Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignant tumor of the liver, with a mortality rate that
ranks second globally (1,2). Various etiologies of HCC have been
reported, including chronic hepatitis virus infection, alcohol
consumption and abnormal metabolism, and treatment is complex
(3,4). Although surgical resection may be
curative under certain conditions, the majority of patients are
diagnosed at a late stage when surgery is no longer effective
(5). A specific biomarker for HCC
has yet to be identified (6,7), thus the early diagnosis of HCC and the
discovery of predictive biomarkers have important clinical
implications (2,8,9).
Investigating the pathogenesis of HCC by identifying abnormally
expressed genes or proteins may considerably improve the diagnosis
and treatment of the disease.
The association between abnormal lipid metabolism
and tumorigenesis has attracted increasing attention (8–11). Tumor
cells have altered proliferative and metabolic abilities compared
with normal cells (12,13), and the enzymes associated with fatty
acid synthesis are upregulated in tumor tissues; this increases
fatty acid synthesis and provides the necessary materials and
energy to facilitate rapid tumor growth (14,15). Of
the associated enzymes, elongation of very long-chain fatty acids
family member 6 (ELOVL6) is a highly conserved member of the
endoplasmic reticulase family, which is involved in the formation
of long-chain fatty acids (16,17).
Previous studies have found that the ELOVL6 gene serves an
important role in the development and progression of breast cancer
by regulating the metabolism of intracellular lipid components
(18,19). Further studies have revealed that HCC
cells exhibit abnormal lipid metabolism (20,21).
Although the liver is the primary site of lipid metabolism
(22,23), there are currently no reports of the
expression and significance of ELOVL6 in HCC, a cancer closely
associated with metabolic disorders. ELOVL6 is a long chain fatty
acid elongase, which may contribute to fatty acid storage. Until
now, the study of ELOVL6 expression in HCC has remained limited.
Hence, the present study aimed to investigate the expression levels
of ELOVL6 in human HCC tissues, and to determine the relationship
between the expression of ELOVL6 and the prognosis of patients with
HCC.
Subjects and methods
Subjects
A total of 377 paraffin-embedded HCC tissues were
collected from the Jining No. 1 People's Hospital (Shandong, China)
between January 2000 and July 2010. None of the patients received
chemotherapy or radiotherapy prior to surgery. Patients received
serological and imaging examinations to exclude recurrence or
metastasis, and serum alpha-fetoprotein (AFP) level was determined.
Abdomen ultrasonography, computed tomography and magnetic resonance
imaging were utilized for physical examinations. Patients with
missing data were excluded. The follow-up period was defined as the
time interval between the date of surgery and that of death or the
last follow-up. The mean follow-up interval was 30.89±28.18 months
(range, 3.12–146.58 months); patient characteristics are outlined
in Table I. The mean age of the
patients with 48.94±12.78 (range, 28–77 years). The present study
was approved by the Medical Ethics Committee of Jining No. 1
People's Hospital, and as a retrospective study, the requirement
for informed patient consent was waived.
 | Table I.Clinical variables in patients with
hepatocellular carcinoma exhibiting low or high ELOVL6 expression
levels. |
Table I.
Clinical variables in patients with
hepatocellular carcinoma exhibiting low or high ELOVL6 expression
levels.
|
| ELOVL6 expression
level |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Sample size | 256 | 121 |
|
| Age, years |
|
| 0.014 |
|
>50 | 134 (52.3%) | 47 (38.8%) |
|
|
≤50 | 122 (47.7%) | 74 (61.2%) |
|
| Sex |
|
| 0.101 |
|
Male | 217 (84.8%) | 110 (90.9%) |
|
|
Female | 39 (15.2%) | 11 (9.1%) |
|
| AFP, ng/ml |
|
| 0.069 |
|
<20 | 45 (17.6%) | 31 (25.6%) |
|
|
≥20 | 211 (82.4%) | 90 (74.4%) |
|
| Cirrhosis |
|
| 0.670 |
|
Yes | 216 (84.4%) | 100 (82.6%) |
|
| No | 40 (15.6%) | 21 (17.4%) |
|
| Tumor size, cm |
|
| 0.039 |
|
<5 | 59 (23.0%) | 40 (33.1%) |
|
| ≥5 | 197 (77.0%) | 81 (66.9%) |
|
|
Differentiation |
|
| 0.347 |
|
Well-moderate | 20 (7.8%) | 13 (10.7%) |
|
|
Poor-undifferentiated | 236 (92.2%) | 108 (89.3%) |
|
| TNM stage |
|
| 0.454 |
|
I–II | 112 (43.8%) | 48 (39.7%) |
|
|
III–IV | 144 (56.3%) | 73 (60.3%) |
|
| Vascular
invasion |
|
| 0.005 |
|
Yes | 53 (20.7%) | 15 (12.4%) |
|
| No | 203 (79.3%) | 106 (87.6%) |
|
Tissue microarray (TMA) and
immunohistochemistry (IHC)
Following surgery, all specimens were immediately
embedded in paraffin and stored at room temperature. Each tissue
core (diameter, 0.6 mm) was perforated and re-embedded from the
labeled area using a tissue array (MiniCore) per the manufacturer's
protocol. The expression level of ELVOL6A was detected in 377
tissue-pairs (cancerous and matched-noncancerous tissues); the
specimens were fixed with 4% paraformaldehyde overnight at room
temperature (RT), and subsequently processed using the biotin
blocking Kit (Dark, Germany) at RT for 15 min. The tissues were
incubated with an anti-ELOVL6 antibody (1:1,000; cat. no. ab69857;
Abcam) in a humid chamber at 4°C overnight. The tissues were washed
3 times with PBS and incubated with biotinylated goat anti-rabbit
antibodies (1:200; cat. no. S0001; Affinity Biosciences) for 1 h at
37°C. The sections were then stained with hematoxylin at room
temperature for 10 min, and observed under a light microscope
(Olympus) at ×4 and ×20 magnification.
Semi-quantitative IHC was used to detect ELOVL6
protein expression levels according to the following intensity
score criteria: 0, negative staining; 1, weak staining; 2, moderate
staining; and 3, strong staining. The final scores were calculated
as a percentage of positive expression multiplied by the intensity
score. The median IHC score was used as a cut-off to differentiate
between high and low expression levels.
Oncomine database analysis
Oncomine™ (http://www.oncomine.org) is a web-based data-mining
platform aimed to facilitate novel discoveries via genome-wide
expression analysis (24,25). The ELOVL6 gene was queried in the
database and the results were filtered by selecting ‘HCC’ and
‘Cancer’ vs. ‘Normal Analysis’. Comparisons between ELOVL6 mRNA
expression levels in the HCC and adjacent normal tissues of 377
patients were analyzed using the Student's t-test.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 13; SPSS Inc.). The paired Student's t-test or
χ2 test was used to assess the association between
ELOVL6 expression level and clinicopathological variables. A
survival curve was generated using the Kaplan-Meier method
(log-rank test), and the multivariate Cox proportional hazards
regression model was used to assess the independence of ELOVL6 as a
predictive factor for HCC. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of ELOVL6 in the HCC
TMA
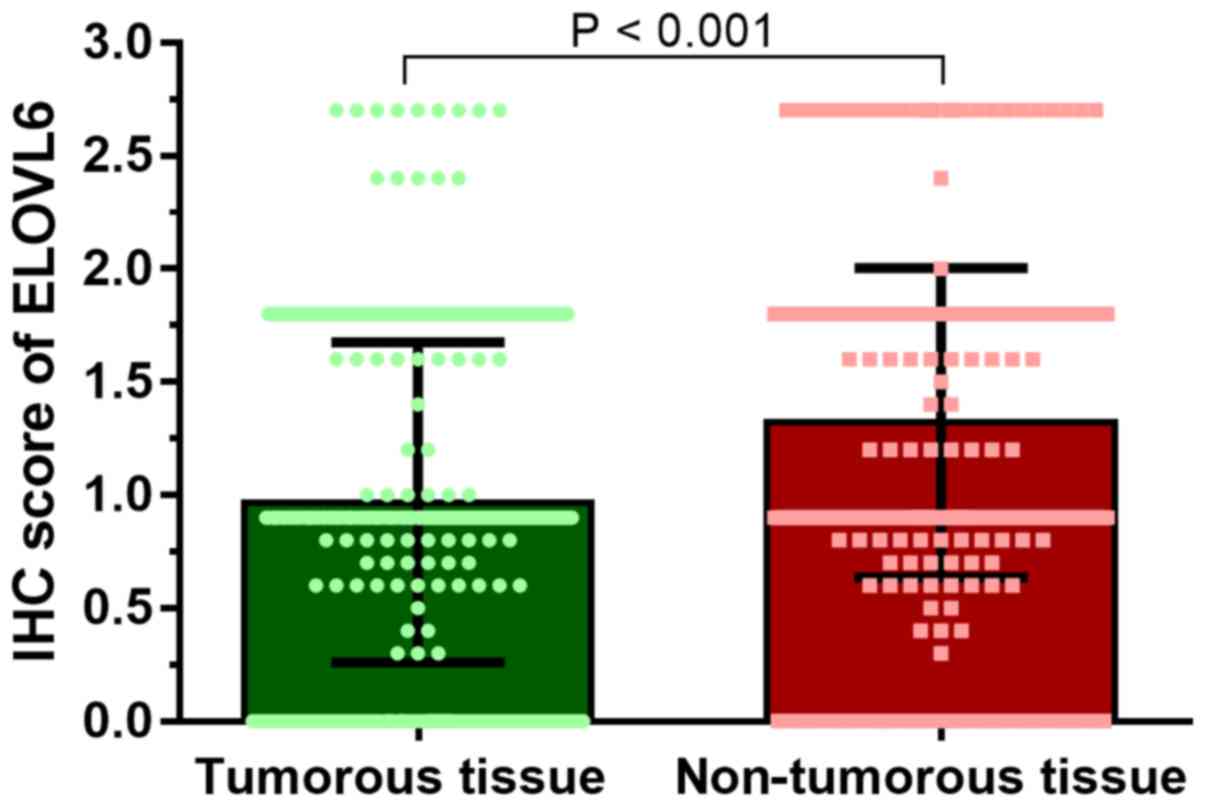
The HCC TMA (n=377) was used to determine ELOVL6
expression levels in HCC tissues. ELOVL6 was predominantly
expressed in the cytoplasm of HCC cells. The ELOVL6 IHC score for
HCC tissues was 0.97±0.71, significantly lower than that of matched
normal tissues (1.32±0.68; P<0.001; Fig. 1). HCC data (Guichard Liver 2 Data)
was also downloaded from the Oncomine Database (https://www.oncomine.org/resource/login.html) as
validation data. These results also showed a reduction in ELOVL6
expression level in HCC tissues (supplementary Fig. 1).
Association between cytoplasmic ELOVL6
and HCC clinical features
To determine the clinical relevance of ELOVL6
expression in HCC, the association between ELOVL6 expression level
and the clinical features of patients with HCC was evaluated. The
median IHC score of the tumor tissues was 0.9 and a low level of
ELOVL6 expression was observed in 67.9% (256/377) of the cases. The
median age (P=0.014) and tumor size (P=0.039) were greater in
patients with low levels of ELOVL6 expression, compared with those
with high expression levels. The proportion of patients exhibiting
vascular invasion was significantly higher in the low ELOVL6
expression group compared with patients in the high ELOVL6
expression group (P=0.005) (Table
I).
Association between ELOVL6 expression
level and the outcome of patients with HCC
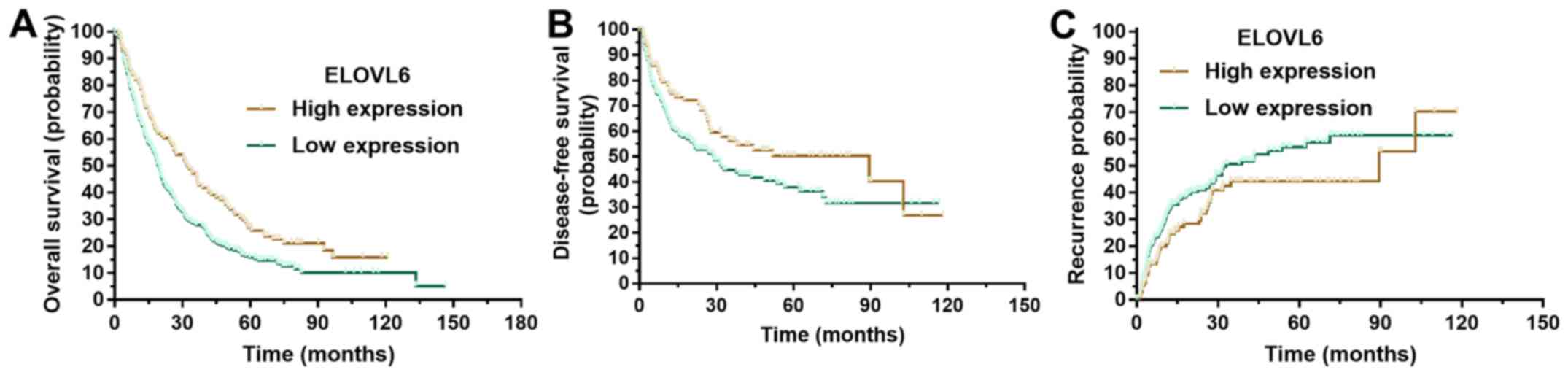
To determine the prognostic value of ELOVL6
expression level in patients with HCC, Kaplan-Meier survival
analysis was conducted using data from the 377 enrolled patients.
Kaplan-Meier analysis revealed that patients with low ELOVL6
expression levels had significantly poorer overall survival times
(Fig. 2A; P<0.001). Similarly,
compared with patients with high ELOVL6 expression levels,
disease-free survival time was shorter (Fig. 2B; P=0.029) and the probability of
recurrence was higher (Fig. 2C;
P=0.044) in those with low ELOVL6 expression levels.
Univariate and multivariate analyses
of prognostic variables in HCC
To evaluate whether ELOVL6 expression was an
independent risk factor for patient outcome in HCC, univariate and
multivariate analyses were conducted. The tumor size, TNM stage,
vascular invasion status and ELOVL6 expression were all shown to be
prognostic variables for overall survival in patients with HCC.
Multivariate analysis showed that only vascular invasion
(P<0.001), TNM stage (P<0.001) and ELOVL6 expression
(P=0.001) were independent prognostic variables for overall
survival (Table II).
 | Table II.Univariate and multivariate analyses
of hepatocellular carcinoma patient variables for overall
survival. |
Table II.
Univariate and multivariate analyses
of hepatocellular carcinoma patient variables for overall
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 0.992 | 0.983–1.001 | 0.097 |
|
|
|
| Sex | 0.816 | 0.578–1.151 | 0.247 |
|
|
|
| AFP | 1.097 | 0.835–1.442 | 0.505 |
|
|
|
| Cirrhosis | 0.946 | 0.698–1.282 | 0.721 |
|
|
|
| Tumor size, cm | 1.578 | 1.211–2.055 | 0.001 |
|
|
|
|
Differentiation | 1.472 | 0.984–2.204 | 0.060 |
|
|
|
| TNM stage | 1.807 | 1.432–2.282 | <0.001 | 1.591 | 1.244–2.036 | <0.001 |
| Vascular
invasion | 3.266 | 2.463–4.331 | <0.001 | 2.678 | 1.992–3.600 | <0.001 |
| ELOVL6
expression | 1.476 | 1.152–1.891 | 0.002 | 1.509 | 1.174–1.939 | 0.001 |
The risk factors associated with disease-free
survival (Table III) and HCC
recurrence were investigated further (Table IV). Univariate analysis revealed
that age, TNM stage, vascular invasion status and ELOVL6 expression
were risk factors associated with disease-free survival.
Multivariate analysis showed that vascular invasion (P=0.032) and
ELOVL6 expression (P=0.041) were independent risk factors
associated with disease-free survival. Only vascular invasion
(P=0.019) and ELOVL6 expression (P=0.045) were independent risk
factors for HCC recurrence.
 | Table III.Univariate and multivariate analyses
of hepatocellular carcinoma patient variables for disease-free
survival. |
Table III.
Univariate and multivariate analyses
of hepatocellular carcinoma patient variables for disease-free
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 0.982 | 0.969–0.995 | 0.005 |
|
|
|
| Sex | 0.805 | 0.510–1.271 | 0.352 |
|
|
|
| AFP | 1.244 | 0.852–1.817 | 0.258 |
|
|
|
| Cirrhosis | 1.066 | 0.707–1.607 | 0.760 |
|
|
|
| Tumor size, cm | 1.406 | 1.001–1.975 | 0.050 |
|
|
|
|
Differentiation | 1.626 | 0.924–2.862 | 0.411 |
|
|
|
| TNM stage | 1.442 | 1.065–1.952 | 0.018 |
|
|
|
| Vascular
invasion | 1.954 | 1.332–2.868 | 0.001 | 1.475 | 1.089–1.998 | 0.032 |
| ELOVL6
expression | 1.441 | 1.036–2.004 | 0.030 | 1.478 | 1.062–2.058 | 0.041 |
 | Table IV.Univariate and multivariate analyses
of hepatocellular carcinoma patient variables for recurrence. |
Table IV.
Univariate and multivariate analyses
of hepatocellular carcinoma patient variables for recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 0.836 | 0.612–1.143 | 0.262 |
|
|
|
| Sex | 0.817 | 0.505–1.320 | 0.408 |
|
|
|
| AFP | 1.122 | 0.760–1.654 | 0.563 |
|
|
|
| Cirrhosis | 1.067 | 0.690–1.648 | 0.771 |
|
|
|
| Tumor size, cm | 1.162 | 0.823–1.641 | 0.392 |
|
|
|
|
Differentiation | 1.432 | 0.811–2.528 | 0.215 |
|
|
|
| TNM stage | 1.378 | 1.002–1.897 | 0.049 |
|
|
|
| Vascular
invasion | 1.839 | 1.217–2.778 | 0.004 | 1.773 | 1.196–2.674 | 0.019 |
| ELOVL6
expression | 1.298 | 1.122–1.827 | 0.030 | 1.421 | 1.156–1.983 | 0.045 |
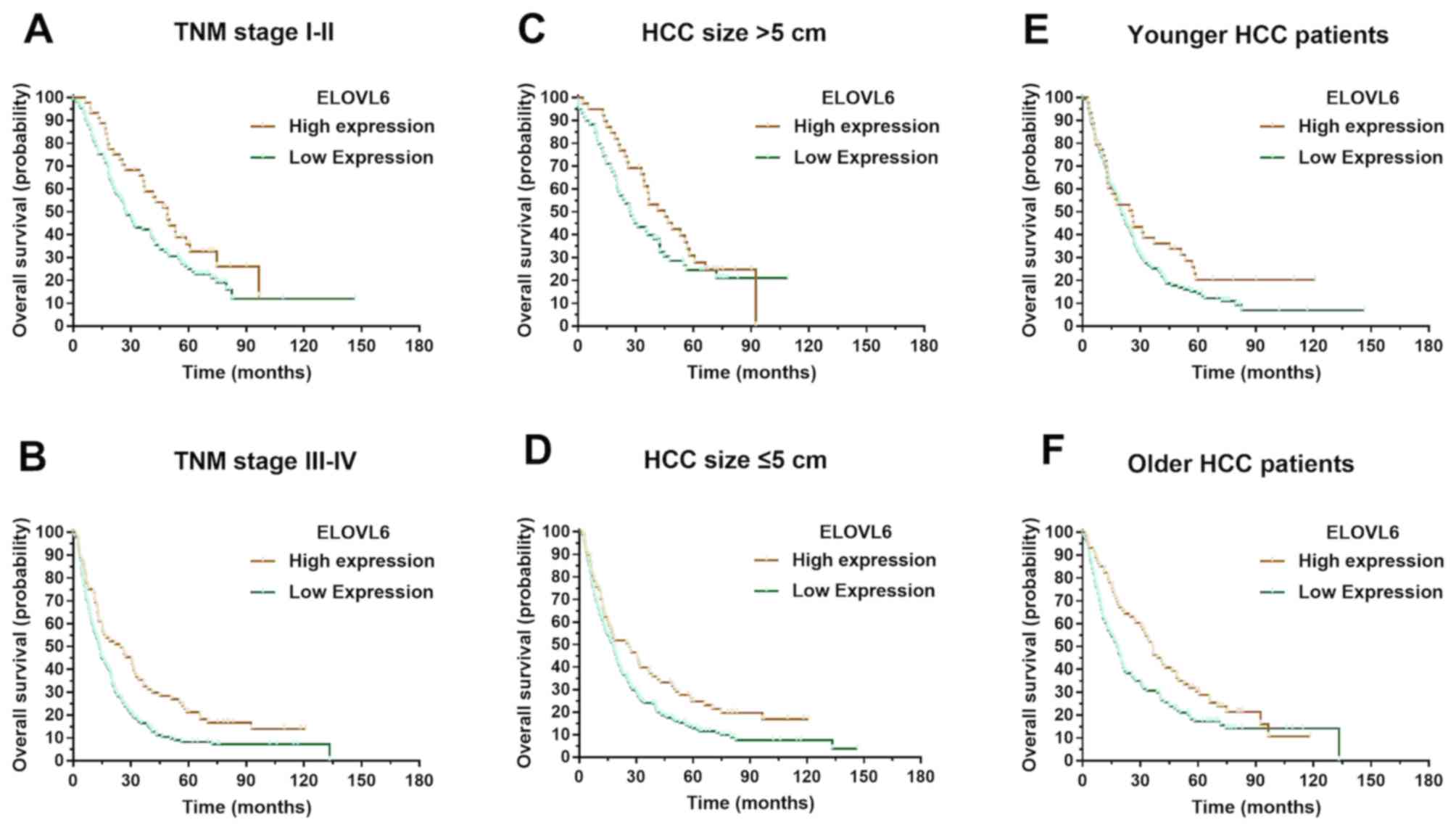
Subgroup analyses of the prognostic
value of cytoplasmic ELOVL6 expression in patients with HCC
Stratified survival analysis was conducted to reveal
the prognostic implication of ELOVL6 expression in patients with
HCC. Kaplan-Meier survival analysis illustrated that ELOVL6
expression was associated with overall survival in both the TNM
stage I–II (P=0.048) and stage III–IV (P=0.003) groups in patients
with tumor size >5 cm (P=0.014) and those with tumor size ≤5 cm
(P=0.042). ELOVL6 expression was also associated with overall
survival in younger (P=0.043) and older (P=0.014) patients with HCC
(Fig. 3).
Discussion
HCC is the most common malignant tumor of the liver
(26–29). The result of ELOVL6 expression on the
proliferation, invasion and metastasis of HCC cells was yet to be
investigated, but was believed to provide insights into novel
treatment options for patients with HCC. The present study
confirmed that the expression level of ELOVL6 was decreased in HCC
tissues, and that ELOVL6 expression was negatively associated with
tumor size. Furthermore, a low expression level of ELOVL6 was
associated with unfavorable outcome in patients with HCC. This
indicates that ELOVL6 is a potential, novel therapeutic target and
prognostic biomarker for HCC.
Fatty acids are essential components of biofilm
lipids, signaling molecules and constituents of energy metabolism
pathways (30–32). Among them, palmitic acid serves a
prominent role in the formation of long-chain fatty acids
containing 16 carbon atoms (C16:0), and studies have reported
excessive accumulation of palmitic acid in breast cancer cells
(33–35). ELOVL6 is a key enzyme in
intracellular lipid metabolism, and has previously been associated
with metabolism in fatty liver and diabetes (36). However, the relationship between
metabolic reprogramming and expression of ELVOL6 in HCC has not
previously been reported. In the present study, it was determined
that tumor size was closely associated with ELOVL6 expression
level. It is also possible that ELOVL6-associated lipid metabolism
is able to promote tumor proliferation, though the specific
mechanisms remain to be determined.
Moon et al (37) found that the conversion of palmitic
acid to stearic acid (C18:0) was inhibited in ELOVL6 knock-out
mice, suggesting that ELOVL6 is indispensable for palmitic acid
metabolism (37). It has been
speculated that ELOVL6 converts excess palmitic acid (C16:0),
serving a role in tumor suppression. Kessler et al (38) found that in a mouse model of
diethylnitrosamine-induced HCC, the expression level of ELVOL6 in
cancerous tissues was lower than that in non-cancerous liver
tissues. The present study was consistent with these results, where
ELOVL6 expression level was also significantly reduced in HCC
tissues. In addition, ELOVL6 expression was negatively associated
with tumor size.
Previous studies have revealed that the level of
palmitic and stearic acid in tumor cells was associated with the
prognosis of cancer patients (39,40).
Bougnoux et al (41) found
that breast cancer patients with high levels of stearic acid in
their tumors had a lower likelihood of these tumors metastasizing.
Further studies also revealed that patients with breast cancer and
elevated palmitic acid levels had a poorer prognosis, and that the
expression of the ELOVL6 gene was significantly downregulated in
these patients (42–44). In the present study, the overall and
disease-free survival time of patients with high ELOVL6 expression
levels was increased. However, the hypothesis that ELOVL6 regulates
intracellular lipid components and influences the prognosis of
patients requires further confirmation. Lipid metabolism is a key
aspect of tumor growth; fatty acids not only serve as an energy
source for tumors, but as a cellular component of rapidly
proliferating tumor cells. ELOVL6 extends the carbon chain of fatty
acids and inhibits their use, which may be detrimental in rapid
tumor proliferation. Additional studies have confirmed that ELOVL6
is involved in both migration and proliferation (45,46), and
in the present study, ELOVL6 expression was associated with
vascular invasion, which is also closely associated with factors
such as vascular endothelial growth factor. The majority of chronic
liver diseases are associated with hypoxic symptoms that come with
with metabolic diseases, such as non-alcoholic fatty liver disease.
Chronic hypoxia can result in the disorder of lipid metabolism and
an increase in vascular endothelial growth factor expression in
hepatocytes, thereby increasing blood flow in the liver to adapt to
the anoxic environment. In HCC, the formation of these microvessels
also increases the migration ability of tumor cells (47,48).
Vascular invasion of tumors is a complex process that utilizes the
motility of tumor cells and the proliferation and migration of
vascular endothelial cells (49,50). The
molecular mechanism of vascular invasion is not fully explained by
ELVOL6 expression; this may explain why vascular invasion was
associated with ELOVL6 expression in the present study, but that
they were also independent prognostic factors. It was demonstrated
that the lower the expression level of ELOVL6, the higher the
probability of vascular invasion, which may be due to the decreased
expression level of ELOVL6 and subsequent increase in tumor cell
migration. However, this theory requires further experimental
confirmation.
Although ELOVL6 is involved in lipid synthesis
(46), in order to meet the
requirements of rapidly proliferating tumor cells, over-activated
lipid-synthesized fatty acids are used to synthesize cell membranes
and other organelles, rather than being stored in lipid droplets
(11,51). In other tumor types, the expression
of ELOVL6 was also found to be decreased (52), but in order to confirm the role of
ELOVL6 in HCC, further in vivo and in vitro
experimentation is required.
There are some limitations to the present study; the
sample size was relatively small, which may have introduced a
degree of bias. The data were also collected from a single
institution, which may also have resulted in enrollment bias. A
multicenter prospective study is warranted to further validate the
role and potential prognostic value of ELOVL6 in HCC.
In summary, the present study highlighted a role for
ELOVL6 in the development and progression of HCC. The data revealed
that ELOVL6 expression level was decreased in HCC tissues, which
was significantly correlated with tumor size. High ELOVL6
expression level correlated with longer survival times in patients
with HCC, and therefore, ELOVL6 may serve as an independent factor
for improved patient outcome. Collectively, the present study
suggested that ELOVL6 may be a promising biomarker for the
prognosis of patients with HCC, and a potential target for HCC
treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD designed the study. HL and MD wrote the
manuscript. XW and JT analyzed and interpreted the patient data. HL
and HZ performed the experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Jining No. 1 People's Hospital. All procedures
followed were in accordance with the ethical standards of the
responsible committee on human experimentation and with the
Helsinki Declaration 2008.
Patient consent for publication
As a retrospective study, the Medical Ethics
Committee waived the need for informed patient consent.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Clavien PA, Lesurtel M, Bossuyt PM, Gores
GJ, Langer B and Perrier A; OLT for HCC Consensus Group, :
Recommendations for liver transplantation for hepatocellular
carcinoma: An international consensus conference report. Lancet
Oncol. 13:e11–e22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al:
Management of hepatocellular carcinoma in Asia: Consensus statement
from the Asian Oncology Summit 2009. Lancet Oncol. 10:1111–1118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai SH, Lu SX, Liu LL, Zhang CZ and Yun
JP: Increased expression of hepatocyte nuclear factor 4 alpha
transcribed by promoter 2 indicates a poor prognosis in
hepatocellular carcinoma. Therap Adv Gastroenterol. 10:761–771.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ou H, Cai S, Liu Y, Xia M and Peng J: A
noninvasive diagnostic model to assess nonalcoholic hepatic
steatosis in patients with chronic hepatitis B. Therap Adv
Gastroenterol. 10:207–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friemel J, Rechsteiner M, Frick L, Böhm F,
Struckmann K, Egger M, Moch H, Heikenwalder M and Weber A:
Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer
Res. 21:1951–1961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Cai S, Li Z, Zheng C, Xue X, Zeng J
and Peng J: Potential effects of telbivudine and entecavir on renal
function: A systematic review and meta-analysis. Virol J.
13:642016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai S, Ou Z, Liu D, Liu L, Liu Y, Wu X, Yu
T and Peng J: Risk factors associated with liver steatosis and
fibrosis in chronic hepatitis B patient with component of metabolic
syndrome. United European Gastroenterol J. 6:558–566. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng J, Cai S, Liu J, Xue X, Wu X and
Zheng C: Dynamic changes in liver stiffness measured by transient
elastography predict clinical outcomes among patients with chronic
Hepatitis B. J Ultrasound Med. 36:261–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
CD36-mediated lipid metabolism promotes
metastasis. Cancer Discov. 7:F122017. View Article : Google Scholar
|
|
11
|
Xiao YB, Cai SH, Liu LL, Yang X and Yun
JP: Decreased expression of peroxisome proliferator-activated
receptor alpha indicates unfavorable outcomes in hepatocellular
carcinoma. Cancer Manag Res. 10:1781–1789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z and Kang Y: Lipid metabolism fuels
cancer's spread. Cell Metab. 25:228–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sunami Y, Rebelo A and Kleeff J: Lipid
metabolism and lipid droplets in pancreatic cancer and stellate
cells. Cancers (Basel). 10(pii): E32017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta S, Santra L, Naskar S, Maurya SK,
Rana M, Ghosh J and Dhara SK: Heterologous expression of porcine
elongase 6 (ELOVL6) gene in a human cell line. Indian J Med Res.
145:563–568. 2017.PubMed/NCBI
|
|
17
|
Su YC, Feng YH, Wu HT, Huang YS, Tung CL,
Wu P, Chang CJ, Shiau AL and Wu CL: Elovl6 is a negative clinical
predictor for liver cancer and knockdown of Elovl6 reduces murine
liver cancer progression. Sci Rep. 8:65862018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng YH, Chen WY, Kuo YH, Tung CL, Tsao
CJ, Shiau AL and Wu CL: Elovl6 is a poor prognostic predictor in
breast cancer. Oncol Lett. 12:207–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamashita Y, Nishiumi S, Kono S, Takao S,
Azuma T and Yoshida M: Differences in elongation of very long chain
fatty acids and fatty acid metabolism between triple-negative and
hormone receptor-positive breast cancer. BMC Cancer. 17:5892017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bosquet A, Guaita-Esteruelas S, Saavedra
P, Rodríguez-Calvo R, Heras M, Girona J and Masana L: Exogenous
FABP4 induces endoplasmic reticulum stress in HepG2 liver cells.
Atherosclerosis. 249:191–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horie Y, Suzuki A, Kataoka E, Sasaki T,
Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, et
al: Hepatocyte-specific Pten deficiency results in steatohepatitis
and hepatocellular carcinomas. J Clin Invest. 113:1774–1783. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai SH, Lv FF, Zhang YH, Jiang YG and Peng
J: Dynamic comparison between Daan real-time PCR and Cobas TaqMan
for quantification of HBV DNA levels in patients with CHB. BMC
Infect Dis. 14:852014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai S, Cao J, Yu T, Xia M and Peng J:
Effectiveness of entecavir or telbivudine therapy in patients with
chronic hepatitis B virus infection pre-treated with interferon
compared with de novo therapy with entecavir and telbivudine.
Medicine (Baltimore). 96:e70212017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen WX, Cheng L, Xu LY, Qian Q and Zhu
YL: Bioinformatics analysis of prognostic value of TRIM13 gene in
breast cancer. Biosci Rep. 39(pii): BSR201902852019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li C, Zhou D, Jiang X, Liu M, Tang H and
Mei Z: Identifying hepatocellular carcinoma-related hub genes by
bioinformatics analysis and CYP2C8 is a potential prognostic
biomarker. Gene. 698:9–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsochatzis EA, Meyer T and Burroughs AK:
Hepatocellular carcinoma. N Engl J Med. 366:92–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
White DL, Thrift AP, Kanwal F, Davila J
and El-Serag HB: Incidence of hepatocellular carcinoma in All 50
United States, From 2000 Through 2012. Gastroenterology.
152:812–820.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai S, Li Z, Yu T, Xia M and Peng J: Serum
hepatitis B core antibody levels predict HBeAg seroconversion in
chronic hepatitis B patients with high viral load treated with
nucleos(t)ide analogs. Infect Drug Resist. 11:469–477. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai S, Yu T, Jiang Y, Zhang Y, Lv F and
Peng J: Comparison of entecavir monotherapy and de novo lamivudine
and adefovir combination therapy in HBeAg-positive chronic
hepatitis B with high viral load: 48-week result. Clin Exp Med.
16:429–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA,
Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, et al:
Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling,
promotes development of human hepatocellular carcinoma.
Gastroenterology. 140:1071–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao D, Song X, Che L, Li X, Pilo MG,
Vidili G, Porcu A, Solinas A, Cigliano A, Pes GM, et al: Both de
novo synthetized and exogenous fatty acids support the growth of
hepatocellular carcinoma cells. Liver Int. 37:80–89. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Caro P, Kishan AU, Norberg E, Stanley IA,
Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, et
al: Metabolic signatures uncover distinct targets in molecular
subsets of diffuse large B cell lymphoma. Cancer Cell. 22:547–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Bahlani S, Al-Lawati H, Al-Adawi M,
Al-Abri N, Al-Dhahli B and Al-Adawi K: Fatty acid synthase
regulates the chemosensitivity of breast cancer cells to
cisplatin-induced apoptosis. Apoptosis. 22:865–876. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sczaniecka AK, Brasky TM, Lampe JW,
Patterson RE and White E: Dietary intake of specific fatty acids
and breast cancer risk among postmenopausal women in the VITAL
cohort. Nutr Cancer. 64:1131–1142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao F, Wang C, Yin H, Yu J, Chen S, Fang
J and Guo F: Leucine deprivation inhibits proliferation and induces
apoptosis of human breast cancer cells via fatty acid synthase.
Oncotarget. 7:63679–63689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoekstra M, van der Sluis RJ, Kuiper J and
Van Berkel TJ: Nonalcoholic fatty liver disease is associated with
an altered hepatocyte microRNA profile in LDL receptor knockout
mice. J Nutr Biochem. 23:622–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moon YA, Ochoa CR, Mitsche MA, Hammer RE
and Horton JD: Deletion of ELOVL6 blocks the synthesis of oleic
acid but does not prevent the development of fatty liver or insulin
resistance. J Lipid Res. 55:2597–2605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kessler SM, Simon Y, Gemperlein K,
Gianmoena K, Cadenas C, Zimmer V, Pokorny J, Barghash A, Helms V,
van Rooijen N, et al: Fatty acid elongation in non-alcoholic
steatohepatitis and hepatocellular carcinoma. Int J Mol Sci.
15:5762–5773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crowe FL, Allen NE, Appleby PN, Overvad K,
Aardestrup IV, Johnsen NF, Tjønneland A, Linseisen J, Kaaks R,
Boeing H, et al: Fatty acid composition of plasma phospholipids and
risk of prostate cancer in a case-control analysis nested within
the European Prospective Investigation into Cancer and Nutrition.
Am J Clin Nutr. 88:1353–1363. 2008.PubMed/NCBI
|
|
40
|
Di Gangi IM, Mazza T, Fontana A, Copetti
M, Fusilli C, Ippolito A, Mattivi F, Latiano A, Andriulli A,
Vrhovsek U and Pazienza V: Metabolomic profile in pancreatic cancer
patients: A consensus-based approach to identify highly
discriminating metabolites. Oncotarget. 7:5815–5829. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bougnoux P, Hajjaji N, Ferrasson MN,
Giraudeau B, Couet C and Le Floch O: Improving outcome of
chemotherapy of metastatic breast cancer by docosahexaenoic acid: A
phase II trial. Br J Cancer. 101:1978–1985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baek JS and Cho CW:
2-Hydroxypropyl-β-cyclodextrin-modified SLN of paclitaxel for
overcoming p-glycoprotein function in multidrug-resistant breast
cancer cells. J Pharm Pharmacol. 65:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao W, Ma Z, Rasenick MM, Yeh S and Yu J:
N-3 poly-unsaturated fatty acids shift estrogen signaling to
inhibit human breast cancer cell growth. PLoS One. 7:e528382012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Conceicao LL, Dias MM, Pessoa MC, Pena GD,
Mendes MC, Neves CV, Hermsdorff HH, Freitas RN and Peluzio MD:
Difference in fatty acids composition of breast adipose tissue in
women with breast cancer and benign breast disease. Nutr Hosp.
33:1354–1360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kikuchi M, Shimada M, Matsuzaka T, Ishii
K, Nakagawa Y, Takayanagi M, Yamada N and Shimano H: Crucial role
of Elovl6 in chondrocyte growth and differentiation during growth
plate development in mice. PLoS One. 11:e1593752016. View Article : Google Scholar
|
|
46
|
Anelli L, Zagaria A, Coccaro N, Tota G,
Impera L, Minervini CF, Pastore D, Minervini A, Casieri P, Specchia
G and Albano F: A novel t(4;16)(q25;q23.1) associated with EGF and
ELOVL6 deregulation in acute myeloid leukemia. Gene. 529:144–147.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Q, Wang D, Li Y, Yan S, Dang H, Yue
H, Ling J, Chen F, Zhao Y, Gou L, et al: LINC00628 suppresses
migration and invasion of hepatocellular carcinoma by its conserved
region interacting with the promoter of VEGFA. J Cell Physiol. Feb
10–2019.doi: 10.1002/jcp.28233 (Epub ahead of print).
|
|
48
|
Cheng SY, Chen NF, Lin PY, Su JH, Chen BH,
Kuo HM, Sung CS, Sung PJ, Wen ZH and Chen WF: Anti-invasion and
Antiangiogenic effects of Stellettin B through inhibition of the
Akt/Girdin signaling pathway and VEGF in Glioblastoma cells.
Cancers (Basel). 11(pii): E2202019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ
and Feng YJ: Perineural invasion of cancer: A complex crosstalk
between cells and molecules in the perineural niche. Am J Cancer
Res. 9:1–21. 2019.PubMed/NCBI
|
|
50
|
Labib PL, Goodchild G and Pereira SP:
Molecular pathogenesis of cholangiocarcinoma. BMC Cancer.
19:1852019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu JB, Cai SH, Pan YH and Yun JP: Altered
epidermal fatty acid-binding protein expression in hepatocellular
carcinoma predicts unfavorable outcomes. Cancer Manag Res.
10:6275–6284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li FJ, Wang HY, Feng XL, Li PP, Shu T,
Zhao XH and Li B: Expression and clinical significance of ELOVL6
gene in high-grade serous ovarian carcinoma. Zhonghua Fu Chan Ke Za
Zhi. 51:192–197. 2016.(In Chinese). PubMed/NCBI
|