Introduction
Although cancer is a disease with high mortality
rate, the current treatments for patients remain limited due to the
complexity of the different types of cancer and the high incidence
of cancer cell metastasis (1). At
present, surgical resection is the most commonly used method to
treat cancer; however, tumor recurrence and metastasis remain the
main cause of cancer-associated mortality in patients (2,3).
Perioperative care and anesthesia management are recognized as
important factors that may affect cancer recurrence, metastasis and
patient survival (4). Previous
retrospective studies reported that inhibition of immune function
caused by postoperative pain could worsen the prognosis of patients
with cancer, whereas intraspinal block, postoperative analgesia,
and the use of non-steroidal anti-inflammatory analgesics can
improve the prognosis of these patients (5,6).
Previous studies demonstrated that local anesthetics can inhibit
the proliferation of liver cancer cells (7) and further induce necrosis of prostate
cancer and ovarian cancer cells (8).
The general anesthetic propofol administered intravenously can
decrease the malignant degree of prostate cancer cells by
inhibiting N-methyl-D-aspartic acid receptors (9). Furthermore, midazolam can induce
apoptosis in a variety of different types of cancer cell (10). However, certain studies reported that
the general anesthetic isoflurane can increase the degree of
malignancy of prostate and renal cancer cells (11,12). In
addition, isoflurane can stimulate glioma stem cell viablity,
thereby increasing the risk of cancer recurrence and metastasis
(11,12). The selection and use of anesthetic
drugs could therefore be associated with cancer cell proliferation
and metastasis.
Tumors can be highly invasive. The infiltration of
cancer cells and metastatic spread towards distant tissues and
organs represent the most direct causes of cancer-associated
mortality in patients (13);
however, the underlying molecular mechanisms remain unclear.
Metastasis comprises multiple discrete steps, as follows: i) Tumor
cell invasion is initiated from the primary tumor site and is
followed by intravasation into the vasculature or lymphatic
circulation; ii) tumor cells lose their initial epithelial
phenotype in order to survive in the circulation; iii)
extravasation of individual tumor cells occurs at the target organ
site; and iv) expansion and colonization of tumor cells at the
secondary site is promoted (13).
Therefore, inhibition of the aforementioned steps may inhibit
cancer metastasis. However, studies on the effects of anesthetics
on tumor metastasis are currently rare, and most of them focus on
the direct effects and underlying mechanisms of drugs on cancer
cell proliferation and tumor growth.
Autophagy is a lysosome-based evolutionarily
conserved and dynamic intracellular catabolic process (14) that serves a crucial role in cancer
cell metastasis (13). The ability
of autophagy to control necrosis and inflammation may limit the
invasion and dissemination of tumor cells from a primary site,
therefore inhibiting metastasis at an early step. However,
autophagy may promote metastasis at later stages by protecting
stressed tumor cells traveling through the vasculature and
colonizing at distant sites (13).
Autophagy can adapt to contextual demands and serve both
prometastatic and antimetastatic roles (13). Regulating autophagy may, therefore,
prevent tumor metastasis.
Autophagy is an important physiological process that
can be triggered by the administration of anesthetics, including
local anesthetics, inhaled general anesthetics, intravenous general
anesthetics and analgesic drugs (15–17).
Autophagy is involved in the effects of anesthetics on tumors. It
has been demonstrated that Propofol induces protective autophagy
and promotes renal fibroblast survival time under hypoxic
conditions (18). Furthermore, the
induction of autophagy protects glioma H4 cells from sevoflurane
toxicity (19). In addition, it has
been reported that fentanyl can trigger autophagy via the reactive
oxygen species/MAPK signaling pathway and decrease lung cancer cell
sensitivity to cisplatin (20).
However, to the best of our knowledge, only a limited number of
studies have examined the effect of anesthetics on the metastasis
of lung cancer through regulating autophagy. The present study
demonstrated that the analgesic sufentanil induced the accumulation
of autophagosomes and impaired the autophagic degradation that may
account from the inhibition of NCI-H460 cell migration. These
findings indicated that sufentanil may be considered as a
preferable analgesic that could be used in patients with lung
cancer.
Materials and methods
Antibodies and agents
The antibody against LC3 (1:2,000; cat. no.
NB100-2220) was purchased from Novus Biologicals, LLC. The antibody
against sequestosome 1 (SQSTM1)/p62 (1:2,000; cat. no. ab56416) was
purchased from Abcam. Antibodies against β-actin (1:1,000; cat. no.
60008-1-Ig), LAMP1 (1:100; cat. no. 21997-1-AP) and Beclin1
(1:1,000; cat. no. 11306-1-AP) were purchased from ProteinTech
Group, Inc. Antibodies against cathepsin D (1:1,000; cat. no.
sc-6486) and goat anti-rabbit immunoglobulin G (IgG)-fluorescein
isothiocyanate (FITC) (1:100; cat. no. SC2-12) were purchased from
Santa Cruz Biotechnology, Inc. Horseradish peroxidase
(HRP)-conjugated anti-rabbit (1:10,000; cat. no. W4011),
HRP-conjugated anti-goat (1:10,000; cat. no. V805A) and
HRP-conjugated anti-mouse (cat. no. W4021) antibodies were
purchased from Promega Corporation. Enhanced chemiluminescence
(ECL) kit was purchased from Biological Industries.
Cell culture
The present study used NCI-H460 as a human large
cell lung carcinoma, cell line, 293 cells known as the Human
Embryonic Kidney 293 cells and HepG2 as a liver cancer cell line.
Cells were obtained from American Type Cell Culture (ATCC,
Manassas, VA) and kindly provided by Professor Longping Wen from
University of Science and Technology of China. NCI-H460 cells were
cultured in RPMI 1640 (SH30809.01, Hyclone) medium, and 293 and
HepG2 cells were cultured in or Dulbecco's Modified Eagle medium
(Hyclone; GE Healthcare Life Sciences) supplemented with 10% fetal
bovine serum (FBS; Biological Industries) at 37°C in the presence
of 5% CO2. Cells were treated with 50 µM chloroquine
(CQ; Sigma-Aldrich; Merck KGaA) or 100 mM trehalose (Tre; autophagy
inducer; Sigma-Aldrich; Merck KGaA) for 24 h (21). Sufentanil was used at the
concentration of 1 nM and incubated with cells for 24 h (22). Sufentanil was purchased from Yichang
Hmanwell Pharmaceutical Co., Ltd.
Immunofluorescence
NCI-H460 cells were fixed with 4% paraformaldehyde
for 10 min, permeabilized with 0.1% Triton X-100 for 10 min and
blocked with 1% FBS for 1 h at room temperature. For LC3 puncta and
lysosomes staining, cells were incubated with antibodies against
LC3 or LAMP1 antibody overnight at 4°C, and with anti-rabbit or
anti-mouse IgG-FITC antibody, respectively, at 37°C for 1 h. Images
were acquired using an Olympus IX71 fluorescence microscope at ×400
magnification (Olympus Corporation). Data were analyzed using Image
J software (version 1.35; National Institutes of Health).
Western blotting
After treatment with drugs and reagents, NCI-H460,
293 and HepG2 cells were lysed with sample lysis buffer (Beyotime
Institute of Biotechnology) at room temperature, and then boiled
for 10 min. Protein concentration was determined using the BCA
protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Proteins (20–40 µg
per lane) were separated via SDS-PAGE (15% gel) and transferred
onto nitrocellulose membranes. The membranes were blocked with 5%
non-fat powdered milk for 1 h at 37°C and incubated with the
relevant primary antibodies at 4°C overnight. The primary
antibodies incubated membranes were washed 5 times with TBST
(Beijing Solarbio Science & Technology Co., Ltd.) and then
incubated with relevant secondary antibodies for 1 h at 37°C.
Eventually, bands were detected using enhanced chemiluminescence
and visualized using a chemiluminescence imaging instrument (GE
Image Quant LAS 4000; GE Healthcare Life Sciences).
Wound healing assay
NCI-H460, 293 or HepG2 cells (1×106
cells/3 ml) were seeded and cultured in FBS-supplemented medium in
a 60 mm dish until they formed a confluent monolayer. The monolayer
was wounded with a manual scratch using a 1 ml pipette tip. Cells
were washed twice with PBS and were incubated with serum-free
medium with or without sufentanil (1 nM) and CQ (50 µM) for 24 h.
Images were subsequently captured using an Olympus IX71 microscope
at ×100 magnification. The wound healing closure was quantified
using ImageJ 1.35 software (National Institutes of Health). The
percentage of wound closure was calculated as follows: [(initial
wound area-remaining wound area)/initial wound area].
Cell invasion assay
Cell invasion was analyzed using Transwell chambers
with 8 µm pore size (Corning Inc.). Prior to the invasion assay,
Transwell were precoated with Matrigel (BD Biosciences) for 1 h at
37°C. NCI-H460, 293 or HepG2 cells (5×105 cells/200 µl)
were seeded into the top chamber and the bottom chamber was filled
with 600 µl DMEM containing 10% FBS. Cells that migrated onto the
bottom surface of the membrane were fixed in 100% methanol for 30
min and stained with 0.5% crystal violet for 20 min at room
temperature after treatment with the specified drugs for 24 h.
Images were subsequently captured using an Olympus IX71
fluorescence microscope at ×100 magnification. Four randomly
selected fields were counted for each experimental group per cell
line.
Cell viability assay
MTT was used to assess the cell viability. Briefly,
NCI-H460, 293 or HepG2 cells were grown in 96-well plates at a
density of ~10,000 cells per well. After the different treatments,
MTT (thiazoyl blue tetrazolium bromide; T0793-500MG, Bio Basic) was
added to the growing cultures at a final concentration of 0.5
mg/ml, incubated for 4 h at 37°C and dissolved in 100 µl
dimethyl sulfoxide (D8370; Solarbio). The absorbance was measured
at 570 nm with a spectrophotometer (Elx800, BioTek).
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Differences among groups were analyzed by one- or
two-way ANOVA followed by Tukey's Post-hoc test or the two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Pearson's co-localization
coefficient of cells was calculated using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc.).
Results
Sufentanil induces autophagosome
accumulation
The present study determined the induction of
autophagy in sufentanil-treated NCI-H460 cells. During the
autophagy process, the protein LC3 is cleaved from LC3-I into the
lower molecular weight LC3-II and aggregates on autophagosome
membranes (23). Beclin1 plays a
central role in autophagy and is considered a marker protein for
autophagy (24). At 1 nM, sufentanil
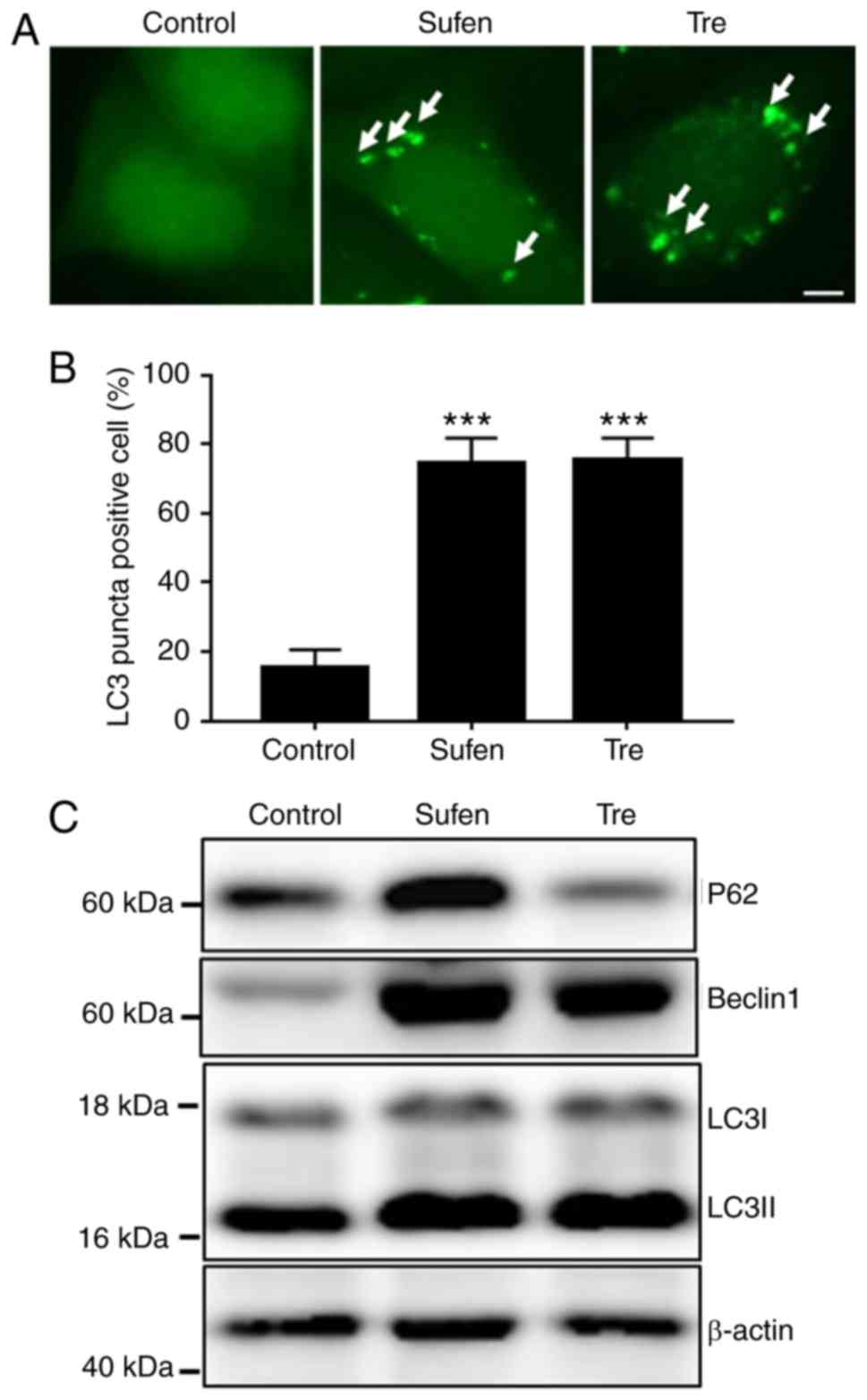
exhibited a mild cytotoxicity in NCI-H460 cells (Fig. S1). The results from
immunofluorescence staining demonstrated that, similarly to
trehalose-treated cells, 1 nM sufentanil induced the generation of
a large number of LC3 puncta (Fig. 1A
and B), which indicated the accumulation of autophagosomes. In
addition, the levels of LC3-II, Beclin1 and p62 in
sufentanil-treated NCI-H460 cells were increased (Fig. 1C), which further confirmed the
accumulation of autophagosomes.
Sufentanil blocks the fusion of
autophagosomes and lysosomes
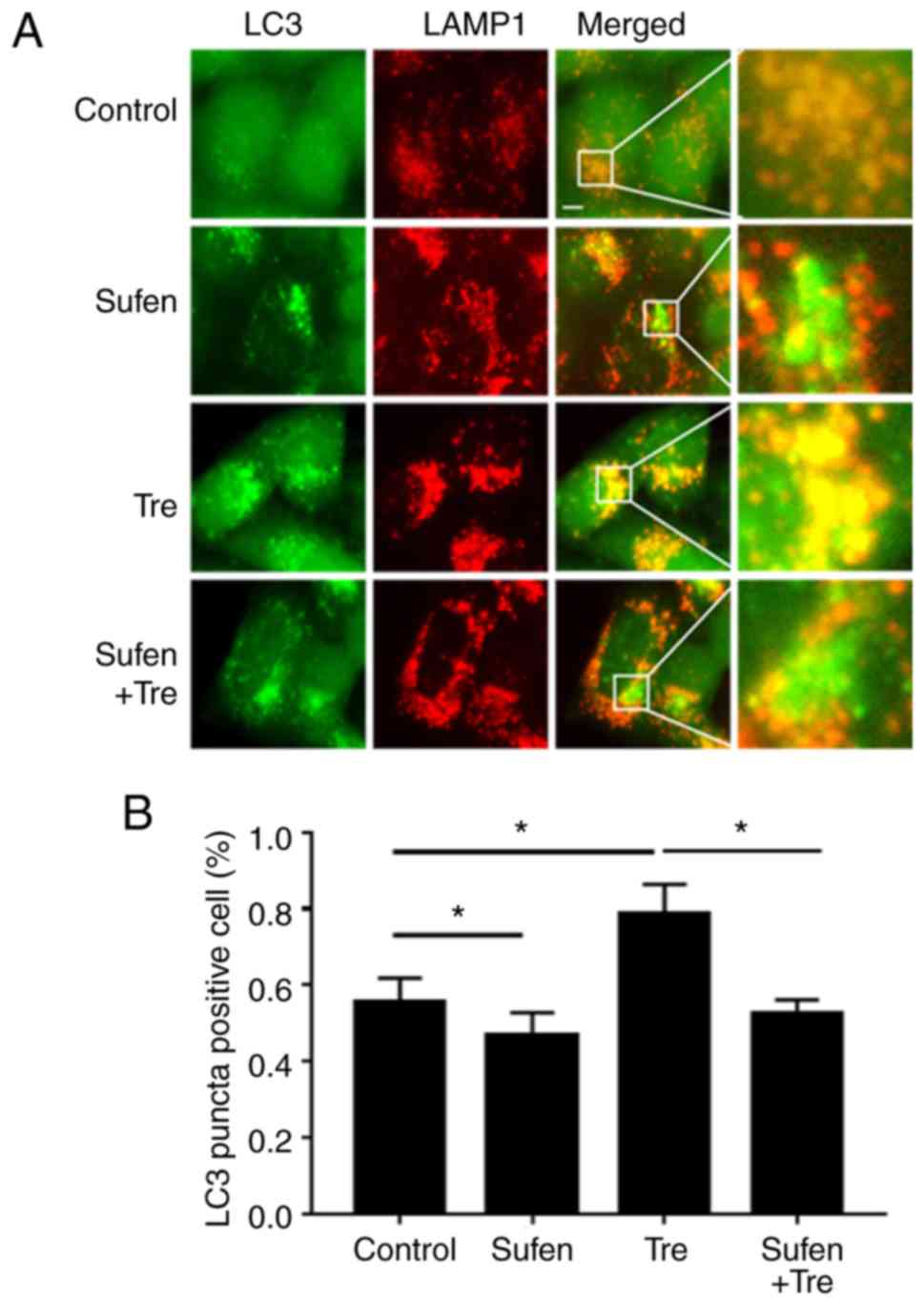
It has been reported that autophagosomes can fuse
with numerous lysosomes to form autolysosomes (23), and that the autophagic content found
in the autolysosomes is degraded (23). LAMP1 is a lysosomal membrane protein
frequently used as a lysosomal marker (25). Autolysosomes can therefore be
identified by assessing the co-localization of LC3 and LAMP1 by
fluorescence microscopy. Trehalose, which is a commonly used
autophagy inducer, was used in the present study as a positive
control. The results demonstrated that few LAMP1 and LC3 were
co-localized in NCI-H460 cells following treatment with 1 nM
sufentanil. However, the majority of LAMP1 signals were overlapped
with LC3 in trehalose-treated cells (Fig. 2A). Statistical analysis of the
co-localization rate between LAMP and LC3 was consistent with the
results from fluorescence microscopy (P=0.04, sufen vs. control;
P=0.03, tre vs. control; P=0.03, tre vs. tre+sufen; Fig. 2B).
Sufentanil gradually impairs
autophagic degradation
Autophagy is a lysosomal-based intracellular
degradation process (14). In the
present study, the ability of autophagosomes to induce degradation
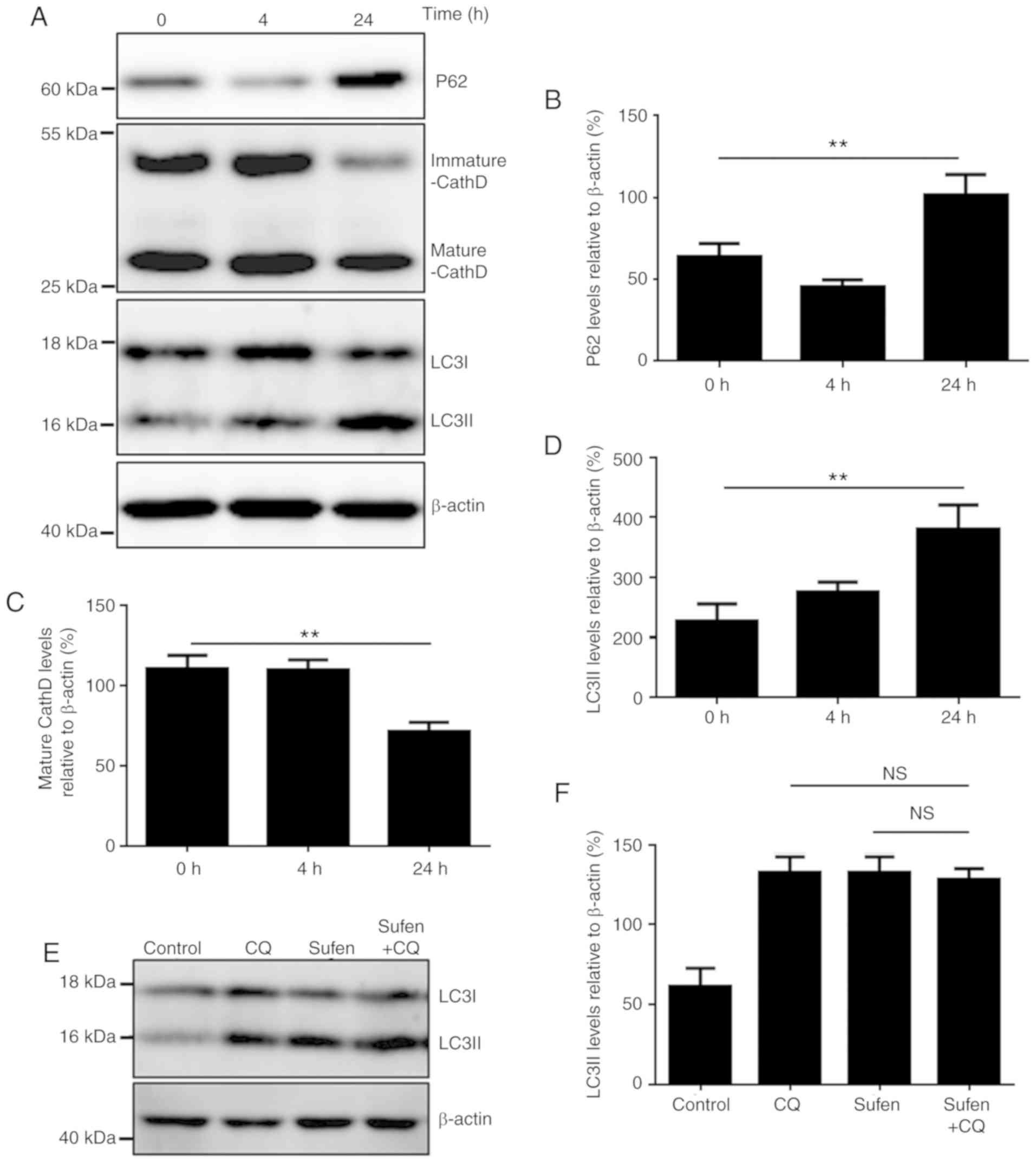
was detected in sufentanil-treated NCI-H460 cells. SQSTM1/p62 is a
protein substrate that is selectively incorporated into the
autophagosomes and degraded during autophagy (26). The results from the present study
demonstrated that the p62 level was slightly decreased after 4 h
treatment, which indicated that sufentanil may induce a complete
autophagy process at early stage of sufentanil treatment. Over
time, sufentanil gradually inhibited autophagy, and after 24 h, p62
level was significantly increased (P<0.01), which suggested that
autophagy may have been blocked (Fig. 3A
and B) (27,28). Furthermore, the protein level of the
autophagic marker LC3-II was significantly increased over time
(Fig. 3A and C). The protein level
of the lysosomal protease cathepsin D, which reflects the lysosomal
function, was also determined (29).
The results demonstrated that the level of the mature form of
cathepsin D after 24 h treatment with sufentanil was significantly
decreased (P<0.01), which suggested that sufentanil may have
disturbed autophagic degradation in NCI-H460 cells (Fig. 3A and D). In addition, p62 and LC3-II
levels in 293 and HepG2 cells were increased following 24 h
treatment with sufentanil (Fig.
S2), suggesting that autophagy may have been impaired.
The former malaria drug CQ is now widely used as an
inhibitor of autophagy in cell culture and in vivo assays
(23,28). CQ is a lysosomotropic weak base,
which diffuses into the lysosome in its monoprotonated form. This
compound is then entrapped in the lysosome and becomes
diprotonated. Protonated CQ can alter the lysosomal pH, thereby
inhibiting the autophagic degradation in the lysosome (28). Similar to CQ treatment, treatment
with 1 nM sufentanil increased the levels of LC3-II, Beclin1 and
p62, and decreased the level of mature cathepsin D in NCI-H460, 293
and HepG2 cells (Figs. 3E, S3 and S4).
No significant difference in LC3-II level was observed between
sufentanil- and CQ-treated cells. Furthermore, additional treatment
with CQ did not further increase LC3-II level in sufentanil-treated
cells (Fig. 3E and F), which
suggested that sufentanil may disrupt autophagic degradation.
Impaired autophagic degradation is
involved in sufentanil- inhibited cell metastasis in vitro
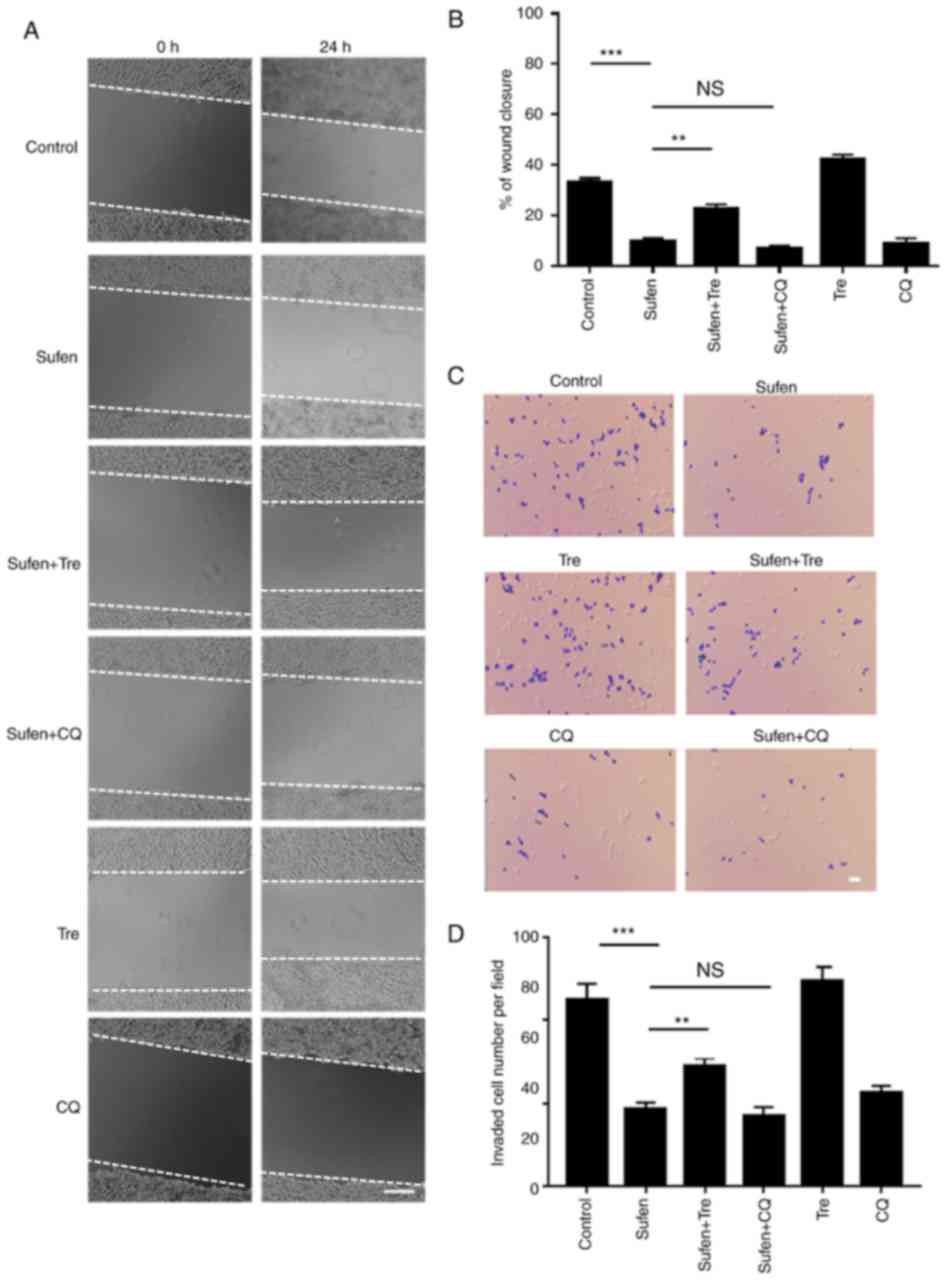
A wound healing assay was used to investigate the
effect of sufentanil on the migratory capability of NCI-H460 cells.
Following 24 h of treatment with 1 nM sufentanil, cell migration
was significantly decreased compared with the control group
(Fig. 4A and B). Furthermore,
additional treatment with CQ did not further decrease the migration
of sufentanil-treated cells. However, the increase in the level of
autophagy (Fig. 1C) following
trehalose treatment significantly increased the wound closure
compared with sufentanil-treated cells. In addition, a lower number
of invasive cells was observed following 1 nM sufentanil treatment
compared with the control group (Fig. 4C
and D). Additional treatment with CQ did not further decrease
the invasive capability of sufentanil-treated cells (Fig. 4C and D). Cell treatment with
trehalose significantly increased the invasive capacity of NCI-H460
cells compared with sufentanil-treated cells (Fig. 4C and D). These results demonstrated
that impaired autophagic degradation may be involved in the
inhibited migration of NCI-H460 cell induced by sufentanil. Similar
results on the migratory and invasive capacities of 293 and HepG2
cell lines following treatment with the aforementioned drugs were
observed (Fig. S5).
Discussion
The results from the present study demonstrated that
cell treatment with sufentanil could inhibit the
autophagosome-lysosome fusion and the disruption of the autophagic
degradation. These findings may explain the inhibition of NCI-H460
cell migratory capacity, and indicated that sufentanil may be
considered as a potential analgesic compound for the treatment of
patients with lung cancer.
Opioids are the most commonly used type of analgesic
for perioperative analgesia (30);
however, whether opioids may favor the prevention of metastasis and
recurrence following cancer surgery remains unclear (30). For example, morphine has been
reported to promote the invasive and migratory capacities of breast
and lung cancer cells via the upregulation of matrix
metalloproteinases (MMPs) (31).
However, a previous study demonstrated that morphine can
significantly decrease the adhesion, invasion and metastasis
capabilities of colon cancer cells via the downregulation of MMPs
(31). The present study
demonstrated that sufentanil inhibited the migration of NCI-H460
cells, which was consistent with previous studies (31,32).
However, additional in-depth and extensive analyses are required in
order to determine the impact of opioids on tumor metastasis.
Numerous retrospective studies reported a lower
incidence of cancer recurrence following post-surgery regional
anesthesia with low doses of opioids in patients with breast
cancer, prostate cancer, colon cancer and melanoma (33,34). It
has been demonstrated that pain can activate the stress response
and suppress the immune system in both animals and humans (35,36),
suggesting that pain could promote tumor recurrence and metastasis.
Analgesics may therefore have the potential to alter tumor
recurrence and metastasis via pain reduction (35). Furthermore, it has been demonstrated
that opioids have extensive immunomodulatory activities, both in
the cellular and humoral immune responses, and are able to modulate
inflammatory cytokine production (37,38),
which suggests that tumor metastasis may be inhibited by opioid
analgesics. The present study aimed to evaluate the effect and
molecular mechanism underlying anesthetics and analgesics on tumor
cells in a simple and economical way, in order to provide some
recommendations for the choice of analgesics in clinical
surgery.
The results from the present study demonstrated that
autophagy may be involved in the inhibited migration of NCI-H460
cell induced by sufentanil. The ability of autophagy to restrict
necrosis and inflammation may limit the invasion and dissemination
of tumor cells from the primary site, inhibiting therefore
metastasis at an early stage (13).
However, autophagy could promote metastasis at later stages by
protecting stressed tumor cells as they travel through the
vasculature and colonize at distant sites (13). Further investigations are required in
order to determine how the impairment of autophagy and cell
migration are associated in sufentanil-treated cells. The present
study demonstrated that inhibition of autophagy was involved in
sufentanil-mediated inhibition of tumor cell migration. Sufentanil
may, therefore, have a beneficial anti-tumor effect in the late
stages of lung cancer, and may be considered as an optimal choice
for surgical analgesia in patients with advanced lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Longping
Wen (University of Science and Technology of China, Hefei, China)
for providing all the cells used in the present study.
Funding
The present study was supported by grants from the
Key Projects of Natural Science Research of the Anhui Colleges and
Universities (grant no. KJ2017A193).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HJ, CW and HW designed the present study, and
analyzed and interpreted the data, HJ and HW wrote the manuscript.
WZ, YH and CC performed the experiments study. CW critically
revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park CG, Hartl CA, Schmid D, Carmona EM,
Kim HJ and Goldberg MS: Extended release of perioperative
immunotherapy prevents tumor recurrence and eliminates metastases.
Sci Transl Med. 10(pii): eaar19162018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moody SE, Perez D, Pan TC, Sarkisian CJ,
Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD and
Chodosh LA: The transcriptional repressor Snail promotes mammary
tumor recurrence. Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sessler DI: Long-term consequences of
anesthetic management. Anesthesiology. 111:1–4. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesthesia. 105:106–115. 2010. View Article : Google Scholar
|
|
6
|
Boland JW and Pockley AG: Influence of
opioids on immune function in patients with cancer pain: From bench
to bedside. Br J Pharmacol. 175:2726–2736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Gac G, Angenard G, Clement B, Laviolle
B, Coulouarn C and Beloeil H: Local anesthetics inhibit the growth
of human hepatocellular carcinoma cells. Anesth Anal.
125:1600–1609. 2017. View Article : Google Scholar
|
|
8
|
Xuan W, Zhao H, Hankin J, Chen L, Yao S
and Ma D: Local anesthetic bupivacaine induced ovarian and prostate
cancer apoptotic cell death and underlying mechanisms in vitro. Sci
Rep. 6:262772016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Wu Q, You L, Chen S, Zhu M and
Miao C: Propofol attenuates pancreatic cancer malignant potential
via inhibition of NMDA receptor. Eur J Pharmacol. 795:150–159.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mishra SK, Kang JH, Lee CW, Oh SH, Ryu JS,
Bae YS and Kim HM: Midazolam induces cellular apoptosis in human
cancer cells and inhibits tumor growth in xenograft mice. Mol
Cells. 36:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang H, Benzonana LL, Zhao H, Watts HR,
Perry NJ, Bevan C, Brown R and Ma D: Prostate cancer cell
malignancy via modulation of HIF-1α pathway with isoflurane and
propofol alone and in combination. Br J Cancer. 111:1338–1349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benzonana LL, Perry NJ, Watts HR, Yang B,
Perry IA, Coombes C, Takata M and Ma D: Isoflurane, a commonly used
volatile anesthetic, enhances renal cancer growth and malignant
potential via the hypoxia-inducible factor cellular signaling
pathway in vitro. Anesthesiology. 119:593–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kenific CM, Thorburn A and Debnath J:
Autophagy and metastasis: Another double-edged sword. Curr Opin
Cell Biol. 22:241–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie Z and Klionsky DJ: Autophagosome
formation: Core machinery and adaptations. Nat Cell Biol.
9:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li R, Ma H, Zhang X, Li C, Xiong J, Lu T,
Mao Y, Dai J, Liu L and Ding Z: Impaired autophagosome clearance
contributes to local anesthetic bupivacaine-induced myotoxicity in
mouse myoblasts. Anesthesiology. 122:595–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Zhou Y, Xu M and Chen G:
Autophagy is involved in the sevoflurane anesthesia-induced
cognitive dysfunction of aged rats. PLoS One. 11:e01535052016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su LY, Luo R, Liu Q, Su JR, Yang LX, Ding
YQ, Xu L and Yao YG: Atg5- and Atg7-dependent autophagy in
dopaminergic neurons regulates cellular and behavioral responses to
morphine. Autophagy. 13:1496–1511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Li LY, Jiang JL, Li K, Su ZB,
Zhang FQ, Zhang WJ and Zhao GQ: Propofol elicits autophagy via
endoplasmic reticulum stress and calcium exchange in C2C12 myoblast
cell line. PLoS One. 13:e01979342018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou YF, Wang QX, Zhou HY and Chen G:
Autophagy activation prevents sevoflurane-induced neurotoxicity in
H4 human neuroglioma cells. Acta Pharmacol Sin. 37:580–588. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao J, Ma C, Gao W, Liang J, Liu C, Yang
H, Yan Q and Wen Q: Fentanyl induces autophagy via activation of
the ROS/MAPK pathway and reduces the sensitivity of cisplatin in
lung cancer cells. Oncol Rep. 36:3363–3370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei PF, Jin PP, Barui AK, Hu Y, Zhang L,
Zhang JQ, Shi SS, Zhang HR, Lin J, Zhou W, et al: Differential ERK
activation during autophagy induced by europium hydroxide nanorods
and trehalose: Maximum clearance of huntingtin aggregates through
combined treatment. Biomaterials. 73:160–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bundscherer A, Malsy M, Gebhardt K,
Metterlein T, Plank C, Wiese CH, Gruber M and Graf BM: Effects of
ropivacaine, bupivacaine and sufentanil in colon and pancreatic
cancer cells in vitro. Pharmacol Res. 95-96:126–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni HM, Bockus A, Wozniak AL, Jones K,
Weinman S, Yin XM and Ding WX: Dissecting the dynamic turnover of
GFP-LC3 in the autolysosome. Autophagy. 7:188–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Margariti A, Li H, Chen T, Martin D,
Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang
Z, et al: XBP1 mRNA splicing triggers an autophagic response in
endothelial cells through BECLIN-1 transcriptional activation. J
Biol Chem. 288:859–872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu L, McPhee CK, Zheng L, Mardones GA,
Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, et al: Termination of
autophagy and reformation of lysosomes regulated by mTOR. Nature.
465:942–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bjorkoy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bjorkoy G, Lamark T, Pankiv S, Overvatn A,
Brech A and Johansen T: Monitoring autophagic degradation of
p62/SQSTM1. Methods Enzymol. 452:181–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pellegrini P, Strambi A, Zipoli C,
Hägg-Olofsson M, Buoncervello M, Linder S and De Milito A: Acidic
extracellular pH neutralizes the autophagy-inhibiting activity of
chloroquine: Implications for cancer therapies. Autophagy.
10:562–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Dai W, Geng P, Zhang L, Tan Q,
Cheng D, Wei P, Yang Z, Zhang L, Gu E, et al: Midazolam enhances
mutant huntingtin protein accumulation via impairment of autophagic
degradation in vitro. Cell Physiol Biochem. 48:683–691. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Connolly C and Buggy DJ: Opioids and
tumour metastasis: Does the choice of the anesthetic-analgesic
technique influence outcome after cancer surgery? Curr Opin
Anaesthesiol. 29:468–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gach K, Szemraj J, Wyrebska A and Janecka
A: The influence of opioids on matrix metalloproteinase-2 and −9
secretion and mRNA levels in MCF-7 breast cancer cell line. Mol
Biol Rep. 38:1231–1236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bimonte S, Barbieri A, Palma G and Arra C:
The role of morphine in animal models of human cancer: Does
morphine promote or inhibit the tumor growth? Biomed Res Int.
2013:2581412013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Biki B, Mascha E, Moriarty DC, Fitzpatrick
JM, Sessler DI and Buggy DJ: Anesthetic technique for radical
prostatectomy surgery affects cancer recurrence: A retrospective
analysis. Anesthesiology. 109:180–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Christopherson R, James KE, Tableman M,
Marshall P and Johnson FE: Long-term survival after colon cancer
surgery: A variation associated with choice of anesthesia. Anesth
Analg. 107:325–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tedore T: Regional anaesthesia and
analgesia: Relationship to cancer recurrence and survival. Br J
Anaesth. 115 (Suppl 2):ii34–ii45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Page GG, Blakely WP and Ben-Eliyahu S:
Evidence that postoperative pain is a mediator of the
tumor-promoting effects of surgery in rats. Pain. 90:191–199. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saurer TB, Ijames SG, Carrigan KA and
Lysle DT: Neuroimmune mechanisms of opioid-mediated conditioned
immunomodulation. Brain Behav Immun. 22:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gong L, Qin Q, Zhou L, Ouyang W and Li Y,
Wu Y and Li Y: Effects of fentanyl anesthesia and sufentanil
anesthesia on regulatory T cells frequencies. Int J Clin Exp
Pathol. 7:7708–7716. 2014.PubMed/NCBI
|