Introduction
Tumors adopt various immune escape strategies to
avoid recognition and elimination by cytotoxic CD8+ T
lymphocytes (CTLs) (1). A major
mechanism by which tumors escape immune recognition is by
downregulating their surface expression of human leukocyte antigen
(HLA) class I molecules. A major explanation for the disappointing
outcome from DNA and peptide-based anti-cancer vaccines is that the
minimal tumoral expression of HLA class I molecules do not allow
for the vaccine activated CTLs to exert their cancer eliminating
cytotoxic effect in the tumor microenvironment (2). Similarly, decreased HLA expression is
also considered to play a deleterious role in the favorable
clinical outcomes of immune checkpoint inhibitor based cancer
immunotherapeutic strategies. A decreased HLA class I expression is
noted in various solid organ tumors including breast cancers
(3). Hence, there is an important
need to develop novel approaches to enhance HLA class I expression
to overcome tumor immune escape and promote tumor rejection.
Further, potential HLA class I recovery in the tumor
microenvironment will complement the clinical outcome of various
anti-tumor immunotherapeutic strategies including vaccine based
approaches.
Active metabolite derivatives of Selenium (Se) have
been suggested to exert an anti-carcinogenic effect on many solid
organ tumors such as prostate and breast cancer (4). Clinical interventional studies
demonstrated that a supra-nutritive intake of Se has positive
effects in the prevention of several solid organ cancers (5). The two key metabolites of Se (Fig. 1A), hydrogen selenide
(H2Se), derived mainly from inorganic selenocompounds
such as selenate or selenite, and methylselenol
(CH3SeH), derived from organic selenocompounds, such as
methyl selenic acid (MSA) and dimethylselenide (DMDSe), have been
shown to be crucial for the biological function of these
selenocompounds (6,7). While the exact mechanisms of action of
this anti-carcinogenic effect of selenium derivatives are unknown,
several studies have suggested that the Se metabolite,
methylselenol, is the active Se compound for anti-carcinogenic
effects while remaining non-cytotoxic on normal terminally
differentiated cells. Murine tumor studies have shown that combined
treatment of MSA with paclitaxel reduced tumor growth of tumor
xenografts with triple negative breast cancer cells (TNBC) compared
to paclitaxel treatment alone (8).
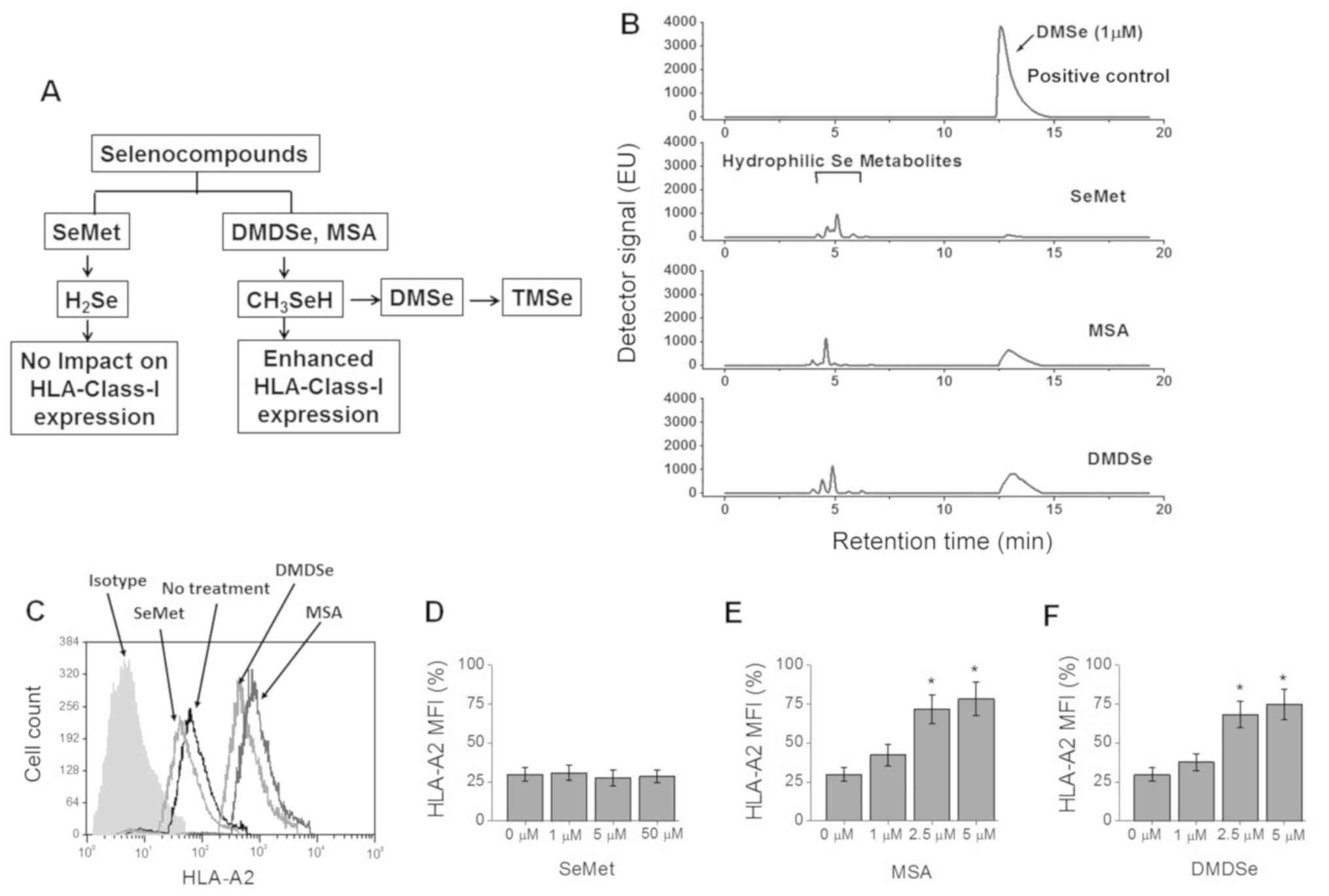 | Figure 1.HLA-A2 expression in THP-1 cells
following treatment with selenocompounds. (A) Schematic of
selenocompound metabolism. (B) Production of DMSe by THP-1 cells
following treatment with SeMet (50 µM), MSA (2.5 µM) and DMDSe (2.5
µM). DMSe could be further methylated to unstable TMSe. Pure DMSe
at 1 µM in cell culture media was analyzed and used as positive
control. (C) HLA-A2 surface expression on THP-1 cells following
treatment with SeMet (50 µM), MSA (2.5 µM) or DMDSe (2.5 µM).
Percent expression was analyzed via isotype labelled negative
control antibody staining. (D-F) Dose response following treatment
(D) SeMet, (E) MSA and (F) DMDSe to assess the expression of
HLA-A2. Data are presented as the mean ± SEM (n=4). Statistical
significance for C, D and E were analyzed via one-way ANOVA.
*P<0.05 vs. 0 µM. HLA, human leukocyte antigen; SeMet,
selenomethionine; MSA, methylselenic acid; DMDSe, methylselenol
derivative dimethyl selenol; TMSe, trimethy selenol; MFI, mean
fluorescence intensity. |
Mammaglobin-A (Mam-A) is a human breast
tumor-associated antigen (TAA) expressed in 40–80% of primary and
metastatic breast cancers (9–13).
Previously, we have demonstrated that Mam-A based vaccination to
stage-IV human breast cancer patients was safe and efficient in
increasing progression free survival in vaccinated patients. Murine
and in vitro studies have demonstrated that
HLA-A2-restricted MamA2.1 peptide (amino acids 83–92 of Mam-A,
LIYDSSLCDL) exerted specific immunodominance towards effector
cytotoxic activation of naïve CD8+ T lymphocytes
(14,15). While we have shown that following
Mam-A vaccination there was some increase in the frequency of
MamA2.1+CD8 T cells, strategies to further enhance HLA class I
expression will provide an additional adjuvant methodology to
enhance vaccine efficiency. Therefore, in this communication, we
studied the role of selenium compounds towards increasing the
cytotoxic efficiency of HLA-A2 restricted Mam-A epitope (MamA2.1)
activated CTLs on Mam-A expressing human breast cancer cells.
Materials and methods
Cell lines and healthy human
CD8+ T lymphocytes
The human breast cancer cell lines were selected
based on the specific expression of antigen presenting class I
HLA-A2 molecule and expression of tumor specific antigen,
mammaglobin-A. The following cell lines:
MAM-A+/HLA-A2+ (AU565 and UACC-812) and
MAM-A−/HLA-A2+ (MCF-7 and MDA-MB-231), and
human monocyte-like HLA-A2+ cell line, THP-1 cells, were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). Human CD8+ T cells from HLA-A2+ healthy
subjects were obtained from StemCell Technologies (Cambridge, MA,
USA). All cell cultures and incubations were performed as per
provider's recommendations and described by us before (16). Briefly, cell were cultured in
RPMI-1640 medium at 37°C in 5% CO2 incubator until they
were 80% confluent. The presence of Mam-A and HLA-A2 expression in
the breast cancer cell lines was confirmed by western blot analysis
(data not shown). The selenocompounds, methylseleninic acid (MSA),
dimethylselenide (DMDSe) and selenomethionine (SeMet) were obtained
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany. The THP-1 cells
were cultured in 24 well plates, 1×105 per well and
stimulated with respective selenocompounds (5 µM) for 24 h. These
cells were later used for various experiments detailed below. For
MamA2.1 peptide stimulation (Peptide 2.0 Inc, Chantilly, VA),
CD8+ T lymphocytes (1×106) were cultured in 2
ml of supplemented RPMI-1640 media in 24-well plates in the
presence of irradiated (5,000 rads) THP-1 cells (1×106)
loaded with Mam-A2.1 in the presence of β2 m (3 µg/ml), CD3 (500
ng/ml), CD28 mAb (500 ng/ml) and recombinant human IL-2 (20 U/ml).
The CD8+ T lymphocytes were isolated by immunomagnetic
separation (MACS Miltenyi Biotec, San Diego, CA) and the resulting
purity was verified to be >95%. The MamA2.1 peptide was custom
synthesized by Peptide 2.0 Inc. (Chantilly, VA) and purified on
HPLC column to >95% purity.
High-performance liquid
chromatography
The supernatant from cell cultures were treated with
methanol (1:1 final concentration) and injected into the HPLC
system. The Agilent 1100 HPLC system was comprised of isocratic
pump (G1310A) and an auto sampler (G1313A). The Gemini C18 (3 µM,
110 Å, 50×1 mm inner diameter) columns were utilized for
chromatography. The mobile phase was 0.1% formaldehyde in 40%
methanol. The flow rate was 100 µl/min. Injection volumes were 10
µl. Data were analyzed with Chemstation software.
Cytotoxicity assay
The cytotoxic efficiency of peptide-activated
CD8+ T cells was investigated by its ability to lyse the
target breast cancer cells by non-irradiative LDH release assay
(Promega Corporation, Madison, WI, USA) and and MTT assay (Life
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The breast cancer cells (1×104 cells) in 100 µl of
complete medium at were plated in triplicate cultures in round
bottom 96-well plates in the presence of varying numbers of
CD8+ T cells (6.25:1 to 50:1) and incubated at 37°C. The
percentage specific lysis was calculated as follows: [(experimental
LDH release-spontaneous LDH release)/(maximum LDH
release-spontaneous LDH release)] ×100.
Flow cytometry
The HLA-A2 expression in cells were analyzed by flow
cytometry using appropriated primary (BB7.2, BioLegend, San Diego,
CA, USA) and FITC-labelled secondary antibody. Samples were
analyzed using a FACS Calibur/LSRII flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). Data were analyzed using BD FACSDiva
software. Gates were set according to isotype controls.
ELISA
The secretory extracellular IFNγ in the cell
supernatant was quantitated by ELISA as per the manufacturer's
protocol (R&D Systems, Minneapolis, MN, USA; catalog# DY285B).
Quantification was performed with a standard curve using the
manufacturer provided standards. Detection at 450 nm was performed
using EMax Plus spectrophotometer and data analysis was carried out
using software provided by the manufacturer (Molecular Devices,
Sunyvale, CA, USA).
Western blotting
Total proteins were extracted from cells with lysis
buffer for western blot analysis as previously described. Total
proteins were separated on a 4–12% sodium dodecyl
sulfate-polyacrylamide gradient gel and transferred onto a
nitrocellulose membrane. All primary and secondary Abs were
obtained from Abcam (Cambridge, MA, USA) or Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The following specific
primary antibodies to TAP-1 (sc-376796, Santa Cruz Biotechnology,
Inc., 1:200), TAP-2 (sc-515576, Santa Cruz Biotechnology, Inc.,
1:200), LMP-2 (ab3328, Abcam, 1:500), LMP-7 (ab3329, Abcam, 1:500),
tapasin (ab196764, Abcam, 1:500), β2-microglobulin (sc-515576,
Santa Cruz Biotechnology, Inc., 1:200) and β-actin (sc-8432, Santa
Cruz Biotechnology, Inc., 1:200). The following specific secondary
antibodies were used based the species of the primary antibody:
Goat anti-mouse-HRP (ab205718, Abcam, 1:2,500) and goat
anti-rabbit-HRP (ab205718, Abcam, 1:2,500). The transferred and
probed nitrocellulose membranes were developed using the
chemiluminescence kit (EMD Millipore, Billerica, MA, USA) and
analyzed on using Bio-Rad Universal Hood II (Hercules, CA, USA).
Morphometric analysis was done using the software provided by the
company.
Reverse transcription-quantitative PCR
(RT-qPCR)
Expression profiles of genes at mRNA level in the
breast cancer cell lines were analyzed using the SyBr-green
detection based RT-qPCR primers (Table
I), obtained from Integrated DNA Technologies (San Jose, CA,
USA). Briefly, total RNA was extracted from 106 cells
using TRIzol reagent (Sigma-Aldrich; Merck KGaA) and analyzed as
mentioned previously. The cycling conditions consisted of an
initial denaturation of 95°C for 15 min, followed by 40 cycles of
95°C for 30 sec, followed by 61°C for 1 min. The final reaction
volume of 50 µl using BioRad CFX96 (Hercules) and analyzed by
2−ΔΔCq method (17).
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Acc. no. | Product lenght
(bp) |
|---|
| Tap1 |
TGCCTAAGAAGCTGGGAAAA |
GTAAGCCAAGGCCTCCTTCT | NM_013683.2 | 203 |
| Tap2 |
CGGTGCTAAAGGAGATCCAG |
CCATCACCCTCCGTATGACT | NM_011530.3 | 204 |
| LMP2 |
TCTTCTGTGCCCTCTCAGGT |
TGGTCCCAGCCAGCTACTAT | NM_013585.2 | 193 |
| LMP7 |
GGAACGCATCTCCGTGTCTG |
CTGCCGGTAACCACTGTCCA | NM_010724.2 | 223 |
| Tapasin |
ACACTGCGAGATGAGCCGCTTC |
TGAGGACGGTCAGCACCACTGT | NM_009318.2 | 221 |
| B2m |
GCCGAACATACTGAACTGCT |
GCCATACTGGCATGCTTAAC | NM_009735.3 | 207 |
| Jak1 |
CCGCATGAGGTTCTACTTTACC |
TCAAATCATACTGTCCCTGTGC | NM_146145.2 | 179 |
| Jak2 |
GCAGATTCATTCAGCAATTCAG |
CGTCCTGTTCTGTCAGTGTCTC | NM_008413.3 | 240 |
| Stat1 |
GACACCTGCAACTGAAG |
CACCAGCATGTTGTACC | NM_001205313.1 | 239 |
| Stat2 |
CTGAAGGAGATGAGTCACATGC |
GGTGAACTTGTTCCCAGTCTTC | NM_019963.1 | 189 |
| Stat3 |
ACAACGCTGGCTGAGAAGCTCC |
TTGTGCTTAGGATGGCCCGCTC | NM_213659.3 | 226 |
| Irf1 |
GAAGATAGCCGAAGACCT |
CTTCATCTCCGTGAAGAC | NM_001159396.1 | 205 |
| Irf2 |
GGTCCTGACTTCAGCTAT |
TTCTGCGTAGGAAGACAG | NM_008391.4 | 220 |
| Irf5 |
GTCAAGACGAAGCTCTTTAGCC |
CTGCTCTACCATGTGGTCTTTG | NM_001252382.1 | 279 |
| Irf7 |
GTTTACGAGGAACCCTATGCAG |
GAAGCGTCTCTGTGTAGTGCAG | NM_001252601.1 | 277 |
| Irf9 |
GCGTTGTAAACCACTCAGACAG |
CATAGATGAAGGTGAGCAGCAG | NM_001159417.1 | 204 |
| GAPDH |
TTGTGCAGTGCCAGCCTCGT |
TCGGCCTTGACTGTGCCGTT | NM_008084.3 | 214 |
| β-actin |
ACTGTCGAGTCGCGTCC |
ATGGCTACGTACATGGCTGG | NM_007393.5 | 487 |
Statistical analysis
Data are expressed as mean ± SEM from four
independent studies. Significant differences between groups were
assessed using Tukey HSD pair-wise comparisons for two groups and
one-way ANOVA for multiple comparisons. A P-value of
<0.05 was considered significant.
Results
Enhanced HLA class I expression
following treatment with methylselenol producing
selenocompounds
As upregulation of HLA class I molecules in the
tumor-infiltrating immune cells is considered critical for cancer
immunotherapy, we first examined the potential impact of the three
selenocompounds, namely methylseleninic acid (MSA),
dimethyldiselenide (DMDSe) and selenomethionine (SeMet), towards
modulation of the surface expression of HLA class I molecules on
THP-1 cells. As the metabolite methylselenol is volatile, we
investigated the production of dimethylselenol (DMSe; Fig. 1A) following treatment with various
selenium compounds (18,19). We first tested for DMSe production by
THP-1 cells following treatment with selenocompounds for 72 h. As
shown in Fig. 1B, there was an
enhanced DMSe production following treatment with MSA and DMDSe,
while there was no production of DMSe following treatment with
SeMet. We next tested the cell surface expression of HLA-A2 on
THP-1 cells following treatment with selenocompounds. As shown in
Fig. 1C-F, DMDSe (2.5 µM) induced a
2.4-fold increased expression of HLA-A2 (from 29.8±4.3%, without
treatment, to 71.7±9.1% following DMDSe treatment,
P<0.05) on THP-1 cells. Further, MSA (2.5 µM) induced a
2.3-fold increased expression of HLA-A2 molecules, while SeMet
(1–50 µM) did not induce any change in HLA-A2 expression on THP-1
cells. These data suggest that MSA and DMDSe induced enhanced
expression of HLA class I molecules which could have a critical
adjuvant role in antigen presentation, eventually leading to
potential immune mediated elimination of tumor cells following
anti-tumor vaccination.
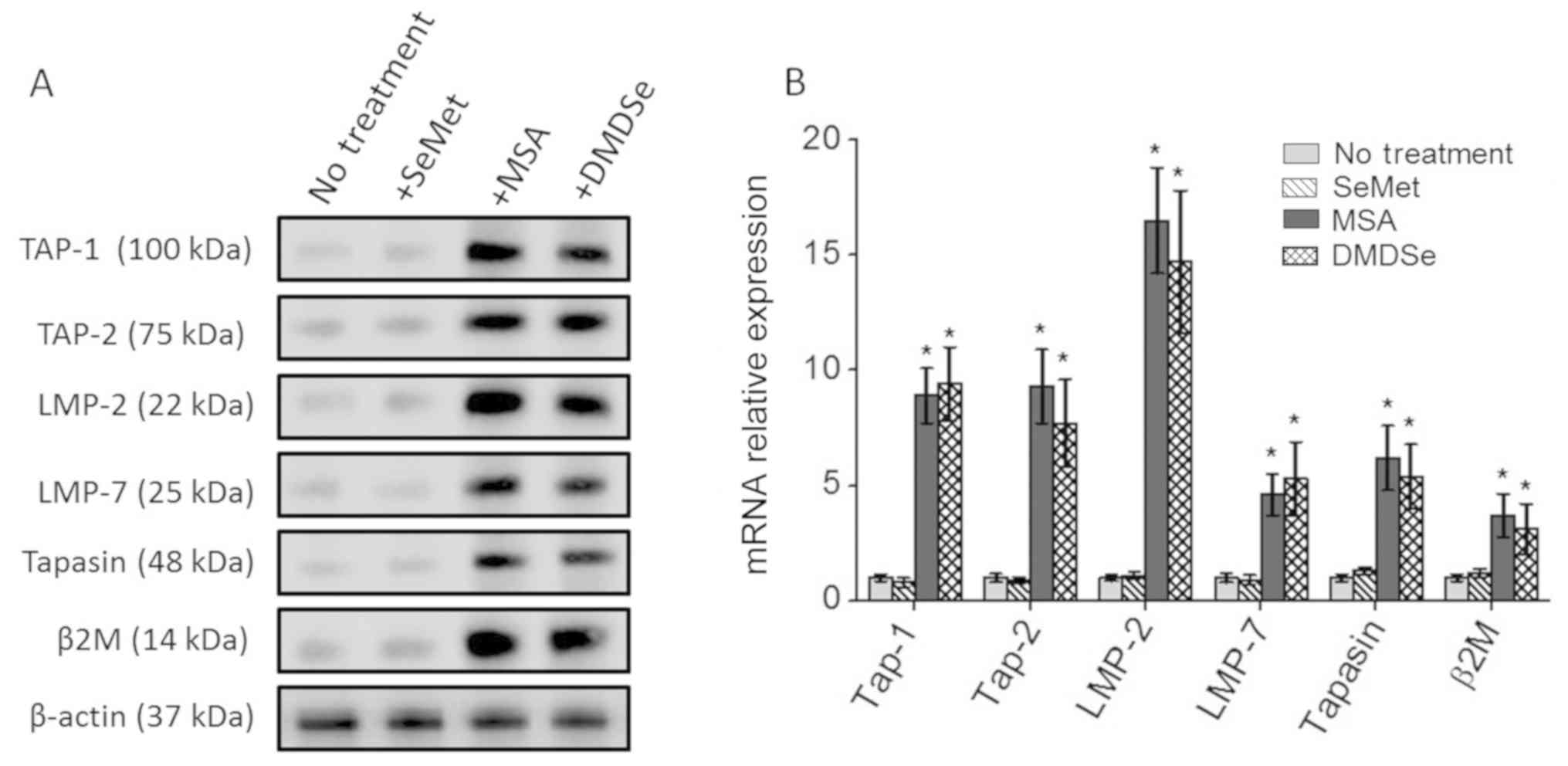
Upregulation of antigen presenting
machinery following treatment with methyl selenol producing
selenocompounds
To determine the molecular mechanisms leading to the
enhanced upregulation of the HLA-A2 surface expression following
treatment with selenocompounds, we analyzed the changes in the
expression of molecules involved in the surface expression of HLA
class I molecules, also known as antigen presentation machinery
(APM). As shown in Fig. 2, treatment
with MSA and DMDSe increased the protein and mRNA transcript levels
of key APM components, namely, TAP-1, TAP-2, LMP-2, LMP-7, tapasin
and β2-microglobulin in THP-1 cells. However, SeMet treatment did
not induce the expression of the APM components. These data
demonstrate that the MSA and DMDSe induced the APM which has a
potential to induce tumor associated antigen specific effector
CD8+ T cell immune responses against cancer cells.
Enhanced activation of Mam-A specific
effector CD8+ T lymphocyte mediated cytotoxic responses
following treatment with selenocompounds
As our data demonstrated that following MSA and
DMDSe treatment there was enhanced HLA-A2 expression on THP-1
cells, we next determined if this enhanced expression enabled HLA
class-I immunodominant MamA2.1 peptide presentation and eventual
antigen-specific CD8+ T cell activation leading to cell
mediated cytotoxicity against breast cancer cells. We therefore
stimulated naïve CD8+ T lymphocytes collected from
healthy HLA-A2+ human subjects with MamA2.1peptide in
the presence of antigen presenting HLA-A2+ THP-1
mononuclear cells pre-treated with selenocompounds (Fig. 3A). We collected these activated
CD8+ T cells by magnetic beads isolation and ascertained
the purity (>95%) by flow cytometry. We investigated if these
MamA2.1 activated CD8+ T cells exerted higher
cytotoxicity against breast cancer cell lines compared to
activation by THP-1 cells without selenocompound treatment. The
breast cancer cell lines (referred to as target cells) and MamA2.1
activated CD8+ T cells (referred to as effector cells)
were co-cultured. The cytotoxic effect was analyzed by LDH release
assay and measured at various effector to target (E:T) ratios
(6.25: 1 to 50:1). As shown in Fig.
3B-E, the MamA2.1-specific CD8+ T lymphocytes
exerted higher cytotoxicity on HLA-A2+/MamA+
AU565 and UACC-812 breast cancer cell lines. The CD8+ T
cells activated by THP-1 cells pre-treated with MSA (72±8%,
P<0.05) and DMDSe (66±11%, P<0.05) exerted higher
cytotoxicity (E:T 50:1) compared to non-selenocompound
pre-treatment (44±9%). However, pre-treatment with SeMet could not
induce any significant additional cytotoxicity (46±8%, P>0.05).
Furthermore, MamA2.1 activated CD8+ T cells did not
induce any cytotoxicity on HLA-A2+/MamA−
MCF-7 (Fig. 3D) and MDA-MB-231
(Fig. 3E) breast cancer cell lines,
thus suggesting that the MamA2.1 activated CD8+ T cells
exerted cytotoxic effector functionality on breast cancer cells in
Mam-A antigen-specific manner. Taken together, these data
demonstrated that MSA and DMDSe induced HLA class I expression
leading enhanced antigen presentation of immunodominant antigenic
peptides following potential anti-cancer vaccination leading to
breast cancer cell elimination.
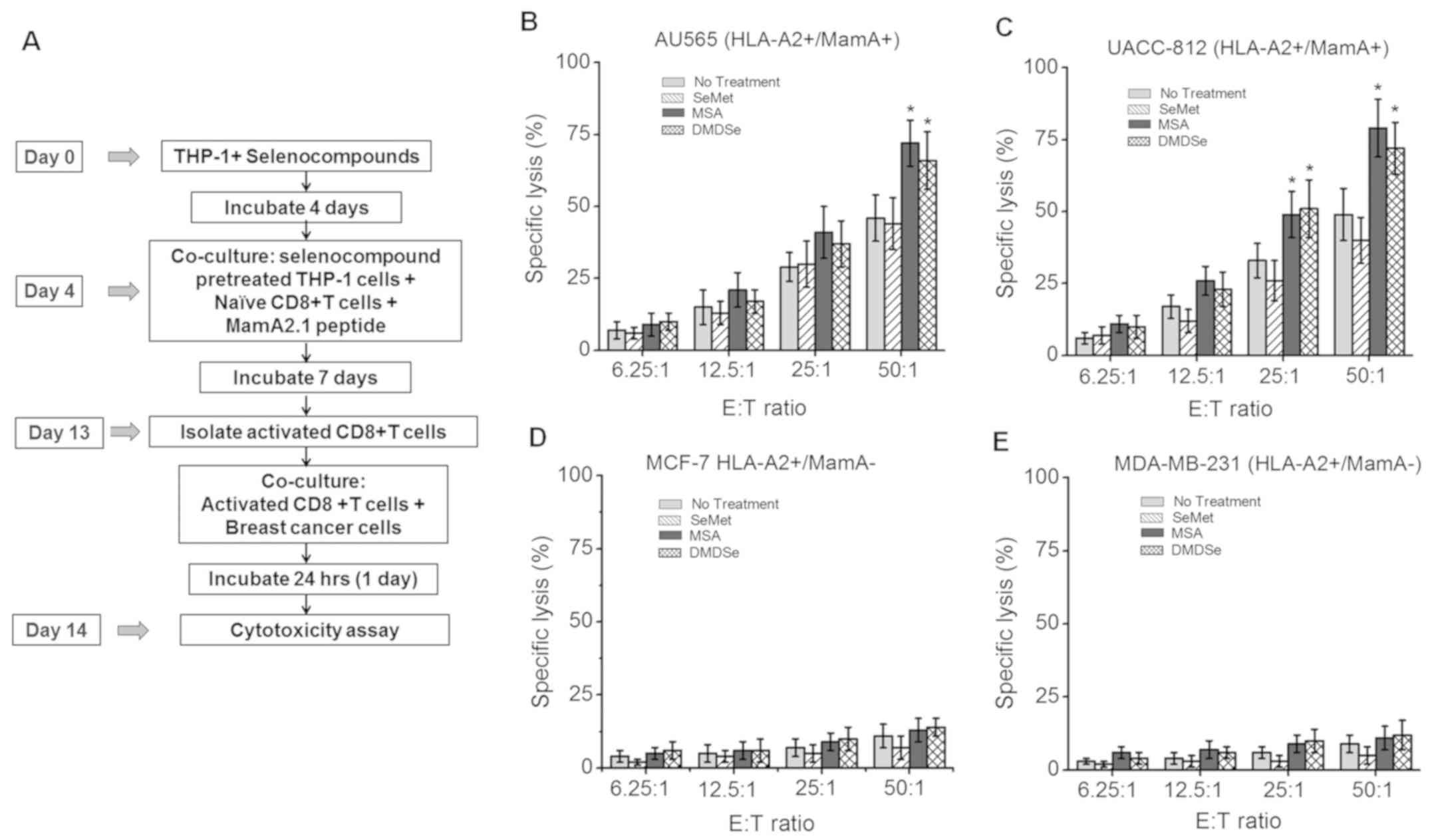 | Figure 3.Cytotoxic potential of CTLs following
activation by MamA2.1 peptide and co-stimulation with
selenocompounds pre-treated THP-1 cells. (A) Experimental design
schematic. Cytotoxicity of CTLs following activation by MamA2.1
peptide and THP-1 cells pre-treated with SeMet (50 µM), MSA (2.5
µM) or DMDSe (2.5 µM) in (B) AU565
(HLA-A2+/Mam-A+), (C) UACC-812
(HLA-A2+/Mam-A+), (D) MCF-7
(HLA-A2+/Mam-A−) and (E) MDA-MB-231
(HLA-A2+/Mam-A−) cells. E:T ratios of 50:1,
25:1, 12.5:1 and 6.25:1 are presented. Data are presented as the
mean ± SEM (n=4). Statistical significance was determined via
one-way ANOVA. *P<0.05 vs. 0 µM. CTLs, CD8+ T
lymphocytes; SeMet, selenomethionine; MSA, methylselenic acid;
DMDSe, methylselenol derivative dimethyl selenol; HLA, human
leukocyte antigen; E:T, effector:target. |
Increased IFNγ signaling in
CD8+ T cells following activation by methylselenol
producing selenocompound pre-treated THP-1 cells
As interferon (IFN)-γ signaling plays a critical
role in cytotoxic CD8+ T lymphocyte responses, we
determined the expression of IFN signaling molecules. Using
methodologies described above, we determined the transcript levels
of IFN signaling molecules in MamA2.1 activated CD8+ T
cells at 4 h following co-culture with AU565 cells at E:T ratio of
50:1. As shown in Fig. 4A,
CD8+ T cell activation by THP-1 cells pre-treated with
MSA and DMDSe induced higher expression of IFN signaling molecules
namely, Jak1, Jak2, Stat1, Stat2, Stat3, Irf-1, Irf-5, Irf-7 and
Irf-9. As these data demonstrated there is increased transcript
levels of IFN signaling molecules, we next determined the secretory
IFNγ protein concentration in the supernatant obtained from the
co-cultures by ELISA. As shown in Fig.
4B, ELISA based analysis of the IFNγ protein expression in the
supernatant collected from the CD8+ T cells activated
under MSA (1179±127 Pg/ml, P<0.05) and DMDSe (983±148 pg/ml,
P<0.05) pre-treatment conditions resulted in enhanced IFNγ
expression compared to no selenocompound pre-treatment (511±73
pg/ml). However, CD8+ T cells activated by SeMet
pre-treatment conditions (549±91 pg/ml, P>0.05) did not induce
significant change in IFNγ expression. Furthermore, we determine
the direct effector role of the expressed IFNγ on cytotoxic
functionality by performing blocking studies. As shown in Fig. 4C, blocking with IFNγ monoclonal
antibodies (mAb) and HLA-A2 mAb significantly inhibited the
cytotoxicity of MamA2.1 activated CD8+ T lymphocytes,
thus suggesting that the cytotoxic effector role CD8+ T
cells is dependent upon selenocompound mediated upregulation of
IFNγ signaling. Taken together, our current data demonstrated that
MSA and DMDSe induced HLA class I expression and presentation of
HLA-A2 restricted antigenic epitope for specific peptide
vaccine-like activation of CD8+ T cell leading to
enhanced breast cancer cell cytotoxicity.
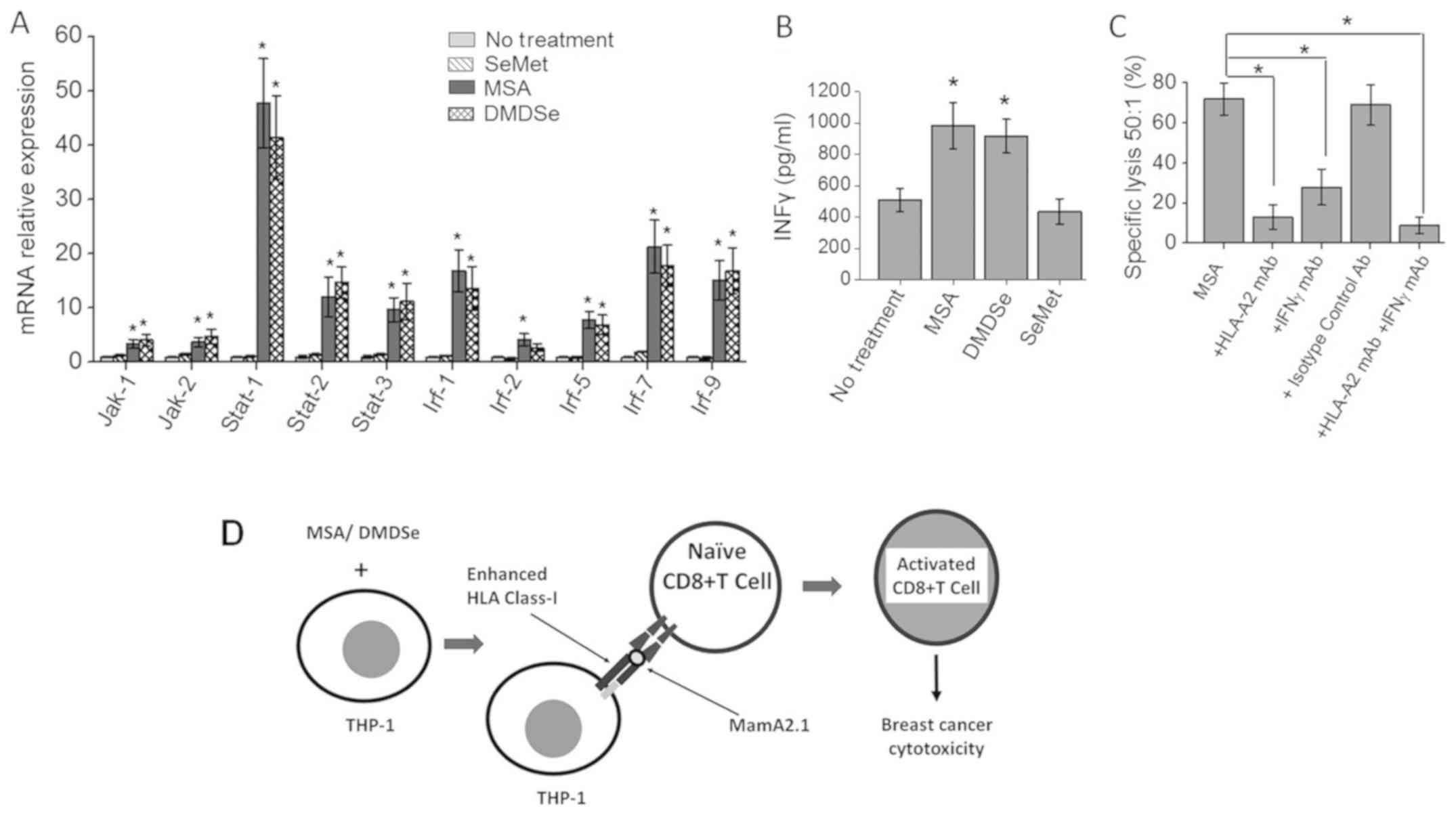 | Figure 4.Upregulation of IFNγ signaling in
CTLs following activation by the MamA2.1 peptide and co-stimulation
with selenocompound pre-treated THP-1 cells. (A) Expression of
components involved in the IFNγ signaling pathway molecules in
CD8+ T cells following activation with MamA2.1 peptide,
and in THP-1 cells pre-treated with SeMet (10 µM), MSA (2.5 µM) or
DMDSe (2.5 µM). (B) Quantity of IFNγ protein secreted in the
supernatant following the 4 h incubation of MamA2.1 activated CTLs
and AU565 cells at an E:T ratio of 50:1, as determined via ELISA.
(C) Cytotoxicity of CTLs on AU565 at an E:T ratio 50:1 upon
blocking of IFNγ and HLA-A2 by specific monoclonal antibodies. (D)
Schematic of the mechanism of action of MSA and DMDSe towards CTL
activation. Data are presented as the mean ± SEM (n=4). Statistical
significance between groups were assessed using Tukey HSD pair-wise
comparisons (A) and one-way ANOVA for multiple comparisons (B and
C) *P<0.05 vs. 0 µM. IFNγ, interferon-γ; CTLs, CD8+ T
lymphocytes; SeMet, selenomethionine; MSA, methylselenic acid;
DMDSe, methylselenol derivative dimethyl selenol; HLA, human
leukocyte antigen; Rel, relative; E:T, effector:target. |
Discussion
Peptide and DNA based cancer vaccines have many
advantages, including-being inexpensive, convenient acquisition of
clinical-grade peptides, easy administration, higher specificity,
potency due to their stronger compatibility with targeted proteins,
and greater safety with few side effects (20,21).
Previous studies from our laboratory have demonstrated safety and
immune efficiency of mammaglobin-A, breast cancer specific tumor
associated antigen, based DNA vaccine in breast cancer patients
(16,22). The efficiency of these vaccination
strategies have limited success as cancer cells downregulate
surface expression of HLA class I molecules causing loss of
identification of tumor cells by the host CTLs (23).
While the exact mechanism by which the trace element
selenium (Se) exerts anti-cancer potential is unknown, our current
study demonstrates that MSA and DMDSe, synthetic selenocompound and
precursor of methylselenol, induced MHC class I surface expression
on professional antigen presenting-like THP-1 cells. This data is
in line with studies from other laboratories which have
demonstrated enhanced HLA class I expression by these compounds in
other cancer cell lines such as melanoma cells. In contrast, the
SeMet based selenocompounds which do not produce methylselenol
failed to induce HLA class I surface expression. This hypothesis is
in accordance to results by Hagemann-Jensen et al, where in,
they have shown that MSA and DMDSe mediated upregulation of NKG2D
receptor ligands (19).
The antigenic peptides loaded on HLA class I
molecules and identified by CD8+ T lymphocytes generally
originate from the degradation of intracellular proteins by
proteasomes and translocation to the lumen of the endoplasmic
reticulum (ER) by the transporter associated with antigen
processing (TAP)1/2 heterodimeric complex. Defects in this
antigen-processing machinery and, in particular, in TAP subunits,
have been described as a major mechanism used by several tumors to
escape from CTL attack (24).
Evidence for antitumor CTL was provided by isolation of
tumor-specific CTL from peripheral blood or tumor tissue of
patients with diverse cancers, such as melanoma and lung carcinoma
(25). The IFNγ mediated
pro-inflammatory mileu is critical for the priming and
effector responses by CTLs following antigen presentation by HLA
class I molecules (26). The CTLs
can lyse target cells via the perforin granule exocytosis by
the IFNγ mediated JAK/STAT pathway. In our current study we
demonstrated that the induction of HLA-A2 by MSA and DMDSe was
associated with transcriptional upregulation of components of the
APM, including proteasomal subunits and components of the peptide
loading complex, along with upregulation of IFNγ signaling, such as
the upregulation of JAK/STAT/IRF molecules.
Our current study is limited to testing HLA class I
expression in mononuclear tumor-infiltrating antigen presenting
macrophage-like cells. However, the exact efficiency of
selenocompounds towards HLA expression on cancer cells could not be
tested in our current in vitro system. In an in vitro
system cancer cell lines already had enhanced cell surface
expression of HLA class I molecules. To directly test this effect
of selenocompounds on cancer cells, would require in vivo
based studies in murine or other small animal tumor models. Further
we have currently utilized peptide-based model to study adjuvant
effect of selenocompounds, however, future studies with Mam-A
DNA-vaccine based model should be utilized to study the adjuvant
effect of selenocompounds on DNA-based anti-cancer vaccines. Future
studies utilizing in vivo model will provided a stronger
evidence for human clinical application.
In conclusion, our data provides a new adjuvant
approach to further potentiate anti-cancer vaccine strategies.
Further, we have demonstrated that MSA and DMDSe could upregulate
the MHC class I expression in cancer cells which would enable
anti-cancer vaccine induced activated CTLs to inhibit tumor
immune-evasion and promote cancer immune-elimination. Therefore, we
think novel anti-cancer strategies utilizing inclusion of MSA and
DMDSe will improve vaccine therapeutic outcomes by enhancing CTL
mediated immune-surveillance and cancer cell elimination.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Institutes of Health (grant no. NIH-5U54CA163066). The current
study was also supported by the Veterans Affairs Merit Reviews
(grant no. 1I01BX002196) and the National Institutes of Health
(grant nos. R01-DK069921 and P30-DK114809).
Availability of data and materials
The datasets used and/or analyzed are available from
the corresponding author on reasonable request.
Authors' contributions
VT conceived the present study. DB, MZ, MTI, RZ and
VT designed the experiments. DB, MZ and VT performed the
experiments. DB, MZ, RZ and VT analyzed the data. DB, MZ, MTI, RZ
and VT wrote the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beatty GL and Gladney WL: Immune escape
mechanisms as a guide for cancer immunotherapy. Clin Cancer Res.
21:687–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaneko K, Ishigami S, Kijima Y, Funasako
Y, Hirata M, Okumura H, Shinchi H, Koriyama C, Ueno S, Yoshinaka H
and Natsugoe S: Clinical implication of HLA class I expression in
breast cancer. BMC Cancer. 11:4542011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng H and Combs GF Jr: Selenium as an
anticancer nutrient: Roles in cell proliferation and tumor cell
invasion. J Nutr Biochem. 19:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark LC, Combs GF Jr, Turnbull BW, Slate
EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG,
et al: Effects of selenium supplementation for cancer prevention in
patients with carcinoma of the skin. A randomized controlled trial.
Nutritional Prevention of Cancer Study Group. JAMA. 276:1957–1963.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ip C, Thompson HJ, Zhu Z and Ganther HE:
In vitro and in vivo studies of methylseleninic acid: Evidence that
a monomethylated selenium metabolite is critical for cancer
chemoprevention. Cancer Res. 60:2882–2886. 2000.PubMed/NCBI
|
|
7
|
Suzuki KT, Kurasaki K and Suzuki N:
Selenocysteine beta-lyase and methylselenol demethylase in the
metabolism of Se-methylated selenocompounds into selenide. Biochim
Biophys Acta. 1770:1053–1061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi Y, Fu X, Xiong Z, Zhang H, Hill SM,
Rowan BG and Dong Y: Methylseleninic acid enhances paclitaxel
efficacy for the treatment of triple-negative breast cancer. PLoS
One. 7:e315392012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demaria M, Giorgi C, Lebiedzinska M,
Esposito G, D'Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE,
Watson CJ, et al: A STAT3-mediated metabolic switch is involved in
tumour transformation and STAT3 addiction. Aging (Albany NY).
2:823–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mikhitarian K, Gillanders WE, Almeida JS,
Hebert Martin R, Varela JC, Metcalf JS, Cole DJ and Mitas M: An
innovative microarray strategy identities informative molecular
markers for the detection of micrometastatic breast cancer. Clin
Cancer Res. 11:3697–3704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fleming TP and Watson MA: Mammaglobin, a
breast-specific gene, and its utility as a marker for breast
cancer. Ann NY Acad Sci. 923:78–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goedegebuure PS, Watson MA, Viehl CT and
Fleming TP: Mammaglobin-based strategies for treatment of breast
cancer. Curr Cancer Drug Targets. 4:531–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gillanders WE, Mikhitarian K, Hebert R,
Mauldin PD, Palesch Y, Walters C, Urist MM, Mann GB, Doherty G,
Herrmann VM, et al: Molecular detection of micrometastatic breast
cancer in histopathology-negative axillary lymph nodes correlates
with traditional predictors of prognosis: An interim analysis of a
prospective multi-institutional cohort study. Ann Surg.
239:828–840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jaramillo A, Narayanan K, Campbell LG,
Benshoff ND, Lybarger L, Hansen TH, Fleming TP, Dietz JR and
Mohanakumar T: Recognition of HLA-A2-restricted
mammaglobin-A-derived epitopes by CD8+ cytotoxic T
lymphocytes from breast cancer patients. Breast Cancer Res Treat.
88:29–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bharat A, Benshoff N, Fleming TP, Dietz
JR, Gillanders WE and Mohanakumar T: Characterization of the role
of CD8+ T cells in breast cancer immunity following
mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast
Cancer Res Treat. 110:453–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tiriveedhi V, Tucker N, Herndon J, Li L,
Sturmoski M, Ellis M, Ma C, Naughton M, Lockhart AC, Gao F, et al:
Safety and preliminary evidence of biologic efficacy of a
mammaglobin-a DNA vaccine in patients with stable metastatic breast
cancer. Clin Cancer Res. 20:5964–5975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai Z, Dong L, Song C, Zhang Y, Zhu C,
Zhang Y, Ling Q, Hoffmann PR, Li J, Huang Z and Li W:
Methylseleninic acid provided at nutritional selenium levels
inhibits angiogenesis by down-regulating integrin beta3 signaling.
Sci Rep. 7:94452017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagemann-Jensen M, Uhlenbrock F, Kehlet S,
Andresen L, Gabel-Jensen C, Ellgaard L, Gammelgaard B and Skov S:
The selenium metabolite methylselenol regulates the expression of
ligands that trigger immune activation through the lymphocyte
receptor NKG2D. J Biol Chem. 289:31576–31590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amara S and Tiriveedhi V: The five immune
forces impacting DNA-based cancer immunotherapeutic strategy. Int J
Mol Sci. 18:E6502017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slingluff CL Jr: The present and future of
peptide vaccines for cancer: Single or multiple, long or short,
alone or in combination? Cancer J. 17:343–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tiriveedhi V, Fleming TP, Goedegebuure PS,
Naughton M, Ma C, Lockhart C, Gao F, Gillanders WE and Mohanakumar
T: Mammaglobin-A cDNA vaccination of breast cancer patients induces
antigen-specific cytotoxic CD4+ ICOShi T cells. Breast
Cancer Res Treat. 138:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsunaga Y, Fukuma D, Hirata S, Fukushima
S, Haruta M, Ikeda T, Negishi I, Nishimura Y and Senju S:
Activation of antigen-specific cytotoxic T lymphocytes by beta
2-microglobulin or TAP1 gene disruption and the introduction of
recipient-matched MHC class I gene in allogeneic embryonic stem
cell-derived dendritic cells. J Immunol. 181:6635–6643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buonaguro L, Petrizzo A, Tornesello ML and
Buonaguro FM: Translating tumor antigens into cancer vaccines. Clin
Vaccine Immunol. 18:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhat P, Leggatt G, Waterhouse N and Frazer
IH: Interferon-γ derived from cytotoxic lymphocytes directly
enhances their motility and cytotoxicity. Cell Death Dis.
8:e28362017. View Article : Google Scholar : PubMed/NCBI
|