Introduction
Human papillomavirus (HPV) infection is the primary
cause of the majority of cases of cervical cancer (CC) worldwide,
and CC has become one of the leading causes of cancer-associated
death among females in 2018 (1). The
most common type of CC is cervical squamous cell carcinoma (CSCC)
(1). The prognosis of cervical
cancer remains poor as it has a high rate of metastasis and
recurrence (2). Cervical
intraepithelial neoplasia (CIN) are types of precancerous lesions
closely associated with cervical carcinoma, including CIN I–III
which reflect the continuous development of cervical cancer
(3). Therefore, it is crucial to
identify novel prognostic markers for auxiliary assessment of CIN
grading and prognosis of patients with CSCC.
Zinc finger of the cerebellum 1 (ZIC1) was first
identified in cerebellum tissues in 1994 (4). A previous study has shown that ZIC1
serves an important role in the growth and development of the brain
nervous system, muscle cell production and bone development
(5). In addition, methylation of
ZIC1 is associated with the development and progression of cervical
cancer (6). Ectopic ZIC1 expression
was found to suppress the growth of colorectal cancer cells by
reducing phosphorylation of Akt and Erk (7). Furthermore, ZIC1 downregulation has
been associated with metastasis and a poor prognosis in patients
with breast carcinoma or gastric cancer (8,9).
However, a previous study reported that ZIC1 was upregulated in
endometrial cancer in Hong Kong Chinese women (10). Our previous study also revealed that
ZIC1 was upregulated in endometrial cancer compared with
corresponding normal tissue, and was positively correlated with
endometrial carcinogenesis (11).
Although ZIC1 exhibits different functions in neoplasms compared
with normal physiological tissue, ZIC1 is thought to be essential
for carcinogenesis, and may thus serve as a novel target for
treating patients with cervical cancer (10,11).
Based on the reported effects of ZIC1 on cervical
carcinoma, the aim of the present study was to determine the mRNA
expression levels of ZIC1 in normal cervical tissue, CIN samples
and CSCC tissues by using reverse transcription-quantitative PCR
(RT-qPCR), and to compare ZIC1 expression between the three tissue
types. In addition, the protein expression levels of ZIC1 in 80
cases of CSCC with radical hysterectomy were determined using
immunohistochemistry (IHC) to examine the clinicopathological and
prognostic value of ZIC1 in patients with CSCC.
Materials and methods
Tissue samples
The present study was approved by The Ethics
Committee of Kunshan First People's Hospital (Jiangsu, China), and
written informed consent was obtained from each patient for the use
of their tissues for research and publication. A total of 569
fresh-frozen biopsy tissues comprised of normal cervical tissues
(n=400), CIN I (n=50), CIN II (n=36), CIN III (n=38) and CSCC
(n=45) were obtained from Kunshan First People's Hospital and the
mRNA was extracted. These patients were recruited between January
2010 and December 2018, and the mean age of the patients was
53.26±11.08 years. Additionally, 80 patients (the mean age was
57.39±8.64 years) with CSCC at International Federation of
Gynecology and Obstetrics (FIGO) stage IA, IB or IIA underwent
radical hysterectomy at Kunshan First People's Hospital between
January 2009 and January 2013 (12).
The patients included in the study had not received chemotherapy or
radiotherapy prior to surgery. The tumor specimens (mean size,
2.73±1.06 cm) and the corresponding adjacent noncancerous tissues
were fixed in 10% formalin at 20°C for 8 h and then embedded in
paraffin blocks. Complete clinical and pathological data were
available for all the patients. All patients received carboplatin +
paclitaxel treatment regimen following surgery. The mean age of the
patients was 53.10±12.76 years, and the follow-up duration ranged
from 3–60 months, with a mean follow-up duration of 46.313±2.317
months. In addition, the pathological tissues of 320 patients with
CIN III (the mean age was 47.21±9.07 years and recruited between
January 2010 and December 2018) who underwent a loop
electrosurgical excision procedure (Leep) at Kunshan First People's
Hospital were also stored in paraffin blocks for IHC analysis. The
569 cohort of patients, the 80 patients with CSCC and the 320
patients with CIN III were all proven to exhibit high-risk HPV
infection using the Digene Hybrid Capture (r) 2 (HC2) High-Risk HPV
DNA Test (Qiagen GmbH), as previously described (13).
RT-qPCR
After pathological confirmation, total RNA was
extracted using TRIzol® reagent (Thermo Fisher
Scientific, Inc.), and 2 µg RNA was reverse transcribed using the
miScript II RT kit (Thermo Fisher Scientific, Inc.) at 50°C for 15
min then 85°C for 2 min. qPCR was performed using an iQ5 real-time
PCR detection system (Bio-Rad Laboratories, Inc.) using SYBR Premix
Ex Taq™ kit (Takara Bio, Inc.). The sequences of the primers were
as follows: ZIC1 forward, 5′-GCGTCCTTTTGTGGATCTTTAA-3′ and reverse,
5′-AGTAATCACATCTGCTTCTGGG-3′; and GAPDH forward,
5′-GAAGGTGAAGGTCGGAGT-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
The PCR thermocycling conditions consisted of 94°C for 4 min;
followed by 40 cycles of 95°C for 1 min, 60°C for 1 min and 72°C
for 1 min. The relative mRNA expression levels were calculated
using the 2−ΔΔCq method and the formula of ΔΔCq was
(CqTumor ZIC1-CqTumor GAPDH)-(CqNormal
ZIC1-CqNormal GAPDH) (14).
Hematoxylin and eosin staining
After deparaffinization and rehydration, 5 µm
longitudinal sections were stained with hematoxylin solution at
20°C for 5 min followed by 5 dips in 1% acid ethanol and then
rinsed in distilled water. Sections were stained with eosin
solution at 20°C for 3 min, followed by dehydration with graded
alcohol and clearing in xylene. The mounted slides were then
examined and photographed using an inverted light microscope
(magnification ×40, 100 and 400; Nikon Corporation).
IHC and evaluation of
immunohistochemical staining
Immunostaining for ZIC1 was performed using a SP
rabbit and mouse horseradish peroxidase (HRP) kit (CoWin
Biosciences) on tissue sections cut from formalin-fixed
paraffin-embedded CSCC tumor lesions or CIN III samples. The ZIC1
rabbit polyclonal antibody (1:200; cat. no. bs-11609R; BIOSS) was
used as the primary antibody. In addition, PBS without primary
antibodies was used as a negative control. The samples were
incubated with the primary antibody at 4°C overnight and
subsequently, the biotinylated HRP secondary antibody (1:1,000;
incubated at 20°C for 15 min) and streptavidin-HRP conjugates
(CoWin Biosciences) were used to detect ZIC1 expression. An
immunoreactivity score (IRS) was calculated using a
semi-quantitative assessment system for each case by two
pathologists. The semi-quantitative assessment system was obtained
by combining a score for staining intensity with a score for the
staining percentage. The staining intensity score was defined as:
0, no staining; 1, mild staining; 2, moderate staining; and 3,
strong staining. The staining percentage score was defined as: 0,
0%; 1, 1–10%; 2, 11–20%; 3, 21–30%; 4, 31–40%; 5, 41–50%; 6,
51–60%; 7, 61–70%; 8, 71–80%; 9, 81–90%; and 10, 91–100%. IRS was
calculated by multiplying the staining intensity score with the
staining percentage score. Any disagreement in the IRS score
between the two pathologists was resolved by discussion. The mean
IRS score from 80 patients with CSCC was calculated to be 5.36±3.48
and this was used as the cut-off value. Cases with an IRS score ≥5
were included in the high ZIC1 expression group, and cases with an
IRS score <5 were included in the low ZIC1 expression group.
Statistical analysis
Continuous variables were expressed as the mean ±
standard deviation and analyzed by using a one-way ANOVA with a
post-hoc Least Significant Difference test. Spearman's rank
correlation was performed to identify potential correlations in the
IRS score between CSCC and the corresponding adjacent noncancerous
tissues. A Pearson's χ2 test was used to analyze the
association between ZIC1 expression and the clinicopathological
characteristics of patients with CSCC. Cox regression analysis and
Kaplan-Meier curves combined with a Log Rank test were used to
analyze overall survival (OS) and disease-free survival (DFS). SPSS
version 20.0 (IBM, Corp.) and GraphPad version 6.0 (GraphPad
Software, Inc.) were used for statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
ZIC1 mRNA expression levels in biopsy
tissues
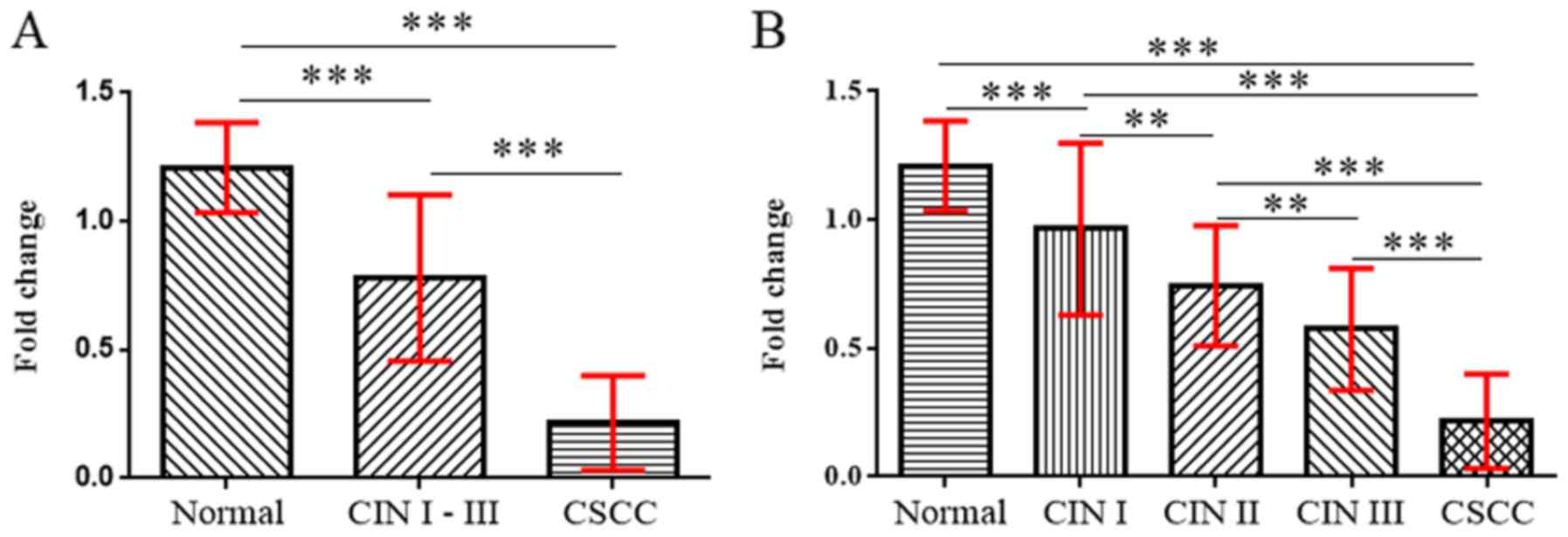
The mRNA expression levels of ZIC1 in CSCC, CIN and
normal cervical tissues were determined using RT-qPCR (Fig. 1). The level of ZIC1 mRNA expression
in CSCC was significantly lower compared with normal cervical
tissues or in CIN I–III (P<0.001; Fig. 1). ZIC1 mRNA expression levels in CIN
I–III were also significantly lower compared with normal cervical
tissues (P<0.001; Fig. 1A). In
addition, ZIC1 mRNA expression levels in CIN were associated with
CIN grade, as lower expression was observed with increasing CIN
grade (P<0.01; Fig. 1B). ZIC1
expression in any CIN grade was significantly lower compared with
normal cervical tissues (P<0.01 or P<0.001; Fig. 1A).
Expression of ZIC1 in CSCC and CIN III
samples
Hematoxylin and eosin staining of noncancerous, CIN
III and CSCC tissues are presented in Fig. 2A, C and E, respectively. ZIC1 was
expressed in the cytoplasm of cells in noncancerous tissues
(Fig. 2B), CIN III samples (Fig. 2D) and CSCC (Fig. 2F). A negative correlation was
observed between the IRS of ZIC1 expression in CSCC with the
corresponding adjacent noncancerous tissues (r=−0.279; P=0.012;
Fig. 2G). The mean ZIC1 IRS in CSCC
was 5.36±3.48, significantly lower compared with that in the
corresponding adjacent noncancerous tissues (11.31±5.68;
P<0.001) and the CIN III samples (10.42±1.54; P<0.001;
Fig. 2H). In addition, the mean IRS
of ZIC1 in noncancerous tissues was significantly higher compared
with CIN III samples (P=0.014; Fig.
2H).
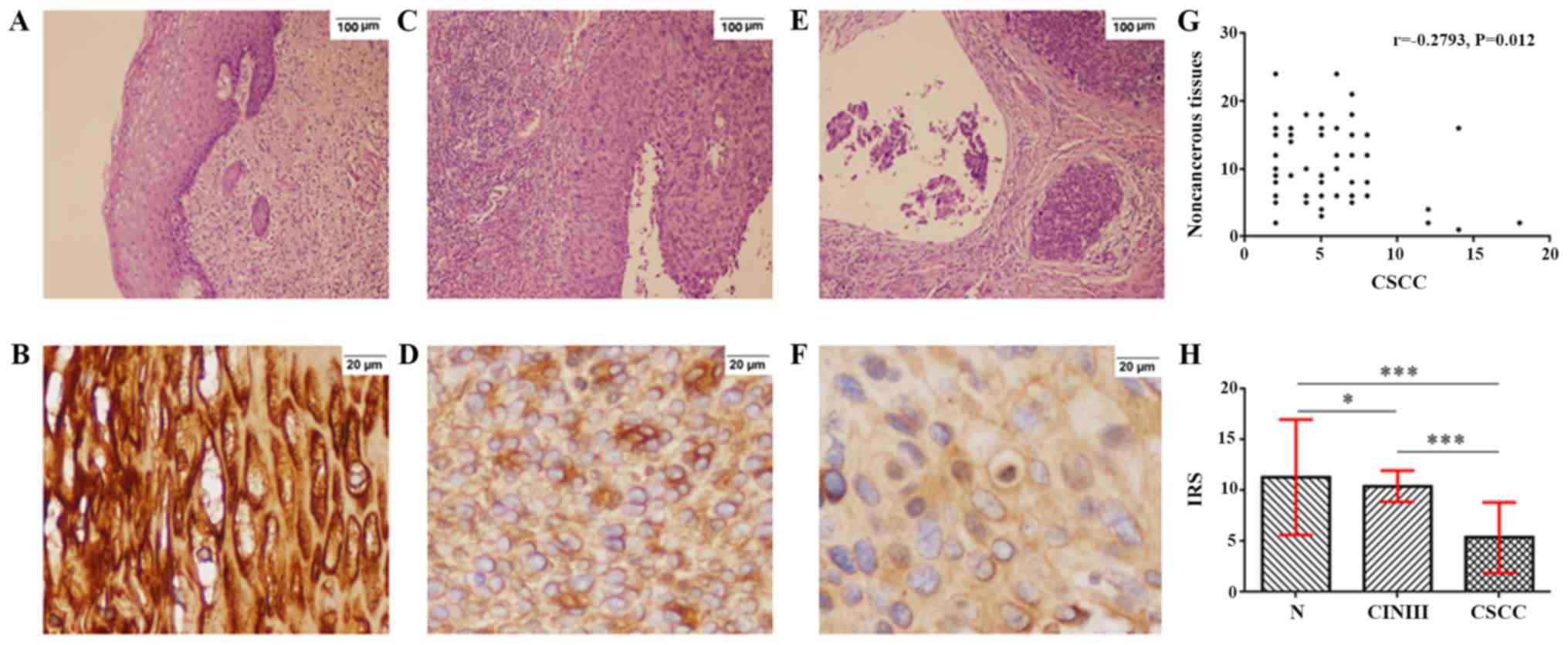 | Figure 2.Expression of ZIC1 protein in CIN III
samples, CSCC and adjacent noncancerous tissues. Hematoxylin and
eosin staining of (A) noncancerous tissues, (C) CIN III and (E)
CSCC tissues (magnification ×100). Immunohistochemistry staining
for ZIC1 protein (B) in noncancerous tissues, (D) CIN III and (F)
CSCC tissues (magnification ×400). (G) There was a negative
correlation between ZIC1 expression in CSCC tissues and expression
in adjacent noncancerous tissues. (H) Statistical comparison of IRS
of ZIC1 staining in CSCC, noncancerous tissues and CIN III samples.
*P<0.05, ***P<0.001. CSCC, cervical squamous cell carcinoma;
N, corresponding adjacent noncancerous tissues; CIN, cervical
intraepithelial neoplasia; ZIC1, zinc finger of the cerebellum 1;
IRS, immunoreactivity score. |
Association between ZIC1 expression
and clinicopathological features in patients with CSCC
As the mean IRS of ZIC1 expression in CSCC was
5.36±3.48, an IRS of 5 was used as the cut-off value. Cases with an
IRS ≥5 were included in the high ZIC1 expression group, and cases
with IRS <5 were included in the low ZIC1 expression group. In
Table I, high expression of ZIC1 was
negatively associated with FIGO stage (P=0.027) and lymph node
metastasis (P<0.001), but there were no significant associations
with any of the other clinicopathological characteristics (age,
tumor size, tumor grading and vascular invasion) between high and
low ZIC1 expression (P>0.05).
 | Table I.Association between ZIC1 expression in
cervical squamous cell carcinoma and clinicopathological variables
of patients. |
Table I.
Association between ZIC1 expression in
cervical squamous cell carcinoma and clinicopathological variables
of patients.
|
|
| ZIC1 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | Higha, n (%) | Lowb, n (%) | χ2
value | P-value |
|---|
| Total | 80 | 46 (100.00) | 34 (100.00) |
|
|
| Age, years |
|
|
| 0.101 | 0.750 |
| ≤53 | 36 | 20 (43.48) | 16 (47.06) |
|
|
|
>53 | 44 | 26 (56.52) | 18 (52.94) |
|
|
| Tumor size, cm |
|
|
| 0.077 | 0.782 |
| ≤2 | 48 | 27 (58.70) | 21 (61.76) |
|
|
|
>2 | 32 | 19 (41.30) | 13 (38.24) |
|
|
| Tumor grading |
|
|
| 0.272 | 0.873 |
| G1 | 22 | 12 (26.09) | 10 (29.41) |
|
|
| G2 | 38 | 23 (50.00) | 15 (44.12) |
|
|
| G3 | 20 | 11 (23.91) | 9 (26.47) |
|
|
| FIGO stage |
|
|
| 7.190 | 0.027 |
| IA | 14 | 10 (21.74) | 4 (11.76) |
|
|
| IB | 37 | 25 (54.35) | 12 (35.30) |
|
|
|
IIA | 29 | 11 (23.91) | 18 (52.94) |
|
|
| Lymph node
metastasis |
|
|
| 14.403 | <0.001 |
| No | 31 | 26 (56.52) | 5 (14.71) |
|
|
|
Yes | 49 | 20 (43.48) | 29 (85.29) |
|
|
| Vascular
invasion |
|
|
| 0.812 | 0.368 |
| No | 52 | 28 (60.87) | 24 (70.59) |
|
|
|
Yes | 28 | 18 (39.13) | 10 (29.41) |
|
|
OS
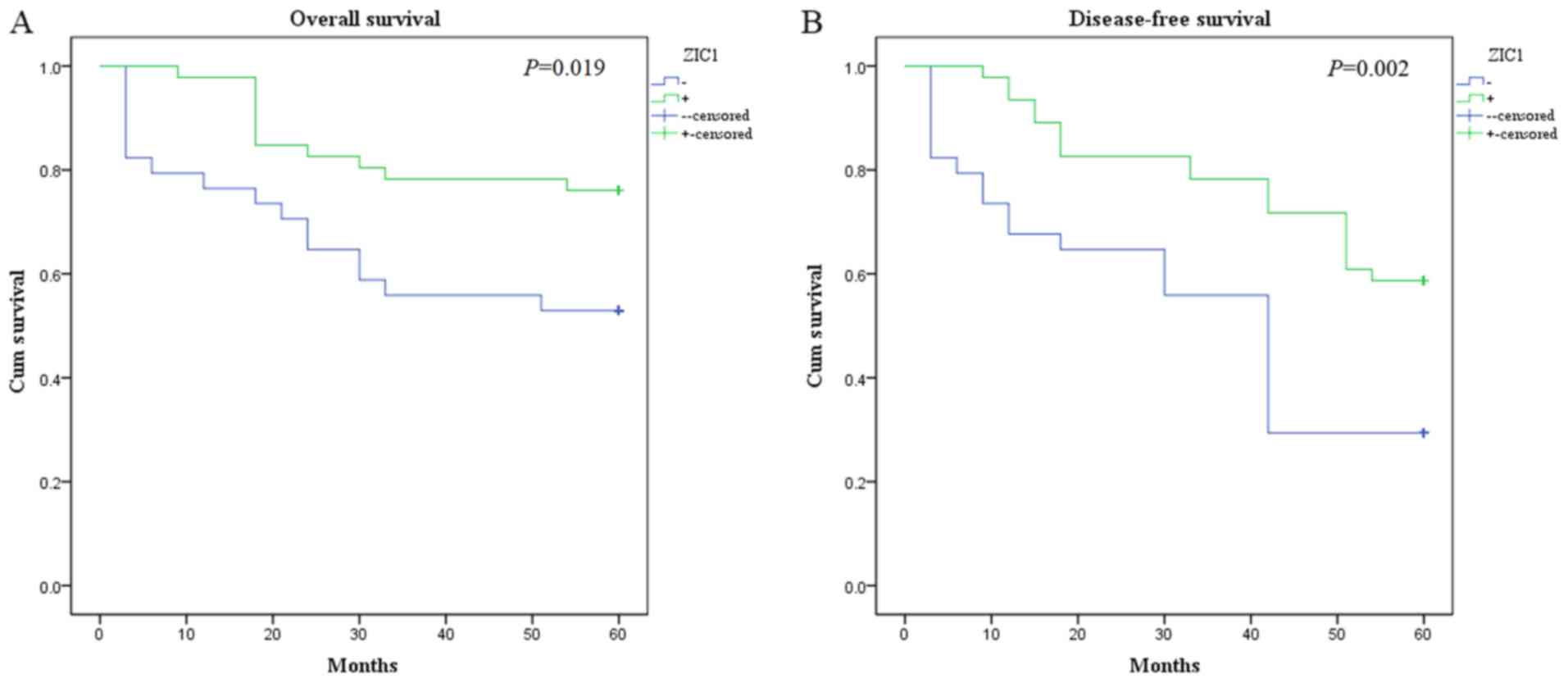
Kaplan-Meier analysis showed that the OS time of the
low ZIC1 expression group (39.62±4.06 months) was significantly
lower compared with the high ZIC1 expression group (51.26±2.44
months; P=0.019; Fig. 3A). In
univariate Cox regression analysis (Table II), ZIC1 (P=0.027), tumor grading
(P=0.020), lymph node metastasis (P=0.003) and FIGO stage
(P<0.001) were associated with OS. In multivariate analysis,
ZIC1 expression (HR, 0.61; 95% CI, 0.40–0.92; P=0.018), FIGO
staging (HR, 3.55; 95% CI, 2.35–5.37; P<0.001) and lymph node
metastasis (HR, 2.50; 95% CI, 1.62–3.86; P<0.001) were
determined to be independent prognostic factors. Of the 320
patients with CIN grade III, there were no deaths in the 5 years
following LEEP treatment.
 | Table II.Prognostic value of ZIC1 expression
and clinicopathological factors for overall survival of patients
with cervical squamous cell carcinoma. |
Table II.
Prognostic value of ZIC1 expression
and clinicopathological factors for overall survival of patients
with cervical squamous cell carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| ZIC1 expression,
high vs. low | 0.42 | 0.20–0.91 | 0.027 | 0.61 | 0.40–0.92 |
0.018a |
| Age, >53 vs. ≤53
years | 1.07 | 0.80–1.44 | 0.652 |
|
|
|
| Tumor size, >2
vs. <2 cm | 1.33 | 0.84–2.11 | 0.217 |
|
|
|
| Tumor grading, G3
vs. G1 and G2 | 1.27 | 1.04–1.56 | 0.020 | – | – | – |
| FIGO stage, IIA vs.
I | 2.32 | 1.68–3.19 | <0.001 | 3.55 | 2.35–5.37 | <0.001 |
| Lymph node
metastasis, yes vs. no | 1.52 | 1.16–2.00 | 0.003 | 2.50 | 1.62–3.86 | <0.001 |
| Vascular invasion,
yes vs. no | 1.05 | 0.81–1.36 | 0.704 |
|
|
|
DFS
In Fig. 3B, the DFS
time of the low ZIC1 expression group (33.88±3.77 months; P=0.002)
was significantly lower compared with the high ZIC1 expression
group (48.65±2.53 months). In Cox regression analysis (Table III), ZIC1 expression (P=0.003),
tumor grading (P=0.022), FIGO stage (P<0.001) and lymph node
metastasis (P=0.006) were four independent factors of DFS. Of the
320 patients with CIN grade III, there were no incidences of
recurrence in the 5 years following LEEP treatment.
 | Table III.Prognostic value of ZIC1 expression
and clinicopathological factors for disease-free survival of
patients with cervical squamous cell carcinoma. |
Table III.
Prognostic value of ZIC1 expression
and clinicopathological factors for disease-free survival of
patients with cervical squamous cell carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| ZIC1 expression,
high vs. low | 0.41 | 0.22–0.76 | 0.005 | 0.69 | 0.55–0.88 | 0.003 |
| Age, >53 vs. ≤53
years | 1.38 | 0.89–2.13 | 0.146 |
|
|
|
| Tumor size, >2
vs. <2 cm | 1.46 | 0.81–2.64 | 0.205 |
|
|
|
| Tumor grading, G3
vs. G1 and G2 | 1.86 | 1.25–2.75 | 0.002 | 1.59 | 1.07–2.35 | 0.022 |
| FIGO stage, IIA vs.
I | 2.02 | 1.36–2.99 | <0.001 | 2.89 | 1.95–4.29 | <0.001 |
| Lymph node
metastasis, yes vs. no | 1.68 | 1.13–2.49 | 0.010 | 2.23 | 1.26–3.94 | 0.006 |
| Vascular invasion,
yes vs. no | 1.12 | 0.73–1.73 | 0.597 |
|
|
|
Discussion
In developing countries, cervical cancer is one of
the most common types of cancer in females, which has significant
repercussions for the medical and academic fields (1). Although the 9-valent HPV vaccine has
the potential to prevent HPV infection and the occurrence of
high-risk HPV cervical cancer, widespread use of the vaccine has
not been adopted due to adverse side effects and economic burdens
(15,16). In addition, cervical cancer is still
one of the leading causes of cancer-associated deaths in females,
and it has a high rate of morbidity and mortality (1). Therefore, novel biomarkers are required
to improve diagnosis and prognosis of patients with CSCC.
The ZIC family of proteins were named as such due to
the high levels of expression of zinc finger proteins in cerebellar
granule cells, and ZIC1 is essential for metabolic and
physiological functions associated with the zinc finger proteins
(4). Recent studies have shown that
ZIC1 is a putative tumor suppressor gene, and its expression is
closely associated with the occurrence and development in a variety
of different types of tumors, such as ovarian cancer, thyroid
cancer and breast cancer (17–20).
Methylation of ZIC1 is frequently observed in patients with ovarian
cancer, and its hypermethylation can contribute to cisplatin
resistance in ovarian cancer cells (17). DNA methylation is significantly
associated with carcinogenesis (21,22). In
addition, ZIC1 expression was found to be significantly reduced in
malignant thyroid cells compared with that in normal thyroid cells,
and was typically associated with methylation of the ZIC1 promoter
(19). Ectopic expression of ZIC1
inhibited the growth of thyroid cancer cell lines (BCPAP, 8305C and
C643 cells) by modulating the PI3K/Akt and MAPK signaling pathways
and the FOXO3a transcription factor (19). In addition, ZIC1 inhibited cell
proliferation, reduced mitochondrial membrane potential and
promoted apoptosis of breast cancer cells by inactivating the
Akt/mTOR/P70S6K pathway, suppressing survivin expression,
modulating the cell cycle and activating the mitochondrial
apoptotic pathway (23).
Additionally, previous studies revealed that expression of ZIC1 was
upregulated in endometrial cancer compared with the corresponding
normal tissue, and was positively correlated with age, disease
stage, Tumor-Node-Metastasis stage and FIGO stage (10,11). The
findings of the current study revealed that the levels of ZIC1 mRNA
in CSCC samples were significantly decreased compared to the levels
in normal cervical tissues and CIN samples. In addition, ZIC1
expression in CIN samples was significantly lower compared with
normal cervical tissues, and ZIC1 expression was found to be
significantly reduced with increased CIN grade. Verlaat et
al (6) showed that methylation
of ZIC1 was associated with the development and progression of CC,
which may explain the effect of downregulation of ZIC1 in CSCC and
CIN samples. Furthermore, ZIC1 protein expression in 80 cases of
CSCC were determined using IHC in the present study. Protein
expression levels were significantly lower compared with the
corresponding adjacent noncancerous tissues or CIN III samples.
ZIC1 expression is disordered in tumors, but not in
normal tissues and its abnormal expression is associated with
malignant biological behaviors (8,9,11). In the present study, ZIC1 expression
in CSCC was significantly lower compared with normal tissues and
high expression of ZIC1 was negatively associated with FIGO stage
and lymph node metastasis. In addition, the OS rate and DFS rate in
patients with low ZIC1 expression were both significantly lower
compared with patients with high ZIC1 expression, and ZIC1
expression was determined to be an independent prognostic marker
for CSCC. Previous research revealed that ZIC1 was a novel
indicator of prognosis in patients with invasive breast cancer and
gastric cancer (8,9). Therefore, in the present study, it was
demonstrated that ZIC1 may additionally be a reliable biomarker for
patients with CSCC. However, the limitation of the current study
was that it was focused only on the prognostic value of ZIC1 in
cervical squamous cell carcinoma. Based on these findings,
functional studies should be performed to ascertain the effect of
ZIC1 expression both in vitro and in vivo in
CSCC.
The present study showed that ZIC1 may be a novel
indicator of clinicopathological features and prognosis in patients
with CSCC. However, it should be noted that only patients
classified with FIGO stage I–IIA were included in the present
study, and patients who had undergone surgery or with a tumor
classified at FIGO IIB-IV CSCC were excluded. Further research is
required to confirm the clinical value of ZIC1 expression in
patients with FIGO stage IIB-III who received chemoradiotherapy
prior to surgery.
In conclusion, ZIC1 expression in CSCC was
significantly lower compared with normal cervical tissues and with
CIN samples. In addition, ZIC1 expression in CIN was significantly
lower compared with expression in normal cervical tissues, and was
associated with CIN grade. High expression of ZIC1 was negatively
associated with FIGO stage and lymph node metastasis, and it was
associated with improved OS and DFS in patients with CSCC. Further
research is required to confirm the clinical value of ZIC1 in
patients with CSCC and to understand the underlying mechanism of
ZIC1 expression in CSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Kunshan
Science and Technology Program of Social Development (grant no.
KS1730).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG and QL conceived and designed the study. XG, XKG
and BHC performed the experiments and wrote the manuscript. XG, XJG
and FC analyzed the data. All authors read and approved the
manuscript and agreed to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Kunshan First People's Hospital (Jiangsu, China), and
consent was obtained from each patient for the use of their tissues
for research and publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HPV
|
human papillomavirus
|
|
CC
|
cervical cancer
|
|
CSCC
|
cervical squamous cell carcinoma
|
|
CIN
|
cervical intraepithelial neoplasia
|
|
ZIC
|
zinc finger of the cerebellum
|
|
IHC
|
immunohistochemistry
|
|
IRS
|
immunoreactivity score
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
Leep
|
loop electrosurgical excision
procedure
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robadi IA, Pharaon M and Ducatman BS: The
importance of high-risk human papillomavirus types other than 16
and 18 in cervical neoplasia. Arch Pathol Lab Med. 142:693–695.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Solís JG and Briones-Torres TI: Prevalence
of intraepithelial lesion in cervical screening cytology in a
First-level Care Unit. Rev Med Inst Mex Seguro Soc. 56:167–172.
2018.(In Spanish; Abstract available in Spanish from the
publisher). PubMed/NCBI
|
|
4
|
Aruga J, Yokota N, Hashimoto M, Furuichi
T, Fukuda M and Mikoshiba K: A novel zinc finger protein, zic, is
involved in neurogenesis, especially in the cell lineage of
cerebellar granule cells. J Neurochem. 63:1880–1890. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aruga J and Millen KJ: ZIC1 Function in
normal cerebellar development and human developmental pathology.
Adv Exp Med Biol. 1046:249–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verlaat W, Snijders PJF, Novianti PW,
Wilting SM, De Strooper LMA, Trooskens G, Vandersmissen J, Van
Criekinge W, Wisman GBA, Meijer CJLM, et al: Genome-wide DNA
methylation profiling reveals methylation markers associated with
3q gain for detection of cervical precancer and cancer. Clin Cancer
Res. 23:3813–3822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gan L, Chen S, Zhong J, Wang X, Lam EK,
Liu X, Zhang J, Zhou T, Yu J, Si J, et al: ZIC1 is downregulated
through promoter hypermethylation, and functions as a tumor
suppressor gene in colorectal cancer. PLoS One. 6:e169162011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han W, Zhang C, Gao XJ, Wang HB, Chen F,
Cao F, Hu YW, Ma J, Gu X and Ding HZ: Clinicopathologic and
prognostic significance of the zinc finger of the cerebellum family
in invasive breast cancer. J Breast Cancer. 21:51–61. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma G, Dai W, Sang A, Yang X and Li Q:
Roles of ZIC family genes in human gastric cancer. Int J Mol Med.
38:259–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS,
Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, et al: Identification of
molecular markers and signaling pathway in endometrial cancer in
Hong Kong Chinese women by genome-wide gene expression profiling.
Oncogene. 26:1971–1982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu X, Liu Q, Yang N, Shen JF, Zhang XG,
Cao F and Ding HZ: Clinicopathological significance of increased
ZIC1 expression in human endometrial cancer. J Huazhong Univ Sci
Technolog Med Sci. 35:898–903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim S, Cho K, Lee S, Lee K, Shin J, Chung
D and Park C: Effect of number of retrieved lymph nodes on
prognosis in FIGO stage IB-IIA cervical cancer patients treated
with primary radical surgery. J Obstet Gynaecol Res. 43:211–219.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ginocchio CC, Barth D and Zhang F:
Comparison of the Third Wave Invader human papillomavirus (HPV)
assay and the digene HPV hybrid capture 2 assay for detection of
high-risk HPV DNA. J Clin Microbiol. 46:1641–1646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guevara A, Cabello R, Woelber L, Moreira
ED Jr, Joura E, Reich O, Shields C, Ellison MC, Joshi A and
Luxembourg A: Antibody persistence and evidence of immune memory at
5years following administration of the 9-valent HPV vaccine.
Vaccine. 35:5050–5057. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martínez-Lavín M and Amezcua-Guerra L:
Serious adverse events after HPV vaccination: A critical review of
randomized trials and post-marketing case series. Clin Rheumatol.
36:2169–2178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang F, Munck J, Tang J, Taverna P, Wang
Y, Miller DF, Pilrose J, Choy G, Azab M, Pawelczak KS, et al: The
novel, small-molecule DNA methylation inhibitor SGI-110 as an
ovarian cancer chemosensitizer. Clin Cancer Res. 20:6504–6516.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du L, Qian X, Dai C, Wang L, Huang D, Wang
S and Shen X: Screening the molecular targets of ovarian cancer
based on bioinformatics analysis. Tumori. 101:384–389. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiang W, Zhao Y, Yang Q, Liu W, Guan H, Lv
S, Ji M, Shi B and Hou P: ZIC1 is a putative tumor suppressor in
thyroid cancer by modulating major signaling pathways and
transcription factor FOXO3a. J Clin Endocrinol Metab.
99:E1163–E1172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Lin Z, Xue M, Si J and Chen S:
Zic1 promoter hypermethylation in plasma DNA is a potential
biomarker for gastric cancer and intraepithelial neoplasia. PLoS
One. 10:e01339062015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Zee RP, Richel O, van Noesel CJM,
Novianti PW, Ciocanea-Teodorescu I, van Splunter AP, Duin S, van
den Berk GEL, Meijer CJLM, Quint WGV, et al: Host cell
deoxyribonucleic acid methylation markers for the detection of
high-grade anal intraepithelial neoplasia and anal cancer. Clin
Infect Dis. 68:1110–1117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baribault C, Ehrlich KC, Ponnaluri VKC,
Pradhan S, Lacey M and Ehrlich M: Developmentally linked human DNA
hypermethylation is associated with down-modulation, repression,
and upregulation of transcription. Epigenetics. 13:275–289. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han W, Cao F, Gao XJ, Wang HB, Chen F, Cai
SJ, Zhang C, Hu YW, Ma J, Gu X and Ding HZ: ZIC1 acts a tumor
suppressor in breast cancer by targeting survivin. Int J Oncol.
53:937–948. 2018.PubMed/NCBI
|