: The
effect of protease inhibitor therapy. AIDS Res Hum Retroviruses.
16:835–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watanabe D, Uehira T, Yonemoto H, Bando H,
Ogawa Y, Yajima K, Taniguchi T, Kasai D, Nishida Y and Shirasaka T:
Sustained high levels of serum interferon-gamma during HIV-1
infection: A specific trend different from other cytokines. Viral
Immunol. 23:619–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piconi S, Trabattoni D, Fusi ML, Milazzo
F, Dix LP, Rizzardini G, Colombo F, Bray D and Clerici M: Effect of
two different combinations of antiretrovirals (AZT + ddI and AZT +
3TC) on cytokine production and apoptosis in asymptomatic HIV
infection. Antiviral Res. 46:171–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Inoue Y, Matsuura S, Kurabe N, Kahyo T,
Mori H, Kawase A, Karayama M, Inui N, Funai K, Shinmura K, et al:
Clinicopathological and survival analysis of Japanese patients with
resected non-small-cell lung cancer harboring NKX2-1, SETDB1, MET,
HER2, SOX2, FGFR1, or PIK3CA gene amplification. J Thorac Oncol.
10:1590–1600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boulware DR, Meya DB, Bergemann TL,
Williams D, Vlasova-St Louis IA, Rhein J, Staddon J, Kambugu A,
Janoff EN and Bohjanen PR: Antiretroviral therapy down-regulates
innate antiviral response genes in patients with AIDS in
sub-saharan Africa. J Acquir Immune Defic Syndr. 55:428–438. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Massanella M, Singhania A,
Beliakova-Bethell N, Pier R, Lada SM, White CH, Pérez-Santiago J,
Blanco J, Richman DD, Little SJ and Woelk CH: Differential gene
expression in HIV-infected individuals following ART. Antiviral
Res. 100:420–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maggiorella L, Wen B, Frascogna V, Opolon
P, Bourhis J and Deutsch E: Combined radiation sensitizing and
anti-angiogenic effects of ionizing radiation and the protease
inhibitor ritonavir in a head and neck carcinoma model. Anticancer
Res. 25:4357–4362. 2005.PubMed/NCBI
|
|
38
|
Ulrike K, Markus H, Thomas H, Ellen H,
Barbara S, Rainer F and Distel LV: NNRTI-based antiretroviral
therapy may increase risk of radiation induced side effects in
HIV-1-infected patients. Radiother Oncol. 116:323–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borcoman E and Le Tourneau C:
Pembrolizumab in cervical cancer: Latest evidence and clinical
usefulness. Ther Adv Med Oncol. 9:431–439. 2017. View Article : Google Scholar : PubMed/NCBI
|