Introduction
The cancer immunoediting theory explains cancer
recurrence in terms of the balance between the tumorigenic
potential of cancer cells and the ability of the host immune system
to remove the cancer cells (1,2). This
theory attempts to explain in three essential phases. These three
phases are commonly called the ‘3 Es’; namely, elimination,
equilibrium, and escape. When the immune system has the upper hand,
tumor cells can be removed by immune system and this situation is
called elimination. Escape defines the situation in which the
cancer develops clinically beyond the immune system. Equilibrium is
a state in which the cancer cells do not grow but are still present
in balance with the immune system. This theory can explain the
limitation of research focusing on cancer cell pathophysiology
alone. We recently evaluated tumor-free lymph nodes harvested
during neck dissection of patients with cancer of the oral cavity
and found that expression of many genes related to T cell
activation is down-regulated in patients with poor prognosis. Among
these markers, decreased cluster of differentiation (CD)40 ligand
(L) expression showed independent significance in Cox regression
multivariate analysis related to disease-free survival (3). CD40L is expressed on activated CD4+ T
helper cells; this finding suggests that patients with impaired CD4
T cell immunity may have a higher risk of recurrence after
treatment. This result has important value in a clinical setting.
Even with aggressive surgery or chemoradiation therapy, microscopic
cancer cells can remain. Recent work on circulating cancer cells
has provided evidence of the presence of numerous microscopic
cancer cells after local treatment (4). Therefore, removal of these remaining
cancer cells is important for complete cure of such malignancies, a
process in which host immunity plays an important role.
An orthotopic tongue cancer model using nude mice
was previously used for the evaluation of targeted therapy
(5). As this animal model was based
on athymic nude mice, which have no T cells, the model cannot be
used to evaluate the role of host immunity. A syngeneic mouse
cancer model could be another option as the mouse has an intact
immune system. However, in this model, the cancer cells develop
from mouse and differ from the cancer cell we are targeting. There
have been efforts to develop a xenograft human tumor model in
immunocompetent mice. Basel et al, injected melanoma cancer
cells into E14 fetuses to desensitize the mice and re-injected
melanoma cells after the mice had matured to succeed in inducing
cancer (6). However, this kind of
model has also a weakness because the immune system is desensitized
to cancer cells, thus preventing the evaluation of the role of the
immune system. Therefore, we injected a human cancer cell line into
immunocompetent mice to assess the effect on the immune system.
Human cancer cells in immunocompetent mice will eventually be
eliminated by the immune system. We used this mouse model to
evaluate the changes in immune cells during this elimination.
Additionally, depletion of CD4 and/or CD8 cells by monoclonal
antibodies was used to investigate the role of subpopulations of T
cells during the elimination of heterogeneous cells. CD4 or CD8
depletion by monoclonal antibodies in Balb/C mice is a method
frequently used for the evaluation of the infectious process or
cancer cell treatments (7–9). A similar trial was reported using human
lymphocytic or epithelial cell lines in the model of human viral
infection or transplant rejection (10). Finally, we assessed the effectiveness
of immunotherapy in conditions of CD4 deficiency.
Materials and methods
CD4- and CD8-depleted mouse
models
The immunocompetent BALB/C mice and athymic nude
mice were purchased from Central Lab. Animal Inc., and were raised
in a specific-pathogen-free (SPF) environment with standard
conditions, controlled at a temperature of 20–25°C with 40–50%
humidity with a 12-h light/dark cycle. There was no specific
limitation for food or water. Mice with 6–8-week-old female with
body weight ranging 18–20 g were used. For the depletion of CD4
and/or CD8 T cells in immunocompetent BALB/C mice, 0.5 mg of the
anti-mouse CD4 clone GK1.5 or anti-mouse CD8 clone 53–6.72
(BioXCell) mixed with 0.5 ml of phosphate-buffered saline (PBS) was
injected intraperitoneally for three consecutive days. Thereafter,
the depletion status was maintained with a twice-weekly peritoneal
injection of the corresponding antibodies. Five nude mice were used
as comparison group, and 47 BALB/C mice were used. (control; 12,
CD8 depletion; 13, CD4 depletion; 13, CD4+8 depletion; 9)
Orthotopic tongue cancer mouse
model
SNU1041 cell lines were established at our institute
and provided to us by Ku et al (11). The cell lines were maintained in
advanced RPMI1640 (Gibco) medium supplemented with 10% fetal bovine
serum, 2 mM l-glutamine, and penicillin (100 IU/ml)/streptomycin
(100 µg/ml). Animal studies were performed in accordance with the
protocol approved by our Institutional Animal Care and Use
Committee. First, 1×106 cells in 15 µl of PBS were
injected to the lateral tongue one week after starting the
injections of CD4 and/or CD8 monoclonal antibody for depletion.
Tongue mass and body weight were measured 2–3 times weekly. The
mice were sacrificed at one or four weeks after cancer cell
injection and their tongues were harvested for pathological
evaluation. Each of four conditions (control, CD4 depletion, CD8
depletion, and CD4+8 depletion) included 9–13 mice to produce data
from at least three mice for each time point (before SNU1041 cell
line injection, one and four weeks after injection of cancer cell
line).
Agonistic CD40 and antagonistic PD1
antibody treatment
Treatment was begun 10 days after cancer cell line
injection after confirmation of tongue mass development. Treatments
were administered twice weekly for 2 weeks. Rat IgG2a isotype
control (100 µg) and InVivoPlus Polyclonal Armenian Hamster IgG
(100 µg) were injected intraperitoneally in the control group.
Agonistic CD40 treatment was performed with the injection of 100 µg
of rat anti-mouse CD40 monoclonal Ab clone FGK 4.5 (BioXCell) and
100 µg of InVivoPlus polyclonal Armenian hamster IgG. The PD1
antagonist treatment used InVivoPlus polyclonal anti-mouse PD1
(BioXCell) and rat IgG2a as the isotype control. The combined
agonistic CD40 and antagonistic PD1 treatment included rat
anti-mouse CD40 monoclonal Ab clone FGK 4.5 and InVivoPlus
polyclonal anti-mouse PD1. Each group included 4–5 mice (five mice
for PD1 antagonist group and four mice for other groups). During
treatment, body weight and tongue tumor size were checked twice
weekly and tongue tissue, blood, lymph nodes, and spleen were
harvested after animal sacrifice.
Flow cytometric analysis
The mice were sacrificed before cell line injection,
one and four weeks after injection. At this time, blood, lymph
nodes in the neck and inguinal area, and spleens were harvested.
The lymph nodes and spleens were mechanically dissociated to make
single-cell suspensions. The blood was centrifuged and the serum
extracted for another study. After red blood cell (RBC) lysis, the
precipitate was suspended in flow cytometry buffer. Anti-mouse FITC
CD4, PreCP-Cy5.5 CD8, APC CD40, PE CD40L, PerCP-Cy5.5 CD11b, and
FITC CD19 (all from BD Bioscience) were used for flow
cytometry.
Cytokine analysis
Levels of CD40L, IFNγ, TNFα, IL-1β, IL-10, IL-2,
IL-4, IL-5, and IL-6 were measured using a Mouse Th17 Magnetic Bead
Panel (EMD Millipore Corp, MI, USA) from serum collected at the
time of sacrifice.
Statistical analysis
All statistical analyses were performed using IBM
SPSS for Windows, version 20.0 (IBM Corp.). One-way analysis of
variance (ANOVA) with Bonferroni post hoc test was used for
comparison of the means.
Results
CD4 and CD8 depletion in Balb/C
mice
Flow cytometry was performed 4 days after three
consecutive peritoneal injections of anti-mouse CD4 clone GK1.5 or
anti-mouse CD8 clone 53–6.72 to verify depletion of the
corresponding markers. CD40L is expressed mainly by CD4-positive
cells (CD4+ T cells) and depletion of CD4 cells results in
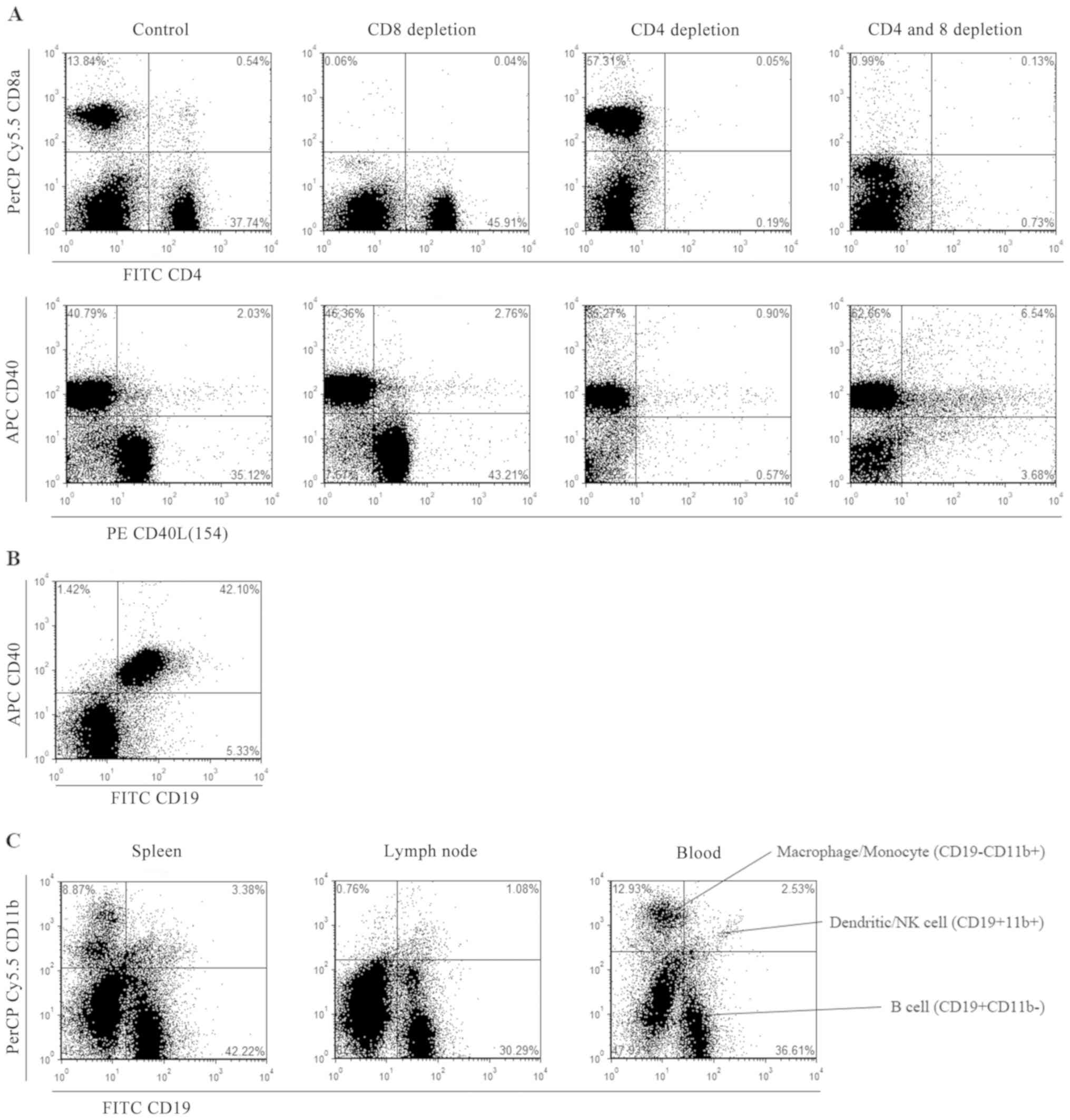
simultaneously decrease in CD40L expression (Fig. 1A). Expression of CD40 is coincident
with that of CD19 (Fig. 1B).
According to the CD marker handbook from BD Bioscience, the
CD19-CD11b+ subset indicated macrophages or monocytes while the
CD19+CD11b+ subset indicated dendritic cells or natural killer (NK)
cells. Most CD19+ cells were CD11b- and considered to be presented
by B cells (Fig. 1C).
Cancer elimination in CD4 or CD8
depleted mice
Tumorigenesis of the SNU1041 cell line was confirmed
in nude mice, with 100% tumor formation in a previous study
(5). When injected into
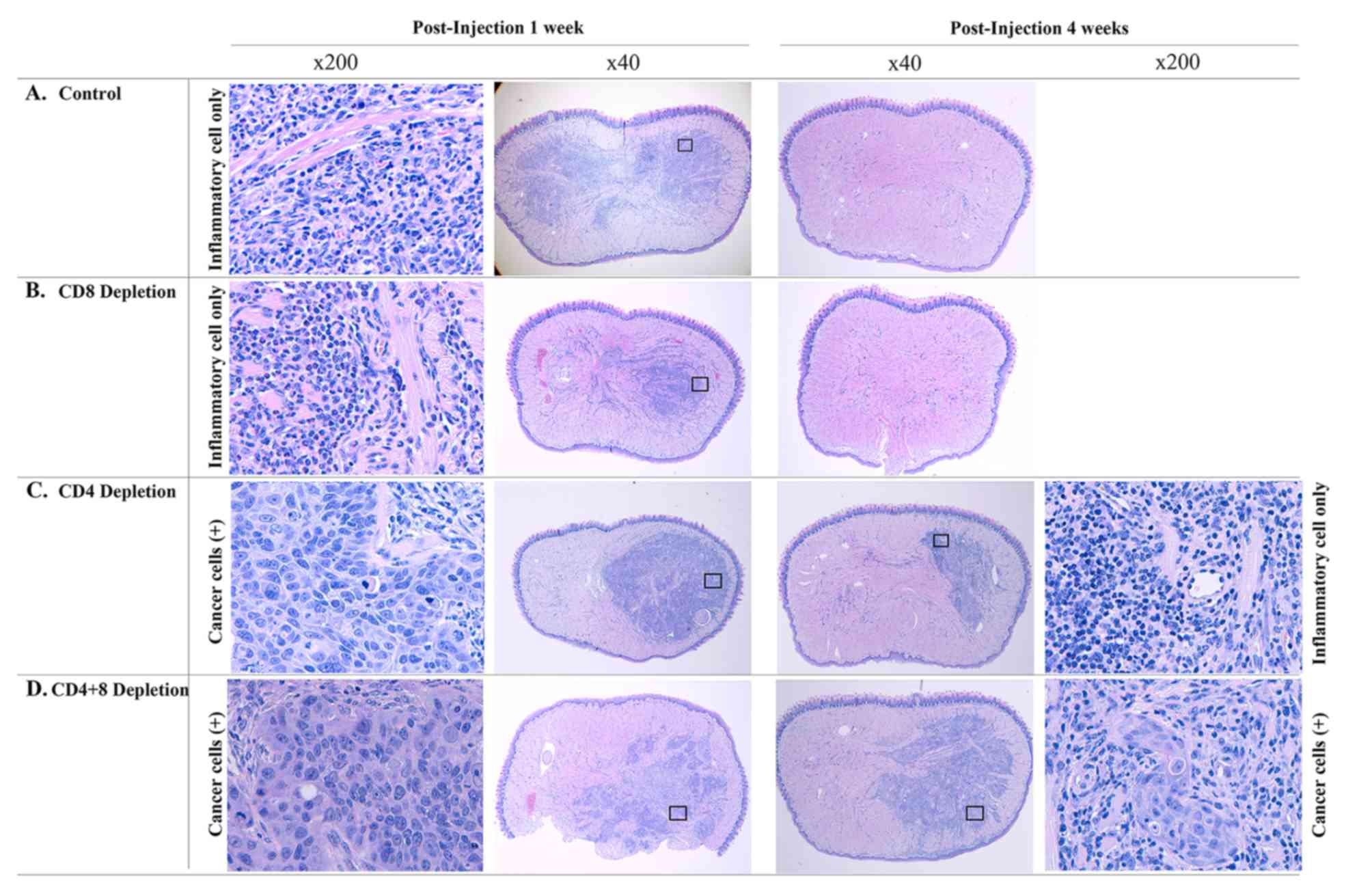
immunocompetent Balb/C mice, tumor growth was not observed in gross
examination of the tongue. Microscopically, inflammatory cells had
infiltrated the entire tongue at one week. However, no viable tumor
cells were found by hematoxylin and eosin (H&E) staining. At 4
weeks, the inflammatory cells were gone, and the tongue is
normalized completely (Fig. 2A). CD8
depleted mice had gross tongue nodules in 50% (2 out of 4 cases) at
one week. H&E staining showed infiltration of inflammatory cell
in half of the tongue without viable tumor cells (Fig. 2B). In CD4 depleted mice, viable
cancer cells could be found with surrounding inflammatory cells by
pathologic evaluation at one week. Four weeks after injection,
66.7% (4 out of 6 cases) showed tumors by gross examination and
pathologic examination showed viable tumor cells in 2 cases
(Fig. 2C). When CD4 and CD8 were
depleted simultaneously, the tumor was growing (Fig. 2D). Table
I describes the number of mice with gross nodule and
pathological findings.
 | Table I.Tongue mass and pathologic findings
according to the depletion of T cells. |
Table I.
Tongue mass and pathologic findings
according to the depletion of T cells.
|
| 1 week | 4 weeks |
|---|
|
|
|
|
|---|
| Group | Nodule | Viable tumor
cell/Visible nodule | Visible
nodule/Mouse | Viable tumor
cell/Visible nodule |
|---|
| Nude mice (N=3) | 1/1 | 1/1 | 2/2 | 2/2 |
| Control (N=9) | 0/4 | – | 0/5 | – |
| CD8 depletion
(N=10) | 2/4 | 0/2 | 0/6 | – |
| CD4 depletion
(N=10) | 4/4 | 4/4 | 4/6 | 2/4 |
| CD4+8 depletion
(N=6) | 3/3 | 3/3 | 2/3 | 2/2 |
Changes in the proportions of immune
cells during cancer cell elimination
To understand the changes in immune cells,
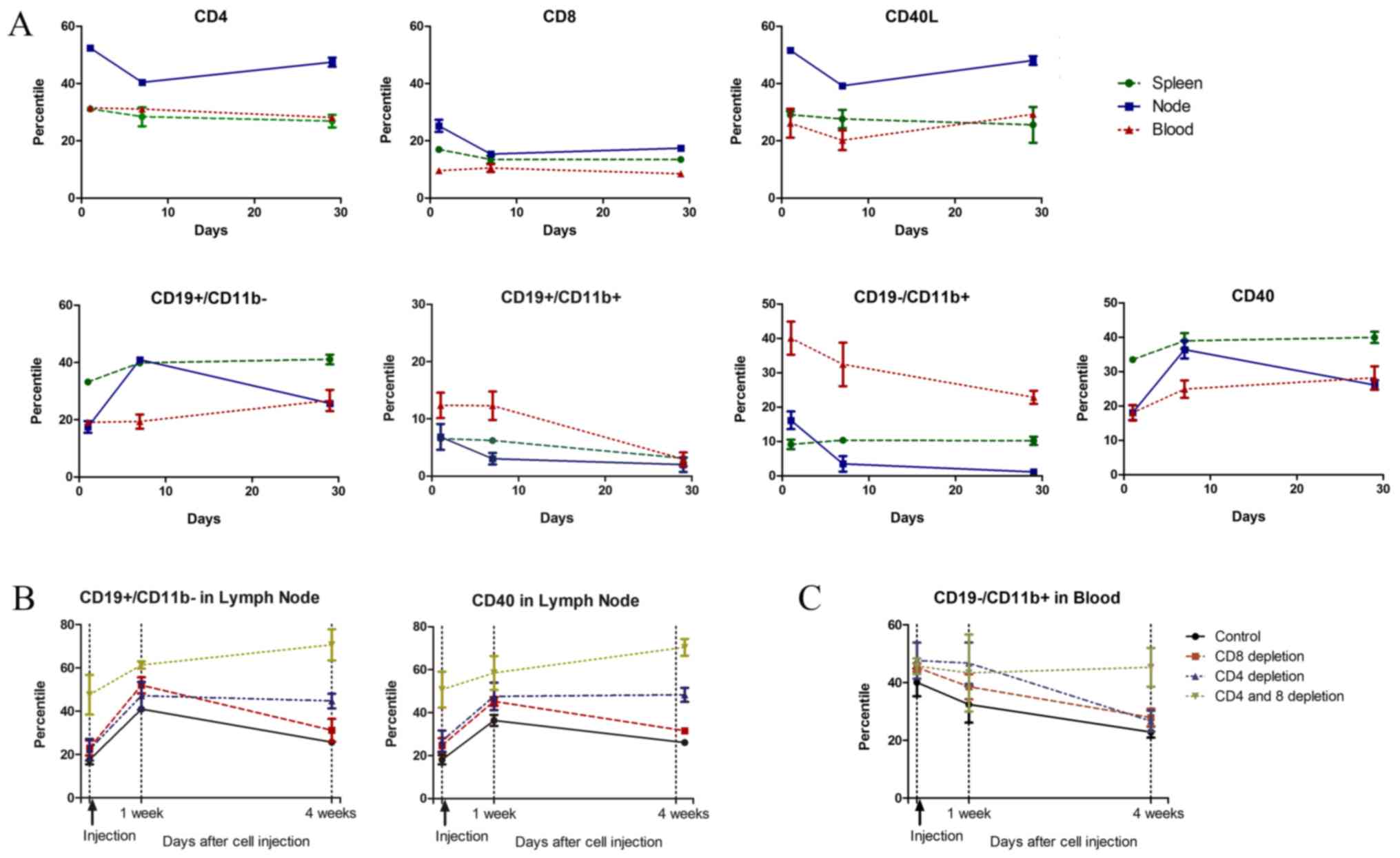
proportional changes were first evaluated in the control group. In
the nodes of the control group, CD4 (CD40L) levels were decreased
at one week after cancer cell injection and returns to the basal
level after four weeks. B cells (CD19+CD11b-) showed an inverse
change from that of CD4, with increased levels in the lymph node at
one week that returned to the basal level at four weeks. In the
blood and spleen, B cell (CD19+11b-) levels increased gradually
until four weeks after injection. Macrophage or monocyte
(CD19-CD11b+) levels decreased in the node and blood through four
weeks after injection of cancer cells (Fig. 3A).
Compared to the control group, the CD8-depleted
group showed similar changing patterns in all immune cells.
However, in the CD4-depleted group, increased levels of B cells
(CD19+CD11b- or CD40+) in the node persisted until four weeks after
the injection. In both the CD4- and CD8-depleted groups, the
proportion of B cells (CD19+CD11b or CD40) continued to increase
through four weeks (Fig. 3B). The
blood levels of macrophages or monocytes (CD19-CD11b+) in the blood
decreased at one week and had further decreased at four weeks in
the control and CD8-depleted groups. However, in the CD4-depleted
group, the blood levels remained high until one week and had
decreased at four weeks. The macrophages or monocytes (CD19-CD11b+)
in the blood did not change in both CD4 and CD8 depleted group
(Fig. 3C). The changes in each
immune cell type in the CD4- and CD8-depleted groups are listed in
detail in Fig. S1. In the lymph
node, the proportion of B cells (CD19+CD11b-) increased while the
proportion of macrophages or monocytes (CD19-CD11b+) decreased
significantly with the depletion of both CD4 and CD8 cells. With
cancer cell injection, the proportions were similar across groups
at one week. After four weeks, the proportion of B cells
(CD19+CD11b-) was significantly increased in the CD4-depleted
groups. In blood, significant differences were observed in the
proportion of B cells (CD19+CD11b-) in CD4-depleted mice and the
proportion of macrophages or monocytes (CD19-CD11b+) in the CD4 and
CD8-depleted groups at four weeks (Fig.
S2).
Changes in cytokine levels
Among the cytokines analyzed, IL-1β, IL-2, IL-4, and
IL-5 were rarely expressed in the samples. In the CD8-depleted
group, CD40L, IFNγ, IL-10, IL-6, and TNFα showed significant
increases at various time points (Table
II).
 | Table II.Changes of cytokines according to the
CD4 or CD8 depletion. |
Table II.
Changes of cytokines according to the
CD4 or CD8 depletion.
| Group | Before injection | P-value | 1 week after
injection | P-value | 4 weeks after
injection | P-value |
|---|
| CD40L |
| 0.354 |
| 0.050 |
| 0.002a |
|
Control | 132.8±78.0 |
| 145.8±109.3 |
| 106.8±75.4 |
|
| CD4
depletion | 69.2±28.2 | 1.000 | 46.3±28.0 | 1.000 | 106.3±75.4 | 1.000 |
| CD8
depletion | 559.6±838.8 | 1.000 | 1556.6±1418.7 | 0.152 | 699.2±370.8 | 0.006a |
| CD4&8
depletion | 46.8±55.1 | 1.000 | 91.1±83.1 | 1.000 | 63.5±47.1 | 1.000 |
| IFNγ |
| 0.495 |
| 0.090 |
|
<0.001a |
|
Control | 0.32±0b |
| 0.32±0b |
| 0.32±0b |
|
| CD4
depletion | 1.0±1.4 | 1.000 | 17.3±18.1 | 0.199 | 0.32±0b | 1.000 |
| CD8
depletion | 0.32±0b | 1.000 | 2.4±3.0 | 1.000 | 67.3±18.6 |
<0.001a |
| CD4&8
depletion | 0.46±0.28 | 1.000 | 0.32±0a | 1.000 | 3.9±2.1 | 1.000 |
| L-10 |
| 0.041a |
| 0.006a |
| 0.178 |
|
Control | 4.1±0.5 |
| 5.0±1.5 |
| 6.2±2.1 |
|
| CD4
depletion | 5.3±0.6 | 0.965 | 3.8±0.3 | 0.890 | 4.92±0 | 1.000 |
| CD8
depletion | 6.7±1.8 | 0.070 | 7.8±1.6 | 0.060 | 157.9±279.8 | 0.395 |
| CD4&8
depletion | 4.3±1.1 | 1.000 | 4.6±0.6 | 1.000 | 4.3±1.1 | 1.000 |
| IL-6 |
|
<0.001a |
| 0.391 |
| 0.098 |
|
Control | 2.9±4.0 |
| 1.1±0.6 |
| 1.1±1.2 |
|
| CD4
depletion | 1.7±0.6 | 1.000 | 5.8±5.0 | 1.000 | 1.4±0 | 1.000 |
| CD8
depletion | 16.2±3.1 | 0.001a | 9.4±11.0 | 0.669 | 91.75±138.5 | 0.206 |
|
CD4&8 depletion | 1.1±1.2 | 1.000 | 3.7±4.3 | 1.000 | 2.2±1.7 | 1.000 |
| TNFα |
|
<0.001a |
| 0.498 |
| 0.313 |
|
Control | 0.12±0b |
| 0.12±0b |
| 2.35±2.7 |
|
| CD4
depletion | 0.12±0b | 1.000 | 0.29±0.3 | 1.000 | 0.69±1.1 | 1.000 |
| CD8
depletion | 3.7±1.5 | 0.001a | 0.92±1.6 | 1.000 | 7.1±13.7 | 1.000 |
|
CD4&8 depletion | 0.12±0b | 1.000 | 0.12±0b | 1.000 | 0.12±0b | 1.000 |
Treatment with agonistic CD40 and
antagonistic PD-1 antibodies in CD4-depleted mice
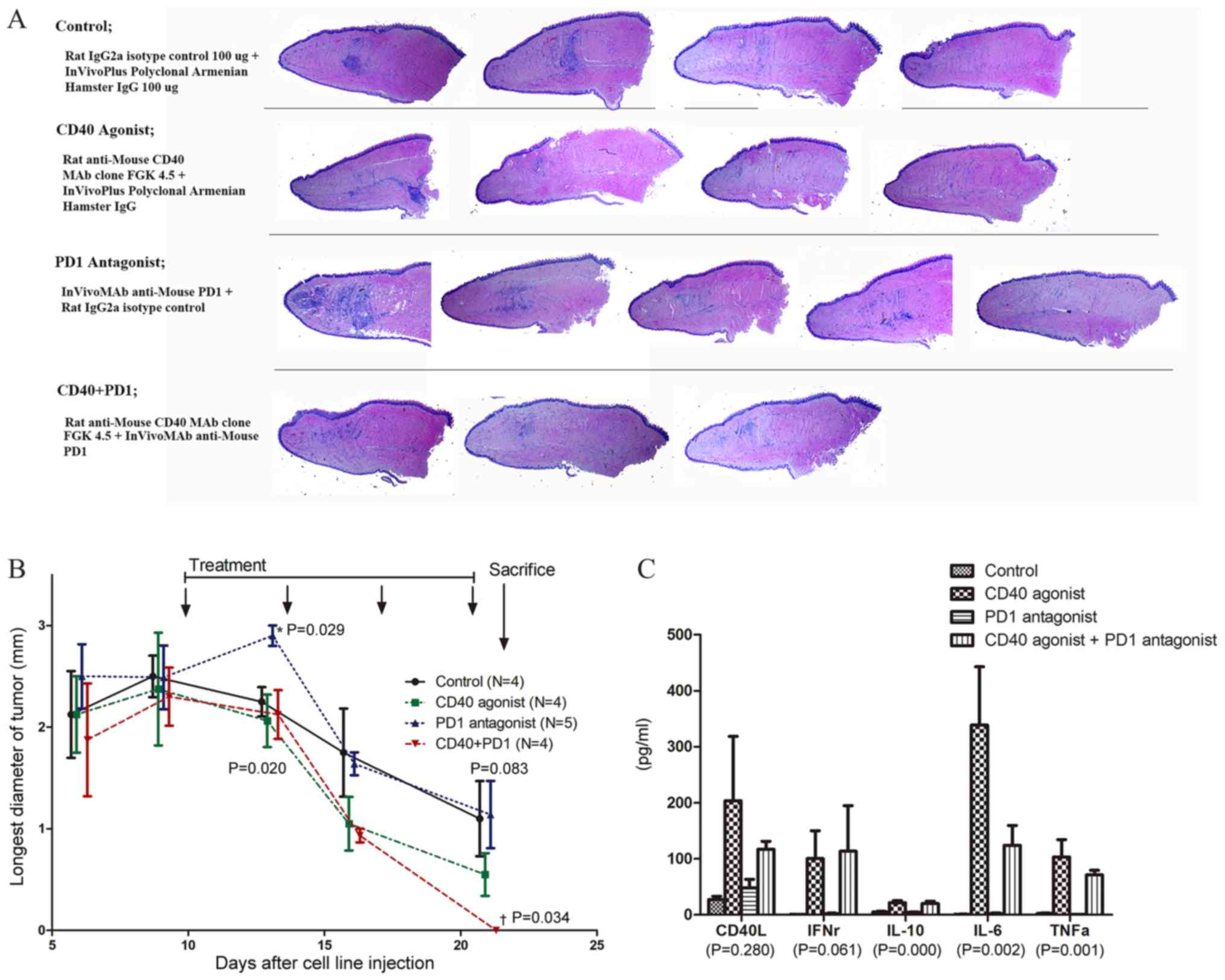
When the mice were sacrificed at 22 days after
SNU1041 cell line injection, viable tumors were observed in only
one out the four control mice. However, gross examination revealed
tongue nodules in all groups (4/4 in the control group, 3/4 in the
agonistic CD40 group, and 4/5 in the antagonistic PD1 group) except
for the combined agonistic CD40 and antagonistic PD1 antibody
treatment group (0/3 mice). The agonistic CD40 single-treatment
group showed the clearest tongue with minimal inflammatory response
in pathologic examination. The antagonistic PD-1 single-treatment
group showed the most prominent inflammatory cell infiltration
(Fig. 4A). The tongue nodules
decreased faster in the treatment group that included agonistic
CD40 (single or in combination) treatment as compared to the other
groups. The combined treatment group also showed a significant
difference in the maximum diameter of the tongue nodules (Fig. 4B).
Evaluation of the changes in CD8, CD40, and PD1 cell
proportions revealed a significant difference only for CD8 in the
spleen. The proportion of CD8 was significantly lower in groups
administered agonistic CD40 treatments (single or in combination).
The lymph node showed the most significant change with treatment.
CD40 is mainly expressed by B cells and CD8 were the only T cells
in this animal model because CD4 was depleted. Therefore, the
proportions of CD8 and CD40 showed reciprocal changes. The blood
level of CD40 was significantly decreased by the administration of
the agonistic CD40 antibody and PD-1 by the antagonistic PD-1
antibody. CD8 cell levels were increased following antagonistic
PD-1 treatment and decreased with combined treatment, similar to
the observations in the lymph node (Fig. S3). Cytokine analysis showed
significant increases in CD40L, IFNγ, IL-10, IL-6, and TNFα levels
in single or combination treatments of an agonistic CD40 antibody
(Fig. 4C).
Discussion
Based on flow cytometry analysis, CD4 and CD40L
expression were co-incident and CD4 depletion resulted in CD40L
depletion. CD40L is expressed by activated CD4 helper cells and
activates B cells through CD40 expressed by B cells. Therefore, the
results of previous studies using human tumor-free lymph nodes,
which showed CD40L to be an independent significant prognostic
factor for disease-free survival (3), could be interpreted as evidence of
decreased CD4 T cell activity. We hypothesize that the recurrence
of macroscopic tumors after surgical resection depends on whether
the microscopic remnant cancer foci can be eliminated by the host
immune system. The purpose of this experiment was to understand the
immune changes during the process of cancer cell elimination.
We injected SNU1041 cells, which were developed from
human squamous cell carcinoma, into the lateral tongue of
immunocompetent Balb/C mice. After one week, the entire tongue was
infiltrated with inflammatory cells, without viable cancer cells,
and had returned to normal at four weeks in the control group.
CD8-depleted mice showed similar finding to those of the controls
except that the inflammation at one week was not as severe as that
in the control and there were some palpable nodules on the tongue.
However, CD4-depleted mice showed viable cancer cells on the tongue
and the inflammatory reaction was very limited at one week.
Moreover, although the size was decreased, viable tumors were
observed in one-third of the mice after four weeks. Both CD4 and
CD8 depletion resulted in a similar situation in athymic nude mice
and tumor progression over time.
The patterns of changes in the immune cells were
nearly identical between the control and CD8-depleted mice. In the
lymph node, the B cell population was increased at one week and had
returned to basal levels at four weeks. In the blood, the
macrophage/monocyte proportions were decreased at one and four
weeks. Compared to the change in the immune cell ratio in the
control or CD8-depleted groups, the change was prolonged in the
CD4-depleted group. The B cell ratio in the lymph node remained
high until four weeks in CD4-depleted group and increased in both
the CD4 and CD8-depleted groups until four weeks (Fig. 3B and C). We hypothesized that the
immune system may become ineffective and that there is a continuous
effort to eliminate tumor cells through B cell activity. Cytokine
analysis showed significant elevation of CD40L, IFNγ, IL-6, IL-10,
and TNFα at various time points in the CD8-depleted group. These
changes may be required to overcome the defect of CD8 T cells.
These results confirm that CD4 activity, as represented by CD40L,
plays a more important role in the effective elimination of
microscopic cancer cells compared to that in CD8 cells.
The second part of the experiment evaluated the
efficacy of PD-1 antagonist and CD40 agonist monoclonal antibodies
for the elimination of cancer cells under CD4-depleted conditions.
An immune checkpoint blocker like PD-1 is currently undergoing
clinical trial at various concentrations, while nivolumab and
pembrolizumab are representative drugs currently in use (12). In contrast, dacetuzumab, a CD40
agonist antibody, has been developed and released, although several
clinical trials in lymphoma, melanoma, and leukemia failed to
produce promising data (13).
However, the selection of appropriate patients is important for the
success of immunotherapy; thus, and immunohistochemistry is
frequently included in the inclusion criteria. For example, PD-1 or
PD-L1 expression are determined by immunohistochemistry and a
proportion score of ≥1% is used as a selection criterion (14). In this experiment, CD4-depleted mice
also showed CD40L depletion. Therefore, we hypothesized that the
CD40 agonist antibody could cover for the CD40L defect. Positive
results were observed in the CD40 agonist treatment group under
these experimental conditions. The inflammatory reaction in the
tongue resolved earlier and the tumor size showed a more rapid
decrease than those in the control or PD-1 antagonist treatment
groups following CD40 agonist treatment. In addition, the serum
levels of cytokines elevated in the CD8-depleted group in our
previous study-CD40L, IFNγ, IL-6, IL-10, and TNFα-were also
significantly elevated after treatment with the CD40 agonist
antibody. Therefore, these cytokines play important roles in making
up for deficits in immune cells. CD4 T cells and macrophages play
the main role in senescence surveillance of pre-malignant lesions
with senescence-associated secretory phenotypes (15). However, CD8 T cells become more
important after malignant cancer is established (16). Our experimental condition mimicked
that in which microscopic cancer cells remain after treatment and
the host can eliminate the cancer cells. These results confirmed
that CD4 T cells are more important in this process and that CD8
depletion could be overcome with activated cytokines. In addition,
the CD40 agonist could facilitate the elimination of cancer cells
with induced cytokine production under CD4-depleted conditions.
Therefore, these results suggest that the host immune status could
be another consideration when selecting immunotherapeutic
agents.
The major limitation of this study was the use of an
animal model. As a human cancer cell line was injected into
immunocompetent mice, it is difficult to say that the changes in
the immune system were specific to the cancer cells. Human cells
are heterogenic and the observed process could be the elimination
of foreign cells rather than that of cancer cells developed in the
body. In addition, it is difficult to determine whether the
findings of this experiment are unique or common to other
challenges because the results observed in the viral infection or
allogeneic graft, which are commonly conducted using this animal
model, were different according to the purpose of study. Therefore,
this animal model is not an ideal system for the evaluation of
cancer treatment. However, it is difficult to find other models for
the elimination of cancer cells and we believe that this experiment
showed the role of the immune system during the elimination of
target cells which should be removed from the body.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science, and Technology
(grant no. NRF-2015R1D1A1A01060660).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHA contributed to the study conception and design,
data interpretation and manuscript drafting and revision. JYC, SDK
and SJP contributed to data acquisition and analysis. HK
contributed to data analysis and interpretation.
Ethics approval and consent to
participate
The animal studies were performed in accordance with
the protocol approved by the Institutional Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rah YC, Ahn JC, Jeon EH, Kim H, Paik JH,
Jeong WJ, Kwon SY and Ahn SH: Low expression of CD40L in tumor-free
lymph node of oral cavity cancer related with poor prognosis. Int J
Clin Oncol. 23:851–859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee CH, Lee SJ, Choi SH, Ahn SH, Son BH,
Lee JW, Yu JH, Kwon NJ, Lee WC, Yang KS, et al: Cancer panel
analysis of circulating tumor cells in patients with breast cancer.
Oncol Lett. 16:612–618. 2018.PubMed/NCBI
|
|
5
|
Ahn SH, Choi JY, Kim DW, Lee DY, Jeon EH,
Jeong WJ and Paik JH: Targeting HIF1α peri-operatively increased
post-surgery survival in a tongue cancer animal model. Ann Surg
Oncol. 22:3041–3048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basel MT, Narayanan S, Ganta C, Shreshta
TB, Marquez A, Pyle M, Hill J, Bossmann SH and Troyer DL:
Developing a xenograft human tumor model in immunocompetent mice.
Cancer Lett. 412:256–263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buller RM, Holmes KL, Hügin A,
Frederickson TN and Morse HC III: Induction of cytotoxic T-cell
responses in vivo in the absence of CD4 helper cells. Nature.
328:77–79. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh S, Sarkar M, Ghosh T, Guha I,
Bhuniya A, Biswas J, Mallick A, Bose A and Baral R: Absence of
CD4(+) T cell help generates corrupt CD8(+) effector T cells in
sarcoma-bearing Swiss mice treated with NLGP vaccine. Immunol Lett.
175:31–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rigo V, Emionite L, Daga A, Astigiano S,
Corrias MV, Quintarelli C, Locatelli F, Ferrini S and Croce M:
Combined immunotherapy with anti-PDL-1/PD-1 and anti-CD4 antibodies
cures syngeneic disseminated neuroblastoma. Sci Rep. 7:140492017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graham BS and Wetherall NT: Growth of
human cell lines in BALB/c mice. Cancer Res. 50:5943–5946.
1990.PubMed/NCBI
|
|
11
|
Ku JL, Kim WH, Lee JH, Park HS, Kim KH,
Sung MW and Park JG: Establishment and characterization of human
laryngeal squamous cell carcinoma cell lines. Laryngoscope.
109:976–982. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cavalieri S, Rivoltini L, Bergamini C,
Locati LD, Licitra L and Bossi P: Immuno-oncology in head and neck
squamous cell cancers: News from clinical trials, emerging
predictive factors and unmet needs. Cancer Treat Rev. 65:78–86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hassan SB, Sørensen JF, Olsen BN and
Pedersen AE: Anti-CD40-mediated cancer immunotherapy: An update of
recent and ongoing clinical trials. Immunopharmacol Immunotoxicol.
36:96–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SY and Wu YL: Ongoing clinical trials
of PD-1 and PD-L1 inhibitors for lung cancer in China. J Hematol
Oncol. 10:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuilman T, Michaloglou C, Vredeveld LC,
Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ and Peeper DS:
Oncogene-induced senescence relayed by an interleukin-dependent
inflammatory network. Cell. 133:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostroumov D, Fekete-Drimusz N, Saborowski
M, Kühnel F and Woller N: CD4 and CD8 T lymphocyte interplay in
controlling tumor growth. Cell Mol Life Sci. 75:689–713. 2018.
View Article : Google Scholar : PubMed/NCBI
|